Submitted:
06 May 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Apple Mature MicroRNAs and ACLSV Genomic Data Source
2.2. Analysis of Multiple mdm-miRNA Target-Pairs in ACLSV Genome
2.3. miRanda
2.4. RNA22
2.5. TAPIR
2.6. psRNATarget
2.7. Discovering Apple mdm-miRNA-Target Interactions
2.8. RNAfold
2.9. RNAcofold
2.10. Statistical Analysis
3. Results
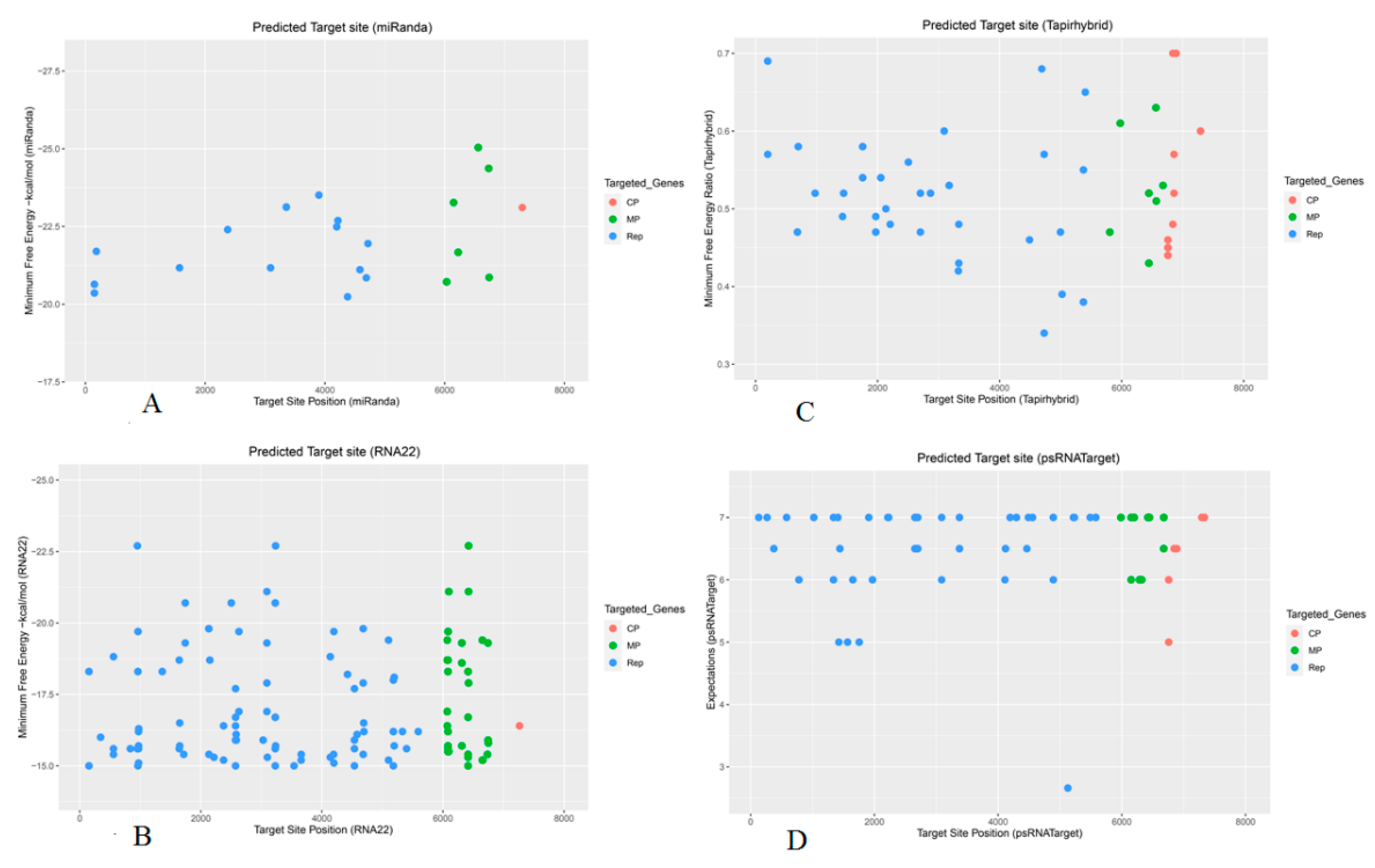
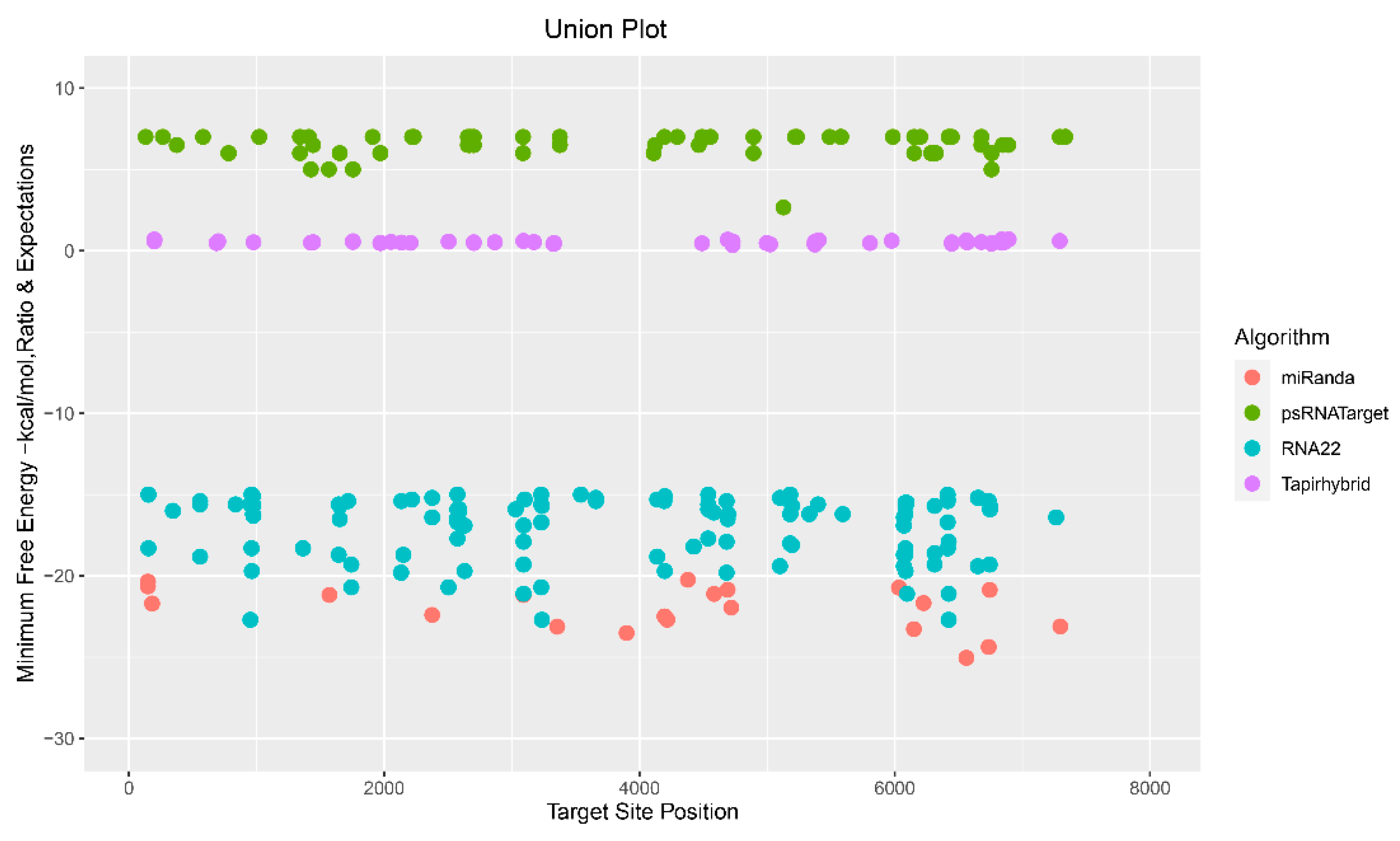
3.1. Apple Genome-Encoded mdm-miRNAs Targeting ACLSV Genome
3.2. Apple mdm-miRNAs Targeting ORF1 that Ecodes Replication-asscoiated Protein
3.3. Apple mdm- miRNAs Targeting ORF2 that Encodes Movement Protein
3.4. Apple mdm- miRNAs Targeting ORF3 that Encodes Coat Protein
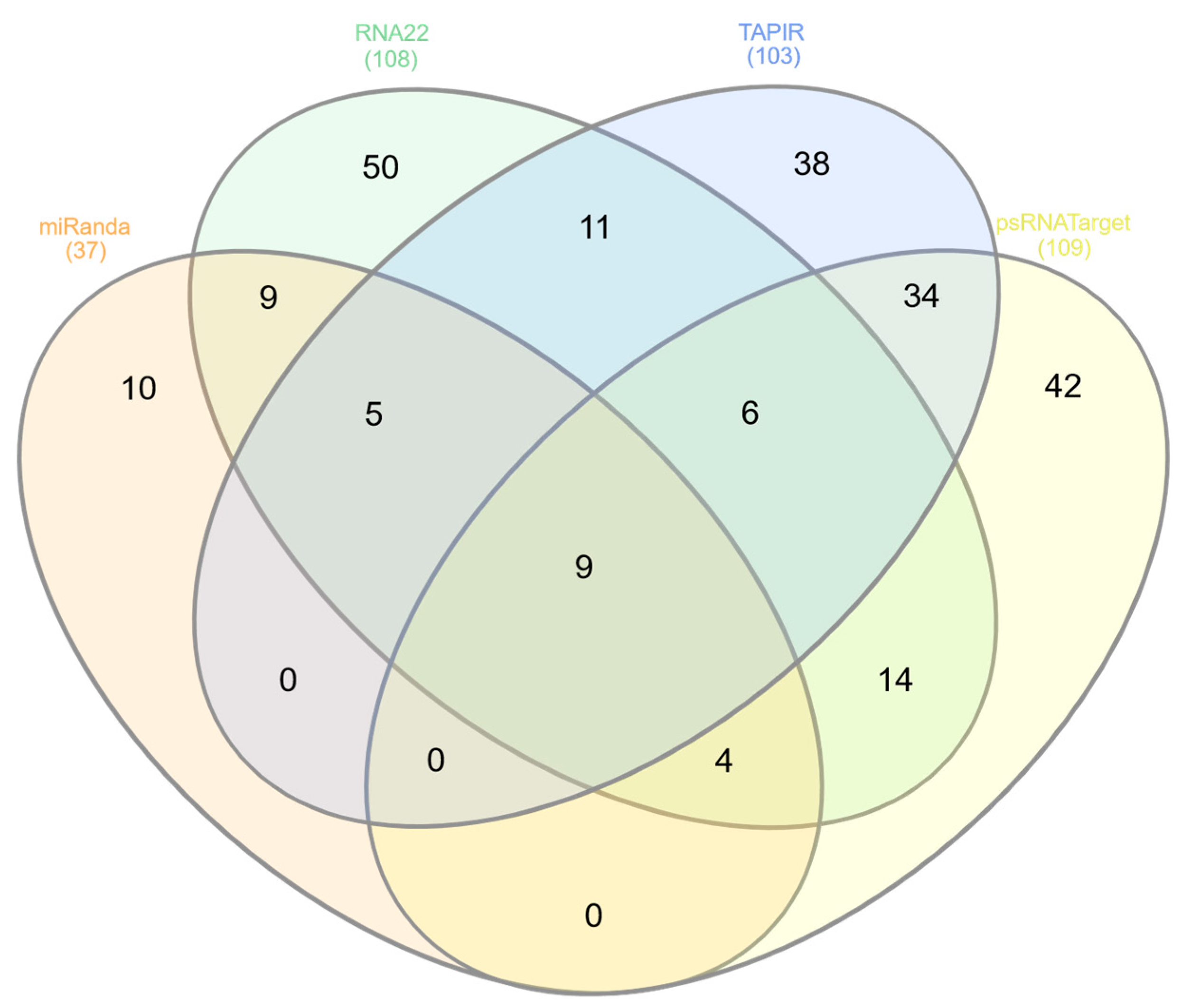
3.5. Evaluation of Common Apple MicroRNAs
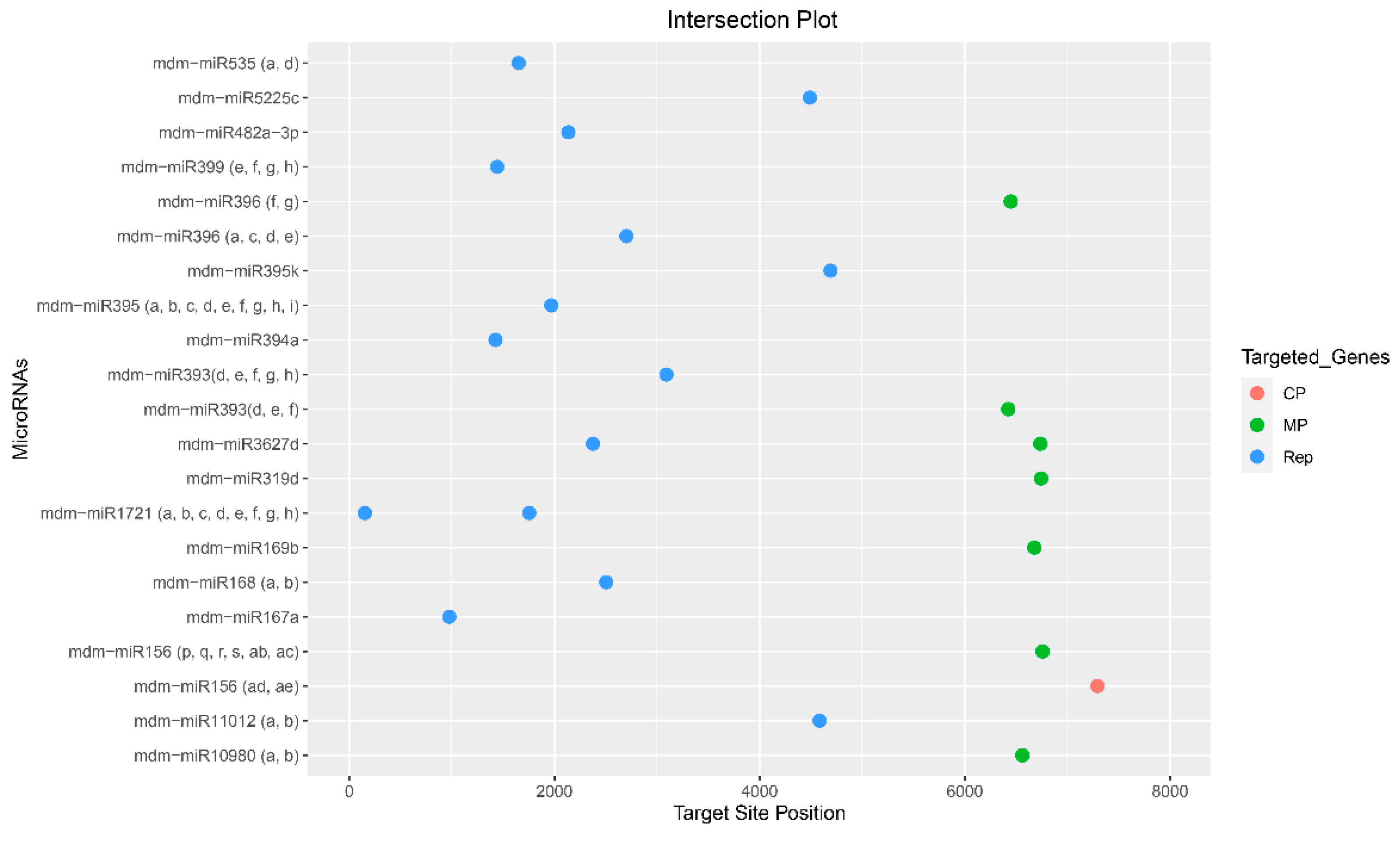
3.6. Evaluation and Identification of Consensual Apple MicroRNAs for ACLSV Silencing
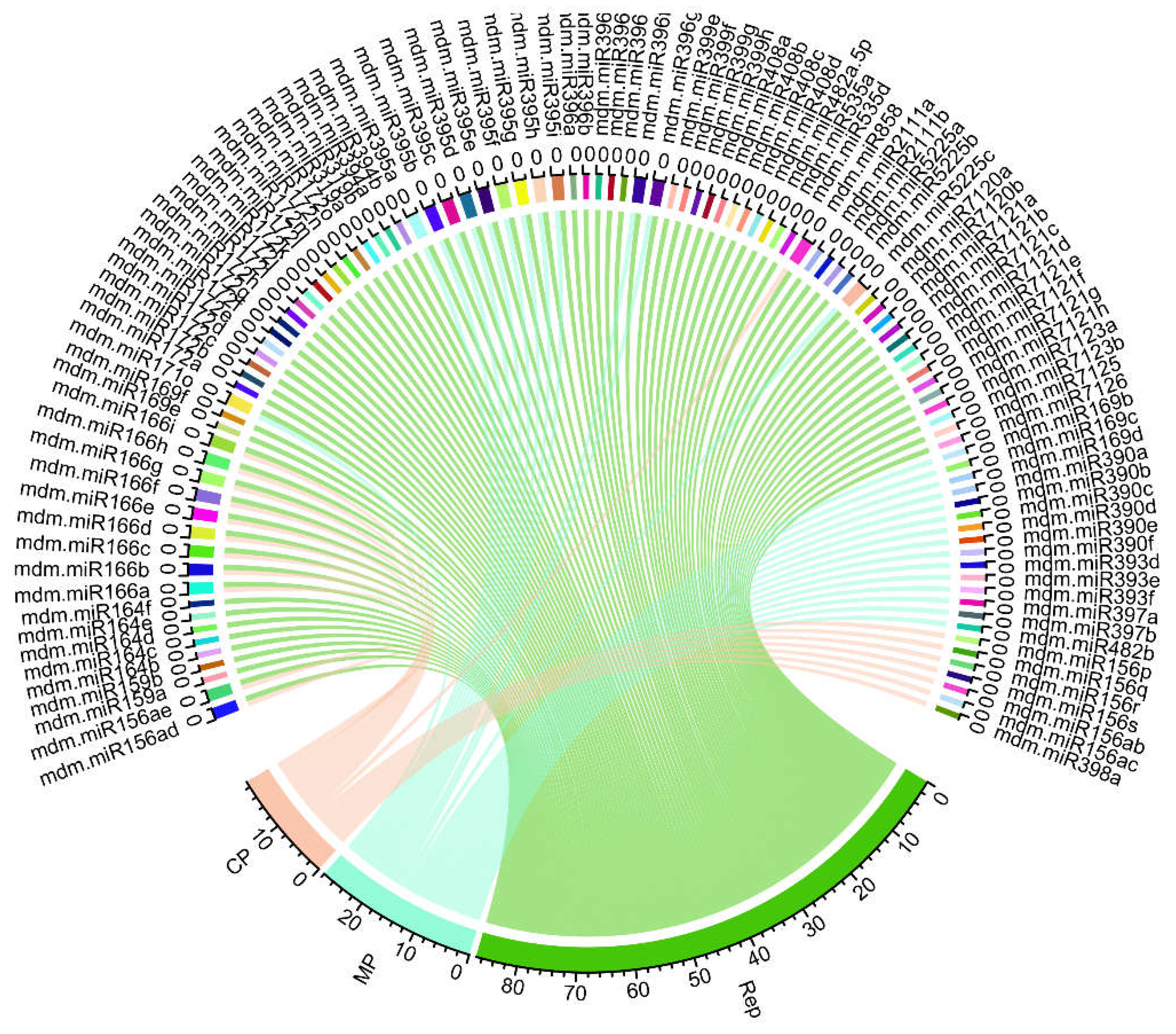
3.7. Construction of Apple mdm-miRNAs Regulatory Network
3.8. Secondary Structures of the Consensual RNA
3.9. Assessment of Free Energy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Na, W.; Wolf, J.; Zhang, F.-s. Towards sustainable intensification of apple production in China—Yield gaps and nutrient use efficiency in apple farming systems. Journal of Integrative Agriculture 2016, 15, 716–725. [Google Scholar]
- Shah, Z.A.; Dar, M.A.; Dar, E.A.; Obianefo, C.A.; Bhat, A.H.; Ali, M.T.; El-Sharnouby, M.; Shukry, M.; Kesba, H.; Sayed, S. Sustainable Fruit Growing: An Analysis of Differences in Apple Productivity in the Indian State of Jammu and Kashmir. Sustainability 2022, 14, 14544. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Liu, W.; Zhang, J.; Wang, N.; Chen, X. Research progress of fruit color development in apple (Malus domestica Borkh.). Plant Physiology and Biochemistry 2021, 162, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D. The genome of the domesticated apple (Malus× domestica Borkh.). Nature genetics 2010, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Martelli, G.; Candresse, T.; Namba, S. Trichovirus, a new genus of plant viruses. 1994.
- Rwahnih, M.A.; Turturo, C.; Minafra, A.; Saldarelli, P.; Myrta, A.; Pallás, V.; Savino, V. Molecular variability of apple chlorotic leaf spot virusin different hosts and geographical regions. Journal of Plant Pathology 2004, 117–122. [Google Scholar]
- Abtahi, F.; Shams-Bakhsh, M.; Safaie, N.; Azizi, A.; Autonell, C.R.; Ratti, C. Incidence and genetic diversity of apple chlorotic leaf spot virus in Iran. Journal of Plant Pathology 2019, 101, 513–519. [Google Scholar] [CrossRef]
- Katsiani, A.; Maliogka, V.; Candresse, T.; Katis, N. Host-range studies, genetic diversity and evolutionary relationships of ACLSV isolates from ornamental, wild and cultivated R osaceous species. Plant pathology 2014, 63, 63–71. [Google Scholar] [CrossRef]
- Yaegashi, H.; Yoshikawa, N.; Candresse, T. Apple chlorotic leaf spot virus in pome fruits. In Virus and Virus-Like Diseases of Pome and Stone Fruits; Hadidi, A., Barba, M., Candresse, T., Jelkmann, W., Eds.; 2011; pp. 17–21. [Google Scholar]
- Guo, W.; Zheng, W.; Wang, M.; Li, X.; Ma, Y.; Dai, H. Genome sequences of three apple chlorotic leaf spot virus isolates from hawthorns in China. PLoS One 2016, 11, e0161099. [Google Scholar] [CrossRef]
- Canales, C.; Morán, F.; Olmos, A.; Ruiz-García, A.B. First detection and molecular characterization of Apple stem grooving virus, apple chlorotic leaf spot virus, and apple hammerhead viroid in loquat in Spain. Plants 2021, 10, 2293. [Google Scholar] [CrossRef]
- Dhir, S.; Zaidi, A.A.; Hallan, V. M olecular C haracterization and R ecombination A nalysis of the C omplete G enome of A pple C hlorotic L eaf S pot V irus. Journal of Phytopathology 2013, 161, 704–712. [Google Scholar] [CrossRef]
- Reinhart, B.J.; Weinstein, E.G.; Rhoades, M.W.; Bartel, B.; Bartel, D.P. MicroRNAs in plants. Genes & development 2002, 16, 1616–1626. [Google Scholar]
- Liu, W.-w.; Meng, J.; Cui, J.; Luan, Y.-s. Characterization and function of microRNA∗ s in plants. Frontiers in plant science 2017, 8, 2200. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Zheng, B.; Yu, Y.; Won, S.Y.; Mo, B.; Chen, X. The role of Mediator in small and long noncoding RNA production in Arabidopsis thaliana. The EMBO journal 2011, 30, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Cui, Y.; Li, Y.; Qi, Y. Transcription and processing of primary microRNAs are coupled by Elongator complex in Arabidopsis. Nature Plants 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Fang, Y.; Spector, D.L. Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Current Biology 2007, 17, 818–823. [Google Scholar] [CrossRef]
- Manavella, P.A.; Koenig, D.; Weigel, D. Plant secondary siRNA production determined by microRNA-duplex structure. Proceedings of the National Academy of Sciences 2012, 109, 2461–2466. [Google Scholar] [CrossRef]
- Trobaugh, D.W.; Klimstra, W.B. MicroRNA regulation of RNA virus replication and pathogenesis. Trends in molecular medicine 2017, 23, 80–93. [Google Scholar] [CrossRef]
- Deng, Z.; Ma, L.; Zhang, P.; Zhu, H. Small RNAs Participate in Plant–Virus Interaction and Their Application in Plant Viral Defense. International Journal of Molecular Sciences 2022, 23, 696. [Google Scholar] [CrossRef]
- Mengistu, A.A.; Tenkegna, T.A. The role of miRNA in plant–virus interaction: a review. Molecular Biology Reports 2021, 48, 2853–2861. [Google Scholar] [CrossRef]
- Zhou, L.; Yuan, Q.; Ai, X.; Chen, J.; Lu, Y.; Yan, F. Transgenic Rice Plants Expressing Artificial miRNA Targeting the Rice Stripe Virus MP Gene Are Highly Resistant to the Virus. Biology 2022, 11, 332. [Google Scholar] [CrossRef]
- Miao, S.; Liang, C.; Li, J.; Baker, B.; Luo, L. Polycistronic artificial microRNA-mediated resistance to cucumber green mottle mosaic virus in cucumber. International journal of molecular sciences 2021, 22, 12237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Zhang, Y.; Wu, C.; Wang, S.; Hao, L.; Wang, S.; Li, T. Md-miR156ab and Md-miR395 target WRKY transcription factors to influence apple resistance to leaf spot disease. Frontiers in Plant Science 2017, 8, 526. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Yan, F.; Meng, R.; Jiang, X.; Yang, H.; Gao, Z.; Dong, Y.; Yang, Y.; Zhao, Z. Identification of microRNAs and their targets associated with fruit-bagging and subsequent sunlight re-exposure in the “Granny Smith” apple exocarp using high-throughput sequencing. Frontiers in plant science 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Li, H.; Jiang, L.; Yan, M.; Li, C.; Geng, D.; Xie, Y.; Yan, Y.; Shen, X.; Chen, P. Genome-wide identification of drought-responsive microRNAs in two sets of Malus from interspecific hybrid progenies. Horticulture research 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, C.; Zhai, Z.; Peng, X.; Wang, Y.; Sun, Y.; Li, J.; Shen, X.; Xiao, Y.; Zhu, S. The apple microR171i-SCARECROW-LIKE PROTEINS26. 1 module enhances drought stress tolerance by integrating ascorbic acid metabolism. Plant physiology 2020, 184, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.M.; Li, S.; Liu, Z.; Fan, L.; Tang, T.; Zhang, X.; Mao, J.; Li, K.; Khan, A.; Shao, Y. Different miRNAs and hormones are involved in PEG-induced inhibition of adventitious root formation in apple. Scientia Horticulturae 2022, 303, 111206. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: from microRNA sequences to function. Nucleic acids research 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Bourexis, D.; Brister, J.R.; Canese, K.; Comeau, D.C.; Funk, K.; Kim, S.; Klimke, W. Database resources of the national center for biotechnology information. Nucleic acids research 2021, 49, D10. [Google Scholar] [CrossRef]
- Enright, A.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D. MicroRNA targets in Drosophila. Genome biology 2003, 4, 1–27. [Google Scholar] [CrossRef]
- John, B.; Enright, A.J.; Aravin, A.; Tuschl, T.; Sander, C.; Marks, D.S. Human microRNA targets. PLoS biology 2004, 2, e363. [Google Scholar] [CrossRef]
- Miranda, K.C.; Huynh, T.; Tay, Y.; Ang, Y.-S.; Tam, W.-L.; Thomson, A.M.; Lim, B.; Rigoutsos, I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell 2006, 126, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Loher, P.; Rigoutsos, I. Interactive exploration of RNA22 microRNA target predictions. Bioinformatics 2012, 28, 3322–3323. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, E.; He, Y.; Billiau, K.; Van de Peer, Y. TAPIR, a web server for the prediction of plant microRNA targets, including target mimics. Bioinformatics 2010, 26, 1566–1568. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhao, P.X. psRNATarget: a plant small RNA target analysis server. Nucleic acids research 2011, 39, W155–W159. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: a plant small RNA target analysis server (2017 release). Nucleic acids research 2018, 46, W49–W54. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: an information aesthetic for comparative genomics. Genome research 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Lorenz, R.; Bernhart, S.; Siederdissen, C.; Tafer, H.; Flamm, C.; Stadler, P.; Hofacker, I. ViennaRNA package 2.0. algorithms for molecular biology. vol 2013, 6, 26–26. [Google Scholar]
- Bernhart, S.H.; Tafer, H.; Mückstein, U.; Flamm, C.; Stadler, P.F.; Hofacker, I.L. Partition function and base pairing probabilities of RNA heterodimers. Algorithms for Molecular Biology 2006, 1, 1–10. [Google Scholar] [CrossRef]
- Gandrud, C. Reproducible research with R and RStudio; Chapman and Hall/CRC, 2018. [Google Scholar]
- German, S.; Candresse, T.; Lanneau, M.; Huet, J.; Pernollet, J.; Dunez, J. Nucleotide sequence and genomic organization of apple chlorotic leaf spot closterovirus. Virology 1990, 179, 104–112. [Google Scholar] [CrossRef]
- Sato, K.; Yoshikawa, N.; Takahashi, T. Complete nucleotide sequence of the genome of an apple isolate of apple chlorotic leaf spot virus. Journal of General Virology 1993, 74, 1927–1931. [Google Scholar] [CrossRef]
- Singh, R.M.; Singh, D.; Hallan, V. Movement protein of Apple chlorotic leaf spot virus is genetically unstable and negatively regulated by Ribonuclease E in E. coli. Scientific Reports 2017, 7, 2133. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Matsuda, H.; Kawamura, T.; Isogai, M.; Yoshikawa, N.; Takahashi, T. Intracellular distribution, cell-to-cell trafficking and tubule-inducing activity of the 50 kDa movement protein of Apple chlorotic leaf spot virus fused to green fluorescent protein. Journal of General Virology 2000, 81, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, H.; Takahashi, T.; Isogai, M.; Kobori, T.; Ohki, S.; Yoshikawa, N. Apple chlorotic leaf spot virus 50 kDa movement protein acts as a suppressor of systemic silencing without interfering with local silencing in Nicotiana benthamiana. Journal of general virology 2007, 88, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Isogai, M.; Yoshikawa, N. Mapping the RNA-binding domain on the Apple chlorotic leaf spot virus movement protein. Journal of general virology 2005, 86, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, H.; Isogai, M.; Tajima, H.; Sano, T.; Yoshikawa, N. Combinations of two amino acids (Ala40 and Phe75 or Ser40 and Tyr75) in the coat protein of apple chlorotic leaf spot virus are crucial for infectivity. Journal of General Virology 2007, 88, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Mazeikiene, I.; Siksnianiene, J.B.; Gelvonauskiene, D.; Bendokas, V.; Stanys, V. Prevalence and molecular variability of Apple chlorotic leaf spot virus capsid protein genes in Lithuania. Journal of Plant Diseases and Protection 2018, 125, 389–396. [Google Scholar] [CrossRef]
- Niu, Q.-W.; Lin, S.-S.; Reyes, J.L.; Chen, K.-C.; Wu, H.-W.; Yeh, S.-D.; Chua, N.-H. Expression of artificial microRNAs in transgenic Arabidopsis thaliana confers virus resistance. Nature biotechnology 2006, 24, 1420–1428. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Ali, B.; Brown, J.K.; Shahid, I.; Yu, N. In Silico Identification of Cassava Genome-Encoded MicroRNAs with Predicted Potential for Targeting the ICMV-Kerala Begomoviral Pathogen of Cassava. Viruses 2023, 15, 486. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Ngah, N.M.F.N.C.; Abas, A.; Talip, N.; Sarian, M.N.; Hamezah, H.S.; Harun, S.; Bunawan, H. Candidate miRNAs from Oryza sativa for Silencing the Rice Tungro Viruses. Agriculture 2023, 13, 651. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Tariq, H.K.; Hu, X.-W.; Khan, J.; Zou, Z. Computational Biology and Machine Learning Approaches Identify Rubber Tree (Hevea brasiliensis Muell. Arg.) Genome Encoded MicroRNAs Targeting Rubber Tree Virus 1. Applied Sciences 2022, 12, 12908. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Feng, X.; Hu, X.; Ashraf, F.; Shen, L.; Iqbal, M.S.; Zhang, S. In silico identification of sugarcane (Saccharum officinarum L.) genome encoded microRNAs targeting sugarcane bacilliform virus. PloS one 2022, 17, e0261807. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Ashraf, F.; Feng, X.; Hu, X.; Shen, L.; Khan, J.; Zhang, S. Potential targets for evaluation of sugarcane yellow leaf virus resistance in sugarcane cultivars: in silico sugarcane miRNA and target network prediction. Biotechnology & Biotechnological Equipment 2021, 35, 1980–1991. [Google Scholar]
- Ashraf, F.; Ashraf, M.A.; Hu, X.; Zhang, S. A novel computational approach to the silencing of Sugarcane Bacilliform Guadeloupe A Virus determines potential host-derived MicroRNAs in sugarcane (Saccharum officinarum L.). PeerJ 2020, 8, e8359. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, Y.Z.A.; Ziebell, H. Novel targets for engineering Physostegia chlorotic mottle and tomato brown rugose fruit virus-resistant tomatoes: in silico prediction of tomato microRNA targets. PeerJ 2020, 8, e10096. [Google Scholar] [CrossRef] [PubMed]
- Petchthai, U.; Yee, C.S.L.; Wong, S.-M. Resistance to CymMV and ORSV in artificial microRNA transgenic Nicotiana benthamiana plants. Scientific reports 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, N.; Shen, W.; Li, J.-F. Engineered artificial microRNA precursors facilitate cloning and gene silencing in arabidopsis and rice. International Journal of Molecular Sciences 2019, 20, 5620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, D.; Chen, S.L.; Gong, B.-Q.; Guo, Y.; Xu, L.; Zhang, X.-N.; Li, J.-F. Engineering artificial microRNAs for multiplex gene silencing and simplified transgenic screen. Plant physiology 2018, 178, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Motawaa, M.; Wang, Q.; Zhang, X.; Khalid, A.; Cai, X.; Li, F. Simple webserver-facilitated method to design and synthesize artificial miRNA gene and its application in engineering viral resistance. Plants 2022, 11, 2125. [Google Scholar] [CrossRef]
- Ali, I.; Amin, I.; Briddon, R.W.; Mansoor, S. Artificial microRNA-mediated resistance against the monopartite begomovirus Cotton leaf curl Burewala virus. Virology journal 2013, 10, 1–8. [Google Scholar] [CrossRef]
- Duan, C.-G.; Wang, C.-H.; Fang, R.-X.; Guo, H.-S. Artificial microRNAs highly accessible to targets confer efficient virus resistance in plants. Journal of virology 2008, 82, 11084–11095. [Google Scholar] [CrossRef]
- Dweep, H.; Sticht, C.; Gretz, N. In-silico algorithms for the screening of possible microRNA binding sites and their interactions. Current genomics 2013, 14, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Thody, J.; Moulton, V.; Mohorianu, I. PAREameters: a tool for computational inference of plant miRNA–mRNA targeting rules using small RNA and degradome sequencing data. Nucleic Acids Research 2020, 48, 2258–2270. [Google Scholar] [CrossRef]
- Pinzón, N.; Li, B.; Martinez, L.; Sergeeva, A.; Presumey, J.; Apparailly, F.; Seitz, H. microRNA target prediction programs predict many false positives. Genome research 2017, 27, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.; Iovino, N.; Unnerstall, U.; Gaul, U.; Segal, E. The role of site accessibility in microRNA target recognition. Nature genetics 2007, 39, 1278–1284. [Google Scholar] [CrossRef]
- Golyshev, V.; Pyshnyi, D.; Lomzov, A. Calculation of Energy for RNA/RNA and DNA/RNA Duplex Formation by Molecular Dynamics Simulation. Molecular Biology 2021, 55, 927–940. [Google Scholar] [CrossRef]
- Ghoshal, A.; Shankar, R.; Bagchi, S.; Grama, A.; Chaterji, S. MicroRNA target prediction using thermodynamic and sequence curves. BMC genomics 2015, 16, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kaja, E.; Szcześniak, M.W.; Jensen, P.J.; Axtell, M.J.; McNellis, T.; Makałowska, I. Identification of apple miRNAs and their potential role in fire blight resistance. Tree Genetics & Genomes 2015, 11, 1–11. [Google Scholar]
- Liu, X.-X.; Luo, X.-F.; Luo, K.-X.; Liu, Y.-L.; Pan, T.; Li, Z.-Z.; Duns, G.J.; He, F.-L.; Qin, Z.-D. Small RNA sequencing reveals dynamic microRNA expression of important nutrient metabolism during development of Camellia oleifera fruit. International journal of biological sciences 2019, 15, 416. [Google Scholar] [CrossRef]
- M Witkos, T.; Koscianska, E.; J Krzyzosiak, W. Practical aspects of microRNA target prediction. Current molecular medicine 2011, 11, 93–109. [Google Scholar] [CrossRef]
| Algorithms | Parameter | Features | Availability | |
|---|---|---|---|---|
| miRanda | Score threshold= 140, Free energy=−20 Kcal/mol, Gap open penalty=−9.00 Gap extend penalty=−4.00 |
Seed-based interaction, Target site accessibility, free energy of RNA-RNA duplex, conservation |
http://www.microrna.org/ (accessed 26 January 2023) |
|
| RNA22 | Folding energy=−15 Kcal/mol Number of paired-up bases= 12, Sensitivity (63%), Specificity (61%), |
Non-seed based interaction, Site complementarity, Target site multiplicity, Pattern recognition, Folding energy of heteroduplex |
https://cm.jefferson.edu/rna22/Interactive/ (accessed on 22 October 2022) |
|
| TAPIR | Free energy=−20 Kcal/mol, Hit per target= 1 |
Seed paring, Free energy of duplex, Multiple target sites, |
http://bibiserv.techfak.uni-bielefeld.de/rnahybrid (accessed on 9 November 2022) |
|
| psRNATarget | Expectation Score= 6.5, HSP size= 19, Penalty for G:U pair= 0.5 Penalty for opening gap= 2 |
Multiplicity of target site, Translation inhibition, Target accessibility, Complementarity scoring |
https://www.zhaolab.org/psRNATarget/analysis?function=2 (accessed on 9 November 2022) |
|
| Apple miRNAs |
Position miRanda |
Position RNA22 | Position TAPIR |
Position psRNATarget |
MFE * miRanda |
MFE ** RNA22 |
MFE Ratio TAPIR |
Expectation psRNATarget |
|---|---|---|---|---|---|---|---|---|
| mdm-miR156 (p, q, r, s) | 6758 | 6758 | 0.44 | 6.00 | ||||
| mdm-miR156 (ab, ac) | 6758 | 6758 | 0.46 | 5.00 | ||||
| mdm-156 (ad, ae) | 7293 | 7293 | 0.60 | 7.00 | ||||
| mes-miR167a | 975 | 976 | −16.30 | 0.52 | ||||
| mdm-miR168 (a, b) | 2504 | 2505 | −20.70 | 0.56 | ||||
| mdm-miR169b | 6678 | 6678 | 0.53 | 6.50 | ||||
| mdm-miR319d | 6744 | 6744 | −20.86 | −18.10 | ||||
| mdm-miR393 (d, e, f) | 6423 | 6423 | −19.30 | 7.00 | ||||
| mdm-miR393 (d, e, f) | 3092 | 3091 | −22.70 | 0.60 | ||||
| mdm-miR393 (g, h) | 3091 | 3092 | −21.17 | −21.11 | ||||
| mdm-miR394 (a, b) | 1426 | 1426 | 0.49 | 5.00 | ||||
| mdm-miR395 (a, b, c, d, e, f, g, h, i) | 1970 | 1970 | 0.47 | 6.00 | ||||
| mdm-miR395k | 4691 | 4691 | 4691 | −20.85 | −18.00 | 0.68 | ||
| mdm-miR396 (a, c, d, e) | 2702 | 2702 | 0.52 | 6.50 | ||||
| mdm-miR396 (f, g) | 6447 | 6447 | 0.43 | 7.00 | ||||
| mdm-399 (e, f, g, h) | 1443 | 1443 | 0.52 | 6.50 | ||||
| mdm-482a-3p | 2135 | 2136 | −18.30 | 0.48 | ||||
| mdm-482b | 2207 | 2207 | 0.34 | 6.00 | ||||
| mdm-535a | 1652 | 1652 | −18.60 | 6.00 | ||||
| mdm-535b | 1652 | 1652 | −17.90 | 6.50 | ||||
| mdm-miR3627d | 2376 | 2376 | −22.40 | −19.30 | ||||
| mdm-miR3627d (1) | 6736 | 6736 | −24.37 | −19.80 | ||||
| mdm-5225c | 4490 | 4490 | 0.46 | 7.00 | ||||
| mdm-7121 (a, b, c) | 149 | 153 | 1755 | 1755 | −20.63 | −22.40 | 0.58 | 5.00 |
| mdm-miR7121 (d, e, f, g, h) | 1755 | 1755 | 0.58 | 5.00 | ||||
| mdm-miR10980 (a, b) | 6561 | 6561 | −25.04 | 0.63 | ||||
| mdm-miR11012 (a, b) | 4585 | 4582 | −21.11 | −18.82 |
| miRNA ID | Accession IDs |
Length Precursor |
MFE */Kcal/mol | AMFE ** | MFEI *** | (G+C)% |
|---|---|---|---|---|---|---|
| mdm-MIR5225c | MI0023156 | 119 nt | −51.30 | −43.10 | −0.85 | 50.42 |
| mdm-MIR395k | MI0035639 | 168 nt | −43.27 | −25.75 | −0.68 | 37.50 |
| mdm-MIR7121a | MI0023144 | 132 nt | −49.40 | −37.42 | −0.79 | 46.97 |
| mdm-MIR7121b | MI0023145 | 172 nt | −70.60 | −41.04 | −0.85 | 48.26 |
| mdm-MIR7121c | MI0023146 | 135 nt | −71.30 | −52.81 | −1.09 | 48.15 |
| mdm-MIR7121d | MI0023147 | 121 nt | −67.50 | −55.78 | −1.08 | 51.24 |
| mdm-MIR7121e | MI0023148 | 121 nt | −67.50 | −55.78 | −1.08 | 51.24 |
| mdm-MIR7121f | MI0023149 | 88 nt | −39.90 | −45.34 | −0.79 | 56.82 |
| mdm-MIR7121g | MI0023150 | 100 nt | −45.80 | −45.80 | −0.89 | 51.00 |
| mdm-MIR7121h | MI0023151 | 121 nt | −67.50 | −55.78 | −1.08 | 51.24 |
| Apple mature miRNA ID | Accession ID | Mdm-miRNA-Target Sequence (5′–3′) | ΔG Duplex (Kcal/mol |
|---|---|---|---|
| mdm-miR5225c | MIMAT0026052 | 5′ UCUGUCGUGGGUGAGAUGGUGC 3′ 5′ GAAGCAGTGTACCCAAGACATA 3′ |
−15.90 |
| mdm-miR395k | MIMAT0043586 | 5’ GUUUCCUCAAACACUUCAUU 3’ 5’ AGGCAGGAGTTTGAGGAAAC 3’ |
−18.30 |
| mdm-miR7121a | MIMAT0026040 | 5′ UCCUCUUGGUGAUCGCCCUGU 3’ 5′ AAAGGGAGTTCATCGAGAGAA 3′ |
−22.10 |
| mdm-miR7121b | MIMAT0026041 | 5′ UCCUCUUGGUGAUCGCCCUGU 3’ 5′ AAAGGGAGTTCATCGAGAGAA 3′ |
−22.10 |
| mdm-miR7121c | MIMAT0026042 | 5′ UCCUCUUGGUGAUCGCCCUGU 3’ 5′ AAAGGGAGTTCATCGAGAGAA 3′ |
−22.10 |
| mdm-miR7121d | MIMAT0026043 | 5′ UCCUCUUGGUGAUCGCCCUGC 3’ 5′ AAAGGGAGTTCATCGAGAGAA 3′ |
−22.10 |
| mdm-miR7121e | MIMAT0026044 | 5′ UCCUCUUGGUGAUCGCCCUGC 3’ 5′ AAAGGGAGTTCATCGAGAGAA 3′ |
−22.10 |
| mdm-miR7121f | MIMAT0026045 | 5′ UCCUCUUGGUGAUCGCCCUGC 3’ 5′ AAAGGGAGTTCATCGAGAGAA 3′ |
−22.10 |
| mdm-miR7121g | MIMAT0026046 | 5′ UCCUCUUGGUGAUCGCCCUGC 3’ 5′ AAAGGGAGTTCATCGAGAGAA 3′ |
−22.10 |
| mdm-miR7121h | MIMAT0026047 | 5′ UCCUCUUGGUGAUCGCCCUGC 3’ 5′ AAAGGGAGTTCATCGAGAGAA 3′ |
−22.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





