Submitted:
05 May 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design, setting and participants
2.2. Data collection
2.3. Variables of the study
2.4. Operational definition of Treatment outcomes.
2.5. Data Processing
2.6. Statistical analysis
3. Results
3.1. Characteristics of the study participants
3.2. Association of the treatment outcome with the period between the start and end of treatment and previous drug history
3.3. Factors associated with treatment outcome
3.4. Socio-demographics of TB patients that were followed up from baseline visits to treatment outcome visits
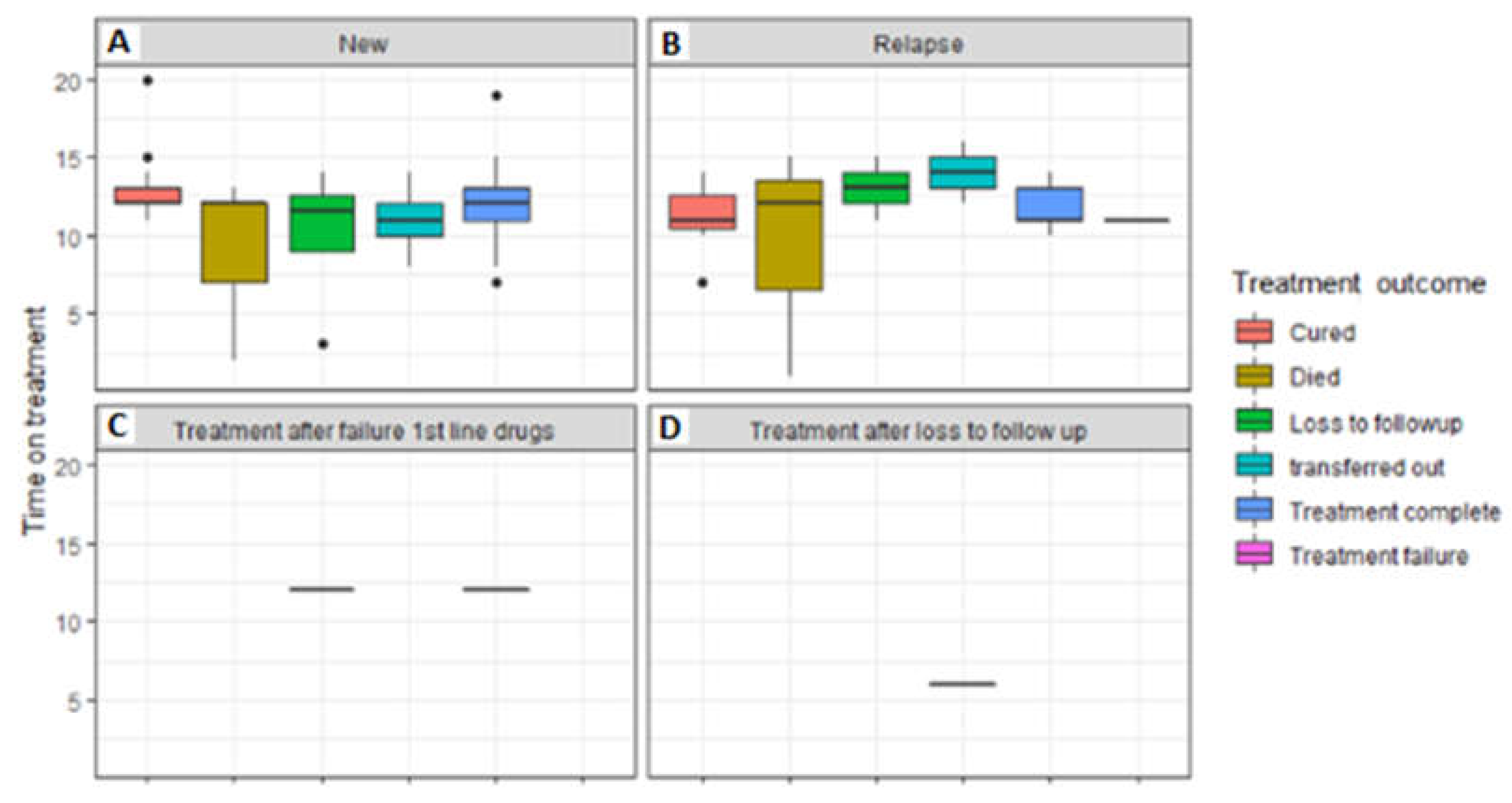
3.5. Treatment outcomes of TB patients that were followed up from baseline visits to treatment outcome visits
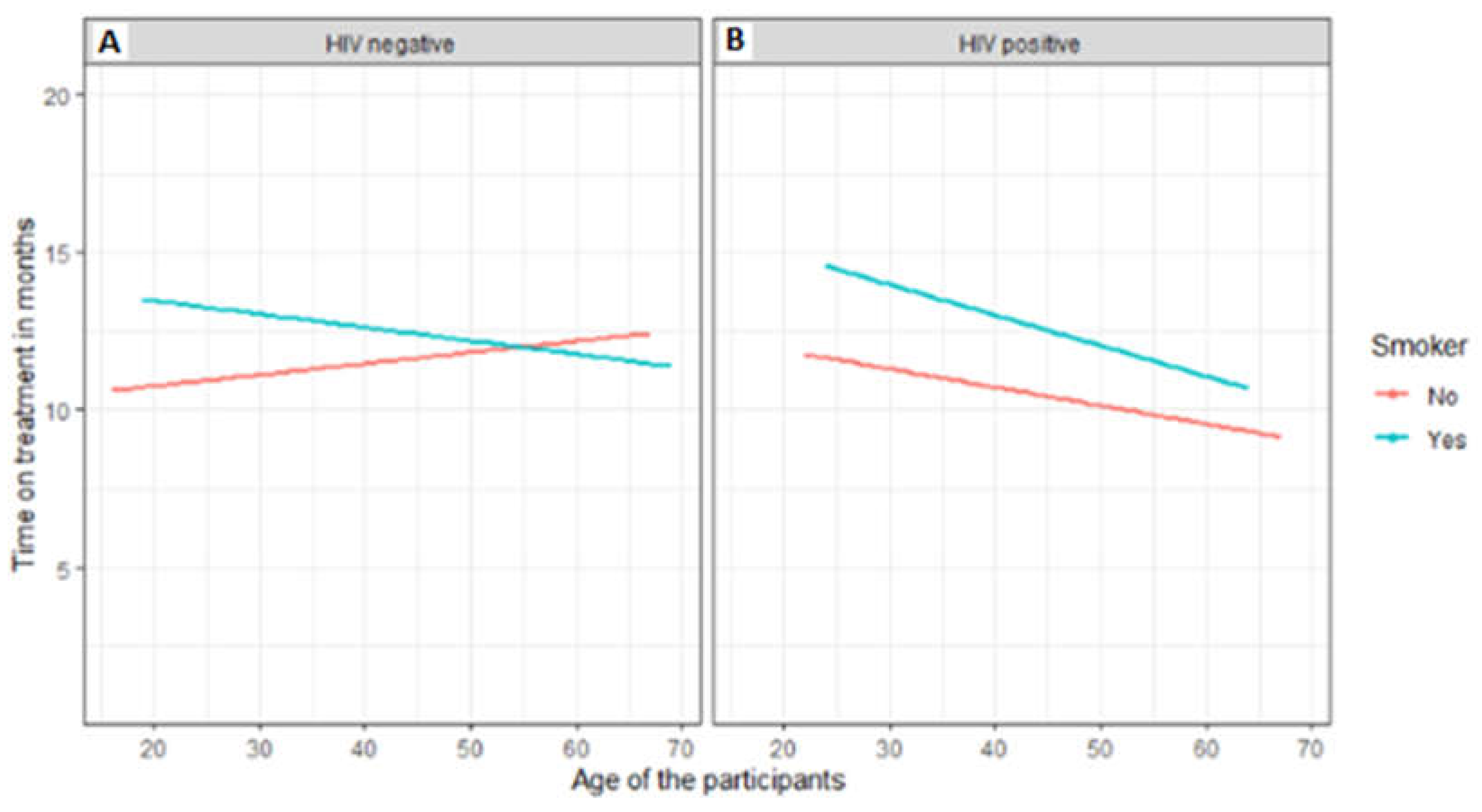
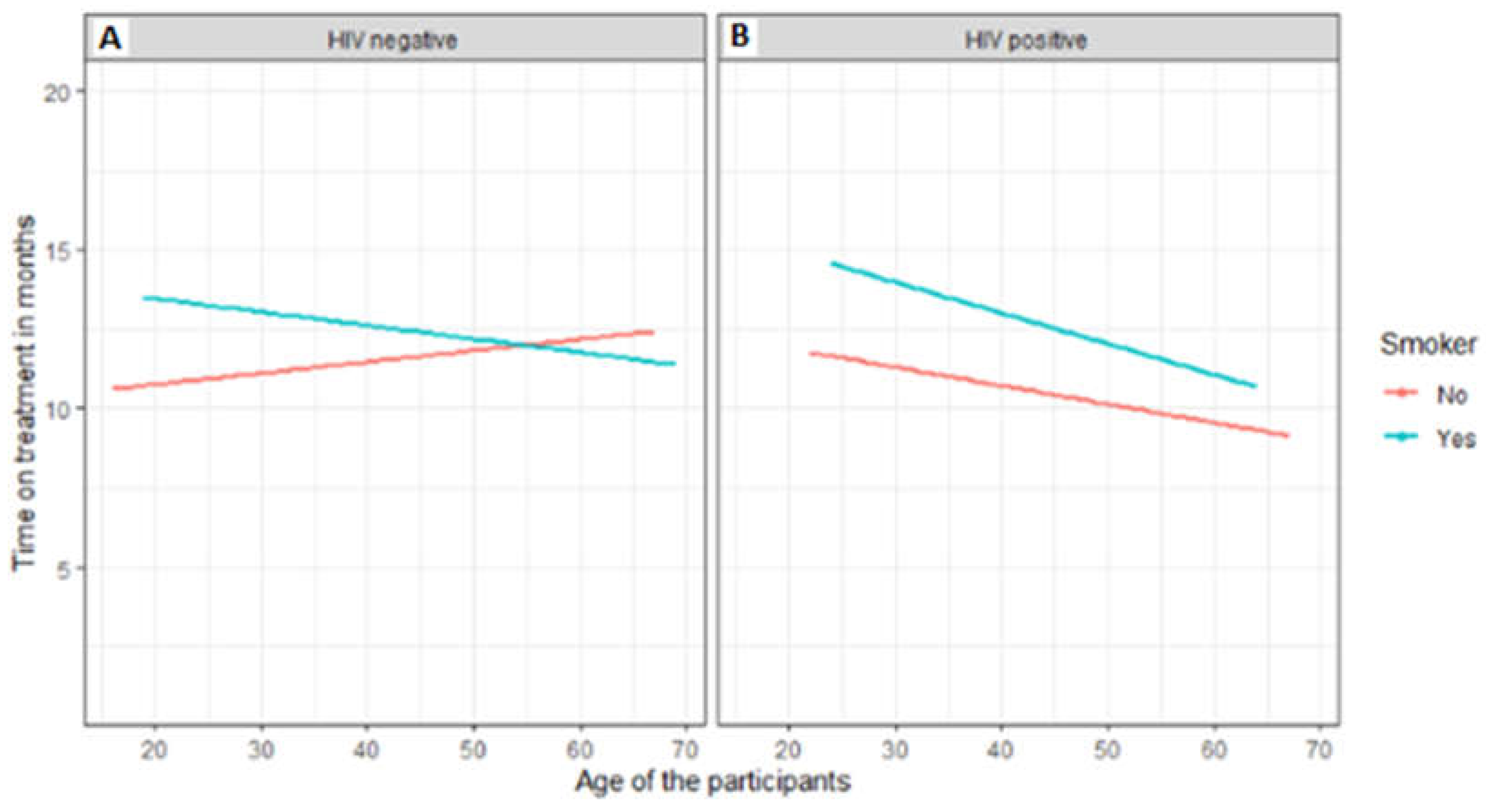
3.6. Factors associated with treatment outcomes of TB patients that were followed up from baseline visits to treatment outcome visits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pillay, S.; Magula, N.P. Treatment outcomes of Gene Xpert positive tuberculosis patients in KwaMashu Community Health Centre, KwaZulu-Natal, South Africa: A retrospective review. S Afr. J. Infect. Dis. 2021, 36, a217. [Google Scholar] [CrossRef] [PubMed]
- Faye, L.M.; Hosu, M.C.; Vasaikar, S.; Dippenaar, A.; Oostvogels, S.; Warren, R.M.; Apalata, T. Spatial Distribution of Drug-Resistant Mycobacterium tuberculosis Infections in Rural Eastern Cape Province of South Africa. Pathogens 2023, 12, 475. [Google Scholar] [CrossRef] [PubMed]
- Ndjeka, N.; Campbell, J.R.; Meintjes, G.; Maartens, G.; Schaaf, H.S.; Hughes, J.; Padanilam, X.; Reuter, A.; Romero, R.; Ismail, F.; Enwerem, M. Treatment outcomes 24 months after initiating short, all-oral bedaquiline-containing or injectable-containing rifampicin-resistant tuberculosis treatment regimens in South Africa: a retrospective cohort study. Lancet Infect. Dis. 2022, 22, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. (2014). Global tuberculosis report 2014. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/137094 (accessed on 20 January 2022).
- Seung, K.J. , Keshavjee, S. and Rich, M.L. Multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis. Cold Spring Harbor perspectives in medicine 2015, 5, a017863. [Google Scholar] [CrossRef] [PubMed]
- Debash, H.; Nega, J.; Bisetegn, H.; Tesfaw, G.; Feleke, D.G.; Ebrahim, H.; Gedefie, A.; Tilahun, M.; Mohammed, O.; Alemayehu, E.; Belete, M.A. Tuberculosis Treatment Outcomes and Its Predictors among Tuberculosis Patients Registered at Tefera Hailu Memorial General Hospital, Sekota Town, Northeast Ethiopia: A Seven-Year Retrospective Study. Can. J. Infect. Dis. Med. Microbiol. 2023, 4212312. [Google Scholar] [CrossRef] [PubMed]
- Lampalo, M.; Jukić, I.; Bingulac-Popović, J.; Safić Stanić, H.; Barišić, B.; Popović-Grle, S. The role of cigarette smoking and alcohol consumption in pulmonary tuberculosis development and recurrence. Acta Clinica Croatica, 2019, 58, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Louwagie, G.; Kanaan, M.; Morojele, N.K.; Van Zyl, A.; Moriarty, A.S.; Li, J.; Siddiqi, K.; Turner, A.; Mdege. N.D.; Omole, O.B.; Tumbo, J.; Bachmann, M.; Parrott, S.; Ayo-Yusuf, O.A. Effect of a brief motivational interview and text message intervention targeting tobacco smoking, alcohol use and medication adherence to improve tuberculosis treatment outcomes in adult patients with tuberculosis: a multicentre, randomised controlled trial of the ProLife programme in South Africa. BMJ Open 2022, 12, e056496. [Google Scholar] [PubMed]
- Wakjira, M.K.; Sandy, P.T.; MavhanduMudzusi, A.H. Treatment outcomes of patients with MDR-TB and its determinants at referral hospitals in Ethiopia. PLoS ONE, 2022, 17, e0262318. [Google Scholar] [CrossRef]
- Wagnew, F.; Alene, K.A.; Kelly, M.; Gray, D. The effect of undernutrition on sputum culture conversion and treatment outcomes among people with multidrug-resistant tuberculosis: A systematic review and meta-analysis. Inter J. Infect. Dis. 2022, 127, 93–105. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714 (accessed on 21 January 2023).
- Zenbaba, D.; Bonsa, M.; Sahiledengle, B. Trends of unsuccessful treatment outcomes and associated factors among tuberculosis patients in public hospitals of Bale Zone, Southeast Ethiopia: A 5-year retrospective study. Heliyon, 2021, 7, e07982. [Google Scholar] [CrossRef]
- Podewils, L.J.; Gler, M.T.S.; Quelapio, M.I.; Chen, M.P. Patterns of treatment interruption among patients with multidrug-resistant TB (MDR TB) and association with interim and final treatment outcomes. PLoS One, 2013, 8, e70064. [Google Scholar] [CrossRef]
- Kurbatova, E.; Caoili, J.C.; Contreras, C.; Ershova, J.; Dalton, T.; Kvasnovsky, C. Loss to follow-up from multidrug-resistant tuberculosis treatment and acquired drug resistance. Presented at: 45th Union World Conference on Lung Health 2014; 2014, Oct 28–Nov 1; Barcelona, Spain.
- Nellums, L.B.; Rustage, K.; Hargreaves, S.; Friedland, J.S. Multidrug-resistant tuberculosis treatment adherence in migrants: a systematic review and meta-analysis. BMC Medicine, 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Toczek, A.; Cox, H.; Du Cros, P.; Cooke, G.; Ford, N. Strategies for reducing treatment default in drug-resistant tuberculosis: systematic review and meta-analysis. Inter. J. Tuberc. Lung Dis. 2013, 17, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Calver, A.D.; Murray, M.; Strauss, O.J.; Streicher, E.M.; Hanekom, M.; Liversage, T.; Masibi, M.; Van Helden, P.D.; Warren, R.M.; Victor, T.C. Emergence of increased resistance and extensively drug-resistant tuberculosis despite treatment adherence, South Africa. Emerg. Infect. Dis. 2010, 16, 264. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.S.; Richardson, J.; Moodley, P.; Moodley, S.; Babaria, P.; Ramtahal, M.; Heysell, S.K.; Li, X.; Moll, A.P.; Friedland, G.; Sturm, A.W. Increasing drug resistance in extensively drug-resistant tuberculosis, South Africa. Emerg. Infect. Dis. 2011, 17. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Keshavjee, S.; Gelmanova, I.Y.; Atwood, S.; Franke, M.F.; Mishustin, S.P.; Strelis, A.K.; Andreev, Y.G.; Pasechnikov, A.D.; Barnashov, A.; Tonkel, T.P. Development of extensively drug-resistant tuberculosis during multidrug-resistant tuberculosis treatment. Am. J. Respir Crit Care Med. 2010, 182, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Muluye, A.B.; Kebamo, S.; Teklie, T.; Alemkere, G. Poor treatment outcomes and its determinants among tuberculosis patients in selected health facilities in East Wollega, Western Ethiopia. PLoS ONE. 2018, 13, p.e0206227. [CrossRef] [PubMed]
- Stats SA. Census 2022; Stats SA: Pretoria, South Africa, 2022. Available online: http://www.statssa.gov.za/publications/P0302/Mid%20year%20estimates%2020 21_presentation.pdf (accessed on 21 April 2023).
- The South African NTCP, 2004. Available online: https://www.kznhealth.gov.za/tbguidelines.pdf (accessed on 21 April 2023).
- Agyare, S.A.; Osei, F.A.; Odoom, S.F.; Mensah, N.K.; Amanor, E.; Martyn-Dickens, C.; Owusu-Ansah, M.; Mohammed, A.; Yeboah, E.O. Treatment Outcomes and Associated Factors in Tuberculosis Patients at Atwima Nwabiagya District, Ashanti Region, Ghana: A Ten-Year Retrospective Study. Tuberc. Res. Treat., 2021, 952806. [Google Scholar] [CrossRef]
- Amede. P.O.; Adedire, E.; Usman, A.; Ameh, C.A.; Umar, F.S.; Umeokonkwo, C.D.; Balogun, M.S. Drug-susceptible tuberculosis treatment outcomes and its associated factors among inmates in prison settings in Bauchi State, Nigeria, 2014–2018. PLoS ONE, 2022, 17, e0270819. [Google Scholar] [CrossRef]
- WHO Global Tuberculosis Report. Geneva: World Health Organization. 2022. License: CC BY-NC-SA 3.0 IGO. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed on 28 March 2023).
- Budgell, E.; Evans, D.; Schnippel, K.; Ive, P.; Long, L.; Rosen, S. Outcomes of treatment of drug-susceptible tuberculosis at public sector primary healthcare clinics in Johannesburg, South Africa: A retrospective cohort study. S Afr Med J. 2016, 106, 1002–1009. [Google Scholar] [CrossRef]
- Osório, D.; Munyangaju, I.; Nacarapa, E.; Nhangave, A.V.; Ramos-Rincon, J.M. Predictors of unfavourable tuberculosis treatment outcome in Bilene District, Gaza Province, Mozambique: A retrospective analysis, 2016-2019. S. Afr. Med. J. 2022, 112, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Daka, S.; Matsuoka, Y.; Ota, M.; Hirao, S.; Phiri, A. Re-evaluated treatment outcomes of bacteriologically positive TB patients registered at a clinic in Lusaka, Zambia in 2018. Public Health Action, 2021, 11, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Matambo, R.; Takarinda, K.C.; Thekkur, P.; Sandy, C.; Mharakurwa, S.; Makoni, T.; Ncube, R.; Charambira, K.; Zishiri, C.; Ngwenya, M.; Nyathi, S. Treatment outcomes of multi drug resistant and rifampicin resistant Tuberculosis in Zimbabwe: a cohort analysis of patients initiated on treatment during 2010 to 2015. PLoS ONE 2020, 15, e0230848. [Google Scholar] [CrossRef] [PubMed]
- Mamo, A.; Mama, M.; Solomon, D.; Mohammed, M. Treatment outcomes and predictors among tuberculosis patients at Madda Walabu University Goba Referral Hospital, Southeast Ethiopia. Infect. Drug Resist. 2021, 13, 4763–4771. [Google Scholar] [CrossRef] [PubMed]
- Berry, K.M.; Rodriguez, C.A.; Berhanu, R.H.; Ismail, N.; Mvusi, L.; Long, L.; Evans, D. Treatment outcomes among children, adolescents, and adults on treatment for tuberculosis in two metropolitan municipalities in Gauteng Province, South Africa. BMC Public Health, 2019, 19, 973. [Google Scholar] [CrossRef] [PubMed]
- Nugus, G.G.; Irena, M.E. Determinants of active tuberculosis occurrences after ART initiation among adult HIV-positive clients in West Showa Zone Public Hospitals, Ethiopia: a case-control study. Adv. Public Health, 2020, 237928. [Google Scholar] [CrossRef]
- Getaneh, T.; Negesse, A.; Dessie, G. , Desta, M. The impact of tuberculosis co-infection on virological failure among adults living with HIV in Ethiopia: a systematic review and meta-analysis. J. Clin. Tuberc. Other Mycobact. Dis., 2022, 100310. [Google Scholar] [CrossRef]
- Monde, N., Zulu, M., Tembo, M., Handema, R., Munyeme, M.; Malama, S. Drug Resistant Tuberculosis in the Northern Region of Zambia: A Retrospective Study. Front. Trop. Dis. 2021, 2, 735028. [CrossRef]
- van de Water, B.J.; Fulcher, I.; Cilliers, S.; Meyer, N.; Wilson, M.; Young, C.; Gaunt, Y.; le Roux, K. Association of HIV infection and antiretroviral therapy with the occurrence of an unfavorable TB treatment outcome in a rural district hospital in Eastern Cape, South Africa: A retrospective cohort study. PLoS ONE, 2022, 17, e0266082. [Google Scholar] [CrossRef]
- Sariem, C.N.; Odumosu, P.; Dapar, M.P.; Musa, J.; Ibrahim, L.; Aguiyi, J. Tuberculosis treatment outcomes: a fifteen-year retrospective study in Jos-North and Mangu, Plateau State, North-Central Nigeria. BMC Public Health, 2020, 20, 1224. [Google Scholar] [CrossRef]
- Khan, F.U.; Rehman, A.u.; Khan, F.U.; Hayat, K.; Khan, A.; Ahmad, N.; Chang, J.; Malik, U.R.; Fang, Y. Assessment of Factors Associated with Unfavorable Outcomes among Drug-Resistant TB Patients: A 6-Year Retrospective Study from Pakistan. Int. J. Environ. Res. Public Health, 2022, 19, 1574. [Google Scholar] [CrossRef] [PubMed]
- Kamara, R.F.; Saunders, M.J.; Sahr, F.; Losa-Garcia, J.E.; Foray, L.; Davies, G.; Wingfield, T. Social and health factors associated with adverse treatment outcomes among people with multidrug-resistant tuberculosis in Sierra Leone: a national, retrospective cohort study. Lancet Glob. Health, 2022, 10, e543–e554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Meng, Q.; Chen, S.; Zhang, M.; Chen, B.; Wu, B.; Yan, G.; Wang, X.; Jia, Z. Treatment outcomes of multidrug-resistant tuberculosis patients in Zhejiang, China, 2009–2013. Clin. Microbiol. Infect, 2018, 24, 381–388. [Google Scholar] [CrossRef] [PubMed]
- South African National Department of Health TB Program. The First National TB Prevalence Survey. Pretoria, 2018. p. 28. Available online: https://www.nicd.ac.za/wp-content/uploads/2021/02/TB-Prevalence-survey-report_A4_SA_TPS-Short_Feb-2021.pdf (accessed on 28 April 2023).
- Dodd, P.J.; Looker, C.; Plumb, I.; Bond, V.; Schaap, A.; Shanaube, K.; Muyoyeta, M.; Vynnycky, E.; Godfrey-Faussett, P.; Corbett, E.L.; Beyers, N. Age- and Sex-specific social contact patterns and incidence of Mycobacterium tuberculosis infection. Am J. Epidemiol. 2016, 183, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Auld, A.F.; Tuho, M.Z.; Ekra, K.A.; Kouakou, J.; Shiraishi, R.W.; Adjorlolo-Johnson, G. , Marlink, R.; Ellerbrock, T.V. Tuberculosis in human immunodeficiency virus-infected children starting antiretroviral therapy in Cote d'Ivoire. Inter J. Tuberc. Lung Dis., 2014, 18, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Bor, J.; Rosen, S.; Chimbindi, N.; Haber, N.; Herbst, K.; Mutevedzi, T.; Tanser, F.; Pillay, D.; Bärnighausen, T. Mass HIV treatment and sex disparities in life expectancy: demographic surveillance in rural South Africa. PLoS Med. 2015, 12, e1001905. [Google Scholar] [CrossRef]
- Baluku, J.B.; Nakazibwe, B.; Naloka, J.; Nabwana, M.; Mwanja, S.; Mulwana, R.; Sempiira, M.; Nassozi, S.; Babirye, F.; Namugenyi, C.; Ntambi, S. Treatment outcomes of drug resistant tuberculosis patients with multiple poor prognostic indicators in Uganda: a countrywide 5-year retrospective study. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 23, 100221. [Google Scholar] [CrossRef]
- Johnston, J.C.; Shahidi, N.C.; Sadatsafavi, M.; Fitzgerald, J.M. Treatment Outcomes of Multidrug-Resistant Tuberculosis: A Systematic Review and Meta-Analysis. PLoS ONE, 2009, 4, e6914. [Google Scholar] [CrossRef]
- Romaino, S.M.N.; Naing, N.N.; MJ, M.Z. Factors associated with tuberculosis treatment success among tuberculosis and human immunodeficiency virus co-infected patients in Kelantan. Med. J. Malaysia, 2022, 77, 696–703. [Google Scholar]
- El Hamdouni, M.; Bourkadi, J.E.; Benamor, J.; Hassar, M.; Cherrah, Y.; Ahid, S. Treatment outcomes of drug-resistant tuberculosis patients in Morocco: Multi-centric prospective study. BMC Infect. Dis. 2019, 19, 316. [Google Scholar] [CrossRef]
- Patel, S.V.; Nimavat, K.B.; Alpesh, P.B.; Shukla, L.K.; Shringarpure, K.S.; Mehta, K.G.; Joshi, C.C. Treatment outcome among cases of multidrug-resistant tuberculosis (MDR TB) in Western India: A prospective study. J. Infect. Public Health, 2015, 9, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Chaaba, E.; Bwembya, J.; Nyambe, E.; Kumar, R.; Thior, I.; Seraphine, K.; Chongwe, G.; Makwambeni, V.; Musonda, V.; Kasese-Chanda, P.; Mwinga, A. Mortality among persons receiving tuberculosis treatment in Itezhi-Tezhi District of Zambia: A retrospective cohort study. PLOS Glob. Public Health, 2023, 3, e0001234. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Ghazali, S.M.; Kee, C.C.; Lim, K.K.; Chan, Y.Y.; The, H.C.; Yusoff, A.F.M.; Kaur, G.; Zain, Z.M.; Mohamad, M.H.N. Epidemiology of smoking among Malaysian adult males: prevalence and associated factors. BMC Public Health. 2013, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Sulaiman, S.A.S.; Hassali, M.A.; Khan, K.U.; Ming, L.C.; Mateen, O.; Ullah, M.O. Effect of smoking on treatment outcome among tuberculosis patients in Malaysia; a multicenter study. BMC Public Health, 2020, 20, 854. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. The health consequences of smoking-50 years of progress: a report of the Surgeon General. Atlanta, GA: USA. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014.
- Wang, E.Y.; Arrazola, R.A.; Mathema, B.; Ahluwalia, I.B.; Mase, S.R. The impact of smoking on tuberculosis treatment outcomes: a meta-analysis. Inter. J. Tuberc. Lung Dis., 2020, 24, 170–175. [Google Scholar] [CrossRef]
- Shin, S.S. , Modongo, C., Baik, Y., Allender, C., Lemmer, D., Colman, R.E., Engelthaler, D.M., Warren, R.M. and Zetola, N.M., 2018. Mixed Mycobacterium tuberculosis–strain infections are associated with poor treatment outcomes among patients with newly diagnosed tuberculosis, independent of pretreatment heteroresistance. J. Infect. Dis. 2018, 218, 1974–1982. [Google Scholar]
- Pereira, C.; Larsson, J.; Hjort, K.; Elf, J.; Andersson, D.I. The highly dynamic nature of bacterial heteroresistance impairs its clinical detection. Commun. Biol, 2021, 4, 521. [Google Scholar] [CrossRef]
- Cohen, T.; Chindelevitch, L.; Misra, R.; et al. Within-host heterogeneity of Mycobacterium tuberculosis infection is associated with poor early treatment response: a prospective cohort study. J Infect Dis. 2016, 213, 1796–1799. [Google Scholar] [CrossRef]
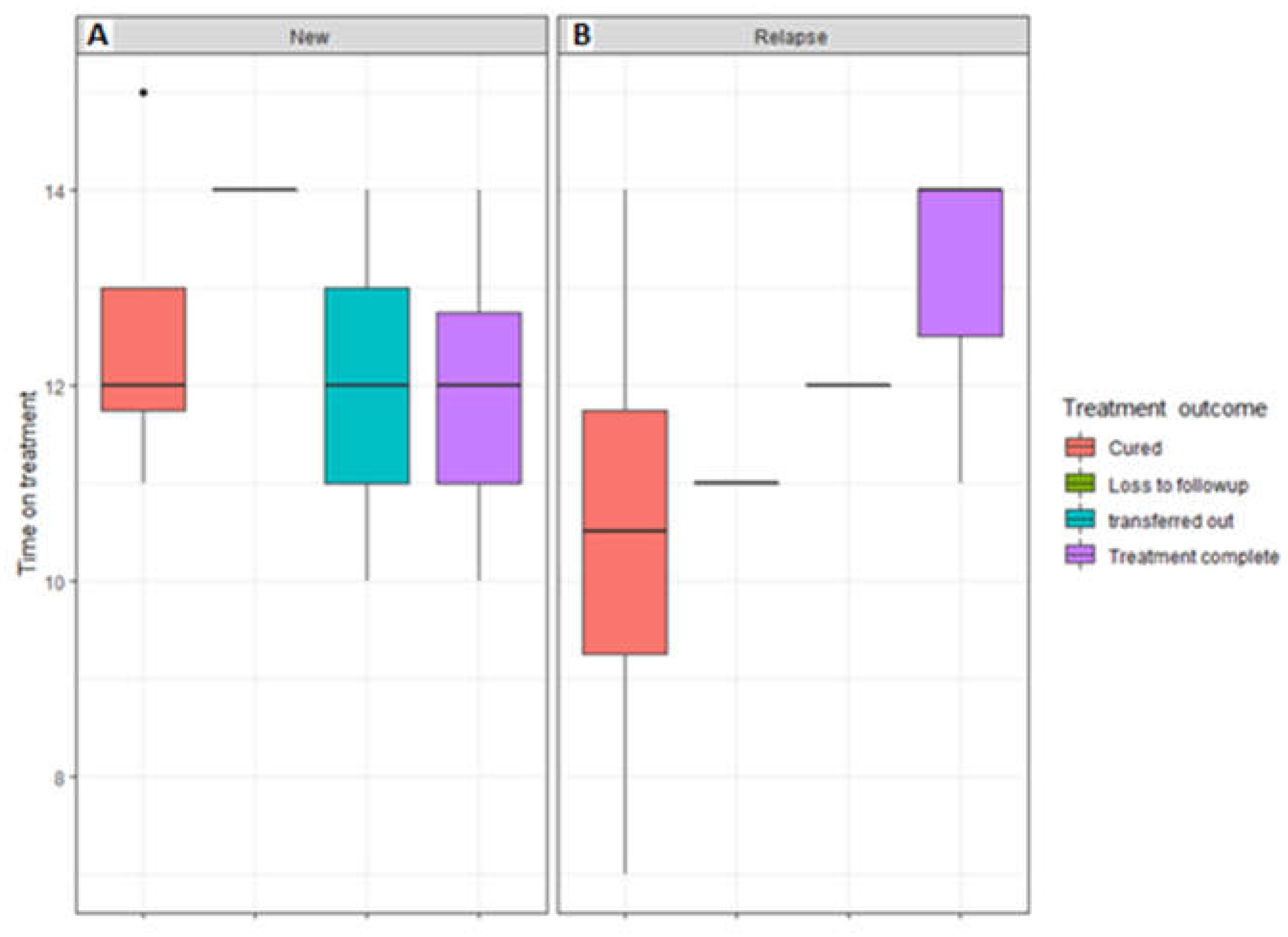
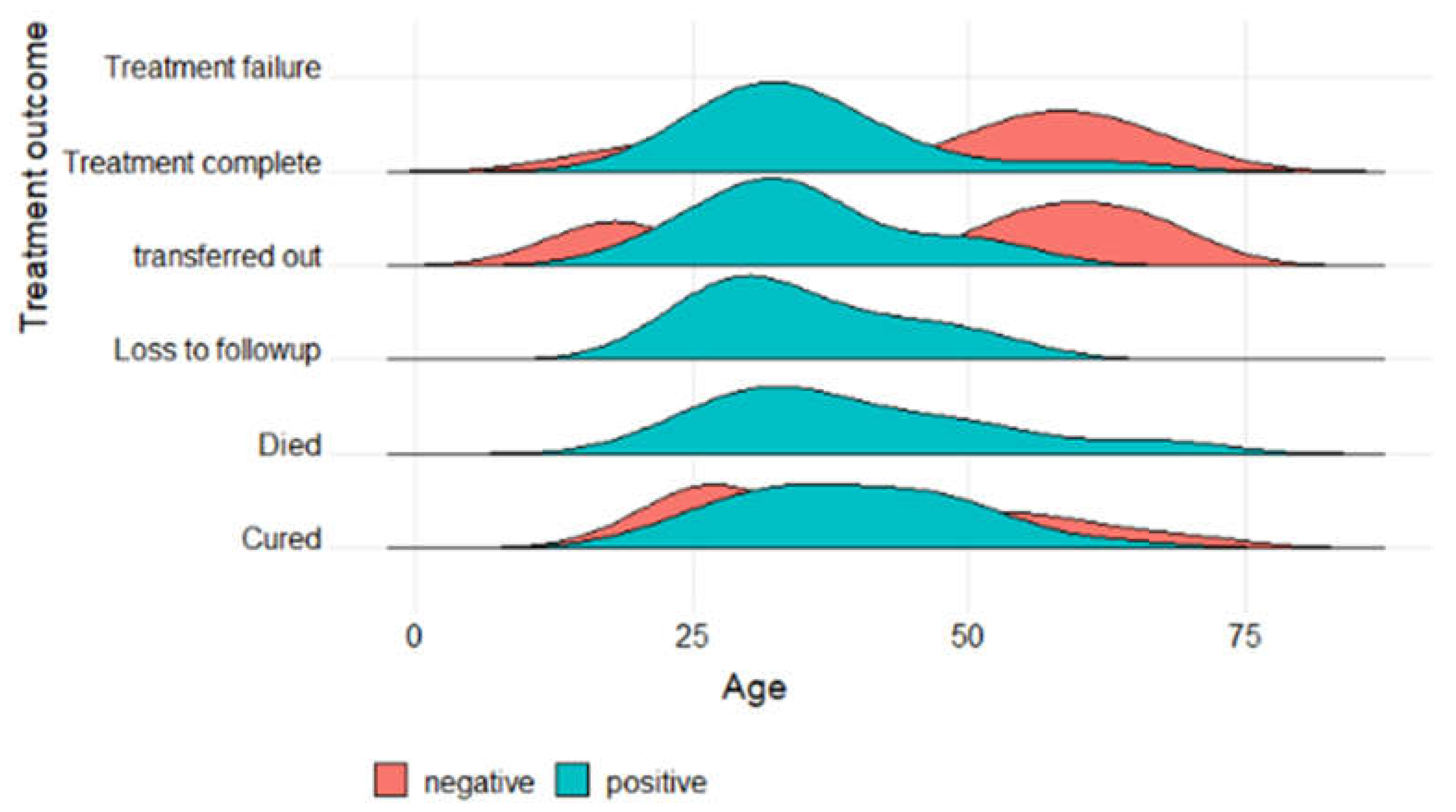
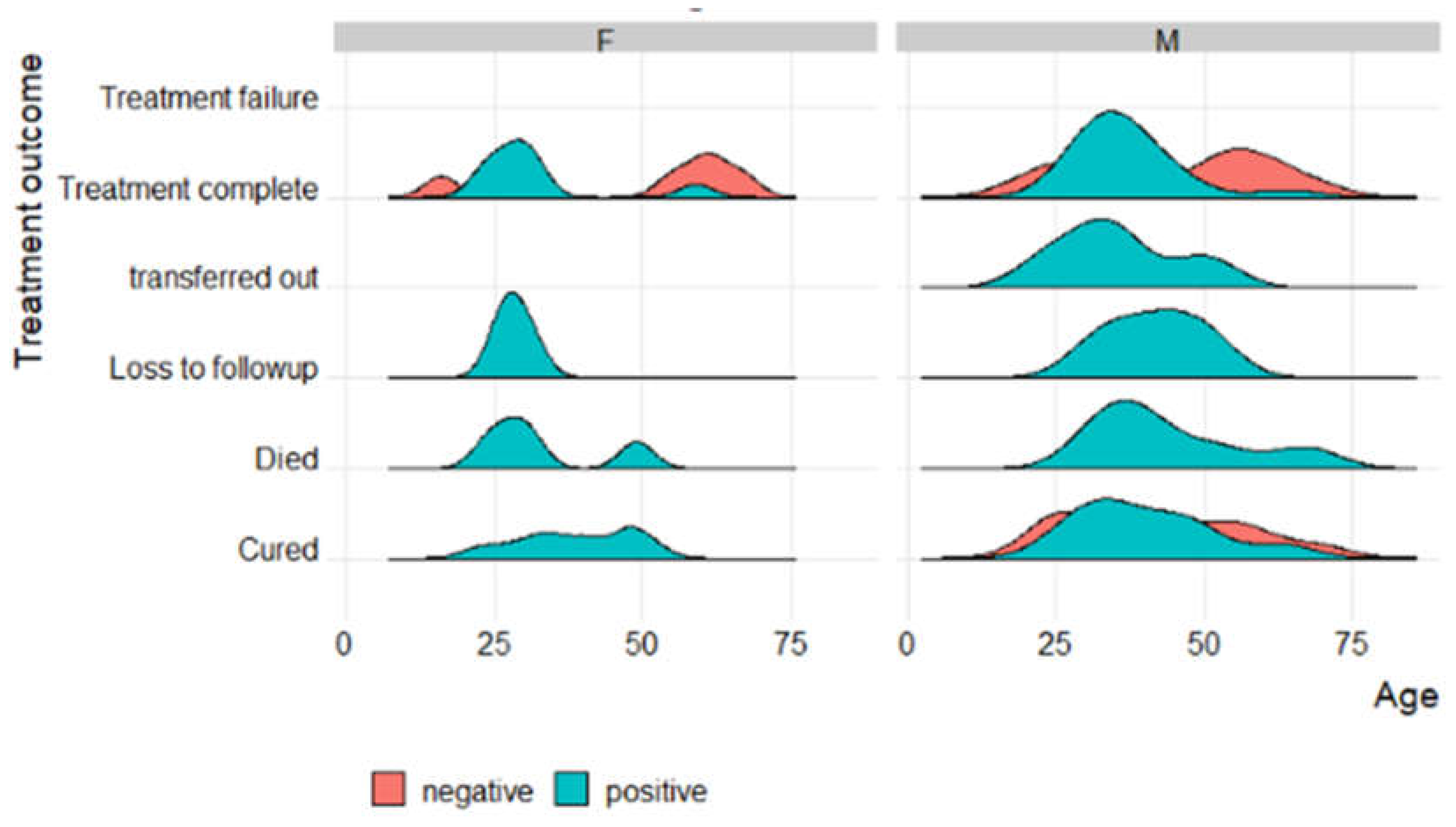
| Patient characteristics | Successful TB Treatment Outcome (N=281) Ave or No. (Min or %) |
Unsuccessful TB Treatment Outcome Ave or No. Min or % |
p-value | |
|---|---|---|---|---|
| Age (years) | 38.19 (14.96) | 36.3 (15.4) | 0.23 | |
| Gender (M and F) | 130 (46.3) | 59 (40.4) | 0.25 | |
| Period between TSD and TED | 326.5 (95.3) | 186 (82.6) | <0.001 | |
| Previous drug history | New PT1 PT2 UNK |
160 (56.9) 110 (39.1) 11 (3.9) 0 (0.0) |
63 (43.2) 57 (39.0) 25 (17.1) 1 (0.7) |
<0.001 |
| Type of resistance | ||||
| Mono-resistance Poly-resistance |
130 (46.3) 151 (53.7) |
71 (48.6) 75 (51.4) |
0.64 | |
| Type of TB drug resistance |
RR MDR Pre-XDR XDR INH-R |
|||
| 131 (46.6) 130 (46.3) 7 (2.5) 8 (2.8) 5 (1.8) |
74 (50.7) 51 (34.9) 12 (8.2) 8 (5.5) 1 (0.7) |
0.012 | ||
| HIV Status | ||||
| Positive Negative |
172 (61.2) 109 (38.8) |
97 (66.4) 49 (33.6) |
0.186 |
|
| Age category |
<=21 >21:<=40 >40:<=55 >55:<=75 >75:<=90 |
|||
| 27 (9.6) 151 (53.7) 56 (19.9) 40 (14.2) 5 (1.8) |
23 (15.8) 73 (50.0) 32(21.9) 17 (11.6) 1 (0.7) |
0.32 |
| TB treatment outcome | Odds ratio (95% CI) | Std err | Z | P>|z| |
|---|---|---|---|---|
| Treatment days | 0.984 (0.981-0.987) | 0.002 | -10.22 | 0.000 |
| Drug history | 1.842 (1.232-2.753) | 0.378 | 2.98 | 0.003 |
| _cons | 12.629 (4.651-34.289) | 6.436 | 4.98 | 0.000 |
| TB treatment outcome | Odds ratio (95% CI) | Std err | P>|z| |
|---|---|---|---|
| Treatment days | 0.983 (0.980-0.987) | 0.002 | -10.24 |
| Drug history | 1.841 (1.228-2.759) | 0.38 | 2.96 |
| Drug resistance | 1.339 (0.984-1.823) | 0.211 | 1.86 |
| _cons | 8.465 (2.893-24.770) | 4.637 | 3.9 |
| TB Treatment outcome | Odds ratio (95% CI) | Std error | Z | P>|z| |
|---|---|---|---|---|
| Treatment days | 0.984 (0.980-0.986) | 0.002 | -10.27 | 0.0000 |
| HIV status | 1.415 (0.787-2.544) | 0.424 | 1.16 | 0.246 |
| Drug resistance | 1.246 (0.825-1.880) | 0.262 | 1.04 | 0.296 |
| Resistance type | 1.266 (0.634-2.529) | 0.447 | 0.67 | 0.503 |
| Drug history | 1.984 (1.296-3.036) | 0.431 | 3.15 | 0.002 |
| Age | 1.025 (0.974-1.079) | 0.027 | 0.95 | 0.340 |
| Gender | 0.708 (0.410-1.223) | 0.198 | -1.24 | 0.126 |
| Age category | 0.963 (0.918-1.011) | 0.024 | -1.53 | 0.126 |
| _cons | 11.109 (1.934-63.8111) | 9.991 | 2.70 |
| Variable | Frequency (N) | Percentage (%) | |
|---|---|---|---|
| Age | 16 – 35 years 36 – 60 years ≥ 60 years |
52 40 9 |
51.5 39.6 8.9 |
| Sex | Male Female |
65 36 |
64.4 35.6 |
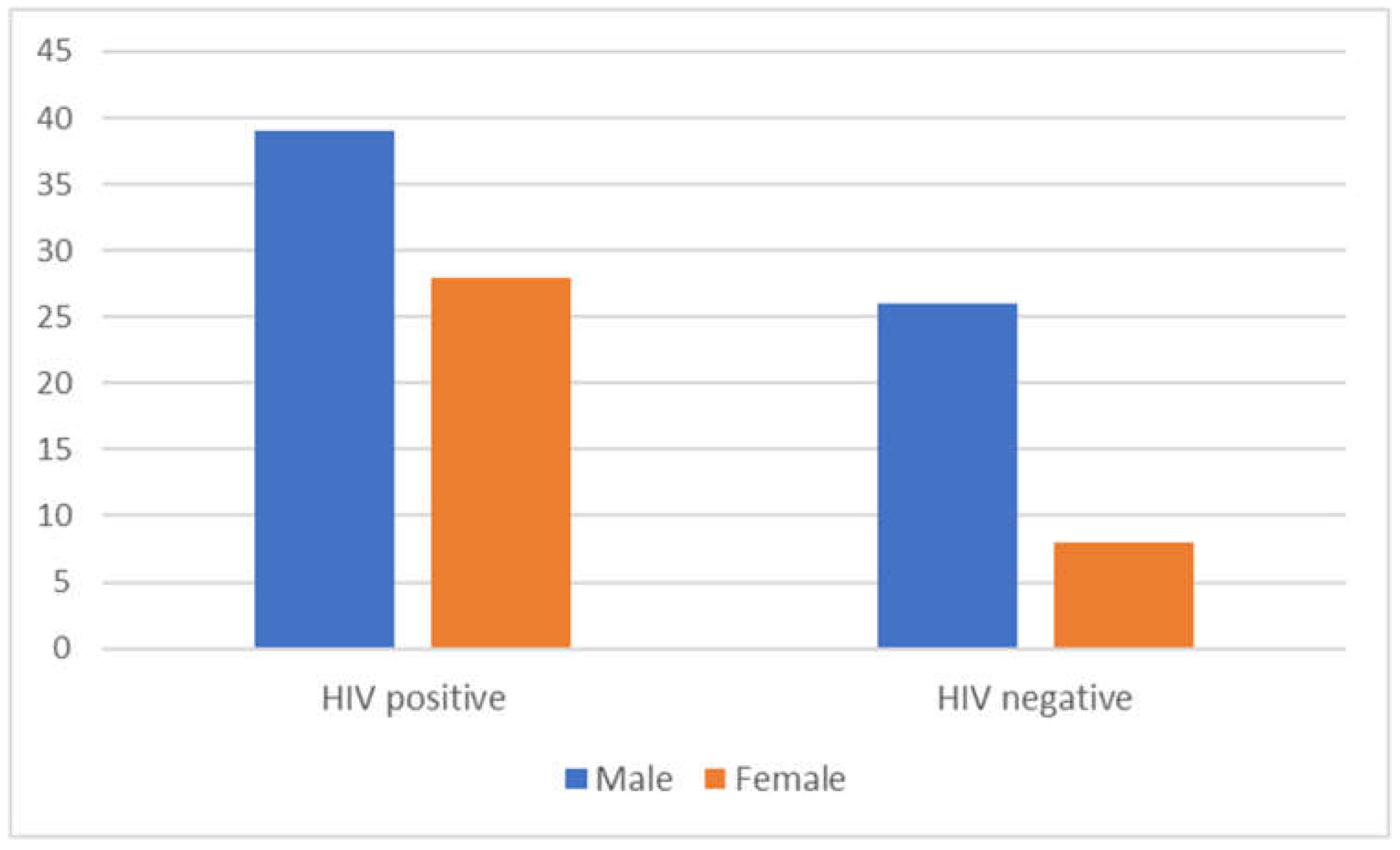
| HIV status | Positive Negative |
67 34 |
66.3 33.7 |
| Social History | Smoker Non-smoker |
35 66 |
34.7 65.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





