Submitted:
08 May 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1.1. Precursors investigation
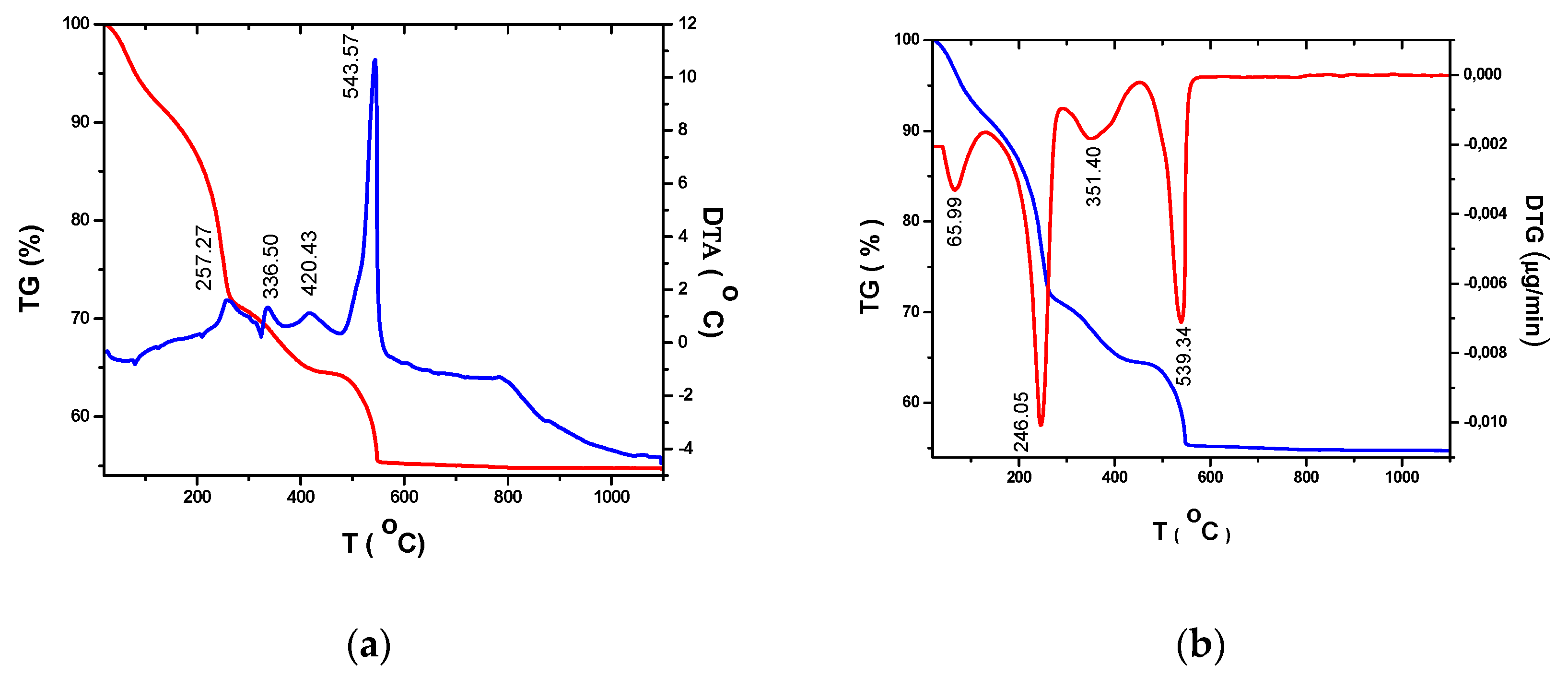
2.2. Structural, morphologic and optical properties
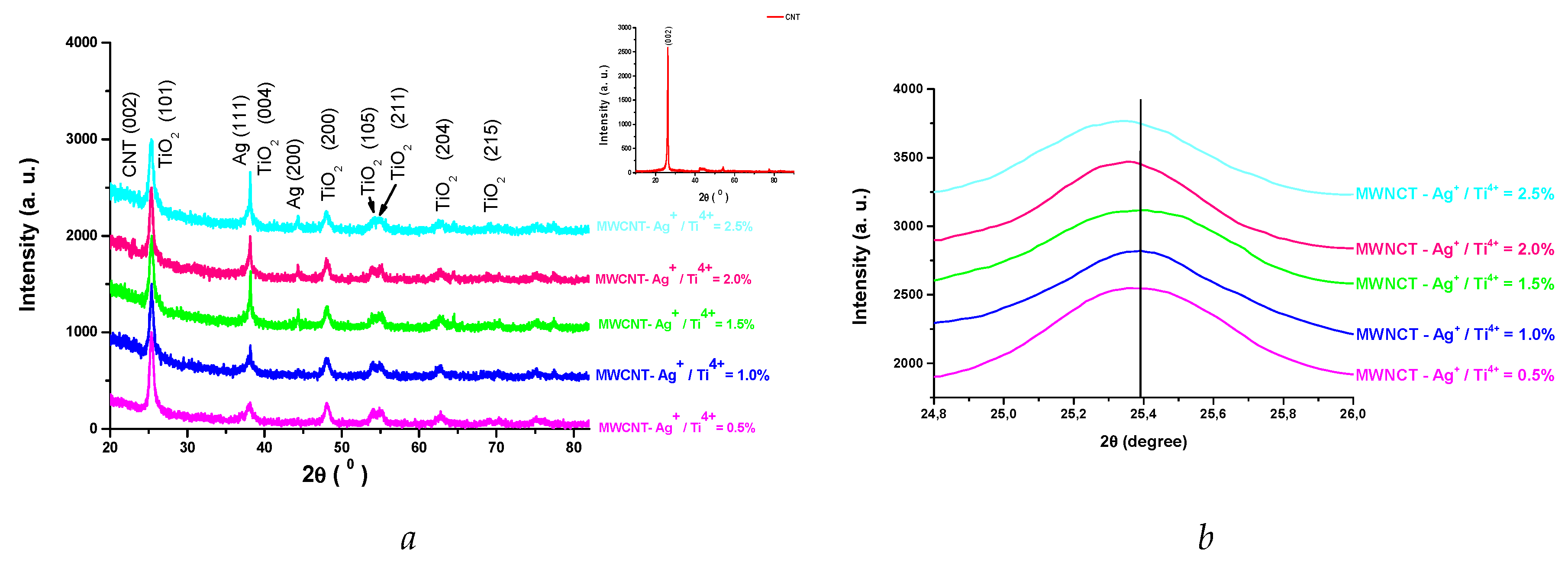
| Sample | Unit cell parameter |
Cell volume [Å3] |
(nm) | x 103 | |
| a [Å] | c [Å] | ||||
| MWCNT - Ag+/ Ti4+ = 0.5% | 3.7717 | 9.4603 | 134.576 | 14.21 | 11.3 |
| MWCNT - Ag+/ Ti4+ = 1.0% | 3.7824 | 9.4821 | 135.656 | 14.08 | 12.44 |
| MWCNT - Ag+/ Ti4+ = 1.5% | 3.7892 | 9.4888 | 136.240 | 13.86 | 13.86 |
| MWCNT - Ag+/ Ti4+ = 2.0% | 3.7920 | 9.4842 | 136.375 | 13.35 | 14.2 |
| MWCNT - Ag+/ Ti4 += 2.5% | 3.7951 | 9.5046 | 136.892 | 13.18 | 15.6 |
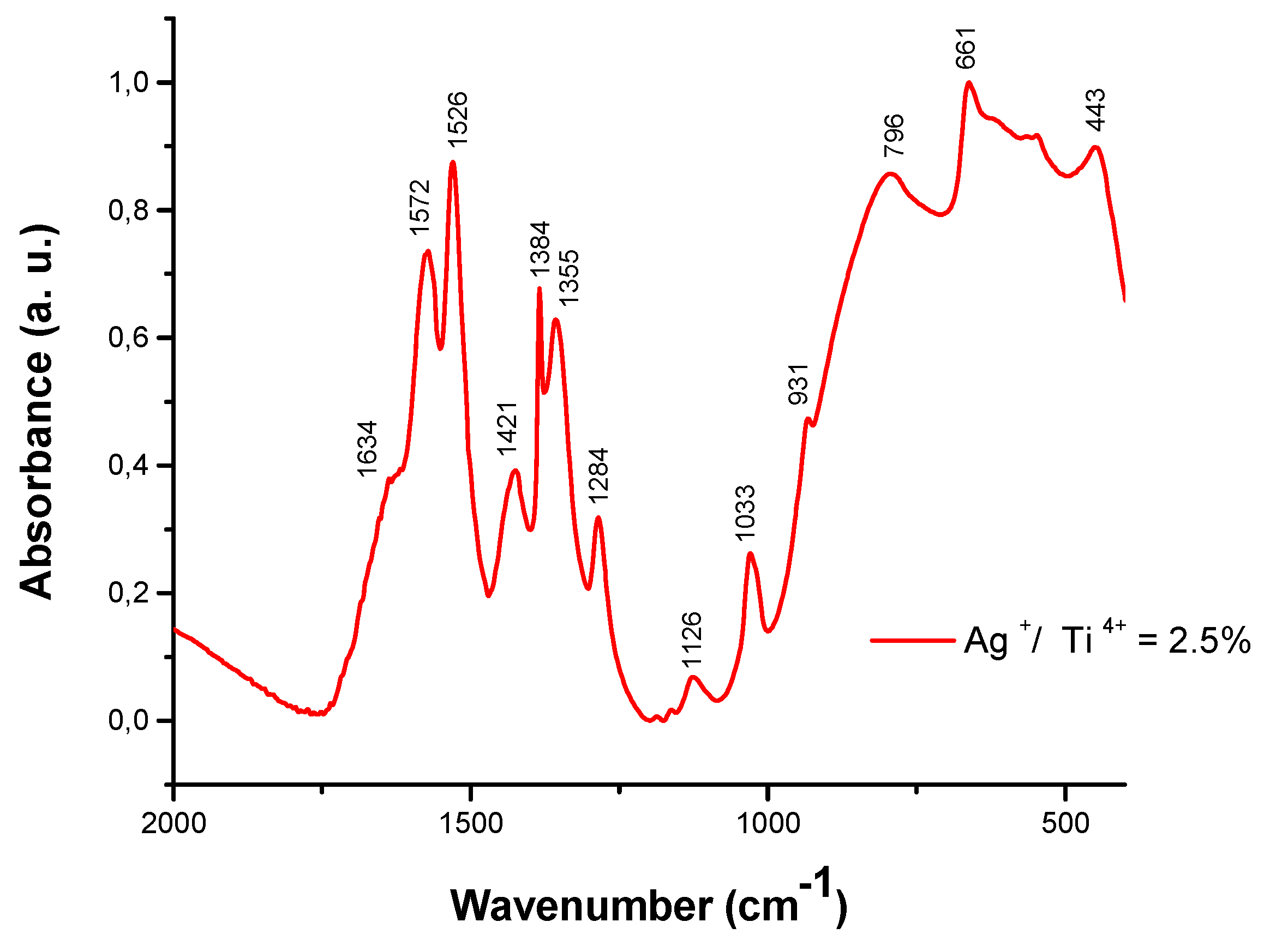
2.3. FT – IR spectroscopy
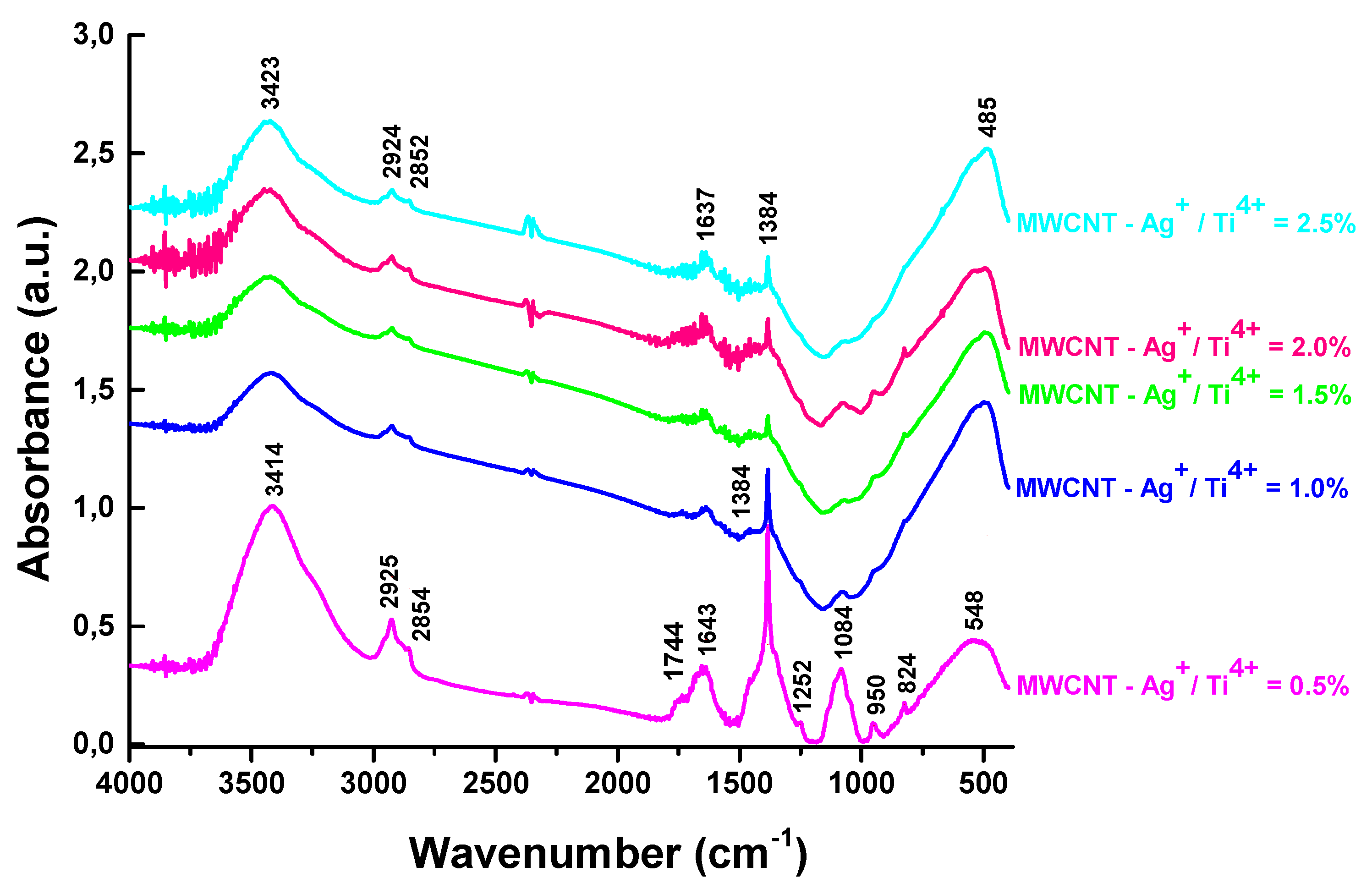
2.4. Raman spectroscopy
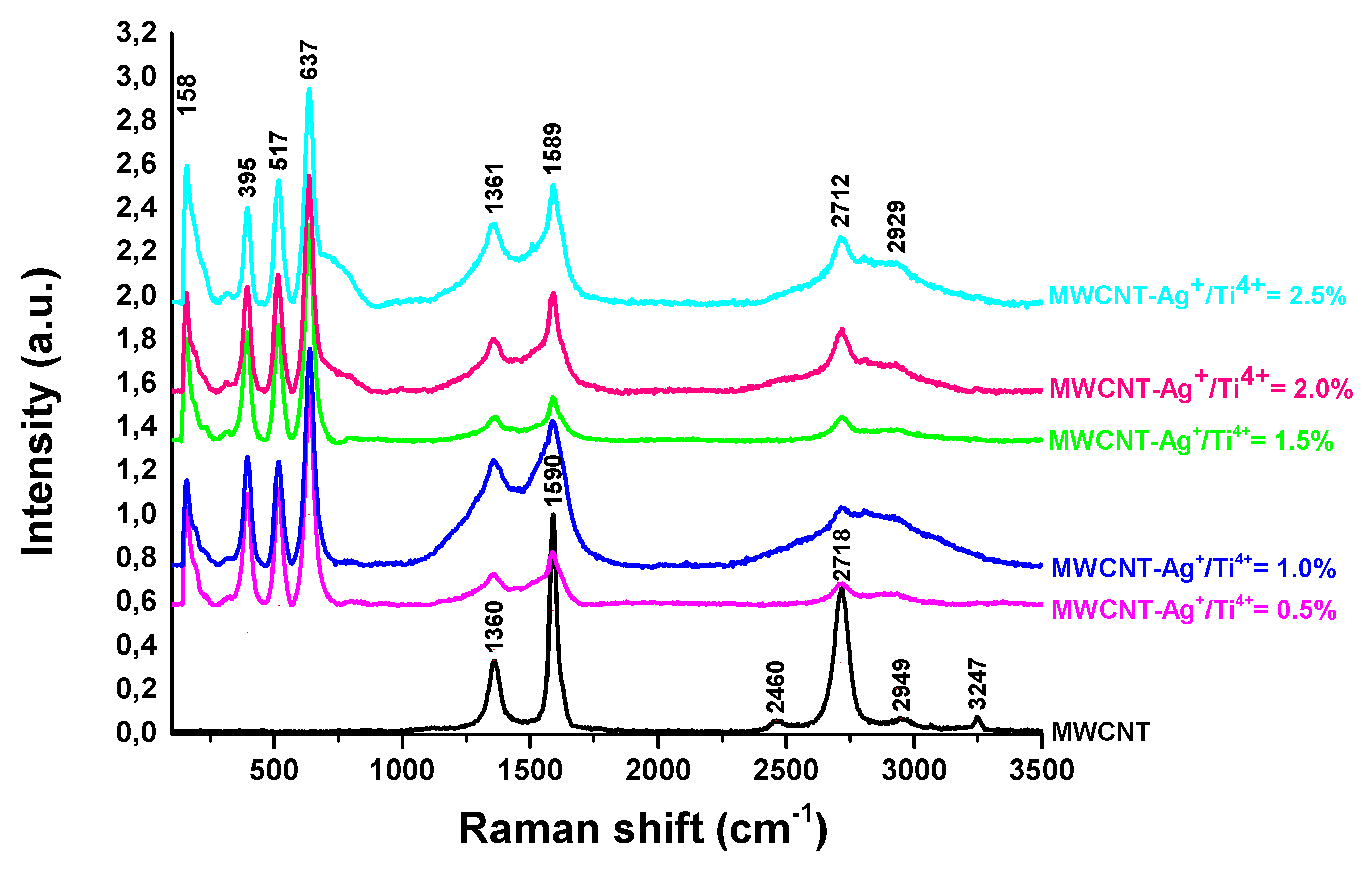
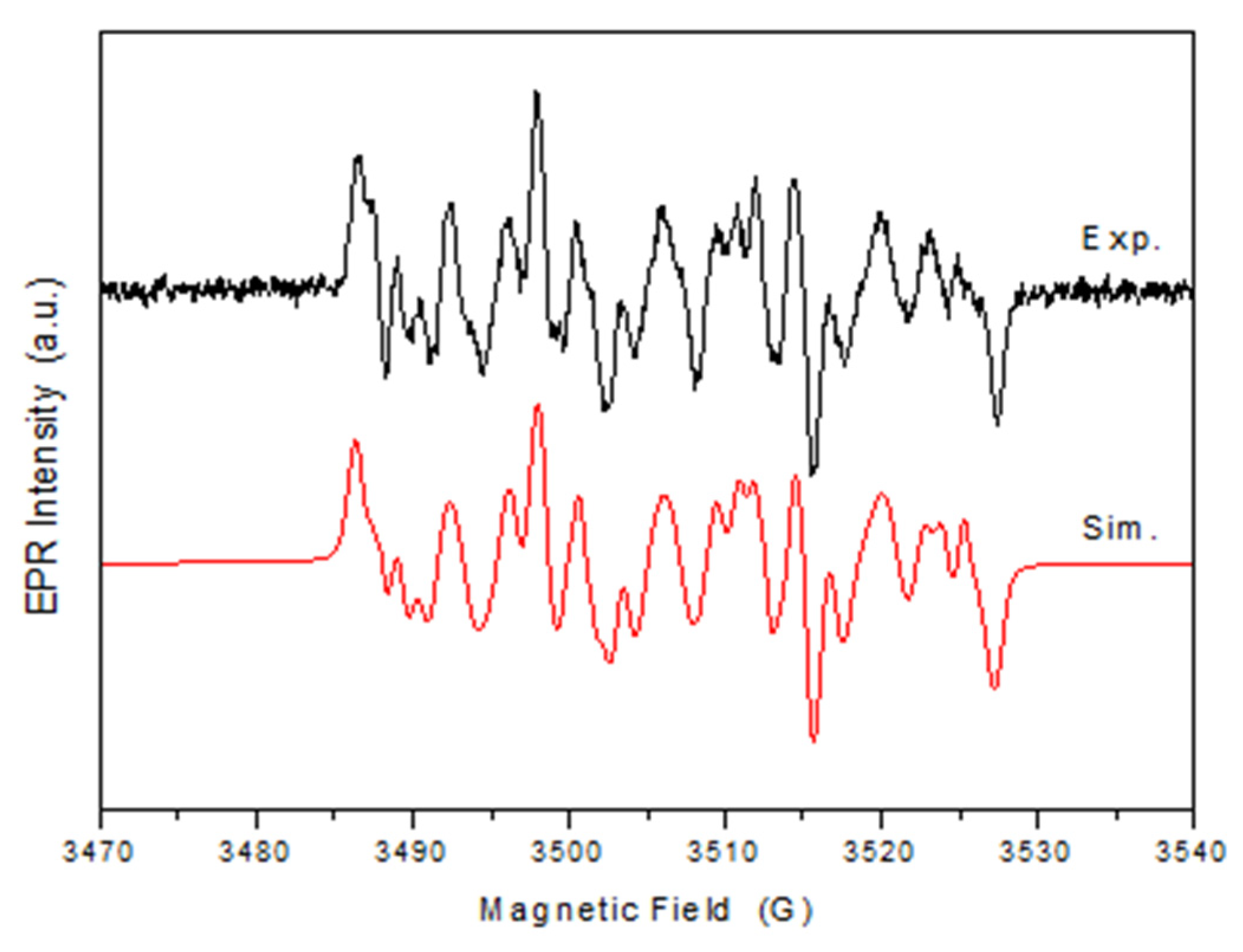
2.5. Electron spin resonance measurements
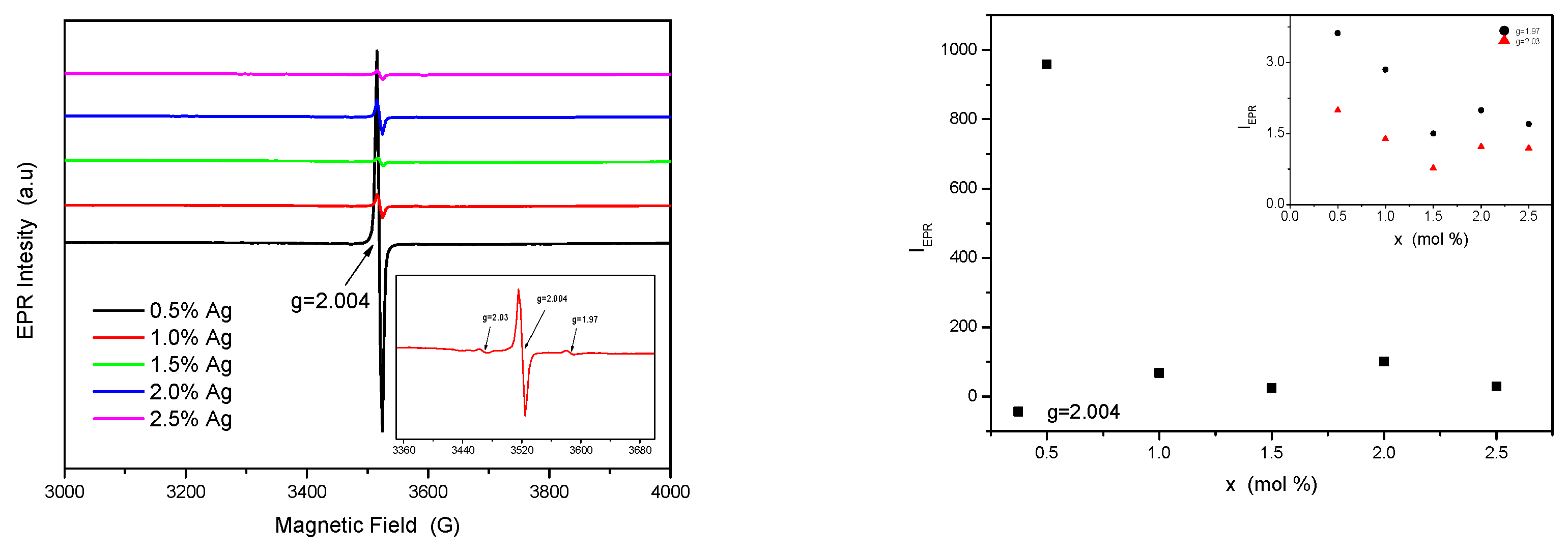
2.6. UV – ViS spectroscopy
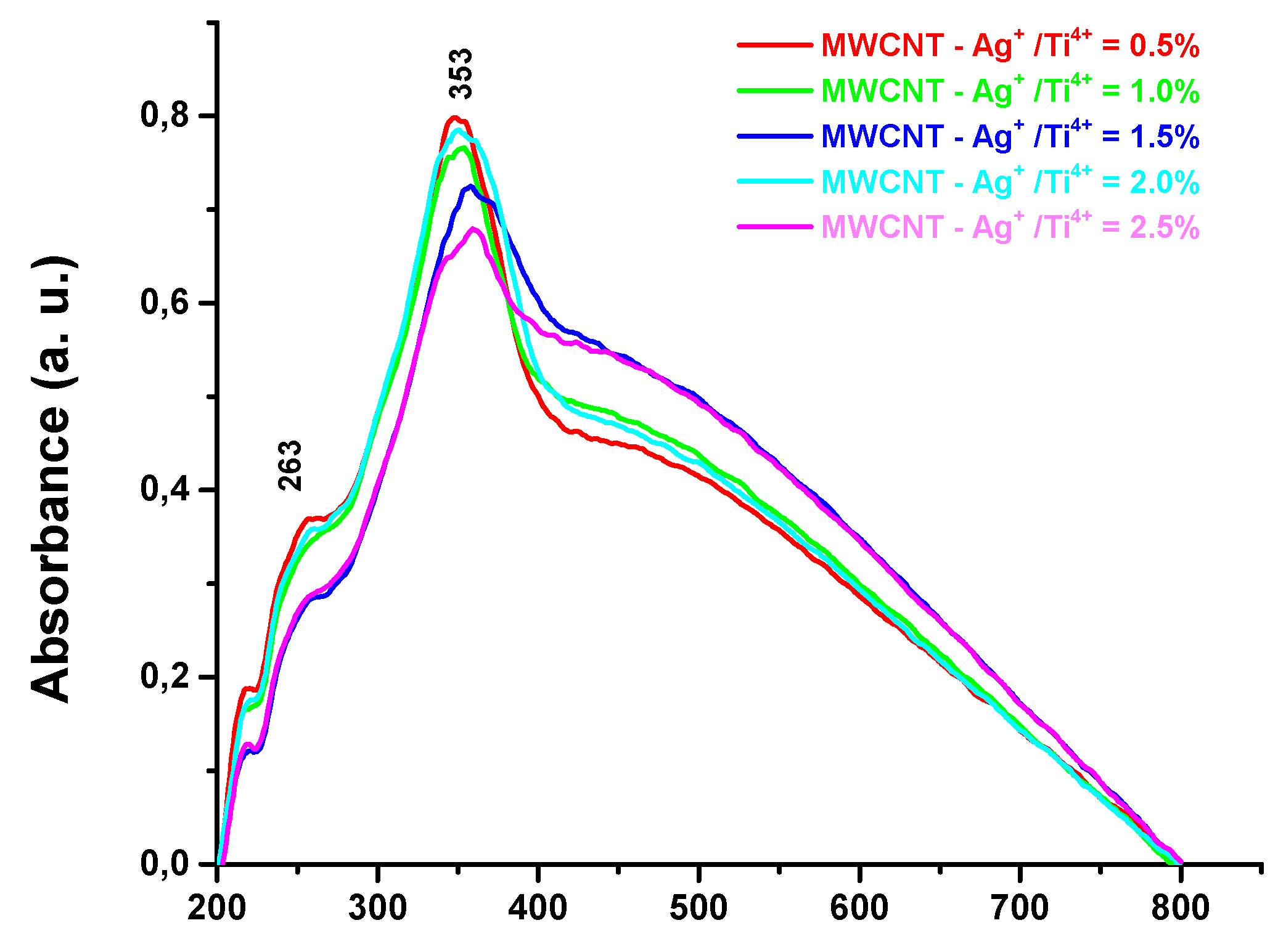
2.7. Optical band gap energy, Eg determination
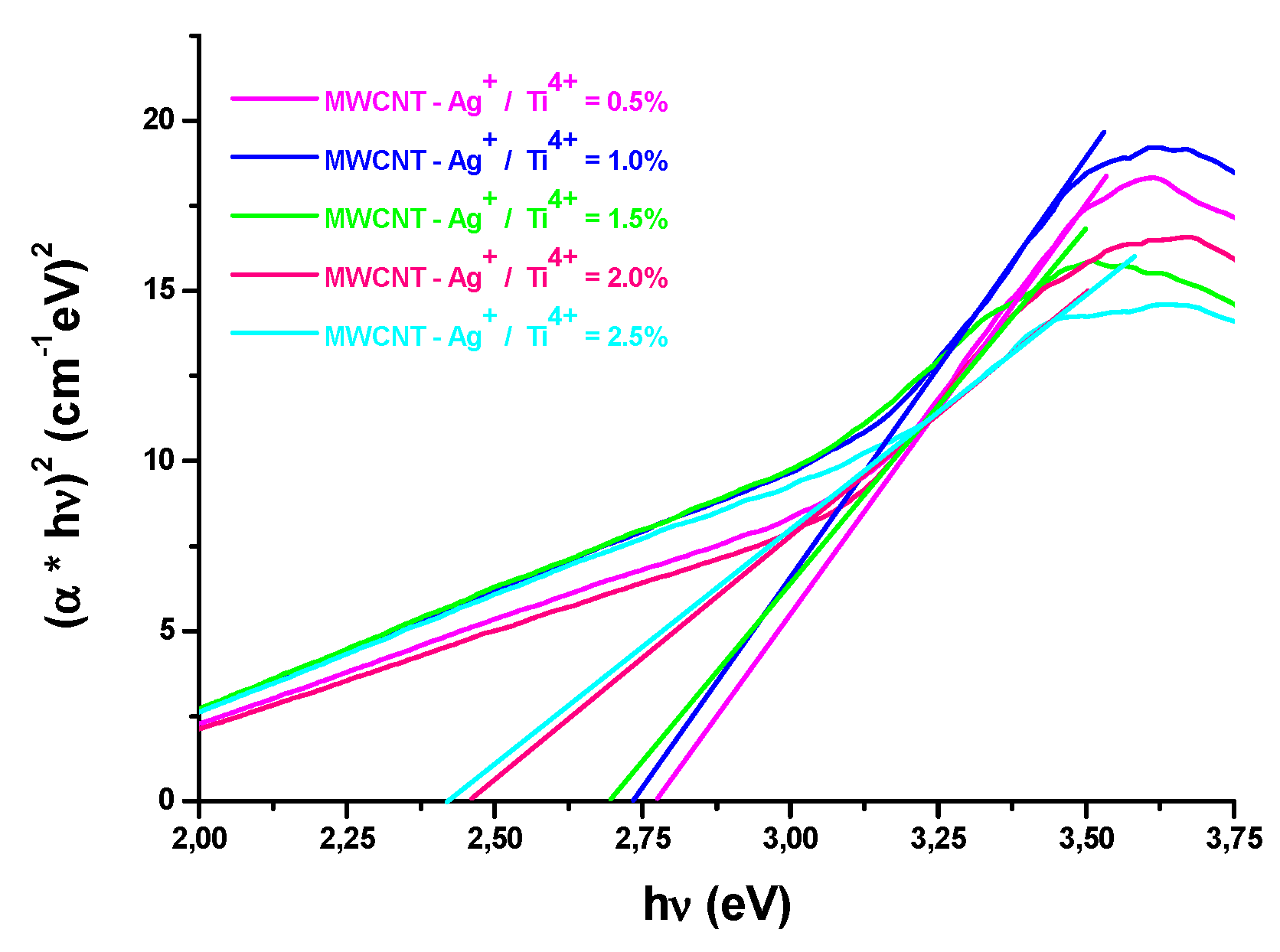
| Sample | Eg – direct transition |
|---|---|
| MWCNT - Ag+/ Ti4+ = 0.5% | 2.77 |
| MWCNT - Ag+ / Ti4+ = 1.0% | 2.73 |
| MWCNT - Ag+ / Ti4+ = 1.5% | 2.69 |
| MWCNT - Ag+ / Ti4+ = 2.0% | 2.46 |
| MWCNT - Ag+ / Ti4+ = 2.5% | 2.41 |
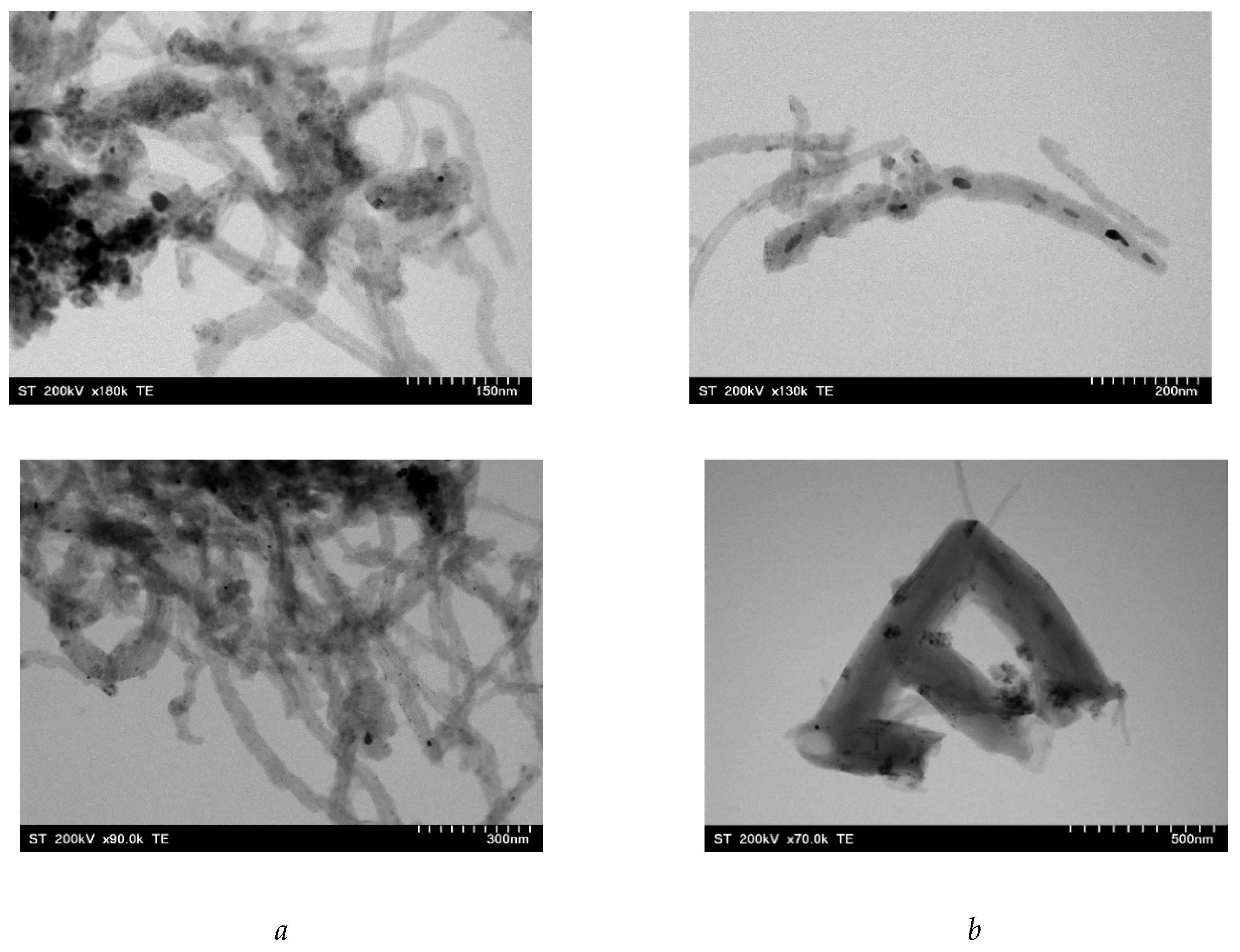
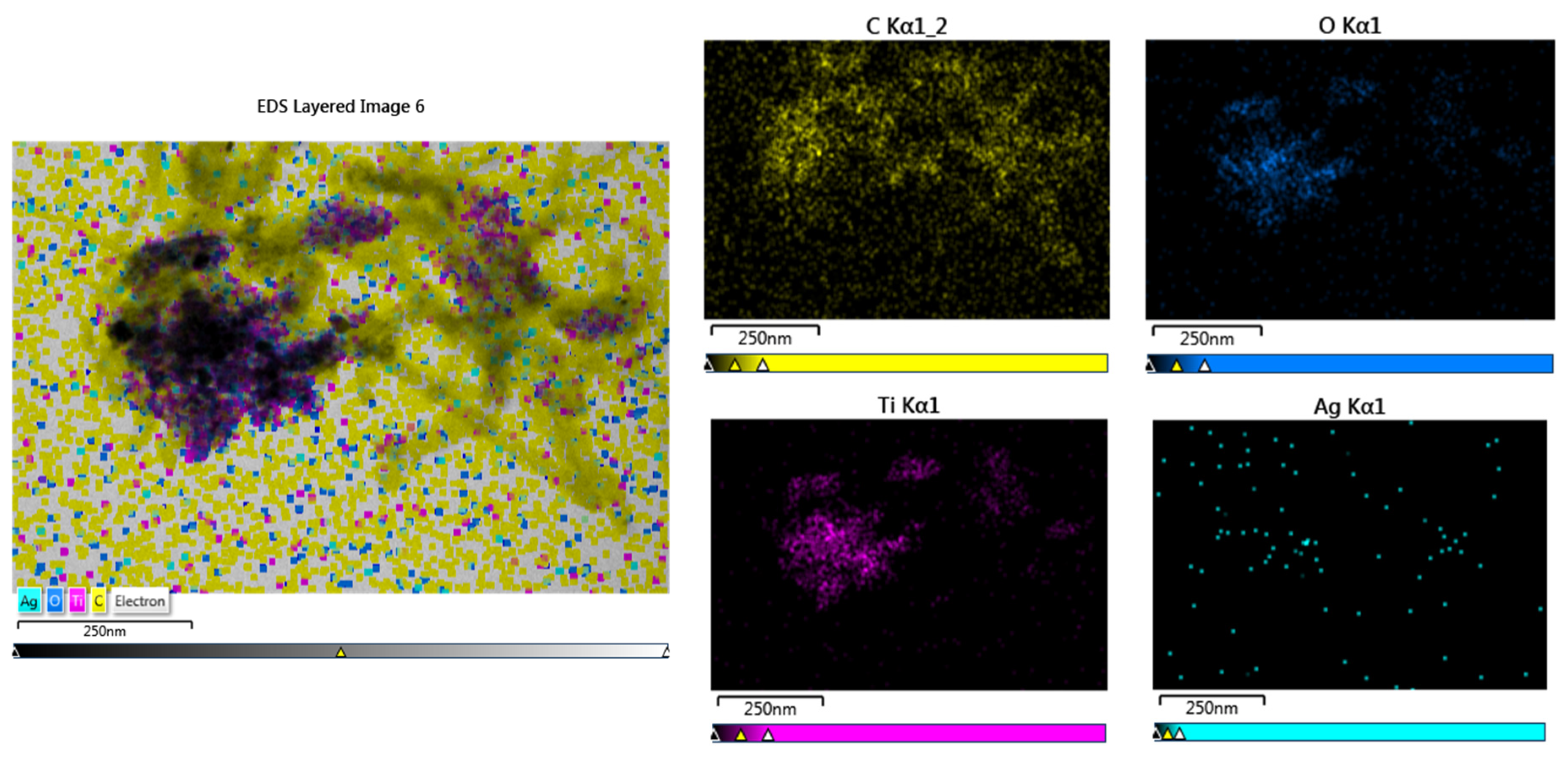
2.8. Morphology of nanocomposites
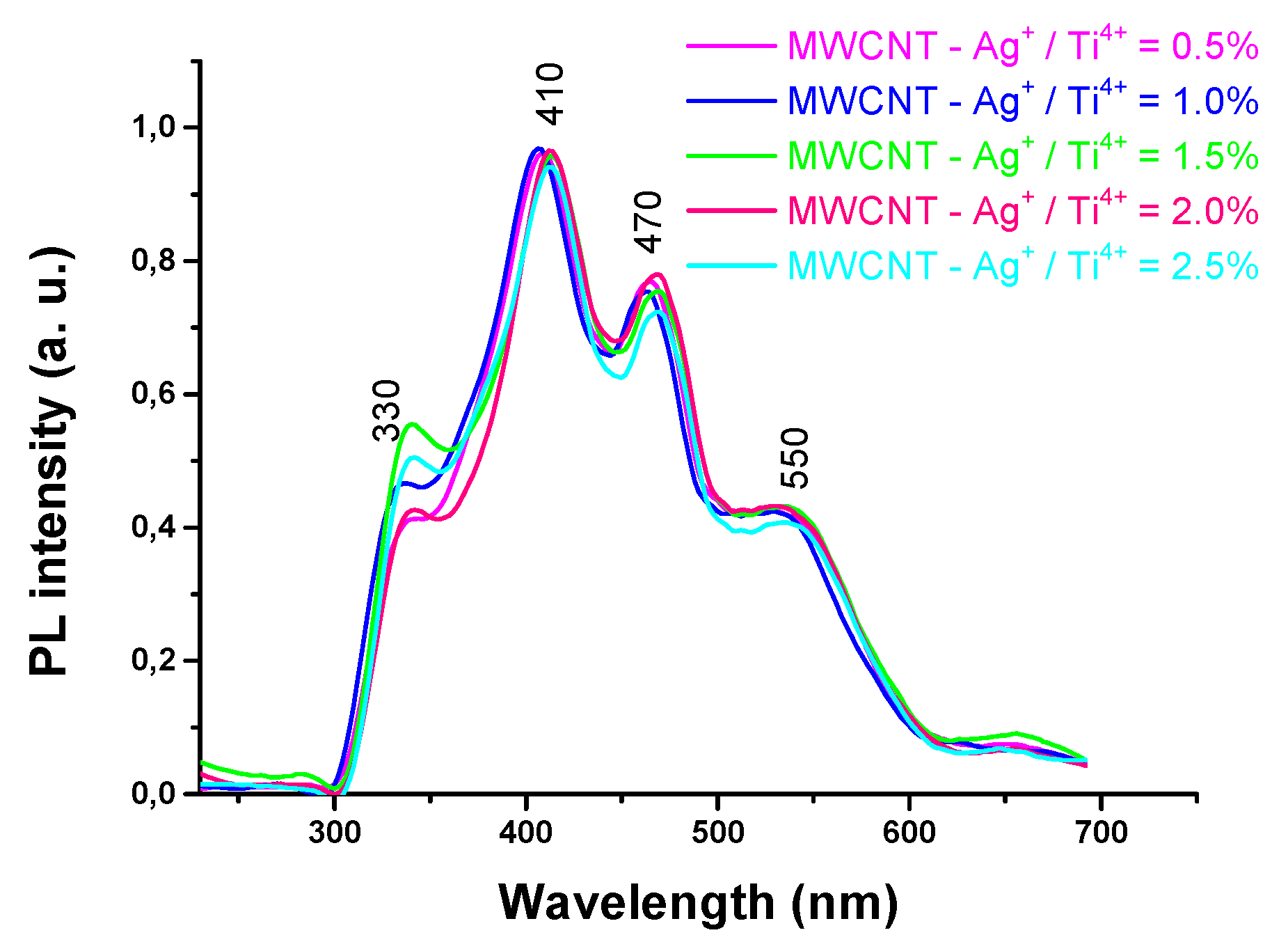
2.11. Photoluminescence spectroscopy.
2.12. Photocatalytic activity.
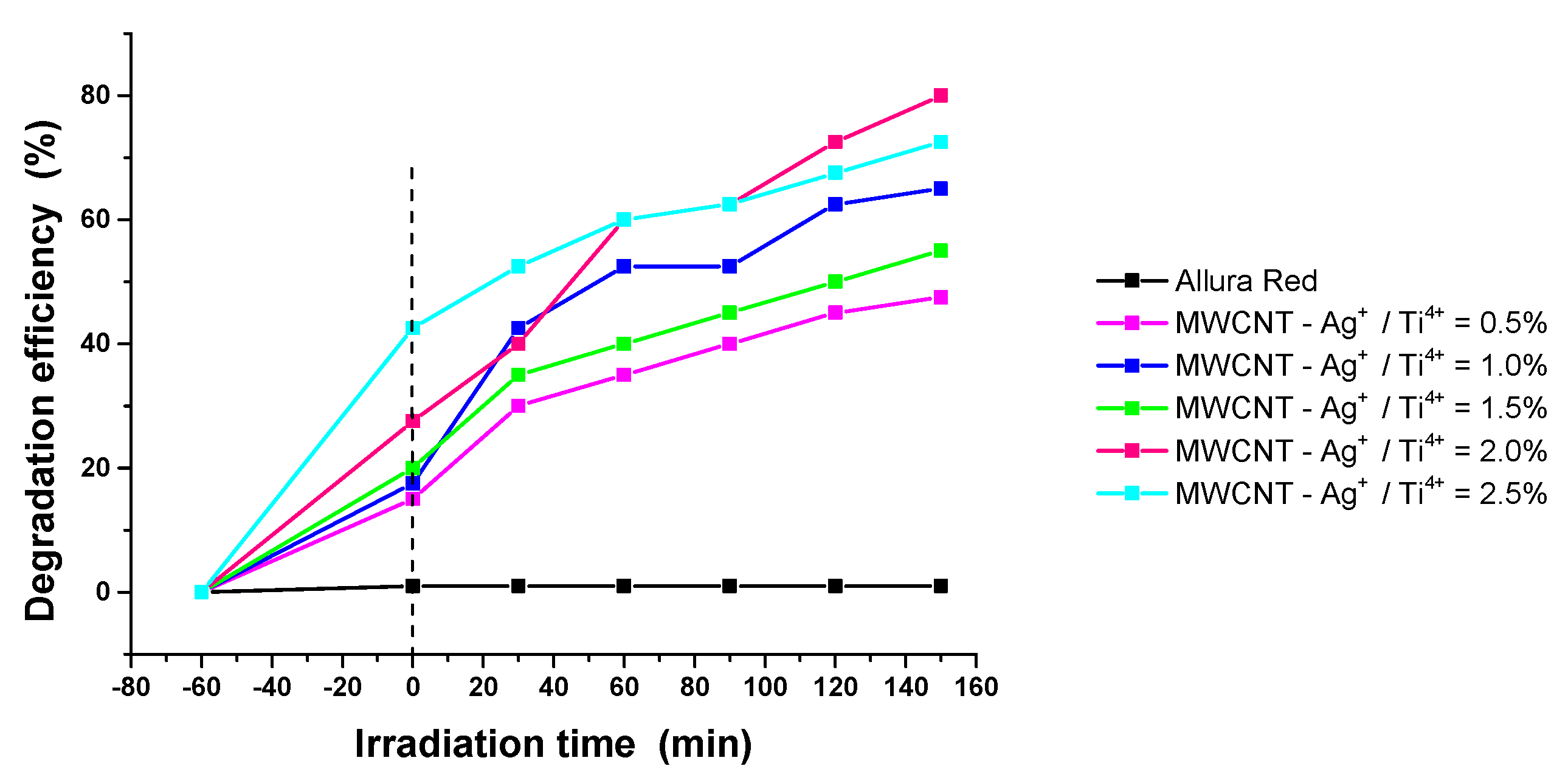
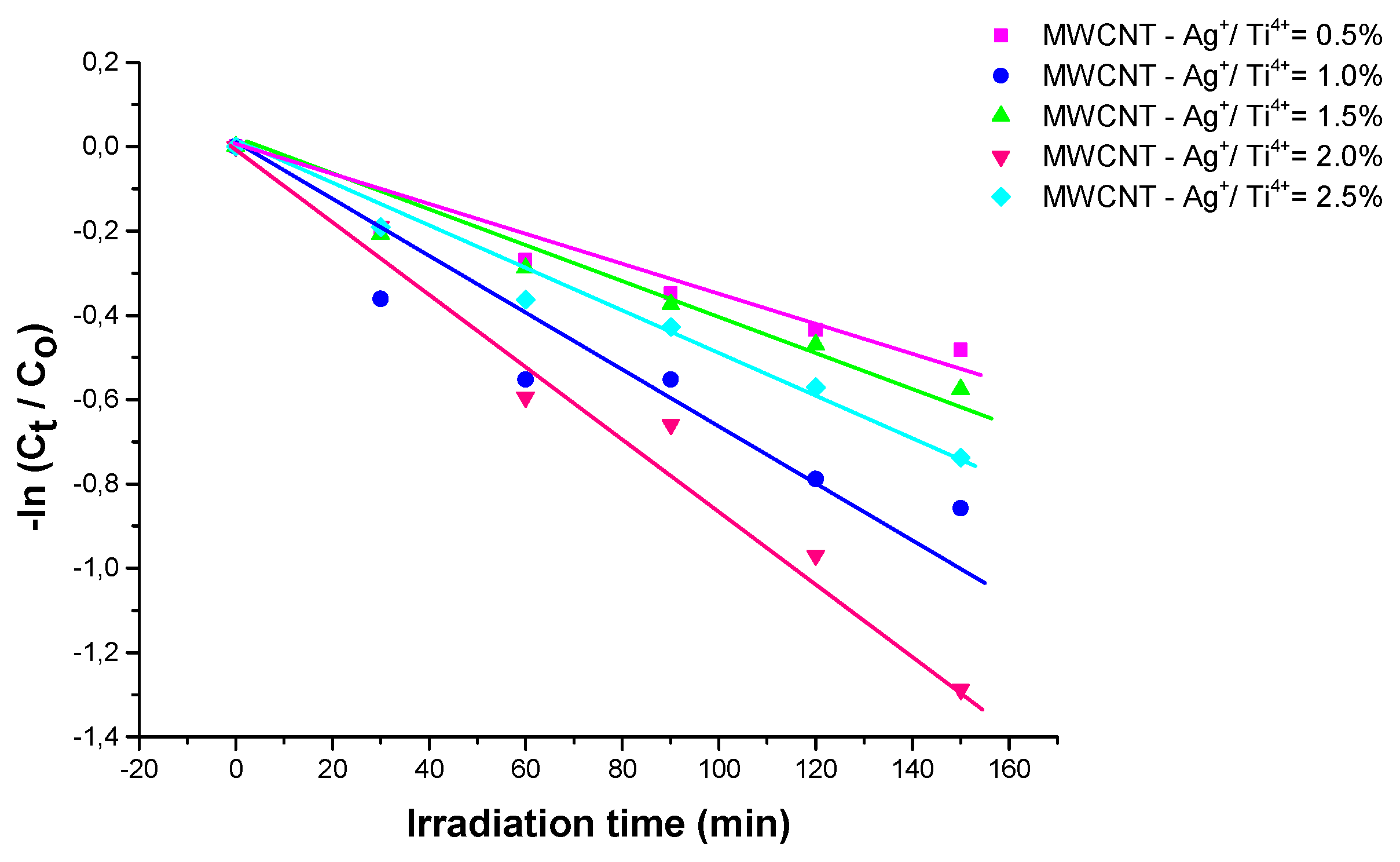
| Sample | Photodegradation rate (%) |
|
R2 | |
| MWCNT - Ag+/ Ti4+ = 0.5% | 47.5 | 3. 06 | 0.95097 | |
| MWCNT - Ag+/ Ti4+ = 1.0% | 65 | 5.30 | 0.91623 | |
| MWCNT - Ag+/ Ti4+ = 1.5% | 55 | 3.57 | 0.97206 | |
| MWCNT - Ag+/ Ti4+ = 2.0% | 80 | 8.42 | 0.98006 | |
| MWCNT - Ag+/ Ti4 += 2.5% | 72.5 | 4. 66 | 0.98538 |
4. Materials and Methods
4.1. Materials.
4.2. Sample preparation.
4.2.1. Functionalization of MWCNTs
4.2.2. Synthesis of TiO2 modified by Ag
4.3. Decoration of MWCNT with TiO2 modified by Ag
4.4. Characterisation
5. Conclusions
Conflicts of Interest
References
- Zhao, D.X.; Cai, C. Cerium-based UiO-66 metal-organic framework for synergistic dye adsorption and photodegradation: A discussion of the mechanism. Dyes Pigments 2021 185 (3) 108957. [CrossRef]
- Xiang, C.; Wang, W.; Liu, S; Huang, Y., Li; M.; Wang, D. Humidity-stimulated film actuator with dual-responsive of bending deformation and discoloration. Sens Actuators B Chem 2023 380 133344. [CrossRef]
- Rajakani, V.; Sahaya Shajan, X., Arulgnanam, A.; Sumithraj Premkumar, P. Photovoltaic studies on iodine incorporated titania aerogel nanocomposites. Results in Optics 2023, 10, 100346. [CrossRef]
- Mandić, V.; Panžić, I.; Brnardić, I.; Jajčinović, I.; Mičetić, M. Lateral and vertical evolution of the compositional and morphological profiles in nanostructured photocatalytic titania thin films. Appl Surf Sci 2023 613 56047. [CrossRef]
- Kausar, F.; Varghese A.; Pinheiro, D.; Sunaja, D. K. R. Recent trends in photocatalytic water splitting using titania based ternary photocatalysts-A review. Int J Hydrog Energy 2022 47 (53) 22371-22402. [CrossRef]
- Sadia, M.; Naz, R.; Khan, J.; Zahoor, M.; Ullah, R.; Khan, R.; Naz, S.; Almoallim, H. S.; Alharbi, S. A. Metal doped titania nanoparticles as efficient photocatalyst for dyes degradation. J King Saud Univ Sci. 2021 33 (2) 101312. [CrossRef]
- Ahmed, D. S.; Mohammed, M. K. A.; Mohammad, M. R. Sol–gel synthesis of Ag-doped titania-coated carbon nanotubes and study their biomedical applications. Chem Zvesti 2020 74 197–208. [CrossRef]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R. P.; Gopinath, K. P.; Lichtfouse, E. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environ Chem Lett 2023 21 339 – 362. [CrossRef]
- Cringoli, M. C.; Perathoner, S.; Fornasiero, P.; Marchesan, S. Carbon nanostructures decorated with titania: Morphological control and applications. Appl Sci 2021 11 6814. [CrossRef]
- Rajakani, V.; Sahaya, X.; Shajan, A.; Arulgnanam, Sumithraj Premkumar, P. Studies on the silver incorporated titania aerogel nanostructure as a photoanode in quasi solid dye- sensitized solar cells. Mater Today Proc 2022 65 (5) 2473-2479. [CrossRef]
- Topolski, A. Functionalization of titania nanotubes surface with platinum(II) complexes. Polyhedron 2023 230 116218. [CrossRef]
- Paszkiewicz, O.; Wang, K.; Rakoczy, R.; Kordas, M.; Leniec, G.; Kowalska, E.; Markowska-Szczupak, A. Antimicrobial properties of pristine and Pt-modified titania P25 in rotating magnetic field conditions. Chem Eng Process 2022 178 109010. [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A. el kacem; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalyticand bactericidal behaviors. Environ Sci Pollut Res 2021 28 44638–44666. [CrossRef]
- Sohrabi, L.; Taleshi, F.; Sohrabi, R. Effect of carbon nanotubes support on band gap energy of MgO nanoparticles. J Mater Sci: Mater Electron 2014 25 4110–4114. [CrossRef]
- Sameera, I.; Bhatia, R.; Prasad, V. Preparation, characterization and electrical conductivity studies of MWCNT/ZnO nanoparticles hybrid. Physica B: Condensed Matter 2010 405 1709-1714. [CrossRef]
- Younas, M.; Gondal, M.,A.; Dastageer, M.,A.; Harrabi, K. Efficient and cost-effective dye-sensitized solar cells using MWCNT-TiO2 nanocomposite as photoanode and MWCNT as Pt-free counter electrode. Solar Energy 2019 188 1178-1188. [CrossRef]
- Hosny, N. M.; Gomaa, I.; Elmahgary, M. G. Adsorption of polluted dyes from water by transition metal oxides: A review, Appl Surf Sci Adv 2023 15 100395. [CrossRef]
- Li, W.;, Wang, B.;Yuan, Y. ; Wang, S. Spatiotemporal distribution patterns and ecological risk of multi-pesticide residues in the surface water of a typical agriculture area in China, Sci Total Environ 2023 870 161872. [CrossRef]
- Jia, W.-L.; Song, C.; He, L. -Y.; Wang, B.; Gao, F. - Z.; Zhang, M.; Ying. G.- G. Antibiotics in soil and water: Occurrence, fate, and risk, Curr Opin Environ Sci Health 2023 32100437. [CrossRef]
- Barboux – Doeuff, S.; Sanchez, C. Synthesis and characterization of titanium oxide – based gels synthesized from acetate modified titanium butoxide precursors. Mat Res Bull 1990 29 41-13. [CrossRef]
- Devi Gamathi, L., Nagaraj, B., Eraiah Rajashekhar, K. Synergetic effect of Ag concentration and nitrogen doping in TiO2 for the degaradtion of phenol under solar irradiation in presence of electron acceptor. Chem Eng J 2012 181 – 182 259 -266. [CrossRef]
- Bouazza, N.; Ouzzine, M.; Lillo – Rόdenas, M. A.; Eder, D.; Linares – Solano, A. TiO2 nanotubes and CNT – TiO2 hybrid materials for the photocatalytic oxidation of propene at low concentration. Appl Catal B – Environ 2009 92 377 – 383. [CrossRef]
- Akbarzadeh, R.; Ghaedi; M., Kokhdan, S. N.;Vashaee, D. Remarkably improved electrochemical hydrogen storage by multi-walled carbon nanotubes decorated with nanoporous bimetallic Fe–Ag/TiO2 nanoparticles. Dalton Trans 2019 48 898. [CrossRef]
- Ashkarran, A. A.; Fakhari, M.; Hamidinezhad H.; Haddadi, H.; Nourani, M. R. TiO2 nanoparticles immobilized on carbon nanotubes for enhanced visible - light photo – induced activity. J Mater Res Technol 2015 4 (2) 126 – 132. [CrossRef]
- Yuliati, L.; Kimi, M.; Shamsuddin, M. High activity of Ag-doped Cd0.1Zn0.9S photocatalyst prepared by the hydrothermal method for hydrogen production under visible-light irradiation. Beilstein J Nanotechnol 2014 5 (587–595. [CrossRef]
- Aldea, N.; Indrea, E. XRLINE, a program to evaluate the crystallite size of supported metal-catalysts by single X-ray profile fourier-analysis. Comput Phys Commun 1990 60 155-159. [CrossRef]
- Indrea, E., Barbu, A. Indirect photon interaction in PbS photodetectors, Appl Surf Sci 1996 106 498. [CrossRef]
- Berkum, J. G. M. van; Vermeulen, A. C.; Delhez, R.; Keijser, T. H. de; Mittemeijer, E. M.. Applicabilities of the Warren-Averbach analysis and an alternative analysis for separation of size and strain broadening, J Appl Cryst 1994 27 345 - 357 . [CrossRef]
- Kraus, W.; Nolze, G. POWDER CELL — a Program for the Representation and Manipulation of Crystal Structures and Calculation of the Resulting X-ray Powder Patterns". Appl Cryst 1996 29 301—309. [CrossRef]
- Thiel, J.; Pakstis, L.; Buzby, S.; Raffi, M.; Ni, C.; Pochan, D. J.; Ismat Shah, S. Antibacterial properties of silver-doped titania, Small 2007 3 (5) 799–803. [CrossRef]
- Soler-Illia, G. J. de A. A.; Louis, A.; Sanchez, C. Synthesis and characterization of mesostructured titania-based materials through evaporation-induced self-assembly. Chem Mater 2002 14 (2) 750–759. [CrossRef]
- Chen, C.S.; Liu, T.G.; Lin, L.W.; Xie, X.D.; Chen, X.H.; Liu, Q.C.; Liang, B.; Yu, W.W.;. Qiu, C. Y. Multi-walled carbon nanotube-supported metal-doped ZnO nanoparticles and their photocatalytic property. J Nano Res 15 2013 1295–1304. [CrossRef]
- Alsharaeh, H. ; Bora, T.; Soliman, A.; Ahmed, F.; Bharath, G.; Ghoniem, M. G.; Abu-Salah, K. M.; Dutta, J. Sol-Gel-Assisted Microwave-Derived Synthesis of Anatase Ag/TiO2/GO Nanohybrids toward efficient visible light phenol degradation, Catalysts 2017 7 (5) 133. [CrossRef]
- Zhang, H.; Wang, X; Xia, J.; Meng, Q.; Ding, J.; Lu, J. Synthesis andcharcterisation of TiO2 / graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate, RSC Adv 2018 8 (60) 34241-34251. [CrossRef]
- Abbasi, S.; Zebarjad, S. M.; Bogdan, S. H. Noe; Yousesfi, A. Synthesis of TiO2 nanoparticles and decorated multi-walled carbon nanotubes with various content of rutile titania. Synth React Inorg M 2015 45 10. [CrossRef]
- Ohsaka, T. Izumi, F., Fujiki, Y. Raman Spectrum of Anatase TiO2. J Raman Spectroscopy 1978 7 (6) 321-324. [CrossRef]
- Misra, K.; Andronenko, S. I.; Tipikin, D.; Freed, J. H.; Somani V.; Prakash, O. Study of paramagnetic defect centers in as-grown and annealed TiO2 anatase and rutile nanoparticles by a variable-temperature X-band and high-frequency (236GHz) EPR. J Magn Magn Mater 2016 401 495–505. [CrossRef]
- Caretti, I.; Keulemans, M.; Verbruggen, S. W.; Lenaerts, S.; Doorslaer, S. Van. Light-Induced Processes in Plasmonic Gold/TiO2 Photocatalysts Studied by Electron Paramagnetic Resonance. Top Catal 2015 58 776–782. [CrossRef]
- Grabowska, E., Marchelek,.M., Klimczuk, T., Trykowski, G., Zaleska-Medynska, A. Noble metal modified TiO2 microspheres: Surface properties and photocatalytic activity under UV–vis and visible light. J Mol Catal A Chem 423 191–206 . [CrossRef]
- Popa, A.; Stefan, M.; Macavei, S.; Muresan, L. E.; Leostean, C.; Floare-Avram, C. V.; Toloman, D. Photoluminescence and photocatalytic properties of MWNTs decorated with Fe-doped ZnO nanoparticles. Materials 2023 16(7) 2858. [CrossRef]
- He, J.; Ichinose, I.; Kunitake, T.; Nakao, A. In situ synthesis of noble metal nanoparticles in ultrathin TiO2-gel films by a combination of ion-exchange and reduction processes, Langmuir 2002 18(25) 10005-10010. [CrossRef]
- Nguyen, M. T.; Nguyen, C. K.; Vu, T. M. P.; Duong, Q.V.; Pham, T. L.; Nguyen. T. C.. A study on carbon nanotube titanium dioxide hybrids: Experiment and calculation. Nanosci Nanotechnol 2014 5 045018. [CrossRef]
- Siwach, O. P.; Sen, P. Fluorescence properties of Ag nanoparticles in water, methanol and hexane. J Lumin 2009 129 (1) 6 - 11. [CrossRef]
- Chrysicopoulou, P.; Davvazoglou, D.; Trapalis, Chr.; Kordas, L. G. Optical properties of very thin (<100 nm) sol–gel TiO2 films. Thin Solid Films 1998 323 188 -193. [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium, Phys Status Solidi 1966 15 627–637 . [CrossRef]
- Li, Y.; Ma, M.; Chen, W., Li. L.; Zen, M. Preparation of Ag – doped TiO2 nanoparticles by a miniemulsion method and their photoactivity in visible light illuminations. Mat Chem Phys 2011 129 501-505. [CrossRef]
- Chen, X.; Luo, W. Optical spectroscopy of rare earth ion-doped TiO2 nanophosphors. J Nanosci Nanotechno 2010 10 482–1494. [CrossRef]
- Selvam, K.; Swaminathan, M. Cost effective one-pot photocatalytic synthesis of quinaldines from nitroarenes by silver loaded. TiO2. J Mol Catal A - Chem 2011 351 52–61. [CrossRef]
- Lipping, W.; Baoshun, L.; Chao, L.; Xiujian, Z. Preparation, characterisation and photocatalytic property of Ag-loaded TiO2 powers using photodeposition method, J. Wuhan Univ Techno.-Mat Sci Edit 2009 24 258–263. [CrossRef]
- Zu, J.; Xiong J.; ChenB.; Liu, S. Fabrication and characterization of Ag–TiO2 multiphase nanocomposite thin films with enhanced photocatalytic activity. Applied Catal B 2005 60 211-221. [CrossRef]
- Falaras, P.; Arabatzis, I. M.; Stergiopoulos T.; Bernard, M. C. Enhanced activity of silver modified thin-film TiO2 photocatalysts. Int J Photoenergy 2003 5 123 – 130. [CrossRef]
- Sobczyński, A.. Photoassisted hydrogen production from a methanol-water mixture on platinized Cr2O3-doped TiO2. J Mol Cat 1987 39 45 – 53. [CrossRef]
- Klein, S. M.; Cohen, G.; Cederbaum, A. I. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical-generating systems. Biochemistry 1981 20 6006–6012. [CrossRef]
- Alyani, S.J.; Pirbazari, A.E.; Khalilsaraei, F. E.; Kolur, N.A.; Gilani. N. Growing Co-doped TiO2 nanosheets on reduced graphene oxide for efficient photocatalytic removal of tetracycline antibiotic from aqueous solution and modeling the process by artificial neural network. J Alloy Compd 2019 799 169–182. [CrossRef]
- Diaz-Uribe, C. E.; Daza, M.; Martínez, C.; Páez-Mozo, F.; Guedes; E. A.; Mauro, C. L. B.; Di. E. Visible light superoxide radical anion generation by tetra(4-carboxyphenyl) porphyrin /TiO2: EPR characterization. J Photochem Photobiol A 2010 215 172–178. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





