1. Introduction
Polymyalgia rheumatica (PMR) is the most common inflammatory rheumatic disease in elderly people of north European ancestry. It affects people over the age of 50 (2-3 times more common among women), with an average age of onset just over 70 and a higher prevalence in Scandinavian countries and people of northern European descent. Patients with PMR usually present proximal muscle pain and stiffness of acute or subacute onset in the neck, shoulders, upper arms, hips, and thighs, resulting in a pronounced morning stiffness with difficulties in performing some daily activities such as turning over in bed, rising from a bed or a chair, or getting dressed [
1,
2]. Patients often report constitutional symptoms such as asthenia, anorexia, weight loss and low-grade fever [
3,
4,
5].
Whole-body cryostimulation (WBC) is a physical treatment that exposes the entire body to cryogenic temperatures (-110°C to -140°C) for a short period of time (2-3 minutes). Exposure to these temperatures is able to reduce pain and inflammatory status improving several metabolic parameters such as thermogenesis, lipid profile, insulin sensitivity and glucose utilisation [
6,
7], but also depression, anxiety [
8] and sleep quality [
9]. Moreover, cycles of WBC are able to reduce fatigue and disease activity in patients with several conditions such as multiple sclerosis [
10], post-COVID-19 condition (PCC) [
11,
12], rheumatoid arthritis [
13], ankylosing spondylitis [
14] and fibromyalgia [
6,
15,
16]. To the best of our knowledge, no studies have so far investigated the effects of WBC on PMR. Given the physiological effects of WBC and the underlying scientific evidence, we exposed a 74-year-old woman diagnosed with PMR to 10 sessions of WBC, in order to evaluate its effectiveness on symptom management.
1.1. Case Presentation
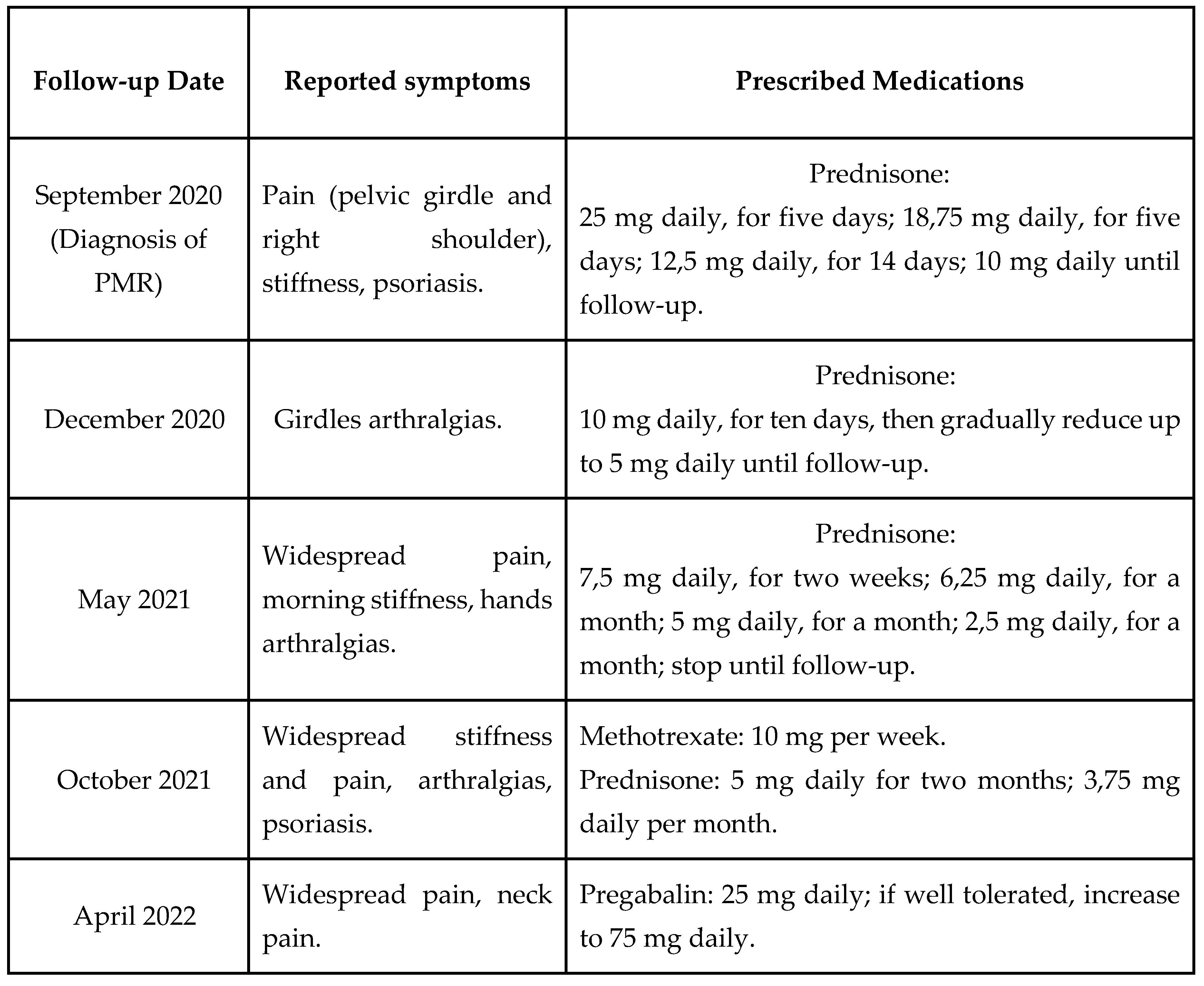
In September 2022, due to the persistence of myalgia, R.A., a 74-year-old Italian woman, BMI of 20,9 kg/m2, diagnosed with PMR, osteoporosis and psoriasis two years earlier (September 2020), came to our outpatient facility at San Giuseppe Hospital (Istituto Auxologico, Piancavallo, Italy) to undergo 10 sessions of WBC.
She was undergoing the following pharmacological therapy: prednisone 3,75 mg daily, alendronate 70 mg per week and vitamin D therapy (50000 UI, monthly).
Because the patient reported pain at the pelvic girdle and the right shoulder and night-morning stiffness, a radiological examination was performed, and no major pathological findings were detected.
The patient underwent four successive follow-ups in which the cortisone dose was adjusted each time by the physician based on the patient's reported symptoms, until replacement with a steroid-sparing agent (methotrexate) and a gamma-amino-butyric acid analog (pregabalin) and final reintroduction (June 2022) due to their ineffectiveness in relieving persistent widespread pain, stiffness, and myalgia (
Table 1).
Before PMR diagnosis and in the four follow-ups thereafter (December 2020, May 2021, October 2021, April 2022), complete blood count (red and white blood cells, haemoglobin, hematocrit and platelets), chemistry parameters (glucose, cholesterol and triglycerides) and levels of thyroid stimulating hormone and free thyroxine (FT4) were within normal ranges (data not shown).
In addition, prior to starting the WBC sessions, the patient reported a moderately active life that consisted, on average, of daily walks and light exercise activities 2 to 3 times a week, although she was experiencing persistent pain and reduced energy.
2. Materials and Methods
Whole body cryostimulation. From 19 September to 23 September 2022, R.A. underwent 10 total WBC sessions. The treatments were carried out twice a day, the first one at 9 a.m. and the second at 12 p.m., inside a cryo-chamber (Artic, CryoScience, Rome) where she was exposed to extremely cold, dry air at -110°C for two minutes. Before starting the treatment, the patient was instructed to remove glasses and metal accessories and to wear a T-shirt, running shorts, an earmuff band, gloves, socks (pulled up to the knee) and rubber slippers.
Primary outcome measures. The patient was asked to complete some questionnaires to assess her physical condition before undergoing WBC (September 2022) and five months after completing the treatments (February 2023). Fibromyalgia Impact Questionnaire (FIQ), in its Italian validation [
17], was used to measure patient’s disease status, progress and outcomes. Total scores range from 0 to 100, where higher scores indicate a more severe impact of FM. Fatigue Severity Scale (FSS) [
18] is a 9-item questionnaire used to assess the severity of fatigue symptoms, whose items are scored on a 7-point scale (1 = strongly disagree, 7 = strongly agree), where a higher score corresponds to greater symptom severity. A Visual Analogue Scale (VAS) [
19] ranging from 0 to 10 was used to measure pain intensity, where high scores indicated high levels of pain intensity. A Pain Management Questionnaire [
20] was administered to assess the medications taken and their dosages. Pittsburgh Sleep Quality Index (PSQI) was used to evaluate sleep quality and disturbances over a 1-month time interval. It consists of nineteen individual items which generate seven "component" scores (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction), whose sum yields one global score [
21]. The International Physical Activity Questionnaire-Short Form (IPAQ-SF) [
22] is widely used to assess physical activity and consists of questions about an individual’s physical activity (PA) during daily life over the past 7 days. It is classified into three categories: low intensity activity (walking of any kind), moderate intensity activity (breathing somewhat harder than normal) and vigorous activity (hard physical effort which makes breathing much harder than normal). The last questions ask about the time that the individual remains seated during a weekday and a weekend day. Total weekly PA at inclusion was estimated by weighting the reported minutes per week within each PA category by a metabolic equivalent of task (MET) energy expenditure estimate assigned to each activity (MET intensity) and by their frequency per week. A global PA score was calculated by summing the MET minutes per week (MET.min/week) for each intensity category. Three categories of patients were then defined depending on their global physical score:
inactive: < 600 MET.min/week (< 150 min of moderate intensity PA);
moderately active: > 600 MET.min/week but < 3000 MET.min/week (between 150 and 750 min of moderate intensity PA);
highly active: > 3000 MET.min/week (> 750 min of moderate intensity PA).
3. Results
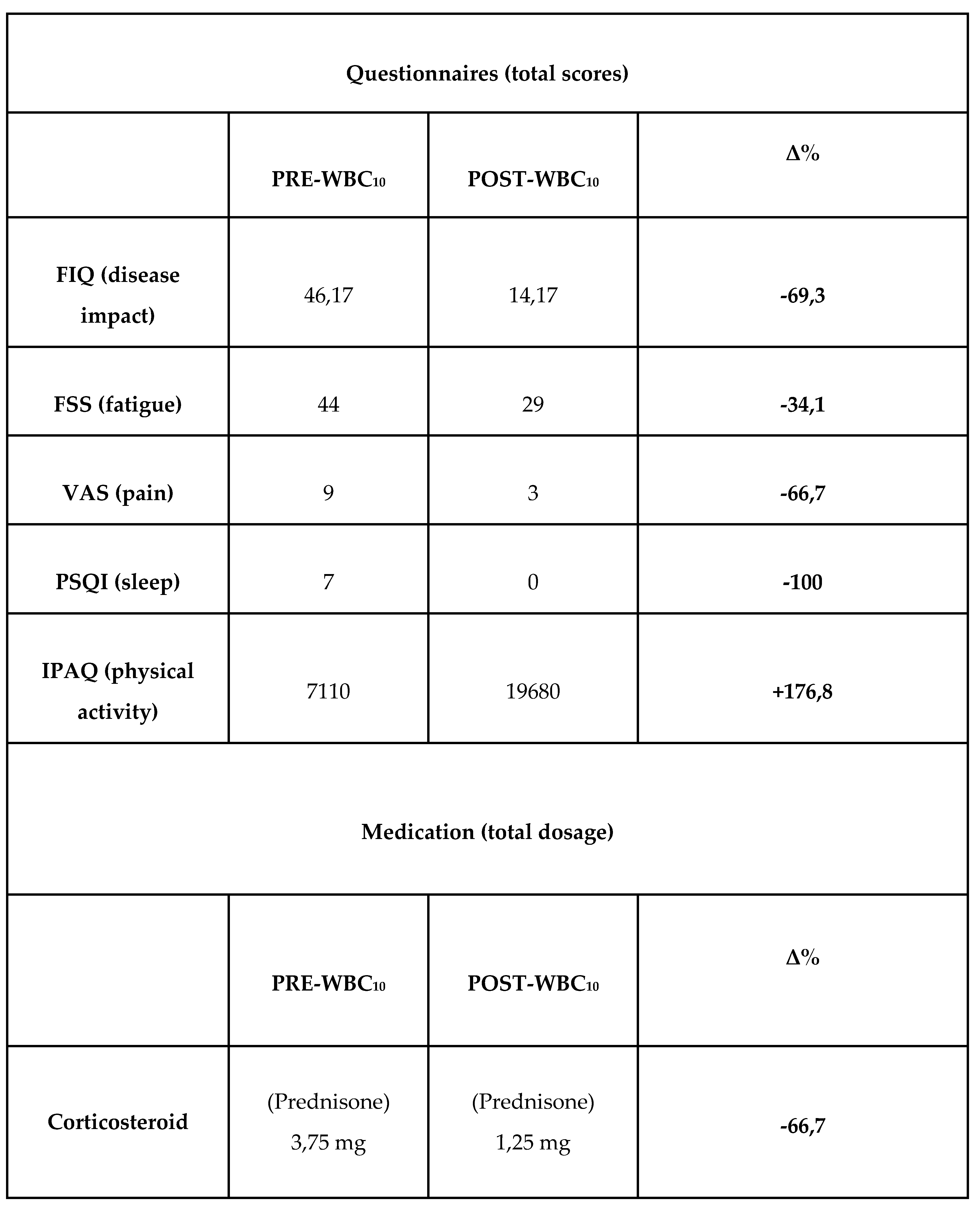
This Questionnaire scores completed by the patient immediately before undergoing WBC and then 5 months after concluding the treatments were compared (
Table 2). FIQ and FSS scores showed a clear improvement in the patient's disease impact (pre-WBC score = 46.17, post-WBC score = 14.17), and fatigue symptoms (pre-WBC score = 44, post-WBC score = 29). The patient also reported an improvement in pain symptoms (VAS pre-WBC score = 9, post-WBC score = 3), to the extent that she reduced the corticosteroid to 1.25 mg per day. In addition, the patient reported better sleep quality following WBC treatments, supported by PSQI total score going from 7 (pre-WBC) to 0 (post-WBC). Finally, IPAQ shows that the patient was very active before and after WBC (> 3000 MET.min/week), but a clear increase in total PA MET.min/week was observed over the 5 months of the study (total IPAQ score pre-WBC = 7110, post-WBC = 19680). Notably, the patient did not report vigorous activity, but her moderate activity increased from 1440 to 9600 MET.min/week, while her walking score increased from 5670 to 10080 MET.min/week.
Complete blood count (red and white blood cells, haemoglobin, hematocrit and platelets) and chemistry (glucose, cholesterol and triglycerides) performed by the patient four months after WBC treatments (January 2023) were within normal ranges (data not shown).
4. Discussion
The main goals of rehabilitation treatments are to control the disease and prevent relapses, which are quite common. To date, despite the availability of new and costly drugs, corticosteroids (oral prednisone/prednisolone constitutes) remain the cornerstone of treatment and remain unsurpassed in terms of resolution of symptoms and control of inflammation [
3,
4].
However, it is well known that prolonged use of corticosteroids can lead to a wide array of adverse effects ranging from mild to severe [
23], and therefore finding therapeutic alternatives to their use becomes crucial.
To date, no studies have investigated WBC as an adjuvant option for the treatment of PMR. Given the known effects of WBC on reducing pain and disease impact in patients with fibromyalgia, and on improving the inflammatory profile in patients with RA [
24,
25,
26], in this study we assessed the effect of 10 WBC treatments on a patient suffering from PMR. The results show that the patient reduced her daily drug intake by 67% following WBC treatments. Moreover, she reported that the effects lasted over time, still experiencing benefits 5 months after the end of the treatments. These results are in line with other findings where the effect of WBC was also evaluated at follow-up visits in patients with FM and RA. Specifically, in a study by Vitenet et al. [
16], a group of patients with fibromyalgia diagnosis who underwent 10 sessions of whole body cryostimulation reported an improved health-related quality of life even 1 month after treatment discontinuation. Moreover, in a recent randomised controlled trial [
24], patients with RA who underwent WBC sessions reported lower pain levels compared to baseline, even twelve weeks after the end of the treatments. Interestingly, some of these patients reported at follow-up that they had reduced or discontinued the use of analgesics.
5. Conclusions
PMR is not a rare disease, and its prevalence will continue to increase in the coming years as the number of elderly people in the world continues to rise. Once diagnosed, further proper management of patients it’s crucial to significantly improve the patient’s prognosis. Therefore, as demonstrated in this report, WBC could be a valuable adjuvant for the treatment of PMR that could potentially have a positive effect in cases of poor prescription compliance and in terms of health care costs. Indeed, in this specific case, WBC has been shown to be a scalable and well-tolerated alternative aimed at reducing not only pain but also fatigue, disease impact, drug therapies, and largely improving sleep and physical activity.
As this is the first work on the effects of WBC in patients with PMR, further research is obviously needed to confirm the data obtained. Since WBC treatment was conducted on a single patient with no comorbidities, no overweight problems and a moderately active lifestyle, a larger and more diverse sample will therefore be needed for future studies to confirm the adjuvant role of WBC in the treatment of PMR and other conditions characterised by chronic persistent pain.
Author Contributions
Conceptualization, F.V., P.C. and J.M.F.; methodology, A.S., G.V. and F.V.; validation, F.V. and J.M.F.; formal analysis, F.V.; investigation, F.V.; resources, F.V., A.S., G.V. and P.P.; data curation, F.V. and P.P.; writing—original draft preparation, F.V. and J.M.F.; writing—review and editing, F.V., J.M.F. and P.C.; visualization, F.V. and J.M.F.; supervision, P.C. and J.M.F.; funding acquisition, P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by O2H s.r.l. and partially supported by the Italian Ministry of Health.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Istituto Auxologico Italiano (protocol code 2021_05_18_14, approved 2021-05-18).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Michet, C.J.; Matteson, E.L. Polymyalgia Rheumatica. BMJ 2008, 336, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, I.E.; Sharma, A.; Turesson, C.; Mohammad, A.J. An Update on Polymyalgia Rheumatica. J Intern Med 2022, 292, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Camellino, D.; Giusti, A.; Girasole, G.; Bianchi, G.; Dejaco, C. Pathogenesis, Diagnosis and Management of Polymyalgia Rheumatica. Drugs Aging 2019, 36, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.; García-Castañeda, N.; Prieto-Peña, D.; Martínez-Quintanilla, D.; Vicente, E.F.; Blanco, R.; González-Gay, M.A. Treatment of Polymyalgia Rheumatica. Biochem Pharmacol 2019, 165, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Kniazkova, I.; Shapovalova, L.; Bogun, M. A Case Report of Polymyalgia Rheumatica. Reumatologia 2018, 56, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.M.; Gobbi, M.; Piterà, P.; Giusti, E.M.; Capodaglio, P. Whole-Body Cryostimulation in Fibromyalgia: A Scoping Review. Applied Sciences 2022, 12, 4794. [Google Scholar] [CrossRef]
- Fontana, J.M.; Bozgeyik, S.; Gobbi, M.; Piterà, P.; Giusti, E.M.; Dugué, B.; Lombardi, G.; Capodaglio, P. Whole-Body Cryostimulation in Obesity. A Scoping Review. J Therm Biol 2022, 106, 103250. [Google Scholar] [CrossRef]
- Rymaszewska, J.; Ramsey, D.; Chładzińska-Kiejna, S. Whole-Body Cryotherapy as Adjunct Treatment of Depressive and Anxiety Disorders. Arch Immunol Ther Exp (Warsz) 2008, 56, 63–68. [Google Scholar] [CrossRef]
- Bouzigon, R.; Ravier, G.; Dugue, B.; Grappe, F. THE USE OF WHOLE-BODY CRYOSTIMULATION TO IMPROVE THE QUALITY OF SLEEP IN ATHLETES DURING HIGH LEVEL STANDARD COMPETITIONS. Br J Sports Med 2014, 48, 572.1–572. [Google Scholar] [CrossRef]
- Miller, E.; Kostka, J.; Włodarczyk, T.; Dugué, B. Whole-Body Cryostimulation (Cryotherapy) Provides Benefits for Fatigue and Functional Status in Multiple Sclerosis Patients. A Case-Control Study. Acta Neurol Scand 2016, 134, 420–426. [Google Scholar] [CrossRef]
- Piterà, P.; Gobbi, M.; Fontana, J.M.; Cattaldo, S.; Massucci, M.; Capodaglio, P. Whole-Body Cryostimulation: A Rehabilitation Booster in Post-COVID Patients? A Case Series. Applied Sciences 2022, 12, 4830. [Google Scholar] [CrossRef]
- GOBBI, M.; TROTTI, G.; TANZI, M.; KASAP, F.; PITERÀ, P.; CAPODAGLIO, P. POST-COVID SYMPTOMS AND WHOLE-BODY CRYOTHERAPHY: A CASE REPORT. J Rehabil Med Clin Commun 2022, 5, 1000075. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, H.E.; Mikkelsson, M.K.; Kautiainen, H.; Pohjolainen, T.H.; Leirisalo-Repo, M. Effectiveness of Different Cryotherapies on Pain and Disease Activity in Active Rheumatoid Arthritis. A Randomised Single Blinded Controlled Trial. Clin Exp Rheumatol 2006, 24, 295–301. [Google Scholar] [PubMed]
- Romanowski, M.W.; Straburzyńska-Lupa, A. Is the Whole-Body Cryotherapy a Beneficial Supplement to Exercise Therapy for Patients with Ankylosing Spondylitis? J Back Musculoskelet Rehabil 2020, 33, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Varallo, G.; Piterà, P.; Fontana, J.M.; Gobbi, M.; Arreghini, M.; Giusti, E.M.; Franceschini, C.; Plazzi, G.; Castelnuovo, G.; Capodaglio, P. Is Whole-Body Cryostimulation an Effective Add-On Treatment in Individuals with Fibromyalgia and Obesity? A Randomized Controlled Clinical Trial. J Clin Med 2022, 11, 4324. [Google Scholar] [CrossRef] [PubMed]
- Vitenet, M.; Tubez, F.; Marreiro, A.; Polidori, G.; Taiar, R.; Legrand, F.; Boyer, F.C. Effect of Whole Body Cryotherapy Interventions on Health-Related Quality of Life in Fibromyalgia Patients: A Randomized Controlled Trial. Complement Ther Med 2018, 36, 6–8. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Atzeni, F.; Fiorini, T.; Panni, B.; Randisi, G.; Turiel, M.; Carrabba, M. Validation of an Italian Version of the Fibromyalgia Impact Questionnaire (FIQ-I). Clin Exp Rheumatol 2003, 21, 459–464. [Google Scholar]
- Valko, P.O.; Bassetti, C.L.; Bloch, K.E.; Held, U.; Baumann, C.R. Validation of the Fatigue Severity Scale in a Swiss Cohort. Sleep 2008, 31, 1601–1607. [Google Scholar] [CrossRef]
- Ritter, P.L.; González, V.M.; Laurent, D.D.; Lorig, K.R. Measurement of Pain Using the Visual Numeric Scale. J Rheumatol 2006, 33, 574–580. [Google Scholar]
- Robinson-Papp, J.; George, M.C.; Wongmek, A.; Nmashie, A.; Merlin, J.S.; Ali, Y.; Epstein, L.; Green, M.; Serban, S.; Sheth, P.; et al. The Quantitative Analgesic Questionnaire: A Tool to Capture Patient-Reported Chronic Pain Medication Use. J Pain Symptom Manage 2015, 50, 381–386. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A Systematic Review. Int J Behav Nutr Phys Act 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Goyal, A.; Sonthalia, S. Corticosteroid Adverse Effects. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Klemm, P.; Hoffmann, J.; Asendorf, T.; Aykara, I.; Frommer, K.; Dischereit, G.; Müller-Ladner, U.; Neumann, E.; Lange, U. Whole-Body Cryotherapy for the Treatment of Rheumatoid Arthritis: A Monocentric, Single-Blinded, Randomised Controlled Trial. Clin Exp Rheumatol 2022, 40, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Sadura-Sieklucka, T.; Sołtysiuk, B.; Karlicka, A.; Sokołowska, B.; Kontny, E.; Księżopolska-Orłowska, K. Effects of Whole Body Cryotherapy in Patients with Rheumatoid Arthritis Considering Immune Parameters. Reumatologia 2019, 57, 320–325. [Google Scholar] [CrossRef]
- Gizińska, M.; Rutkowski, R.; Romanowski, W.; Lewandowski, J.; Straburzyńska-Lupa, A. Effects of Whole-Body Cryotherapy in Comparison with Other Physical Modalities Used with Kinesitherapy in Rheumatoid Arthritis. Biomed Res Int 2015, 2015, 409174. [Google Scholar] [CrossRef]
Table 1.
List of symptoms and medications prescribed to the patient from September 2020 to subsequent follow-ups.
Table 1.
List of symptoms and medications prescribed to the patient from September 2020 to subsequent follow-ups.
Table 2.
Pre-post WBC10 total scores and percentage change of FIQ (disease impact), FSS (fatigue), VAS (pain), PSQI (sleep), IPAQ (physical activity) questionnaires and medication dosage (Prednisone). The percentage change (Δ%) was calculated according to the formula: Δ% = (POST − PRE)/PRE*100...
Table 2.
Pre-post WBC10 total scores and percentage change of FIQ (disease impact), FSS (fatigue), VAS (pain), PSQI (sleep), IPAQ (physical activity) questionnaires and medication dosage (Prednisone). The percentage change (Δ%) was calculated according to the formula: Δ% = (POST − PRE)/PRE*100...
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).