Submitted:
07 May 2023
Posted:
09 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Study protocol
Search strategy
Study selection
Data extraction
Risk of bias assessment
Statistical analysis
Patient and Public Involvement
3. Results
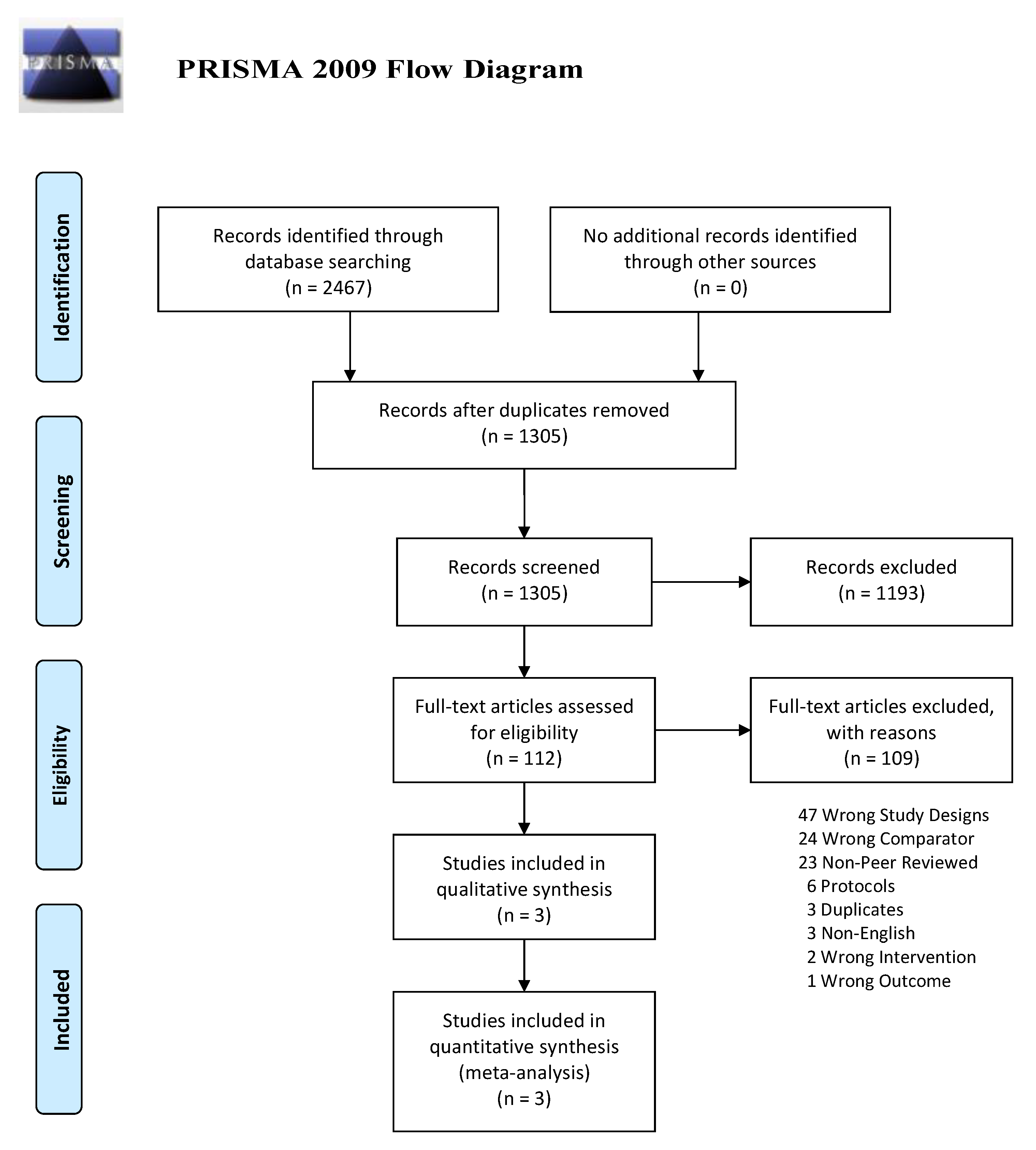
Study selection
Study characteristics
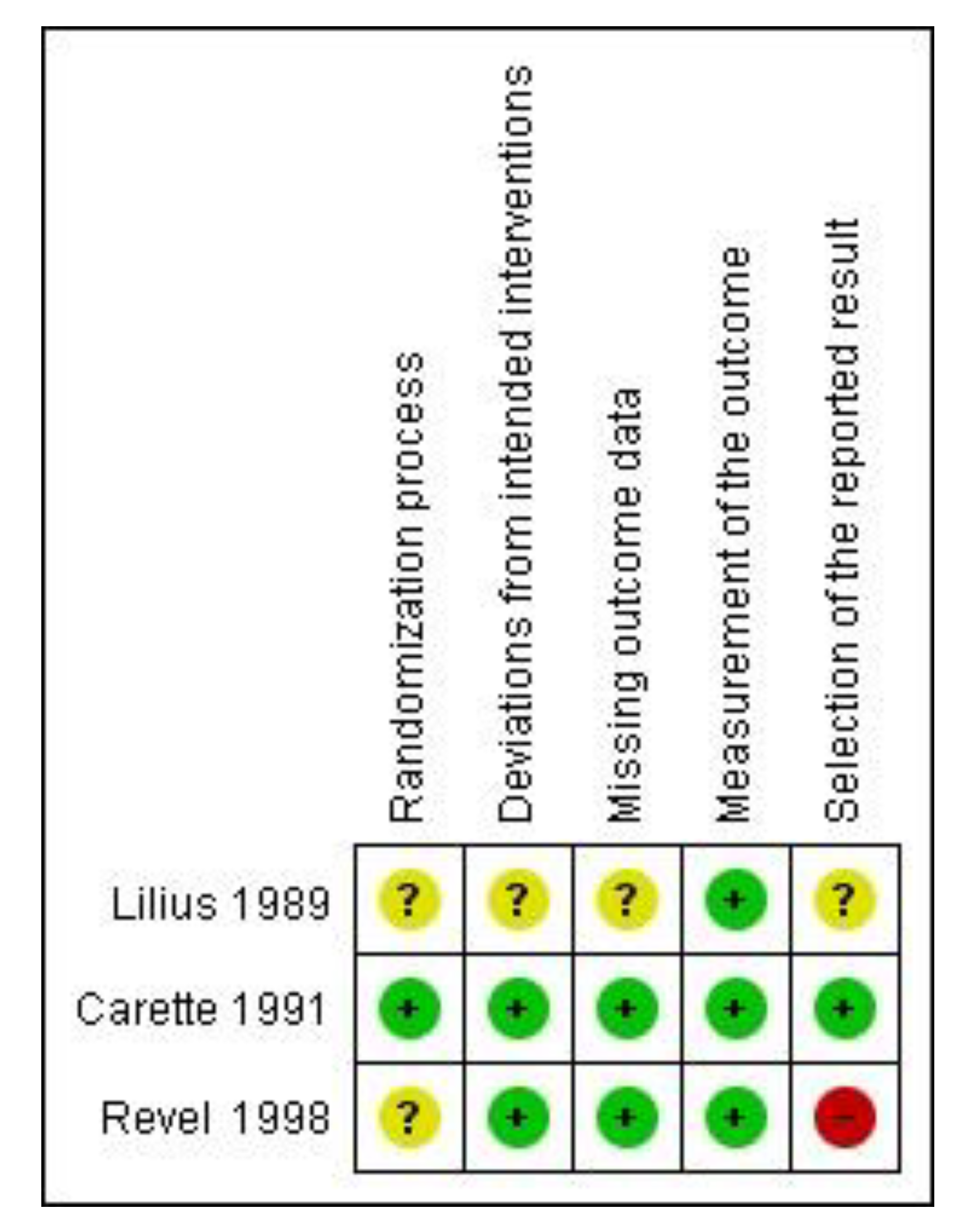
Quality Assessment
Qualitative analysis
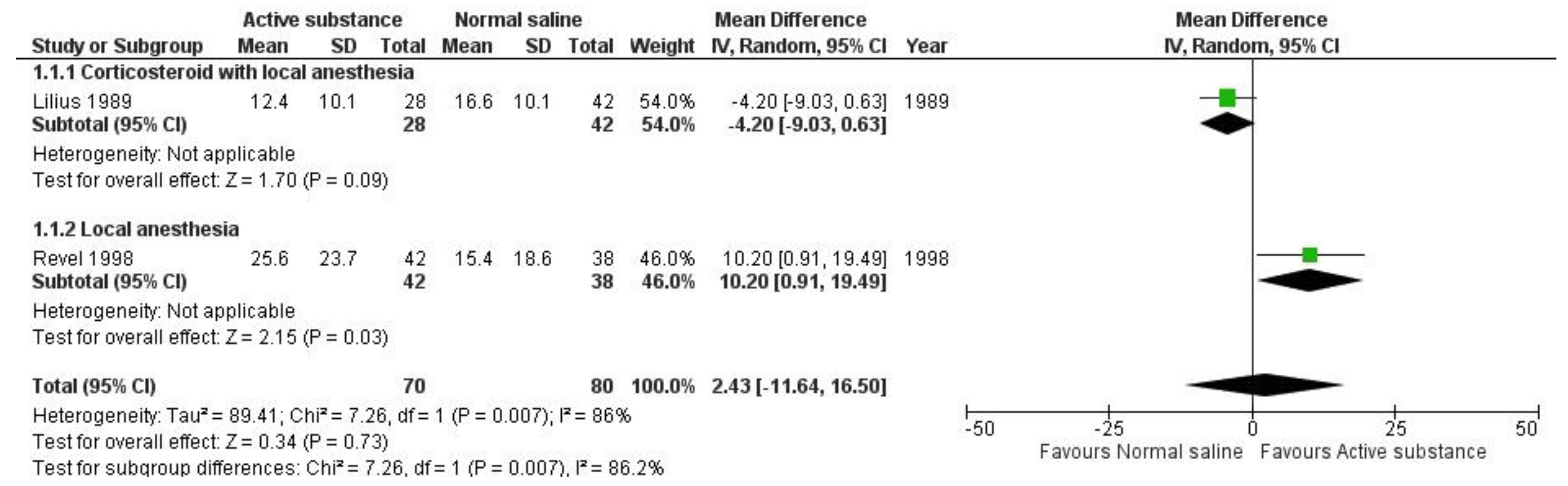
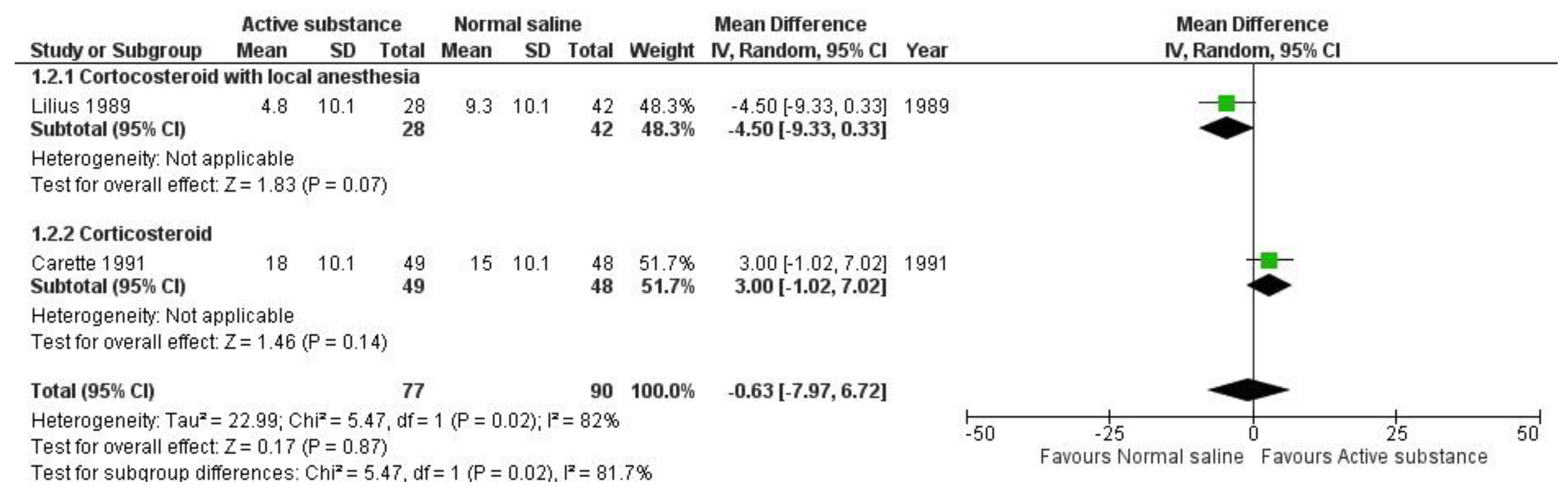
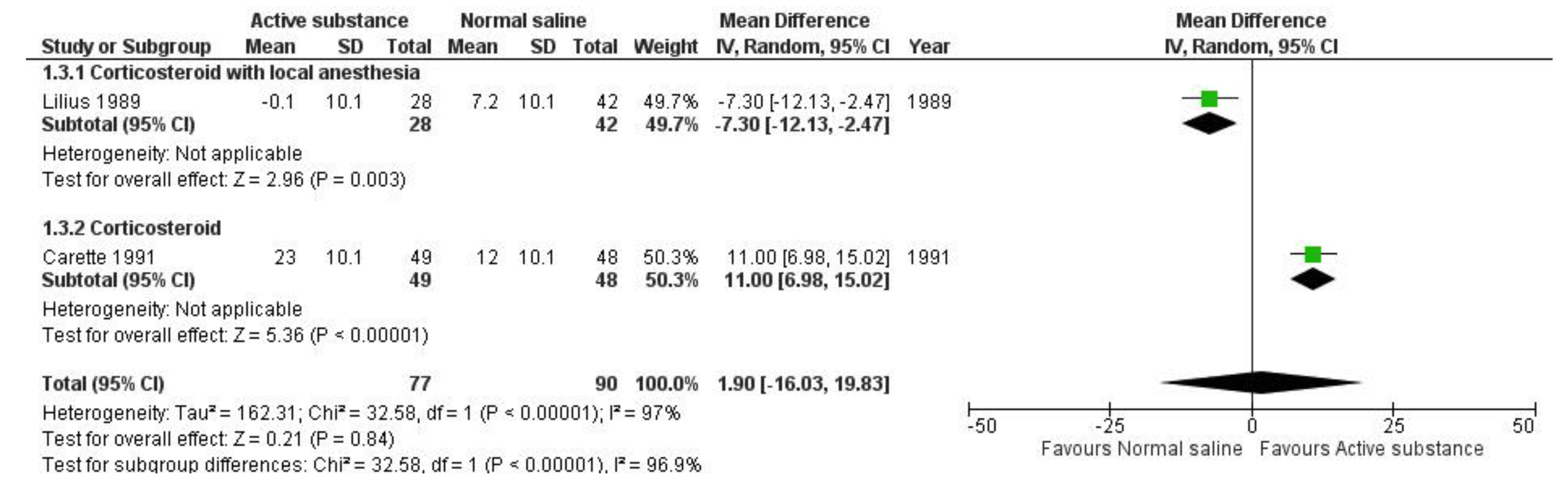
Quantitative analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen S, Chen M, Wu X, Lin S, Tao C, et al. (2021) Global, regional and national burden of low back pain 1990-2019: A systematic analysis of the Global Burden of Disease study 2019. J Orthop Translat 32: 49-58. [CrossRef]
- O'Leary S, Raymer M, Window P, Kelly PS, Lee D, et al. (2020) A multisite longitudinal evaluation of patient characteristics associated with a poor response to non-surgical multidisciplinary management of low back pain in an advanced practice physiotherapist-led tertiary service. BMC Musculoskelet Disord 21: 807. [CrossRef]
- Mannion AF, Brox JI, Fairbank JC (2016) Consensus at last! Long-term results of all randomized controlled trials show that fusion is no better than non-operative care in improving pain and disability in chronic low back pain. Spine J 16: 588-590. [CrossRef]
- Oliveira CB, Maher CG, Pinto RZ, Traeger AC, Lin CC, et al. (2018) Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J 27: 2791-2803. [CrossRef]
- Sayed D, Grider J, Strand N, Hagedorn JM, Falowski S, et al. (2022) The American Society of Pain and Neuroscience (ASPN) Evidence-Based Clinical Guideline of Interventional Treatments for Low Back Pain. J Pain Res 15: 3729-3832. [CrossRef]
- Altman RD, Devji T, Bhandari M, Fierlinger A, Niazi F, et al. (2016) Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: A systematic review and meta-analysis of randomized trials. Semin Arthritis Rheum 46: 151-159. [CrossRef]
- Saltzman BM, Leroux T, Meyer MA, Basques BA, Chahal J, et al. (2017) The Therapeutic Effect of Intra-articular Normal Saline Injections for Knee Osteoarthritis: A Meta-analysis of Evidence Level 1 Studies. Am J Sports Med 45: 2647-2653. [CrossRef]
- Gazendam A, Ekhtiari S, Bozzo A, Phillips M, Bhandari M (2021) Intra-articular saline injection is as effective as corticosteroids, platelet-rich plasma and hyaluronic acid for hip osteoarthritis pain: a systematic review and network meta-analysis of randomised controlled trials. Br J Sports Med 55: 256-261. [CrossRef]
- Suputtitada A, Chen CPC, Pongpirul K (2022) Mechanical Needling with Sterile Water versus Steroids Injection for Facet Joint Syndrome: A Retrospective Observational Study. Pain Res Manag 2022: 9830766. [CrossRef]
- Suputtitada A, Chen CPC, Pongpirul K. Mechanical Needling with Sterile Water Injection versus Lidocaine injection for Lumbar Spinal Stenosis. Global Spine J 21925682221094533. [CrossRef]
- Carette S, Marcoux S, Truchon R, Grondin C, Gagnon J, et al. (1991) A controlled trial of corticosteroid injections into facet joints for chronic low back pain. N Engl J Med 325: 1002-1007. [CrossRef]
- Lilius G, Laasonen EM, Myllynen P, Harilainen A, Grönlund G (1989) Lumbar facet joint syndrome. A randomised clinical trial. J Bone Joint Surg Br 71: 681-684. [CrossRef]
- Revel M, Poiraudeau S, Auleley GR, Payan C, Denke A, et al. (1998) Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia. Proposed criteria to identify patients with painful facet joints. Spine (Phila Pa 1976) 23: 1972-6; discussion 1977. [CrossRef]
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, et al. (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366: l4898. [CrossRef]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, et al. (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355: i4919. [CrossRef]
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N (2006) Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 59: 7-10. [CrossRef]
- Melzack R (1975) The McGill Pain Questionnaire: major properties and scoring methods. Pain 1: 277-299. [CrossRef]
- Nelemans PJ, de Bie RA, de Vet HC, Sturmans F (2000) Injection therapy for subacute and chronic benign low back pain. Cochrane Database Syst Rev (2): CD001824. [CrossRef]
- Sit RWS, Wu RWK, Rabago D, Reeves KD, Chan DCC, et al. (2020) Efficacy of Intra-Articular Hypertonic Dextrose (Prolotherapy) for Knee Osteoarthritis: A Randomized Controlled Trial. Ann Fam Med 18: 235-242. [CrossRef]
- Bar-Or D, Rael LT, Brody EN (2017) Use of Saline as a Placebo in Intra-articular Injections in Osteoarthritis: Potential Contributions to Nociceptive Pain Relief. Open Rheumatol J 11: 16-22. [CrossRef]
- Hirsch C, Ingelmark BE, Miller M (1963) The anatomical basis for low back pain. Studies on the presence of sensory nerve endings in ligamentous, capsular and intervertebral disc structures in the human lumbar spine. Acta Orthop Scand 33: 1-17. [CrossRef]
- Caterini R, Mancini F, Bisicchia S, Maglione P, Farsetti P (2011) The correlation between exaggerated fluid in lumbar facet joints and degenerative spondylolisthesis: prospective study of 52 patients. J Orthop Traumatol 12: 87-91. [CrossRef]
- Annaswamy TM, Armstead C, Carlson L, Elkins NJ, Kocak D, et al. (2018) Intra-articular Triamcinolone Versus Hyaluronate Injections for Low Back Pain With Symptoms Suggestive of Lumbar Zygapophyseal Joint Arthropathy: A Pragmatic, Double-Blind Randomized Controlled Trial. Am J Phys Med Rehabil 97: 278-284. [CrossRef]
- Manchikanti L, Kaye AD, Boswell MV, Bakshi S, Gharibo CG, et al. (2015) A Systematic Review and Best Evidence Synthesis of the Effectiveness of Therapeutic Facet Joint Interventions in Managing Chronic Spinal Pain. Pain Physician 18: E535-E582.
- Manchikanti L, Kaye AD, Soin A, Albers SL, Beall D, et al. (2020) Comprehensive Evidence-Based Guidelines for Facet Joint Interventions in the Management of Chronic Spinal Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines Facet Joint Interventions 2020 Guidelines. Pain Physician 23: S1-S127.
- Wu J, Zhou J, Liu C, Zhang J, Xiong W, et al. (2017) A Prospective Study Comparing Platelet-Rich Plasma and Local Anesthetic (LA)/Corticosteroid in Intra-Articular Injection for the Treatment of Lumbar Facet Joint Syndrome. Pain Pract 17: 914-924. [CrossRef]
- Dagenais S, Yelland MJ, Del Mar C, Schoene ML (2007) Prolotherapy injections for chronic low-back pain. Cochrane Database Syst Rev 2007: CD004059. [CrossRef]
- Chen CPC, Chen JL, Ho CS, Suputtitada A (2020) Ultrasound-guided Medial Branch Blocks, Facet Joint, and Multifidus Muscle Injections: How It Is Done under One Needle Insertion Point! Anesthesiology 132: 582-583. [CrossRef]
- Chen CPC, Chen CK, Chen JL, Chen HM, Suputtitada A (2021) Ultrasound-Guided Lumbar Spine Medial Branch Blocks for the Treatment of Low Back Pain. Am J Phys Med Rehabil 100: e73-e74. [CrossRef]
| Country | Interventions | Sample size | Disease duration (months) | Female; n (%) | Age; mean (range) | Imaging technique | Patient-reported outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain | Disability | Numbness | Quality of Life | Adverse events | ||||||||
| Lilius [12] | Finland |
|
70 | NR | 39 (56%) | 44 (19-64) | Fluoroscopy | VAS | Objectivedisabilityscore(Only overall result) | NR | NR | 7 Overall (5 men,2 women) |
| Carette [11] | Canada |
|
97 |
|
44 (45%) | 43 | Fluoroscopy | VAS | NR | NR | SIPs | No adverse events occurred |
| Revel [13] | France |
|
80 | Overall mean 19.6 months | 54 (68%) | 58 (34–87) | Fluoroscopy | VAS | NR | NR | NR | NR |
| NR: Not Reported; VAS: Visual Analog Scale | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





