1. Introduction
Pneumonia is one of the largest killer of children under the age of five worldwide which kills over two million children under the age of five every year [
1,
2]. WHO developed standard guidelines in 2011 for management of patients to reduce the number of people dying from pneumonia [
3].
The guidelines have been used widely in several less-developed countries for many years and recommend five days of oral amoxicillin for treatment of non-severe pneumonia [
4]. In additional, transport to a distant facility can entail serious delays in effective treatment. Many children with severe pneumonia referred for admission to a hospital could die in transit or reach too sick to be saved [
5].
Antimicrobial resistance rate is growing health problem and concern widely, studies that were conducted in different countries addressed such barriers to the recommended treatment of severe pneumonia. [
6].
Furthermore, the study revealed whether oral antibiotics are equivalent to injectable antibiotics when both are given in the hospital. This was an open label equivalency study called Amoxicillin Penicillin Pneumonia International Study (APPIS), which was a large multicenter randomized controlled trial comparing injectable penicillin versus oral amoxicillin given for 7 days to children in the hospital [
7].
In addition, New Outpatient Short-Course Home Oral Therapy for Severe Pneumonia Study (NOSHOTS) was a randomized, open-label equivalency trial done at seven study sites in Pakistan and compared initial hospitalization and parenteral ampicillin for 48 h followed by 3 days of oral amoxicillin at home, to 5 days of home-based treatment with oral amoxicillin [
8]. NOSHOTS showed home treatment with high dose oral amoxicillin is equivalent to hospital-based treatment with parenteral ampicillin in selected children aged 3–59 months with WHO defined severe pneumonia [
8].
Later, another study called Multicenter Amoxicillin Severe Pneumonia Study (MASS) showed that clinical treatment failure and adverse event rates among children with severe pneumonia treated at home with oral amoxicillin did not substantially differ across geographic areas (Bangladesh, Ghana, Vietnam, and Egypt) and hence home-based therapy of severe pneumonia could possibly be applied to a wide variety of settings [
9].
The Lancet Series on Childhood Pneumonia and Diarrhea has reported that case management is one of the three most effective interventions to reduce pneumonia deaths in children but also noted that the cost effectiveness of these interventions in national health systems needs urgent assessment [
10].
The conflict in Yemen has devastated the health system, with only 51% of health facilities classified as fully functional and 19.7 million people lacking access to health care. To address the urgent need for primary health care services in rural communities, [
11] This lead to increase the acute respiratory infections (ARI) which is a leading cause of mortality and morbidity among children under 5 of age in Yemen. In a recent epidemiological survey, 49% of this age group had cough and 25% had cough with difficult breathing during the 2 weeks prior to the survey. [
11,
12].
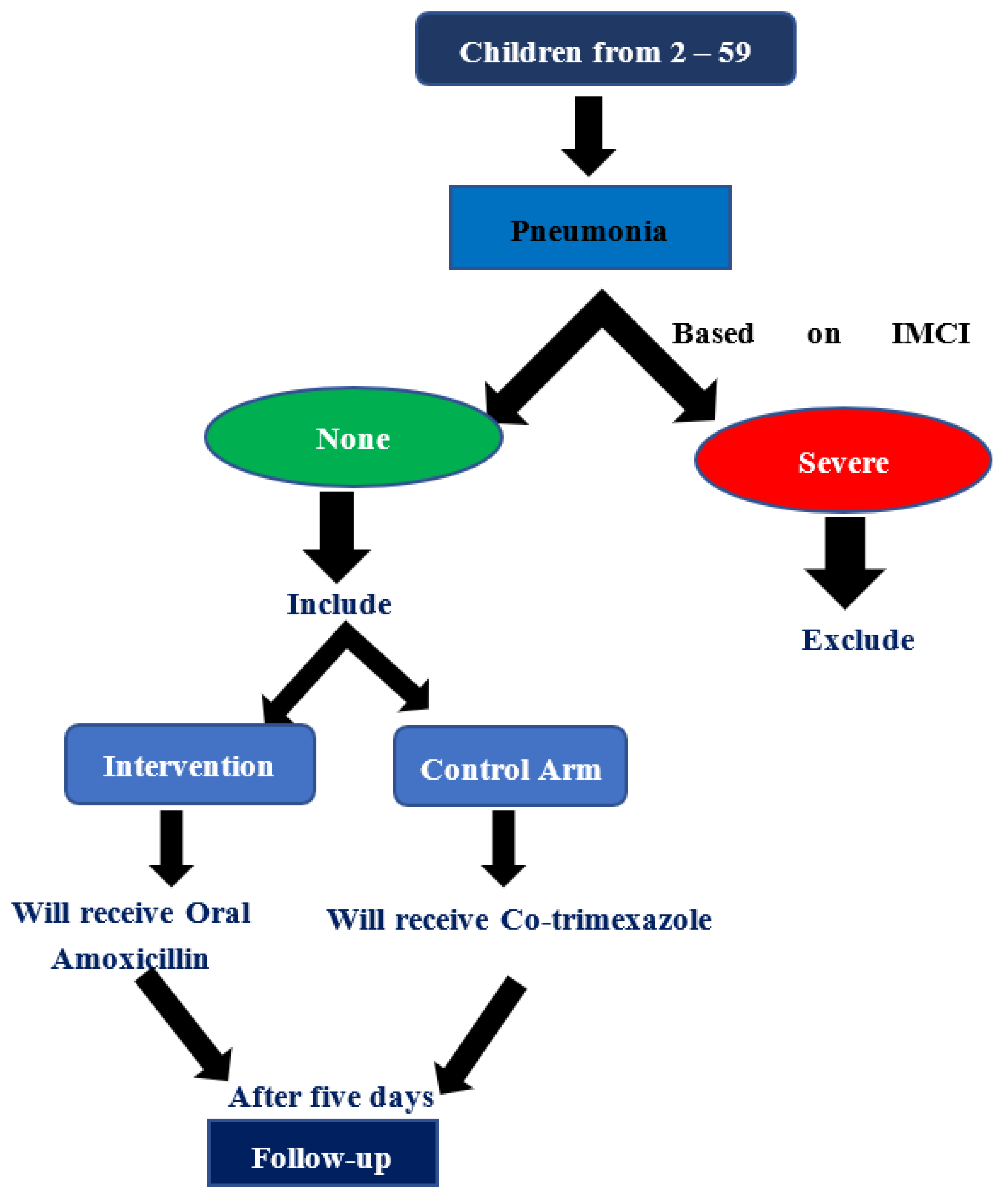
Therefore, our study hypothesis (null) was the clinical failure rate with Amoxycillin as the first line based on the Integrated Management Childhood Illness (IMCI) strategy or Co-trimoxazole antibiotic treatment would be similar in children with non-severe pneumonia.
General objective:
To assess the IMCI strategy in the primary health care centers (PHCCs) is beneficial for use of amoxicillin in treatment of childhood non-sever pneumonia.
Specific objectives:
1. To determine the effectiveness of antibiotic amoxicillin for treatment of childhood non-severe pneumonia.
2. To identify the risk factors associated with clinical failure.
3. Provide policy makers with evidence to make a decision.
2. Materials and Methods
2.1. Study design
A randomized controlled trail was conducted from August to October 2021.
2.2. Study setting and population.
This study was conducted at PHCCs in Sana’a governorate, which supported by World Bank. All children with age group of 2 months to 5 years, who attending the PHCCs for non-severe pneumonia as IMCI classify strategy were included in the study.
2.3. Definitions and selection criteria:
• Exposed arm: All cases with non-sever pneumonia who were treat with Oral Amoxicillin.
• Non-exposed arm: All cases with non-sever pneumonia who were treat with oral co-trimoxazole instead of amoxicillin.
• The clinical cure was defined as respiratory rate of less than 50/minute between 2 months to 12 months of age and less than 40/minute between 1 year to 5 years of age and absence of any of clinical signs of treatment failure.
• The treatment failure was defined as occurrence of any signs of WHO defined of severe pneumonia, increase respiratory rate of more than or equal 50/minute between 2 months to 12 months of age and more than or equal 40/minute between 1 year to 5 years of age. [
13]
• Inclusion Criteria: based on IMCI strategy, any cases of non-sever pneumonia, who is attending to PHCCs and will receive oral amoxicillin or any other antibiotics.
•
Exclusion Criteria: The cases were excluded if they have WHO signs of severe pneumonia, history of having received antibiotics for any illness anywhere 48 hours before coming to the PHCCs, previous history of wheezing including asthma or children who have been prescribed corticosteroids along with bronchodilators, children with congenital heart disease, immunodeficiency (congenital or acquired) including suspected or confirmed HIV infection, any chronic illness including chronic infections like tuberculosis, malignancy, acute/chronic organ disorder, known allergy/ hypersensitivity to penicillin. [
14]
• Follow-up of cases:
The physicians assessed every child in first day of visiting, primary follow up will be after 2 days of starting treatment, while the final follow up will be after 5 days of treatment, treatment was stopped after 5 days if respiratory rate had returned to normal.
2.4. Sample size:
The sample size calculated using open Epi program with based on alpha of 0.05, Confidence Interval (CI) of 95%, power of 80%, old ratio 2.5 and assuming a treatment failure rate of expose and non- expose group according to previous studies (7). The sample size comes to 228. we increased the sample by 10% to be 254 to overcome refusal or loss to follow-up.
2.5. Sampling procedures:
The sample was selected from 30 % of districts in Sana’a governorate through choosing the main PHCCs in each district, who applied the IMCI strategy, the sampling interval will be calculated form the main HFs. Randomization of the intervention and control arms for each targeted health facility by Proportion Probability Size (PPS) which is depending on the average of the last three months reports of the non-severe pneumonia cases, the randomization selection generated by the Open Epi Random Program Version 3 which available at:
www.openepi.com (Annex I).
2.6. Data Collection:
Data were collected through face-to-face interview with parents of the children using IMCI registration form and a predesigned questionnaire. The questionnaire that includes closed and open questions which cover demographic, socioeconomic characteristics, and clinical features with follow up.
One data collector (doctor) that will administer the questionnaire and one sample collector (lab technician) to collect blood sample will be chosen from the staff at each center. Those will be trained on data and sample collection under direct supervision of the principal investigator. All cases were reviewed after 2 days and then five days after starting treatment.
The effectiveness and therapy failure were decided based on clinical, radiological, and complete blood count results.
2.7. Variables
The following variables will be gathered for the study:
• Demographical data such as name, sex, age, place of birth, residency, ...etc.
• Clinical data such as symptoms, signs …etc.
• Dates such as date of diagnosis, date of start onset, date of follow-up, …etc.
• Investigation such as CBC, Nasopharyngeal swab, and chest X-ray result.
• Outcome such as clinically cured, failure of treatment.
2.8. Laboratory testing and procedures:
Three ml whole blood sample was collected twice before treatment and after 5 days of treatment from each case in a labeled plain tube with the unique identifier number. Only CBC will be measured. Regarding the failure of treatment, a nasopharyngeal swab will be taken from the cases after three days from stopping the antibiotics (Amoxicillin or Co-trimoxazole) then send the swab to the national laboratories to do culture and sensitives test.
2.9. Analysis and data management:
Data were entered and analyzed using Epi info 7.2 version. The Chi-squared test will be used for categorical variables and T-test for continuous one. For comparing clinical failure in two interventions, Kaplan Meier survival curve analyses were employed by comparing the means and probability of survival for the two arms (groups) of cases to find any difference in response due to antibiotics therapy, P value <0.05 is considered a statistically significant cut off point.
2.10. Quality assurance and control:
The quality assurance was secured during data collection through proper training of study members on the objectives of the study and how to collect the data through the questionnaire and ensure proper case definition using during samples collection. After collection of data, the investigator will follow up the cases in a daily basis. After collections of the questionnaires, the investigator will check data for completeness and accuracy to make sure that data are correctly taken. After completing data entry, cleaning will be taken by checking frequencies for each variable looking for inconsistencies and out-of-range values.
2.11. Ethical considerations:
Study was submitted to the and approved by the Institutional Review Board of the National Committee for Health and Medical Research at the ministry of Public Health and Population (protocol code 103 and date of approval 13/8/2021). Permission from IMCI will be secured and written consent will be taken from parents of children. Privacy will be maintained during interviews, questionnaires kept in a lockable cabinet and data entered in the computer was password protected.
3. Results
3.1. Cases characteristics (Descriptive Analysis)
A total of 269 children were randomly enrolled to study, 262 cases of them were well-matched to inclusion and exclusion criteria of this study, 97% of them (254 cases) were followed up and committed until the end of the study, of whom 128 cases were treated with five days amoxicillin while 126 cases in the five days co-trimoxazole. as clarified in the trail profile ((
Figure 2).
Table 1 shows the demographic characteristics of childhood non-severe pneumonia cases. Male (64%), farmer jobs of the fathers (61%), children aged less than 12 months (53%), and the primary or secondary education level of fathers (50%) formed the majority of cases. The overall median age of children was 12 months (rang: 2.5 - 59), the overall median wight of children was 8 kilograms (range: 4 - 17.5), and the overall mean monthly income was 30,830 YER (±12,637).
100 % of cases were their mothers' housewife, 35 % of cases enrolled from Bani matter district and 94 % of cases were their own houses. This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
Table 2 show the last 6 months medical characteristics of childhood non-severe pneumonia cases, 122 cases (48%) had a previous history of pneumonia infection during the last 6 months, most of them 85% used antibiotics, 60% of them were syrup in type, the final outcome was cured and represented 89%.
Table 3: shows the clinical presentation, sings, laboratory finding of childhood non-severe pneumonia cases, the mean of temperature, respiratory rate, and T.WBC 37.7, 51.7, and 11.2, respectively. The common symptoms of cases were cough, difficulty of breath and fever 99 %, 97 % and 82 %, respectively. In addition, the common measured signs were tachypnea 100%, fever (axillary 37.2<) 70%, chest indrawing 41%, and under wight (Z score > -2) 40%. The lab findings show abnormal deferential blood count (CBC) which represent 61%, out of 51 failure of treatment cases, 80% conduct nasopharyngeal swab, 49% of them were Staph. aureus while 7% no growth, nasopharyngeal sensitivity significantly show resistance for amoxicillin vs co-trimoxazole (91% vs 9%). However, there were statistically significant differences between two arms in the diarrhea, malnutrition, otitis media, CBC, and nasopharyngeal sensitivity.
3.2. Clinical outcome:
The clinical effectiveness of two arms of antibiotics were shown in (
Table 4), which was the failure of treatment for amoxicillin significantly more than co-trimoxazole based on the IMCI failure criteria (30% vs 10%, p value > 0.001).
3.3. Predictors (Risk factors) associated with clinical failure:
Risk factors associated with clinical failure bivariate analysis of two groups of antibiotics with baseline variables are given in (
Table 5).
The multivariate analysis was done to estimate the treatment effect after considering the imbalance in certain baseline variables. Only pre-infection in the last 6 months, malnutrition, abnormal CBC, and high-grade fever were found to be associated with clinical failure of amoxicillin even after adjustment (p > 0.05) while abnormal CBC and literate mothers associated with clinical failure of co-trimoxazole (p > 0.05) (
Table 6).
There were no deaths and only 3 cases were hospitalization from amoxicillin arm, there were no serious adverse drug reactions in the amoxycillin or co-trimoxazole arm, four cases on co-trimoxazole drop out during follow up while one on amoxicillin.
4. Discussion
Globally, most deaths due to childhood pneumonia occur at the community level (1) and increasing concern over bacterial resistance to amoxicillin, which is recommended by WHO and IMCI strategy as a first-line drug for treating non-severe pneumonia, led to the suggestion that this might not be optimal therapy. (3) However, changing to alternative antimicrobial agents which is the second line drug, such as co-trimoxazole, is more effective. (4)
During the period of study, almost 53% of the cases were infants below the age of one years, and this is in concordance with other trail conduct in India that have shown maximum no. of cases in the age group below one years (9).
Male preponderance was noticed in our study with male: female ratio being 1.7:1. This is like the findings of other studies (8,9,10). The higher incidence among males might be due to their increase exposure to outdoor activities which make them in a high risk to be sick with pneumonia.
Regarding clinical features, cough, fast breathing, and fever are the dominant symptoms in addition to runny nose; all of these are the most common symptoms reported throughout the literature on non-severe pneumonia and agreed with our findings. (8,9,10)
All cases were included in the study based on IMCI classification of clinical criteria of non-severe pneumonia. As tachypnea is the earliest sign of pneumonia before the laboratory evidence of pneumonia occur, is the best method with combination of high sensitivity and specificity to detect pneumonia in children below 5 years of age. (14)
However, in all cases complete blood counts (Hb, T.WBC, Neutrophil were carried out before the treatment and the abnormal cases were repeated after treatment. Our finding shows higher in abnormal findings of CBC (61%) compared to a trail conducted in India (18%). (9)
Our results shows that the clinical efficacy of the amoxicillin is a significantly higher than cotrimoxazole when given in daily basis for five days to treat children with non-severe pneumonia and this is in contrast to study conducted in India (9) but in Pakistan, there was no difference in effectiveness of treating children with non-severe pneumonia with oral co-trimoxazole for 5 days or oral amoxycillin for 3 days. (10)
Relatively high failure rate with amoxicillin in our study might be due to number of factors like the extensive use of newer generations of antibiotics, inappropriate prescribing of antibiotics and use of antibiotics without medical prescription in addition to the availability of those drugs over the count all this lead to use it without return to doctors. (15)
The cure rate among children of intervention group (amoxicillin) was 70% and in control group (co trimoxazole) 90%. Higher cure rate with co-trimoxazole in our study is in contrast to the study conducted in India which was the cure rate of amoxicillin (91%) cases and in co trimoxazole (60%). (9)
The Limitation was the difficulty of the road and the distance of the targeted health facilities from the central laboratory which led to delay to receive the NPS.
5. Conclusions and recommendation
The frequency of treatment failure was higher in Amoxicillin group than in the Co-trimoxazole group among children under 5 years with non-severe pneumonia. The pre-infection in the last 6 months, malnutrition, abnormal CBC, and high-grade fever were the most important associated factors to the failure of treatment of amoxicillin in our study.
Recommendations:
• Oral co-trimoxazole should be used instead of amoxicillin for the treatment of non-severe pneumonia in the IMCI strategy in Yemen.
• Update IMCI strategy is essential in the development of treatment management that are based on clinical outcomes.
• Increase the awareness activities and sessions about antimicrobial resistance.
• Further study to determine the suitable antibiotics instead of amoxicillin as first line for non-severe pneumonia.
Author Contributions
E. Al-Sakkaf contributed to the study conceptualization, collected, analyzed, interpreted the data, and prepared the original draft of manuscript. K. Al-Jamrah and A. Al-Hadi contributed in validation, investigation and data curation, M. Al-Amad contributed in questionnaire design, data analysis and revising the draft, A. Y. Ghaleb contributed in final revising and final approval of the version to be submitted. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by TEPHINET.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the National Committee for Health and Medical Research at the ministry of Public Health and Population (protocol code 103 and date of approval 13/8/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.”.
Data Availability Statement
data is unavailable due to privacy or ethical restrictions.
Acknowledgments
my sincere thanks go to public Health specialist, Data collectors, and Republican Teaching Hospital Authority for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Sample Randomization (N = 250) and flow chart for the data collection
Table 1.
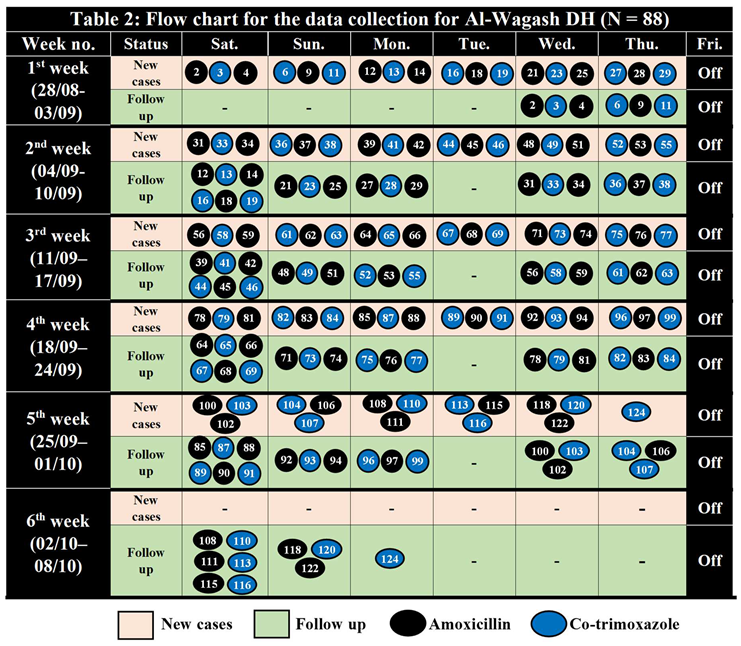
Sample Randomization for Al-Wagash DH (1 – 125) N = 88.
Table 1.
Sample Randomization for Al-Wagash DH (1 – 125) N = 88.
| Intervention Arm (N = 44) |
Control Arm (N = 44) |
| 2 |
31 |
56 |
78 |
100 |
3 |
33 |
58 |
79 |
103 |
| 4 |
34 |
59 |
81 |
102 |
6 |
36 |
61 |
82 |
104 |
| 9 |
37 |
62 |
83 |
106 |
11 |
38 |
63 |
84 |
107 |
| 12 |
39 |
64 |
85 |
108 |
13 |
41 |
65 |
87 |
110 |
| 14 |
42 |
66 |
88 |
111 |
16 |
44 |
67 |
89 |
113 |
| 18 |
45 |
68 |
90 |
115 |
19 |
46 |
69 |
91 |
116 |
| 21 |
48 |
71 |
92 |
118 |
23 |
49 |
73 |
93 |
120 |
| 25 |
51 |
74 |
94 |
122 |
27 |
52 |
75 |
96 |
124 |
| 28 |
53 |
76 |
97 |
|
29 |
55 |
77 |
99 |
|
- 2.
Qa'a Al-Reqah Health Center, Hamdan district
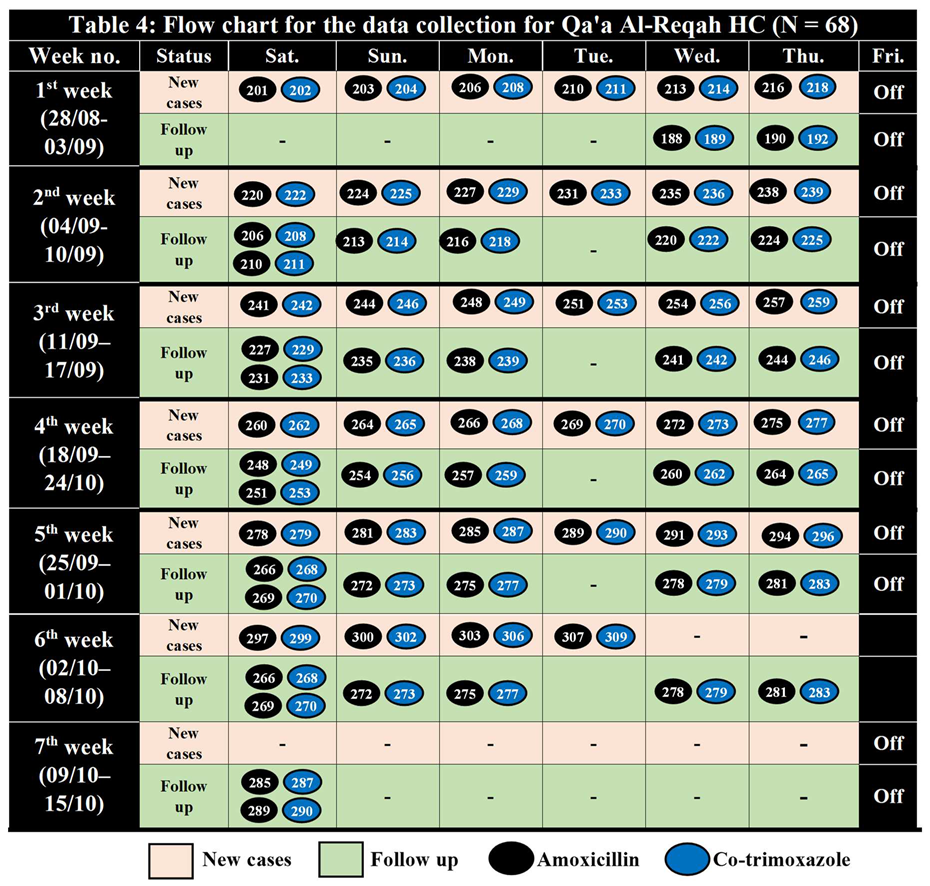
Table 3.
Sample Randomization for Qa'a Al-Reqah HC (200 – 310) N = 68.
Table 3.
Sample Randomization for Qa'a Al-Reqah HC (200 – 310) N = 68.
| Intervention Arm (N = 34) |
Control Arm (N = 34) |
| 201 |
224 |
248 |
269 |
291 |
202 |
225 |
249 |
270 |
293 |
| 203 |
227 |
251 |
272 |
294 |
204 |
229 |
253 |
273 |
296 |
| 206 |
231 |
254 |
275 |
297 |
208 |
233 |
256 |
277 |
299 |
| 210 |
235 |
257 |
278 |
300 |
211 |
236 |
259 |
279 |
302 |
| 213 |
238 |
260 |
281 |
303 |
214 |
239 |
262 |
283 |
306 |
| 216 |
241 |
264 |
285 |
307 |
218 |
242 |
265 |
287 |
309 |
| 220 |
244 |
266 |
289 |
|
222 |
246 |
268 |
290 |
|
- 3.
Bahran Health Center, Sanhan Wa Bni Bahlol District
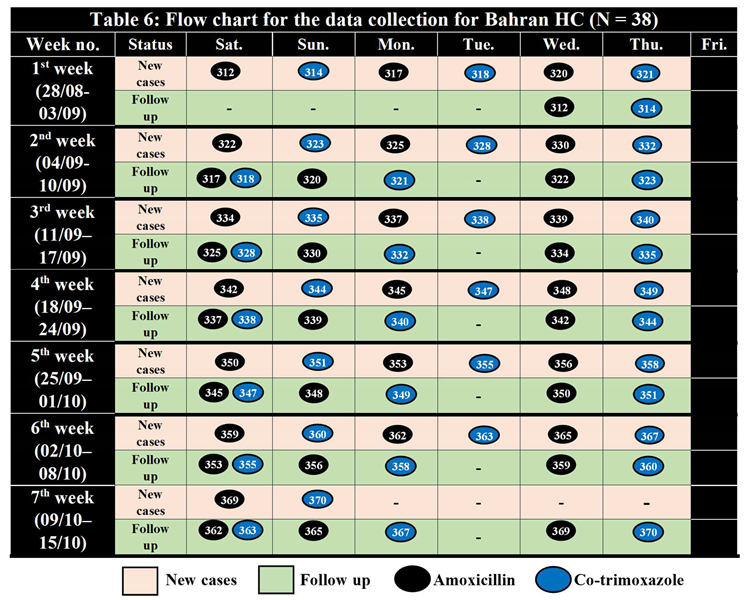
Table 5.
Sample Randomization for Bahran HC (310 – 370) N = 38.
Table 5.
Sample Randomization for Bahran HC (310 – 370) N = 38.
| Intervention Arm (N = 19) |
Control Arm (N = 19) |
| 312 |
325 |
339 |
350 |
362 |
314 |
328 |
340 |
351 |
363 |
| 317 |
330 |
342 |
353 |
365 |
318 |
332 |
344 |
355 |
367 |
| 320 |
334 |
345 |
356 |
369 |
321 |
335 |
347 |
358 |
370 |
| 322 |
337 |
348 |
359 |
|
323 |
338 |
349 |
360 |
|
- 4.
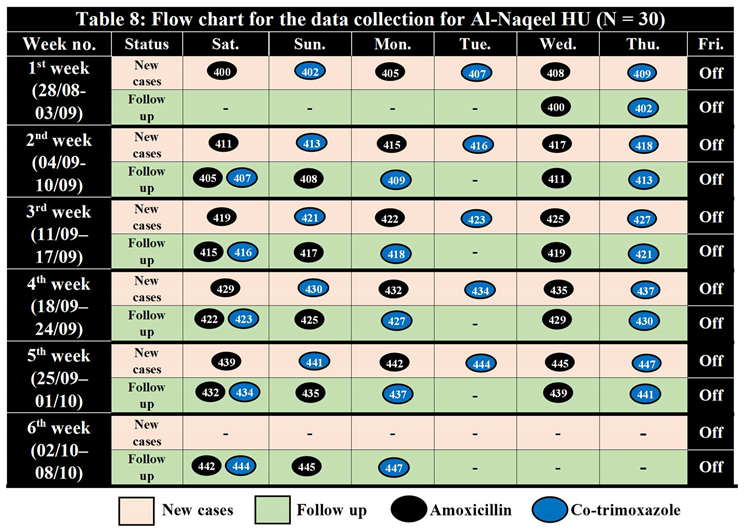
Al-Naqeel Health Unit, Blad Al-Rous District
Table 7.
Sample Randomization for Al-Naqeel HU (400 – 450) N = 30.
Table 7.
Sample Randomization for Al-Naqeel HU (400 – 450) N = 30.
| Intervention arm (N = 15) |
Control arm (N = 15) |
| 400 |
411 |
419 |
429 |
439 |
402 |
413 |
421 |
430 |
441 |
| 405 |
415 |
422 |
432 |
442 |
407 |
416 |
423 |
434 |
444 |
| 408 |
417 |
425 |
435 |
445 |
409 |
418 |
427 |
437 |
447 |
- 5.
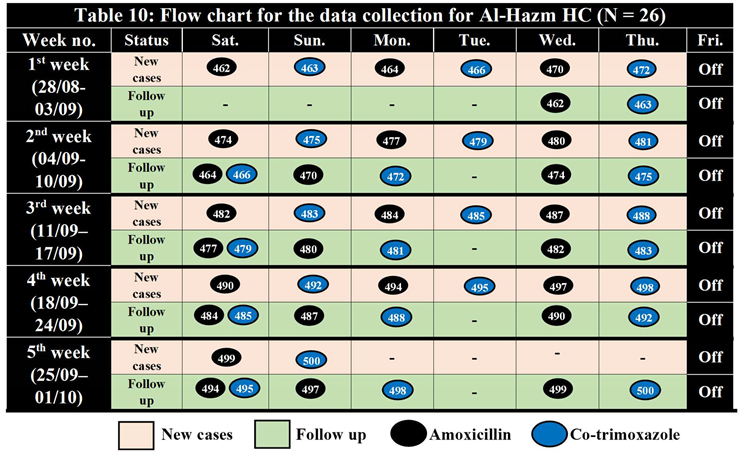
Al-Hazm Health Center, Arhab District
Table 9.
Sample Randomization for Al-Hazm HC (460 – 500) N = 26.
Table 9.
Sample Randomization for Al-Hazm HC (460 – 500) N = 26.
| Intervention arm (N = 13) |
Control arm (N = 13) |
| 462 |
474 |
482 |
490 |
499 |
463 |
475 |
483 |
492 |
500 |
| 464 |
477 |
484 |
494 |
|
466 |
479 |
485 |
495 |
|
| 470 |
480 |
487 |
497 |
|
472 |
481 |
488 |
498 |
|
References
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhea. Lancet. 2013;381:1405–16. [CrossRef]
- Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology, and etiology of childhood pneumonia. Bulletin of the World Health Organization. 2008;86:408–16. [CrossRef]
- International Institute for Population Sciences (IIPS) and ORC Macro. National Family Health Survey (NFHS-3), 2005–06, vol. 1. Mumbai: IIPS; 2007. p. 234–6.
- World Health Organization: Technical bases for the WHO recommendations on the management of pneumonia in children at first-level health facilities. Geneva: World Health Organization; 1991. Available at: http://www.who.int/maternal_child_adolescent/documents/ ari_91_20/en/. Accessed 17 November 2015.
- World Health Organization: Home treatment of pneumonia safe and effective, finds study. Available at: http://www.who.int/mediacentre/news/ releases/2008/pr01/en/. Accessed 17 November 2015.
- World Health Organization. ANTIMICROBIAL RESISTANCE Global Report on Surveillance [Internet]. 2014 [cited 2022 Jan 29]. Available from: https://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf.
- Addo-Yobo E, Chisaka N, Hassan M, Hibberd P, Lozano JM, Jeena P, et al. Oral amoxicillin versus injectable penicillin for severe pneumonia in children aged 3 to 59 months: a randomised multicentre equivalency study. Lancet. 2004;364:1141–8. [CrossRef]
- Hazir T, Fox LM, Nisar YB, Fox MP, Ashraf YP, MacLeod WB, et al. Ambulatory short-course high-dose oral amoxicillin for treatment of severe pneumonia in children: a randomised equivalency trial. Lancet. 2008;371:49–56. [CrossRef]
- Addo-Yobo E, Anh DD, El-Sayed HF, Fox LM, Fox MP, MacLeod W, et al. Outpatient treatment of children with severe pneumonia with oral amoxicillin in four countries: the MASS study. Trop Med Int Health. 2011;16: 995–1006. [CrossRef]
- Bhutta ZA, Das JK, Walker N, Rizvi A, Campbell H, Rudan I, et al. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet. 2013;381:1417–29. [CrossRef]
- Miller NP, Zunong N, Al-Sorouri TA, Alqadasi YM, Ashraf S, Siameja C. Implementing integrated community case management during conflict in Yemen. Journal of Global Health. 2020 Dec;10(2). [CrossRef]
- Goodarzi, E., Sohrabivafa, M., Darvishi, I., Naemi, H. and Khazaei, Z., 2021. Epidemiology of mortality induced by acute respiratory infections in infants and children under the age of 5 years and its relationship with the Human Development Index in Asia: an updated ecological study. Journal of Public Health, 29(5), pp.1047-1054. [CrossRef]
- Scott, J.A.G., Wonodi, C., Moïsi, J.C., Deloria-Knoll, M., DeLuca, A.N., Karron, R.A., Bhat, N., Murdoch, D.R., Crawley, J., Levine, O.S. and O’Brien, K.L., 2012. The definition of pneumonia, the assessment of severity, and clinical standardization in the Pneumonia Etiology Research for Child Health study. Clinical infectious diseases, 54(suppl_2), pp.S109-S116. [CrossRef]
- World Health Organization. Revised WHO classification and treatment of childhood pneumonia at health facilities. Geneva: World Health Organization. 2014:6-14.
- Currie CJ, Berni E, Jenkins-Jones S, Poole CD, Ouwens M, Driessen S, et al. Antibiotic treatment failure in four common infections in UK primary care 1991-2012: longitudinal analysis. BMJ [Internet]. 2014 Sep 23 [cited 2022 Jan 29];349. Available from: https://www.bmj.com/content/349/bmj.g5493. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).