1. Introduction
Osteoarthritis of the hand (HOA) is one of the most common musculoskeletal diseases that frequently affects the hand, predominately of elder female [
1,
2]. Imaging techniques are crucial when clinical symptoms are suspicious for OA diagnosis. The clinical spectrum comprises clinical courses from less symptomatic to severe impairments of quality of life [
3]. However, interpretation of imaging is not always straightforward: the prevalence of HOA differs strongly according to the modality used for assessment, e.g. clinical examination, conventional radiography (CR), magnetic resonance imaging (MRI) or US. Because of the different forms and patterns of HOA, there is no uniform standardized procedure for diagnosing HOA.
Clinically, symptomatic HOA can be classified according to clinical American College of Rheumatology (ACR) criteria if hard tissue enlargement can be found in two or more selected finger joints out of 10, including at least two DIP joints, as well as fewer than three MCP swellings [
4].
Radiographically, the Kellgren and Lawrence criteria allow the imaging recognition of HOA. These criteria are commonly used in epidemiological studies. The severity of HOA is classified based on an imaging atlas between 0-4, a score ≥2 according to Kellgren and Lawrence is considered HOA positive. In addition to the presence of osteophytes, joint space narrowing, sclerosis and deformities are assessed. HOA can also be detected with other imaging modalities like US or MRI [
4,
5,
6,
7].
Overall, in HOA, most studies have used CR as the imaging modality of choice so far.
Ultrasound (US) plays an increasing role for diagnosis and monitoring and is widely used in daily clinical practice, not only in inflammatory joint diseases. Next to inflammatory lesions such as synovitis or tenovaginitis, structural damage like erosions or osteophytes are recognized reliably by ultrasound [
8]. If osteophytes are detected, the concomitant diagnosis of hand osteoarthritis (HOA) is often considered, especially if there are no potentially inflammatory diseases associated with osteophyte lesions. If and how frequent osteophytes are found by US in a working population that does not refer to a musculoskeletal unit is unknown.
The objectives of this study were to investigate the prevalence and pattern of US findings suggestive of HOA in a population-based cross-sectional study in a population working in the industry without known HOA diagnosis.
2. Materials and Methods
The investigation is part of a screening initiative of referral centers for rheumatologically conditions in an industrialized area(“Rheumazentrum Rhein-Ruhr e.V.”). Employees were visited at their working places in cooperating industrial companies and a voluntarily participation in a structured examination designed to check their individual risk for inflammatory and non-inflammatory musculoskeletal conditions was offered.
US assessment was provided for both hands, scanning 26 finger joints of each participant (carpometacarpal (CMC) 1, metacarpophalangeal (MCP) 2-5, proximal interphalangeal (PIP) 2-5 and distal interphalangeal (DIP) 2-5) using an Ultrasound Esaote Mylab 25 Gold unit with an 18 MHz linear transducer. The participants were examined sitting with their hand placed on a small cushion in front of the examiner. Gray-scale US was performed on the palmar side, except PIP 2 examined additionally from lateral radial, with all joints in neutral position. The lateral images of PIP 2 joint were not included in the evaluation. Static images were stored and evaluated using Esaote Mylab-Desk software to guarantee standardization. Osteophytes were defined as protrusions of the bony surface. One medical student (MG) who completed basic and advanced ultrasound courses and was trained in joint ultrasound at outpatients with musculoskeletal diseases previously performed all ultrasound examinations.
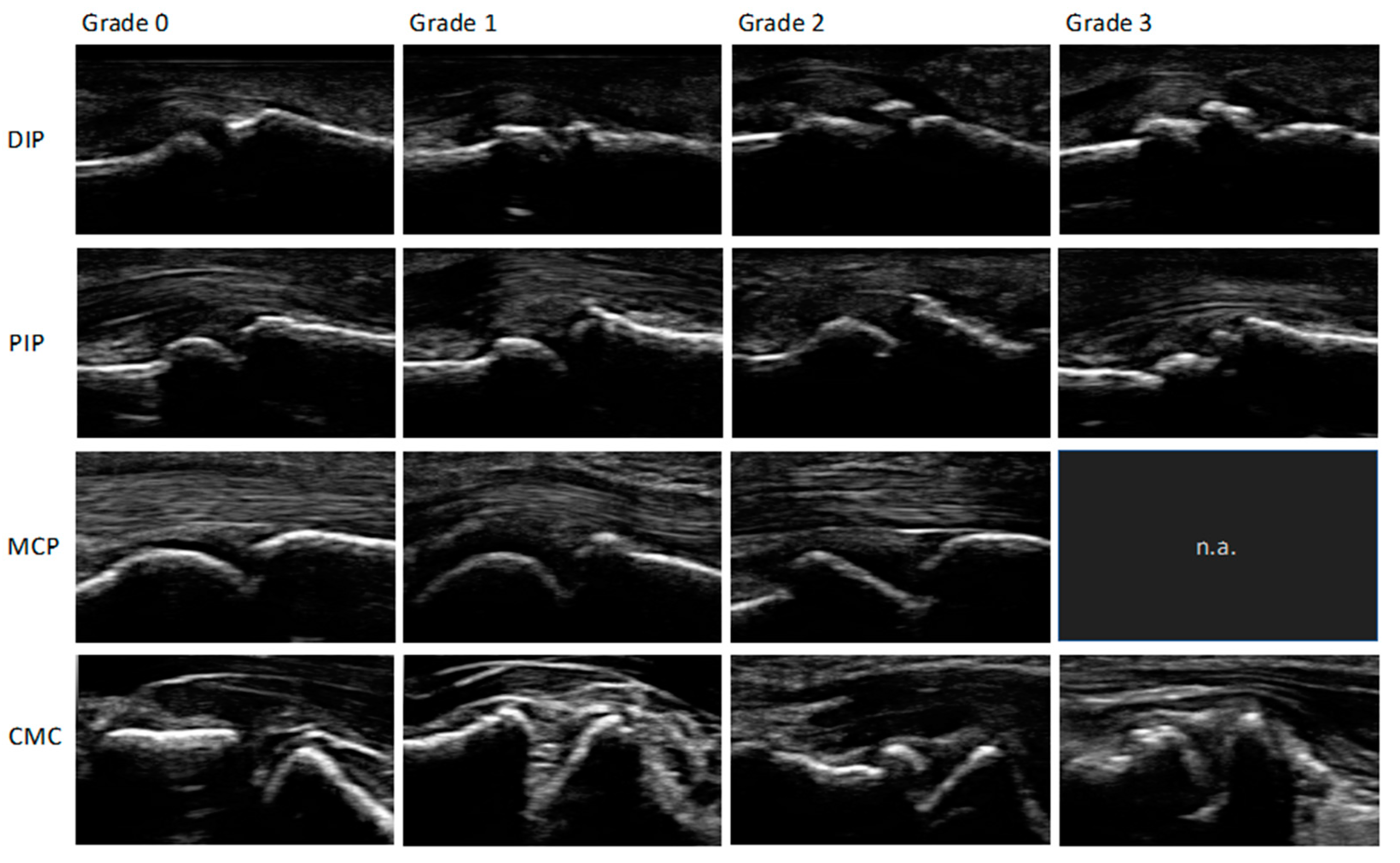
To read the images, a modified (palmar, not dorsal view) semi-quantitative score for osteophytes ranging from 0-3: 0 = no osteophytes, 1 = mild osteophytes, 2 = moderate osteophytes and 3 = severe osteophytes was used. An increase in the score describes an increase in the severity of the osteophytes found. The biggest osteophyte at each joint was scored. The score was previously described by Keen et al., Kortekaas et al., Mathiessen et al. and was evaluated and recommended by the Outcome measures in Rheumatology ultrasonography (OMERACT) group [
9,
10,
11,
12,
13]. If one or more joints of the participant showed osteophyte values ≥1, they were positively defined of showing HOA signs. The pictures were scored by a medical student (MG) in a consensus read with an experienced rheumatology resident (PS).
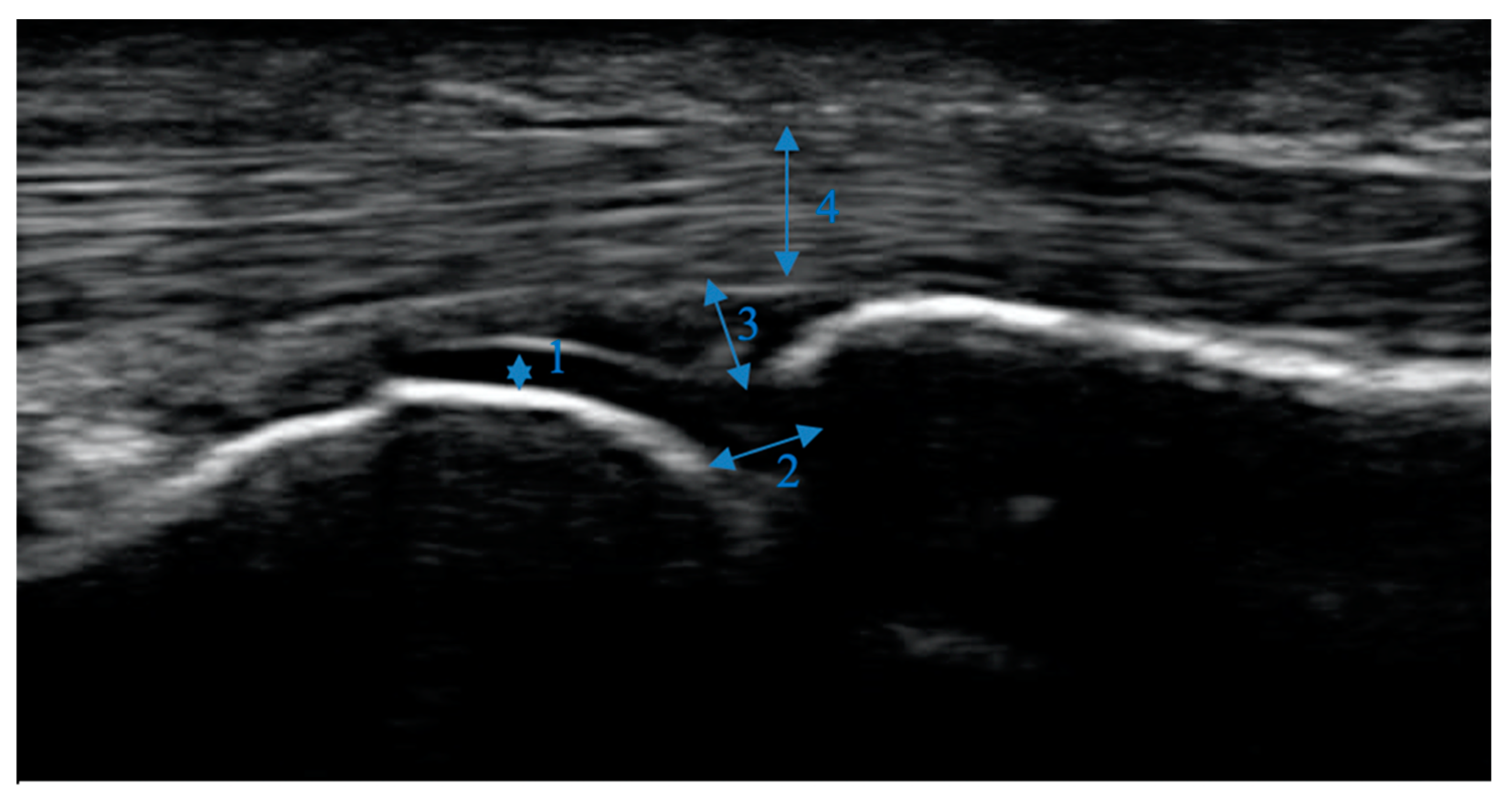
Figure 1 shows a normal MCP joint without any major pathologies.
Figure 2 shows an example of the different grades of osteophytes for each joint group in palmar view.
In addition, for assessment of both prevalence and severity of the presence of osteophytes, a sum score was calculated by adding all individual osteophyte-scores of the 26 joints assessed for each participant.
Besides osteophytes, joint structures and pathological changes were seen and defined as following: Definitions for effusion and synovitis were used according to the OMERACT group [
10]. Erosions were defined as cortical discontinuity that can be visualized in two planes perpendicular to each other according to the OMERACT criteria [
14]. Joint space was defined as area between two bones and was measured perpendicular to the joint surface [
15]. Cartilage was defined as normally anechoic with a white band on top when assessed by the right angle as described by Torp-Pedersen et al [
16,
17].
Descriptive analyses for continuous variables are given as mean and standard deviation. Discrete variables are given in frequency tables and percentages. Linear regression modeling was used to investigate the association between the number of osteophytes (dependent variable) and age and sex (independent variables). Confidence intervals for regression coefficients are calculated using the direct formulas based on the t-values. P-values below 0.05 were regarded as significant. Calculations were performed using the statistical software R, 3.4.1 (The R Foundation for Statistical Computing).
3. Results
29 industrial locations in 22 cities (Bochum, Recklinghausen, Werne, Muenster, Hamburg, Lingen, Hamm, Essen, Gladbeck, Wesel, Muelheim an der Ruhr, Amsberg, Siegen, Frechen, Bergheim, Grevenbroich, Niederzier, Eschweiler, Trier, Saffig, Biblis and Grundremmingen) with different working modalities like office work or heavy industrial work were covered. 427 participants mean age 53.5 years, range 20-79 years (15.7% women and 84.3% men) were enrolled for the standardized ultrasound examination. While 116 images were excluded due to insufficient image quality, a total of 11,840 images of joints were stored. In more detail, images were available from 837 CMC1, 3,386 MCP, 4,239 PIP and 3,378 DIP joints. As 840 images of PIP 2 joints, taken from lateral view, were not included 11,000 images were scored.
Prevalences of osteophytes in various joints are detailed in
Table 1 with the highest prevalence in DIP joints with 61% (2057/3378), followed by PIP with 48% (1620/3399), CMC1 with 39% (325/837) and MCP joints with 16% (544/3386) of participants. Overall DIP 3 on the right-hand side was the most frequently involved joint followed by DIP 3 on the left-hand side and DIP 2 and DIP 4 on the right-hand side and DIP 2 on the left-hand side. When only grade 2 and grade 3 osteophytes were considered, 553 joints were affected. DIP joints were the most frequently affected joint group with 9% (301/3378). The ranking was right and left DIP 3 followed by right hand DIP 2 and 4. CMC1 was marked in 6% (46/837), PIP with 5% (179/3399) and MCP joints with 1% (27/3386). Details are shown in
Table 1.
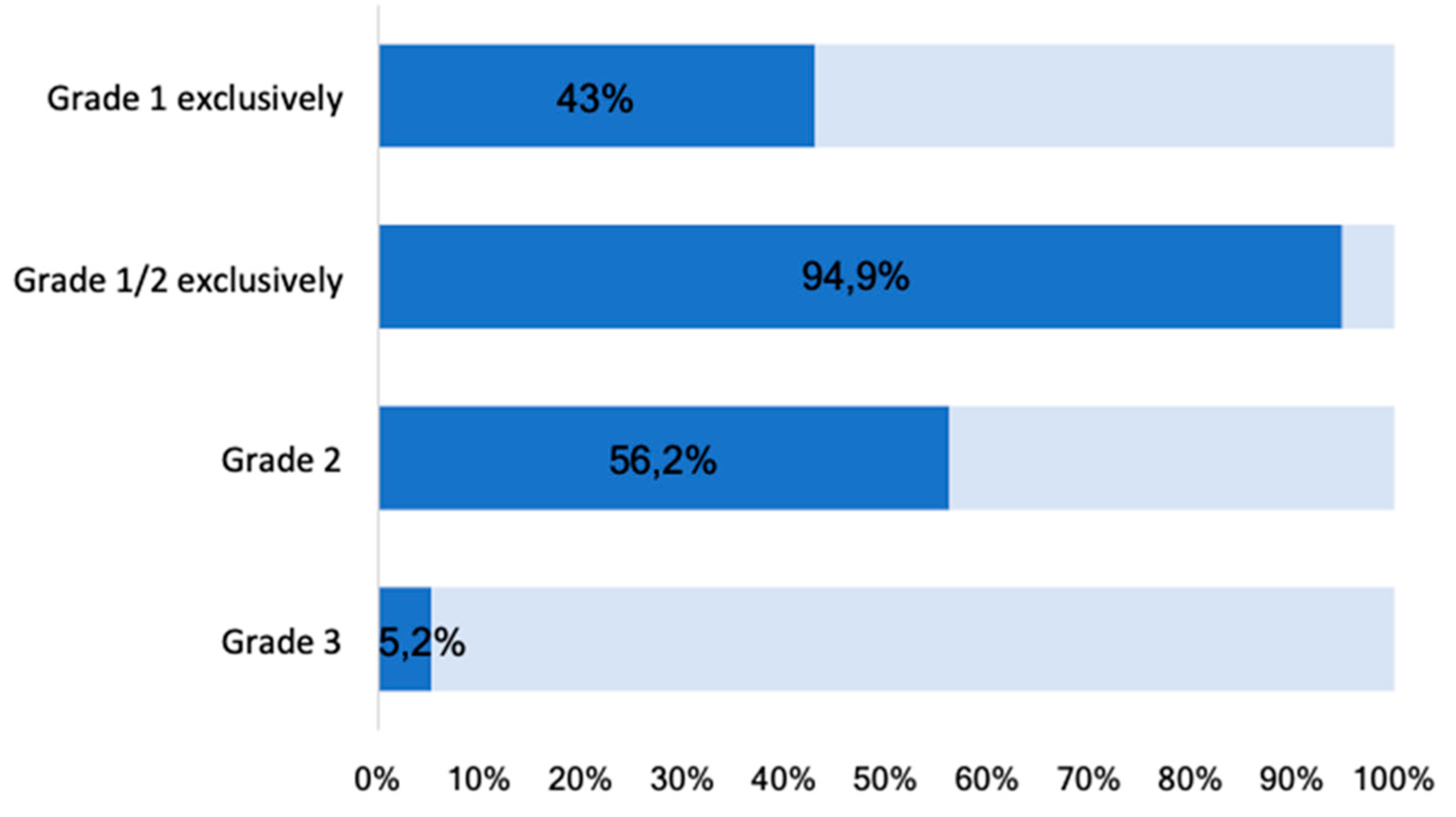
At least one grade 1 osteophyte was found in 426/427 participants (99.8%). Grade 1 osteophytes were present in 184 (43.0%), Grade 2 in 240 (56.2%), and Grade 3 in 22 (5.2%) participants (
Figure 3). No grade 3 osteophyte was found at any MCP joint.
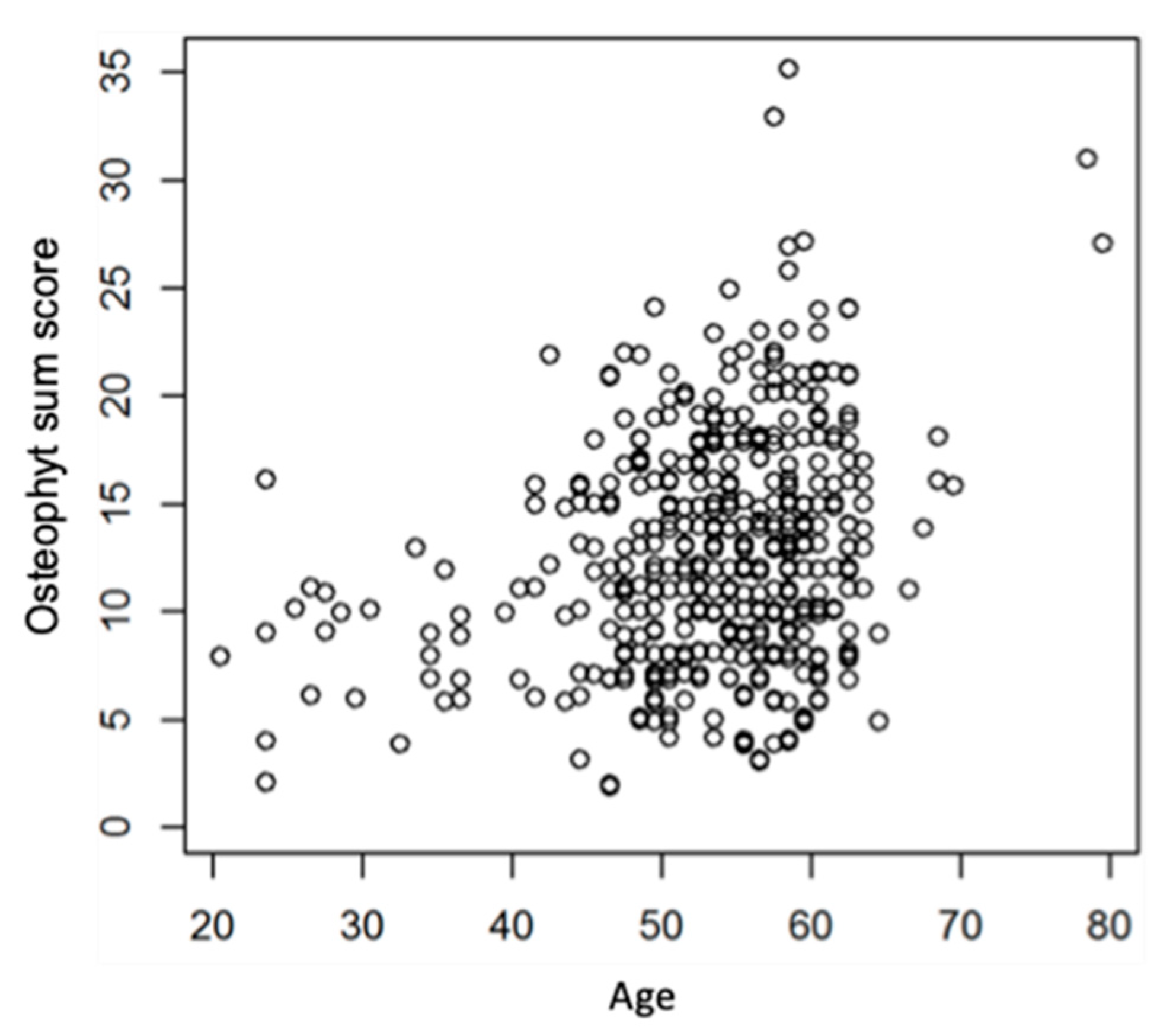
Osteophyte sum scores increased significantly with age but was not influenced by gender (p-value adjusted for age=0.9).
Figure 4 shows the relationship between age and osteophyte sum score.
4. Discussion
With its gripping function, the hand is essential for people and their health. As a surrogate, (good) grip strength is an independent predictor of survival or premature death that has been confirmed by various large cohorts, although the exact relationships are still unclear [
18,
19,
20,
21,
22,
23,
24].
Osteoarthritis of the fingers (HOA) and rheumatoid arthritis are the most common degenerative and inflammatory changes in the hand and lead to a reduction in grip strength. In the population based NAKO cohort of 200,000 adults, 2.68% of men and 9.04% of women reported osteoarthritis of the finger joints [
25]. In the hand examination, 3.79% of men and 8.50% of women had pain in at least one finger joint, and 1.46 and 3.48% had more than 1 swollen finger joint. The frequency increases significantly from the age of 40 [
25]. This evidence motivates for research in the frequency of degenerative changes in the working population and especially a focus on early changes.
Osteophytes are even today by definition one important morphological parameter in various imaging modalities for the confirmation of clinical suspicious HOA. The prevalence of this sign in a population-based study with no previous HOA diagnosis has not yet been evaluated by US systematically. In our study, using US, we found in all but one investigated participants at least one osteophyte, which could result in a clinical diagnosis of HOA. When using CR as the primary imaging modality studies consistently report a lower prevalence, which was expected considering the lower sensitivity of CR in detecting osteophytes in a range from 21% to 92%, with higher prevalence in elder [
12,
15]. For symptomatic HOA, prevalences between 2% and 26% are reported. Of note, most of the published studies examined participants with CR and diagnosed HOA using the Kellgren and Lawrence scoring system, thus osteophytes are one of the main criteria [
5,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38].
Most osteophytes we found are grade 1 osteophytes, a level which are exclusively present in 43.1% participants. If only grade 2 and 3 osteophytes were considered, the prevalence was lower with 56.1% and comparable to other population-based studies [
26,
27,
28,
29,
30,
31,
33,
34,
35,
36,
37,
38]. Only 5.2% of the participants showed severe, larger, osteophytes. Overall, 94.9%, had mild to moderate, grade 1 or 2, osteophytes.
Only a few US studies estimating the prevalence of HOA are published. Recently Abraham et al. showed that about 78% of 311 participants of the Newcastle Thousand Families Study sample had HOA signs in at least one finger joint. All the participants were aged between 61 and 63 [
39]. The lower frequency of HOA reported therein does not contradict our results, however, as only four joints were assessed with US and participants were considered HOA positive if only one osteophyte was found. In our study, 26 joints were assessed. Thus, the chance for at least one osteophyte was much higher in our study.
Based on the results of the present analysis and due to the extremely high prevalence of grade 1 osteophytes, the question whether these findings may be considered per se pathologic or not must be raised. The most frequently used scoring system by Kellgren and Lawrence for CR leaves some room for interpretation: herein, doubtful osteophytes are considered to be normal and are not scored as osteophytes [
5]. As already mentioned, the resolution and, thus, the sensitivity of high frequency US exceeds CR. This could lead to the consequence that grade 1 osteophytes in US are classified as doubtful in conventional radiographs. According to Hart et al., small osteophytic processes in knees should not be considered normal [
40], the same is also suggested by Mathiessen et al. in a study published in 2017, for finger joints examined by US. In subsequent examinations both studies showed that these doubtful osteophytes can develop into larger ones.[
41,
42] Mathiessen et al. made these observations in previously unaffected joints of patients with HOA. It seems that osteophytes detected by US can predict the incidence of CR and clinically proven HOA five years later [
41]. Questions arise, whether these observations also apply to individuals for whom no HOA is known.
No solitary grade 2 or grade 3 osteophytes were found in our study. This indicates a continuity of development, as osteophytes may develop from lower to higher degrees even in individuals without known HOA. Our investigations show that almost every participant examined has at least one osteophyte. We assume that grade 1 osteophytes can be considered normal in our population-based ultrasound study.
Mechanical stress of the hands is discussed repeatedly in the literature as a main risk factor of HOA. There is evidence that the prevalence of OA and HOA is etiologically linked to occupation [
42]. In a study by Haara et al. a correlation between workload and HOA could only be found in women [
43]. Furthermore, it seems that not heavy mechanical work but a high number of repetitive movements could play a role [
44]. However, in a study by Caspi et al. workload had no influence on HOA expression in a cohort of patients with a relatively high mean age of 79 years [
32]. In our study there was no subgrouping that considered the intensity of the different workloads. The participants came from various working areas such as offices, kitchens, handicraft areas or other industry.
In concordance with our results, DIP joints seem to be the most affected joints in studies using CR and US, too [
27,
28,
39,
45,
46]. The second most frequently affected joint group are PIP joints with 48%. This is in contrast to studies using CR which found out that CMC 1 joints are the second most affected joint group by HOA not PIP joints [
27,
28]. Even a study by Abraham et al. using US to estimate the prevalence found CMC 1 joints to be more often affected in 41% than PIP joints with only 23% [
39]. Different results could be explained by assessing more joints than other US studies and a higher sensitivity of US compared to CR. However, if only grade 2 and grade 3 osteophytes are considered, CMC 1 joints are also the second most frequently affected joint group in our study.
We found a significant correlation between age and an increasing number of osteophyte sum score. Similar results were found in various studies. Kalichman et al. described a strong correlation of osteophytes and age in all joint groups in both women and men [
47]. While other studies showed similar results but not always for every age category or joint group [
27,
28,
32,
45]. The findings underline, that low-grade osteophytes are especially common in older patients and should therefore only be considered pathological with caution. We found no effect of gender on the number of osteophytes. Recent studies in this matter provide different results. Higher prevalence of HOA for women was reported by the NAKO trial [
25] and Jones et al. [
48]. Haugen et al. showed similar results but the data were not significant [
31]. Whereas Caspi et al. found a similar prevalence in men and women [
32]. Regarding that there is a sex difference in clinical obvious HOA in particular low-grade osteophytes seem to occur with equal frequency in both groups in our cohort.
It has to be mentioned that US is a suitable imaging technique for detecting HOA signs, but CR is still the gold standard of imaging modalities for diagnosing HOA as there are clinically accepted and more validated as diagnostic criteria [
9]. Various studies show a greater sensitivity of US for the detection of osteophytes [
11,
15,
49,
50], synovitis [
51,
52,
53], and erosions [
49,
50,
54] compared to CR. Moreover, US performs comparable to MRI in detecting osteophytes in HOA. Compared to CR and clinical examinations, US is more sensitive [
12]. US and MRI offer the additional advantage that cartilage thickness can be directly assessed [
55]. The technical progress of US devices with high image resolution and thus increased sensitivity for the detection of HOA signs is contrasted with evaluation systems for estimating the prevalence, which have been used so far.
Upcoming development of rapid, cheap and save robot performed US hand investigation and interpretation by artificial intelligence will boost hand US as a no more specialist dependent population based screening tool [
56,
57,
58,
59,
60].
Thus, definitions in diagnostic criteria for HOA should recognize increasing sensitivity for osteophytes and adapt new evaluation systems for estimating the prevalence of HOA. So far, only one preliminary scoring system for HOA has been introduced which comprises osteophytes and synovitis in grey scale and power Doppler (PD) mode if present [
10]. The experts of the OMERACT group headed by Hammer et al. 2014 recommend the use of a semi-quantitative scoring system to detect and evaluate osteophytes using US like in this study [
13]. Further studies are needed to determine at what number or which degree osteophytes in finger joints are pathological when using US. In the present study, comparable values to those in population-based studies using CR are seen when the presence of grade 2 osteophytes are used as a US criterion to estimate the prevalence of HOA.
As a limitation of our work, interphalangeal 1 (IP1) and MCP 1 joints were not assessed, because we considered standardization of assessment to be difficult in a preliminary study. Images were taken from a palmar view although osteophytes are recommended to be investigated from a dorsal view or by two planes. However, considering our high prevalence rates this recommendation should be reassessed. The palmar view was chosen originally because synovitis, erosions, cartilage thickness, and an approximation of joint space were examined in addition to osteophytes in the data collection, and overall more pathologies were better visualized from palmar. Furthermore, the voluntary nature of participation, the recruitment at work and the larger male cohort may introduce bias by indication. To keep this bias as low as possible, subjects without any complaints were approached and encouraged to participate in the study. Another limitation is that the images were evaluated with a consensus reading, a blinded double reading with a third independent reading in case of discrepancies could certainly make the results more reliable.
5. Conclusions
The presence of osteophytes is a main criterion for the diagnosis of HOA. The presence of osteophytes, particularly low-grade osteophytes, are a normal finding in our population-based US study and may lead to overdiagnosis of HOA. The prevalence is increasing with age. The border to clinically relevant pathology and the risk for general progression of HOA can only be defined by longitudinal observations. Even with those limitations high sensitivity, validated grading and automated performance will increase the relevance of hand US as a population based screening tool in future.
Author Contributions
MG: Acquisition, analysis, and interpretation of data. Draft and design of the work. Revision of the work. RB: Analysis and interpretation of data. Draft and design of the work. Revision of the work. SV: Design and conception of the study. Interpretation and analysis of data. Draft and design of the work. Revision of the work. HA: Design and conception of the study. Draft and design of the work. Revision of the work. JR: Interpretation and analysis of data. Draft and design of the work. Revision of the work CF: Design and conception of the study. Revision of the work. XB: Interpretation of data, revision of the manuscript. BO: Interpretation and analysis of data. Draft and design of the work. Revision of the work MS: Design and conception of the study. Analysis and interpretation of data. Draft and design of the work. Revision of the work. OS: Design and conception of the study. Analysis and interpretation of data. Draft and design of the work. Revision of the work. PS: Interpretation and analysis of data. Draft and design of the work. Revision of the work. All authors have read and approved the manuscript. OS and PS share the last authorship. “Conceptualization, S.V., J.R., B.O., M.S., C.F., and O.S.; methodology, R.B., S.V., J.R., B.O., O.S. and P.S.;; software, H.A., and R.B.; validation, M.G., H.A., P.S., and R.B.; formal analysis, M.G., H.A., and R.B.; investigation, M.G. and P.S.; resources, C.F. and M.S.; data curation, H.A. and J.R.; writing—original draft preparation, M.G.; writing—review and editing, S.V., J.R., B.O., O.S., X.B., and P.S.; visualization, X.X.; supervision, X.B. and O.S.; All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
The study was carried out in accordance with the principles of the Declaration of Helsinki, International Conference of Harmonization Good Clinical Practice guidelines, and all applicable laws and regulations. The local ethics committee at the University of Duesseldorf approved the study and all participants provided informed written consent (trial number: 4336).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Further data could be requested by the authors.
Acknowledgments
We thank the Rheumazentrum Rhein-Ruhr e.V for the continuous support during that study, especially Ms. Martina Brandes. In this work, the results and figures of the dissertation "Prevalence and patterns of ultrasonographic findings of hand osteoarthritis in a working population" by Mario Giulini, presented at the Faculty of Medicine Meeting of the Heinrich Heine University Duesseldorf in 2022, have been included.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haugen, I. Hand osteoarthritis: current knowledge and new ideas. Scand. J. Rheumatol. 2016, 45, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, M.; Bøyesen, P.; Smeets, W.; Haugen, I.; Liu, R.; Visser, W.; van der Heijde, D.M. Report from the OMERACT Hand Osteoarthritis Special Interest Group: Advances and Future Research Priorities. J. Rheumatol. 2014, 41, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Slatkowsky-Christensen, B.; Mowinckel, P.; Loge, J.H.; Kvien, T.K. Health-related quality of life in women with symptomatic hand osteoarthritis: A comparison with rheumatoid arthritis patients, healthy controls, and normative data. Arthritis Care Res. 2007, 57, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Alarcon, G.; Appelrouth, D.; Bloch, D.; Borenstein, D.; Brandt, K.; Brown, C.; Cooke, T.D.; Daniel, W.; Gray, R.; et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990, 33, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Rheumatol. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.; Vignon, E.; Maheu, E. Session 2: Clinical aspects. Clinical assessment of hand OA. Osteoarthr. Cartil. 2000, 8, S38–S40. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T.; Zhang, Y. Epidemiology of Osteoarthritis. Rheum. Dis. Clin. North Am. 2013, 39, 1–19. [Google Scholar] [CrossRef]
- Figueiredo, C.P.; Simon, D.; Englbrecht, M.; Haschka, J.; Kleyer, A.; Bayat, S.; Hueber, A.; Pereira, R.M.R.; Rech, J.; Schett, G. Quantification and Impact of Secondary Osteoarthritis in Patients With Anti–Citrullinated Protein Antibody–Positive Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 2114–2121. [Google Scholar] [CrossRef]
- Zhang, W.; Doherty, M.; Leeb, B.F.; Alekseeva, L.; Arden, N.K.; Bijlsma, J.W.; Dincer, F.; Dziedzic, K.; Hauselmann, H.J.; Kaklamanis, P.; et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Rheumatol. 2009, 68, 8–17. [Google Scholar] [CrossRef]
- I Keen, H.; Lavie, F.; Wakefield, R.J.; D'Agostino, M.-A.; Hammer, H.B.; Hensor, E.; Pendleton, A.; Kane, D.; Guerini, H.; Schueller-Weidekamm, C.; et al. The development of a preliminary ultrasonographic scoring system for features of hand osteoarthritis. Rheumatol. 2008, 67, 651–655. [Google Scholar] [CrossRef]
- Kortekaas, M.C.; Kwok, W.-Y.; Reijnierse, M.; Huizinga, T.W.J.; Kloppenburg, M. Osteophytes and joint space narrowing are independently associated with pain in finger joints in hand osteoarthritis. Rheumatol. 2011, 70, 1835–1837. [Google Scholar] [CrossRef]
- Mathiessen, A.; Haugen, I.K.; Slatkowsky-Christensen, B.; Bøyesen, P.; Kvien, T.K.; Hammer, H.B. Ultrasonographic assessment of osteophytes in 127 patients with hand osteoarthritis: exploring reliability and associations with MRI, radiographs and clinical joint findings. Rheumatol. 2013, 72, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Hammer, H.B.; Iagnocco, A.; Mathiessen, A.; Filippucci, E.; Gandjbakhch, F.; Kortekaas, M.C.; Möller, I.; Naredo, E.; Wakefield, R.J.; Aegerter, P.; et al. Global ultrasound assessment of structural lesions in osteoarthritis: a reliability study by the OMERACT ultrasonography group on scoring cartilage and osteophytes in finger joints. Rheumatol. 2016, 75, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, R.J.; Balint, P.V.; Szkudlarek, M.; Filippucci, E.; Backhaus, M.; D’Agostino, M.-A.; Sanchez, E.N.; Iagnocco, A.; A Schmidt, W.A.; Bruyn, G.A.W.; et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J. Rheumatol. 2005, 32, 2485–2487. [Google Scholar] [PubMed]
- I Keen, H.; Wakefield, R.J.; Grainger, A.J.; A Hensor, E.M.; Emery, P.; Conaghan, P.G. Can ultrasonography improve on radiographic assessment in osteoarthritis of the hands? A comparison between radiographic and ultrasonographic detected pathology. Rheumatol. 2008, 67, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Torp-Pedersen, S.; Bartels, E.M.; Wilhjelm, J.; Bliddal, H. Articular Cartilage Thickness Measured with US is Not as Easy as It Appears: A Systematic Review of Measurement Techniques and Image Interpretation. Ultraschall der Med. - Eur. J. Ultrasound 2011, 32, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Mandl, P.; Supp, G.; Baksa, G.; Radner, H.; Studenic, P.; Gyebnar, J.; Kurucz, R.; Niedermayer, D.; Aletaha, D.; Balint, P.V.; et al. Relationship between radiographic joint space narrowing, sonographic cartilage thickness and anatomy in rheumatoid arthritis and control joints. Rheumatol. 2014, 74, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Martyn, C.N.; Cooper, C.; Sayer, A.A. Grip strength, body composition, and mortality. Leuk. Res. 2006, 36, 228–235. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Arvandi, M.; Strasser, B.; Meisinger, C.; Volaklis, K.; Gothe, R.M.; Siebert, U.; Ladwig, K.-H.; Grill, E.; Horsch, A.; Laxy, M.; et al. Gender differences in the association between grip strength and mortality in older adults: results from the KORA-age study. BMC Geriatr. 2016, 16, 201. [Google Scholar] [CrossRef]
- Kim, Y.; Wijndaele, K.; Lee, D.-C.; Sharp, S.J.; Wareham, N.; Brage, S. Independent and joint associations of grip strength and adiposity with all-cause and cardiovascular disease mortality in 403,199 adults: the UK Biobank study. Am. J. Clin. Nutr. 2017, 106, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.A.; Welsh, P.; Lyall, D.M.; Steell, L.; Petermann, F.; Anderson, J.; Iliodromiti, S.; Sillars, A.; Graham, N.; Mackay, D.F.; et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 2018, 361, k1651. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.H.; Han, X.; Zheng, W.J.; Zhuang, S.F. Low Grip Strength and Increased Mortality Hazard among Middle-Aged and Older Chinese Adults with Chronic Diseases. 2023, 36, 213–221. [CrossRef]
- Cai, Y.; Liu, L.; Wang, J.; Gao, Y.; Guo, Z.; Ping, Z. Linear association between grip strength and all-cause mortality among the elderly: results from the SHARE study. Aging Clin. Exp. Res. 2021, 33, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.O.; Günther, K.-P.; Goronzy, J.; Albrecht, K.; Chenot, J.-F.; Callhoff, J.; Richter, A.; Kasch, R.; Ahrens, W.; Becher, H.; et al. Häufigkeiten muskuloskelettaler Symptome und Erkrankungen in der bevölkerungsbezogenen NAKO Gesundheitsstudie. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz 2020, 63, 415–425. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Nevitt, M.C.; Niu, J.; Goggins, J.P.; Aliabadi, P.; Yu, W.; Lui, L.; Felson, D.T. Lower prevalence of hand osteoarthritis among Chinese subjects in Beijing compared with white subjects in the United States: The Beijing Osteoarthritis Study. Arthritis Rheum. 2003, 48, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Dahaghin, S.; A Bierma-Zeinstra, S.M.; Ginai, A.Z.; Pols, H.A.P.; Hazes, J.M.W.; Koes, B.W. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Rheumatol. 2005, 64, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Wilder, F.; Barrett, J.; Farina, E. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthr. Cartil. 2006, 14, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Szoeke, C.; Cicuttini, F.; Guthrie, J.; Clark, M.; Dennerstein, L. Factors affecting the prevalence of osteoarthritis in healthy middle-aged women: Data from the longitudinal Melbourne Women's Midlife Health Project. Bone 2006, 39, 1149–1155. [Google Scholar] [CrossRef]
- Kwok, W.; Bijsterbosch, J.; Malm, S.; Biermasz, N.; Huetink, K.; Nelissen, R.; Meulenbelt, I.; Huizinga, T.; Klooster, R.v. .; Stoel, B.; et al. Validity of joint space width measurements in hand osteoarthritis. Osteoarthr. Cartil. 2011, 19, 1349–1355. [Google Scholar] [CrossRef]
- Haugen, I.K.; Englund, M.; Aliabadi, P.; Niu, J.; Clancy, M.; Kvien, T.K.; Felson, D.T. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Rheumatol. 2011, 70, 1581–1586. [Google Scholar] [CrossRef]
- Caspi, D.; Flusser, G.; Farber, I.; Ribak, J.; Leibovitz, A.; Habot, B.; Yaron, M.; Segal, R. Clinical, radiologic, demographic, and occupational aspects of hand osteoarthritis in the elderly. Semin. Arthritis Rheum. 2001, 30, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; Li, L.; Batsevich, V.; Malkin, I.; Kobyliansky, E. Prevalence, pattern and determinants of radiographic hand osteoarthritis in five Russian community-based samples. Osteoarthr. Cartil. 2010, 18, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Kodama, R.; Muraki, S.; Oka, H.; Iidaka, T.; Teraguchi, M.; Kagotani, R.; Asai, Y.; Yoshida, M.; Morizaki, Y.; Tanaka, S.; et al. Prevalence of hand osteoarthritis and its relationship to hand pain and grip strength in Japan: The third survey of the ROAD study. Mod. Rheumatol. 2016, 26, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.; Lachance, L.; Hochberg, M.; Jamadar, D. Radiographically defined osteoarthritis of the hand and knee in young and middle-aged African American and Caucasian women. Osteoarthr. Cartil. 2000, 8, 69–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, J.; Kelly-Hayes, M.; Chaisson, C.E.; Aliabadi, P.; Felson, D.T. Prevalence of Symptomatic Hand Osteoarthritis and Its Impact on Functional Status among the Elderly: The Framingham Study. Am. J. Epidemiology 2002, 156, 1021–1027. [Google Scholar] [CrossRef]
- Carmona, L. The burden of musculoskeletal diseases in the general population of Spain: results from a national survey. Rheumatol. 2001, 60, 1040–1045. [Google Scholar] [CrossRef]
- A Andrianakos, A.; Kontelis, L.K.; Karamitsos, D.G.; I Aslanidis, S.; I Georgountzos, A.; O Kaziolas, G.; Pantelidou, K.V.; Vafiadou, E.V.; Dantis, P.C. ; ESORDIG Study Group Prevalence of symptomatic knee, hand, and hip osteoarthritis in Greece. The ESORDIG study.. 2006, 33, 2507–13. [Google Scholar]
- Abraham, A.M.; Pearce, M.S.; Mann, K.D.; Francis, R.M.; Birrell, F. Population prevalence of ultrasound features of osteoarthritis in the hand, knee and hip at age 63 years: the Newcastle thousand families birth cohort. BMC Musculoskelet. Disord. 2014, 15, 162. [Google Scholar] [CrossRef]
- Hart, D.; Spector, T. Kellgren & Lawrence grade 1 osteophytes in the knee—doubtful or definite? Osteoarthr. Cartil. 2003, 11, 149–150. [Google Scholar] [CrossRef]
- Mathiessen, A.; Slatkowsky-Christensen, B.; Kvien, T.K.; Haugen, I.K.; Hammer, H.B. Ultrasound-detected osteophytes predict the development of radiographic and clinical features of hand osteoarthritis in the same finger joints 5 years later. RMD Open 2017, 3, e000505. [Google Scholar] [CrossRef]
- Rossignol, M.; Leclerc, A.; Hilliquin, P.; A Allaert, F.; Rozenberg, S.; Valat, J.-P.; Avouac, B.; Coste, P.; Savarieau, B.; Fautrel, B. Primary osteoarthritis and occupations: a national cross sectional survey of 10 412 symptomatic patients. Occup. Environ. Med. 2003, 60, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Haara, M.M.; Manninen, P.; Kröger, H.; A Arokoski, J.P.; Kärkkäinen, A.; Knekt, P.; Aromaa, A.; Heliövaara, M. Osteoarthritis of finger joints in Finns aged 30 or over: prevalence, determinants, and association with mortality. Rheumatol. 2003, 62, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Zhang, Y.; Nevitt, M.C.; Xu, L.; Niu, J.; Lui, L.-Y.; Yu, W.; Aliabadi, P.; Felson, D.T. Chopstick arthropathy: The Beijing Osteoarthritis Study. Arthritis Rheum. 2004, 50, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Kallman, D.A.; Wigley, F.M.; Scott, W.W.; Hochberg, M.C.; Tobin, J.D. The longitudinal course of hand osteoarthritis in a male population. Arthritis Rheum. 1990, 33, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Egger, P.; Coggon, D.; Hart, D.J.; Masud, T.; Cicuttini, F.; Doyle, D.V.; Spector, T.D. Generalized osteoarthritis in women: Pattern of joint involvement and approaches to definition for epidemiological studies. 1996, 23, 1938–1942.
- Kalichman, L.; Cohen, Z.; Kobyliansky, E.; Livshits, G. Patterns of joint distribution in hand osteoarthritis: Contribution of age, sex, and handedness. Am. J. Hum. Biol. 2004, 16, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Cooley, H.M.; Stankovich, J.M. A cross sectional study of the association between sex, smoking, and other lifestyle factors and osteoarthritis of the hand. . 2002, 29, 1719–24. [Google Scholar]
- Vlychou, M.; Koutroumpas, A.; Malizos, K.; Sakkas, L. Ultrasonographic evidence of inflammation is frequent in hands of patients with erosive osteoarthritis. Osteoarthr. Cartil. 2009, 17, 1283–1287. [Google Scholar] [CrossRef]
- Wittoek, R.; Carron, P.; Verbruggen, G. Structural and inflammatory sonographic findings in erosive and non-erosive osteoarthritis of the interphalangeal finger joints. Rheumatol. 2010, 69, 2173–2176. [Google Scholar] [CrossRef]
- Backhaus, M.; Kamradt, T.; Sandrock, D.; Loreck, D.; Fritz, J.; Wolf, K.J.; Raber, H.; Hamm, B.; Burmester, G.-R.; Bollow, M. Arthritis of the finger joints: A comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 1999, 42, 1232–1245. [Google Scholar] [CrossRef]
- Szkudlarek, M.; Court-Payen, M.; Jacobsen, S.; Klarlund, M.; Thomsen, H.S.; Østergaard, M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 955–962. [Google Scholar] [CrossRef]
- Szkudlarek, M.; Klarlund, M.; Narvestad, E.; Court-Payen, M.; Strandberg, C.; Jensen, K.; Thomsen, H.; Østergaard, M. Ultrasonography of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis: a comparison with magnetic resonance imaging, conventional radiography and clinical examination. Arthritis Res. Ther. 2006, 8, R52. [Google Scholar] [CrossRef]
- Bajaj, S.; Lopez-Ben, R.; Oster, R.; Alarcón, G.S. Ultrasound detects rapid progression of erosive disease in early rheumatoid arthritis: a prospective longitudinal study. Skelet. Radiol. 2006, 36, 123–128. [Google Scholar] [CrossRef]
- Hayashi, D.; Roemer, F.; Guermazi, A. Imaging for osteoarthritis. Ann. Phys. Rehabilitation Med. 2016, 59, 161–169. [Google Scholar] [CrossRef]
- Lautwein, A.; Ostendorf, B.; Vordenbäumen, S.; Liedmann, A.; Brinks, R.; Giulini, M.; Ohrndorf, S.; Backhaus, M.; Acar, H.; Sander, O.; et al. Musculoskeletal ultrasound as a screening-tool for rheumatoid arthritis: results of the “Rheuma-Truck” screening and awareness initiative. Hortic. Bras. 2022, 62, 1. [Google Scholar] [CrossRef]
- Molyneux, P.; Bowen, C.; Ellis, R.; Rome, K.; Frecklington, M.; Carroll, M. Evaluation of osteoarthritic features in peripheral joints by ultrasound imaging: A systematic review. Osteoarthr. Cartil. Open 2021, 3, 100194. [Google Scholar] [CrossRef]
- Frederiksen, B.A.; Schousboe, M.; Terslev, L.; Iversen, N.; Lindegaard, H.; Savarimuthu, T.R.; Just, S.A. Ultrasound joint examination by an automated system versus by a rheumatologist: from a patient perspective. Hortic. Bras. 2022, 62, 1–10. [Google Scholar] [CrossRef]
- Christensen, A.B.H.; Just, S.A.; Andersen, J.K.H.; Savarimuthu, T.R. Applying cascaded convolutional neural network design further enhances automatic scoring of arthritis disease activity on ultrasound images from rheumatoid arthritis patients. Rheumatol. 2020, 79, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.K.H.; Pedersen, J.S.; Laursen, M.S.; Holtz, K.; Grauslund, J.; Savarimuthu, T.R.; Just, S.A. OP0349 NEURAL NETWORKS FOR AUTOMATED SCORING OF JOINT DISEASE ACTIVITY ON DOPPLER ULTRASOUND IMAGES. Annals of the Rheumatic Diseases 2019;78:258.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).