Submitted:
08 May 2023
Posted:
09 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
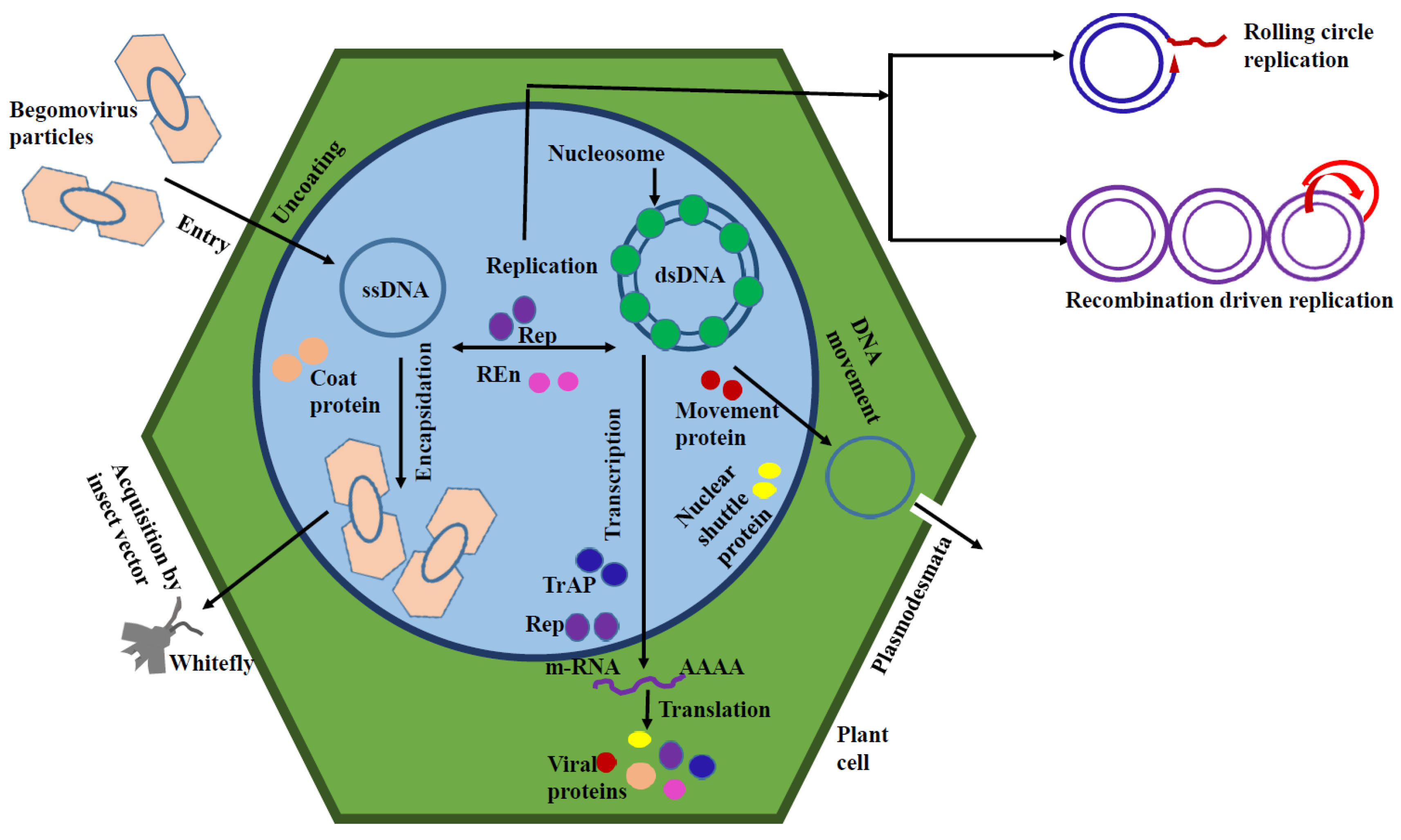
2. Genome Organization and Transmission of Begomovirus
3. Interaction among Plant, Whitefly and Begomovirus
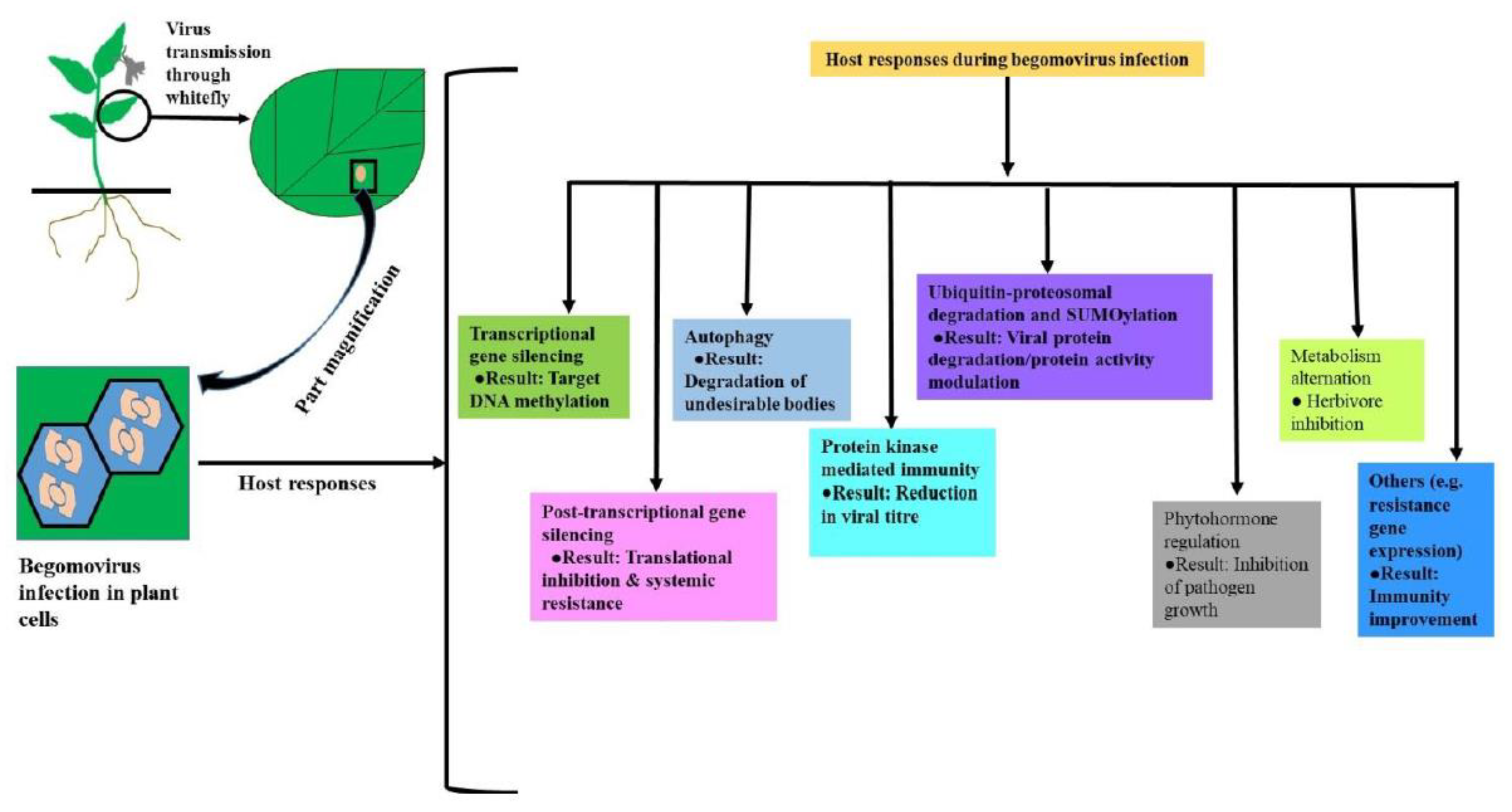
4. Host Plant Responses against the Begomovirus Infection
4.1. Transcriptional Gene Silencing (TGS)
4.2. Post-Transcriptional Gene Silencing (PTGS)
4.3. Autophagy
4.4. Protein Kinase-Mediated Immunity
4.5. Ubiquitin–Proteasomal Degradation and SUMOylation
4.6. Regulation of Phytohormones
4.7. Alternations in Primary and Secondary Metabolism of Plant
5. Future Prospect and Conclusion
Funding
Conflicts of Interest
References
- Fondong, V.N. Geminivirus Protein Structure and Function. Mol. Plant Pathol. 2013, 14, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Beam, K.; Ascencio-Ibáñez, J.T. Geminivirus Resistance: A Minireview. Front. Plant Sci. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Devendran, R.; Kumar, M.; Ghosh, D.; Yogindran, S.; Karim, M.J.; Chakraborty, S. Capsicum-Infecting Begomoviruses as Global Pathogens: Host–Virus Interplay, Pathogenesis, and Management. Trends Microbiol. 2022, 30, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Ansari, W.A.; Jaiswal, D.K. Whitefly-Transmitted Plant Viruses and Their Management. Emerg. Trends Plant Pathol. 2020, 175–195. [Google Scholar] [CrossRef]
- Leke, W.N.; Mignouna, D.B.; Brown, J.K.; Kvarnheden, A. Begomovirus Disease Complex : Emerging Threat to Vegetable Production Systems of West and Central Africa. Agric. Food Secur. 2015, 4, 1–14. [Google Scholar] [CrossRef]
- Jones, D.R. Plant Viruses Transmitted by Whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Nigam, D. Genomic Variation and Diversification in Begomovirus Genome in Implication to Host and Vector Adaptation. Plants 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Zerbini, F.M.; Briddon, R.W.; Idris, A.; Martin, D.P.; Moriones, E.; Navas-Castillo, J.; Rivera-Bustamante, R.; Roumagnac, P.; Varsani, A. ICTV Virus Taxonomy Profile: Geminiviridae. J. Gen. Virol. 2017, 98, 131–133. [Google Scholar] [CrossRef]
- Kumar, Y.; Hallan, V.; Zaidi, A.A. Molecular Characterization of a Distinct Bipartite Begomovirus Species Infecting Tomato in India. Virus Genes 2008, 37, 425–431. [Google Scholar] [CrossRef]
- Venkataravanappa, V.; Lakshminarayana Reddy, C.N.; Swaranalatha, P.; Jalali, S.; Briddon, R.W.; Reddy, M.K. Diversity and Phylogeography of Begomovirus-Associated Beta Satellites of Okra in India. Virol. J. 2011, 8, 555. [Google Scholar] [CrossRef]
- Gray, S.M.; Banerjee, N. Mechanisms of Arthropod Transmission of Plant and Animal Viruses. Microbiol. Mol. Biol. Rev. 1999, 63, 128–148. [Google Scholar] [CrossRef] [PubMed]
- Yadava, P.; Suyal, G.; Mukherjee, S.K. Begomovirus DNA Replication and Pathogenicity. Curr. Sci. 2010, 98, 260–268. [Google Scholar]
- Leonard, A. The Different Faces of Rolling-Circle Replication and Its Multifunctional Initiator Proteins. Front. Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Roumagnac, P.; Varsani, A. Recombination in Eukaryotic Single Stranded DNA Viruses. Viruses 2011, 3, 1699–1738. [Google Scholar] [CrossRef]
- Rodr, E.; Lozano-dur, R.; Piedra-aguilera, A.; Cruzado, L.; Bejarano, E.R.; Castillo, A.G. Geminivirus Rep Protein Interferes with the Plant DNA Methylation Machinery and Suppresses Transcriptional Gene Silencing. New Phytol. 2013, 199, 464–475. [Google Scholar]
- Mansoor, S.; Zafar, Y.; Briddon, R.W. Geminivirus Disease Complexes : The Threat Is Spreading. Trends Plant Sci. 2006, 11, 209–212. [Google Scholar] [CrossRef]
- Luan, J.; Wang, X.; Colvin, J.; Liu, S. Plant-Mediated Whitefly – Begomovirus Interactions : Research Progress and Future Prospects. Bull. Entomol. Res. 2014, 104, 267–276. [Google Scholar] [CrossRef]
- Zhang, T.; Luan, J.; Qi, J.-F.; Huang, C.-J.; Li, Meng; Zhou, Xueping; Liu, S. Begomovirus – Whitefly Mutualism Is Achieved through Repression of Plant Defences by a Virus Pathogenicity Factor. Mol. Ecol. 2012, 21, 1294–1304. [Google Scholar] [CrossRef]
- Wang, J.; Bing, X.; Li, M.; Ye, G.; Liu, S. Infection of Tobacco Plants by a Begomovirus Improves Nutritional Assimilation by a White Fl Y. Entomol. Exp. Appl. 2012, 144, 191–201. [Google Scholar] [CrossRef]
- Ueda, S.; Onuki, M.; Yamashita, M.; Yamato, Y. Pathogenicity and Insect Transmission of a Begomovirus Complex between Tomato Yellow Leaf Curl Virus and Ageratum Yellow Vein Betasatellite. Virus Genes 2012, 44, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Reddy, K.; Bhattacharyya, D.; Chakraborty, S. Plant Responses to Geminivirus Infection : Guardians of the Plant Immunity. Virol. J. 2021, 18, 1–25. [Google Scholar] [CrossRef]
- Jackel, J.N.; Storer, J.M.; Coursey, T.; Bisaro, D.M. Arabidopsis RNA Polymerases IV and V Are Required To Establish H3K9 Methylation, but Not Cytosine Methylation, on Geminivirus. J. Virol. 2016, 90, 7529–7540. [Google Scholar] [CrossRef]
- He, X.; Hsu, Y.; Zhu, S.; Wierzbicki, A.T.; Pontes, O.; Pikaard, S.; Liu, H.; Wang, C.; Jin, H.; Zhu, J. An Effector of RNA-Directed DNA Methylation in Arabidopsis Is an ARGONAUTE 4- and RNA-Binding Protein. Cell 2010, 137, 498–508. [Google Scholar] [CrossRef]
- Wang, B.; Li, F.; Huang, C.; Yang, X.; Qian, Y.; Xie, Y. V2 of Tomato Yellow Leaf Curl Virus Can Suppress Methylation-Mediated Transcriptional Gene. gene Virol. 2013, 1–18. [Google Scholar] [CrossRef]
- Wang, J.; Mei, J.; Ren, G. Plant MicroRNAs : Biogenesis, Homeostasis, and Degradation. Front. Plant Sci. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Chellappan, P.; Vanitharani, R.; Fauquet, C.M. MicroRNA-Binding Viral Protein Interferes with Arabidopsis Development. Proc. Natl. Acad. Sci. 2005, 102, 10381–10386. [Google Scholar] [CrossRef]
- Elvira-matelot, E. Diversity of RNA Silencing Pathways in Plants. Plant Gene Silenc. Mech. Appl. 2022, 1–32. [Google Scholar] [CrossRef]
- Bisaro, D.M. Silencing Suppression by Geminivirus Proteins. Viro 2006, 344, 158–168. [Google Scholar] [CrossRef]
- Li, F.; Huang, C.; Li, Z.; Zhou, X. Suppression of RNA Silencing by a Plant DNA Virus Satellite Requires a Host Calmodulin-Like Protein to Repress RDR6 Expression. PLoS Pathog. 2014, 10, 11–14. [Google Scholar] [CrossRef]
- Haxim, Y.; Ismayil, A.; Jia, Q.; Wang, Y.; Zheng, X.; Chen, T.; Qian, L.; Liu, N.; Wang, Y.; Han, S.; et al. Autophagy Functions as an Antiviral Mechanism against Geminiviruses in Plants. Plant Biol. 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wang, Q.; Xiao, R.; Cao, L.; Wang, Y. Mimic Phosphorylation of a  C1 Protein Encoded by TYLCCNB Impairs Its Functions as a Viral Suppressor of RNA Silencing and a Symptom Determinant. J. Virol. 2017, 91, 1–18. [Google Scholar] [CrossRef]
- Hao, L.; Wang, H.; Sunter, G.; Bisaro, D.M. Geminivirus AL2 and L2 Proteins Interact with and Inactivate SNF1 Kinase. Plant Cell 2003, 15, 1034–1048. [Google Scholar] [CrossRef]
- Patel, A.; Dey, N.; Chaudhuri, S.; Pal, A. Molecular and Biochemical Characterization of a Vigna Mungo MAP Kinase Associated with Mungbean Yellow Mosaic India Virus Infection and Deciphering Its Role in Restricting the Virus Multiplication. Plant Sci. 2017. [Google Scholar] [CrossRef]
- Li, Y.; Qin, L.; Zhao, J.; Muhammad, T.; Cao, H.; Li, H. SlMAPK3 Enhances Tolerance to Tomato Yellow Leaf Curl Virus ( TYLCV ) by Regulating Salicylic Acid and Jasmonic Acid Signaling in Tomato ( Solanum Lycopersicum ). PLoS One 2017, 1–21. [Google Scholar] [CrossRef]
- Gui, X. β C1 Protein Encoded in Geminivirus Satellite Concertedly Targets MKK2 and MPK4 to Counter Host Defense. PLoS Pathog. 2019, 1–23. [Google Scholar]
- Huang, B.; Du, D.; Zhang, R.; Wu, X.; Xing, Z.; He, Y.; Huang, W. Synthesis, Characterization and Biological Studies of Diosgenyl Analogues. Bioorganic Med. Chem. Lett. 2012, 22, 7330–7334. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Carolino, S.M.B.; Mariano, A.C.; Andrade, M.O.; Oliveira, M.L.; Baracat-pereira, M.C.; Brommonshenkel, S.H.; Fontes, E.P.B. Identification of a Novel Receptor-like Protein Kinase That Interacts with a Geminivirus Nuclear Shuttle Protein. Virology 2004, 318, 24–31. [Google Scholar] [CrossRef]
- Florentino, L.H.; Santos, A.; Fontenelle, M.R.; Pinheiro, G.L.; Zerbini, F.M.; Baracat-pereira, M.C.; Fontes, E.P.B. A PERK-Like Receptor Kinase Interacts with the Geminivirus Nuclear Shuttle Protein and Potentiates Viral Infection. J. Virol. 2006, 80, 6648–6656. [Google Scholar] [CrossRef]
- Adams, E.H.G.; Spoel, S.H. The Ubiquitin-Proteasome System as a Transcriptional Regulator of Plant Immunity. J. Exp. Bot. 2018, 0–3. [Google Scholar] [CrossRef]
- Shen, Q.; Hu, T.; Bao, M.; Cao, L.; Zhang, H.; Song, F.; Xie, Q.; Zhou, X. Tobacco RING E3 Ligase NtRFP1 Mediates Ubiquitination and Proteasomal Degradation of a Geminivirus-Encoded b C1. Mol. Plant 2016, 9, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Chatterjee, K.S.; Jha, V.; Das, R.; Shivaprasad, P. V Stability of Begomoviral Pathogenicity Determinant β C1 Is Modulated by Mutually Antagonistic SUMOylation and SIM Interactions. BMC Biol. 2020, 1–24. [Google Scholar]
- Ghosh, D. Molecular Interplay between Phytohormones and Geminiviruses : A Saga of a Never-Ending Arms Race. J. Exp. Bot. 2021, 72, 2903–2917. [Google Scholar] [CrossRef] [PubMed]
- Miozzi, L.; Napoli, C.; Sardo, L.; Accotto, G.P. Transcriptomics of the Interaction between the Monopartite Phloem-Limited Geminivirus Tomato Yellow Leaf Curl Sardinia Virus and Solanum Lycopersicum Highlights a Role for Plant Hormones, Autophagy and Plant Immune System Fine Tuning during Infection. PLoS One 2014, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Weldegergis, B.T.; Li, J.; Jung, C.; Qu, J.; Sun, Y.; Qian, H. Virulence Factors of Geminivirus Interact with MYC2 to Subvert Plant Resistance and Promote Vector Performance. Plant Cell 2014, 26, 4991–5008. [Google Scholar] [CrossRef] [PubMed]
- Oa, G.I.W.; Sozzani, R.; Lee, T.; Chu, T.; Wolfinger, R.D.; Cella, R.; Hanley-bowdoin, L. Global Analysis of Arabidopsis Gene Expression Uncovers a Complex Array of Changes Impacting Pathogen Response and Cell Cycle During. Plant Physiol. 2008, 148, 436–454. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of Convalescent Plasma Therapy in Severe COVID-19 Patients. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Kaltdorf, M.; Dandekar, T. The Nexus between Growth and Defence Signalling : Auxin and Cytokinin Modulate Plant Immune Response Pathways. J. Exp. Bot. 2015, 1–12. [Google Scholar] [CrossRef]
- Vinutha, T.; Vanchinathan, S.; Bansal, N.; Kumar, G.; Permar, V.; Watts, A.; Ramesh, S. V; Praveen, S. Tomato Auxin Biosynthesis / Signaling Is Reprogrammed by the Geminivirus to Enhance Its Pathogenicity. Planta 2020, 1–14. [Google Scholar] [CrossRef]
- Mahmoudabadi, G.; Milo, R.; Phillips, R. Energetic Cost of Building a Virus. Proc. Natl. Acad. Sci. 2017, E4324–E4333. [Google Scholar] [CrossRef]
- Ghosh, S. Factors Determining Transmission of Persistent Viruses by Bemisia Tabaci and Emergence of New Virus – Vector Relationships. Viruses 2021, 13, 1–13. [Google Scholar] [CrossRef]
- Luan, J.; Yao, D. Suppression of Terpenoid Synthesis in Plants by a Virus Promotes Its Mutualism with Vectors. Ecol. Lett. 2013, 16, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.E.I.; Auslane, H.J.M.C.; Schuster, D.J. Repellency of Ginger Oil to Bemisia Argentifolii ( Homoptera : Aleyrodidae ) on Tomato. Ecotoxicology 2004, 1310–1318. [Google Scholar]
- Oa, T.I.W.; Weidner, M.; Schu, S.; Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Both, M.T.J. De; Haring, M.A.; Schuurink, R.C.; Wageningen, A.E. The Role of Specific Tomato Volatiles In. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef]
- Verlaan, M.G.; Hutton, S.F.; Ibrahem, R.M.; Kormelink, R.; Visser, R.G.F.; Scott, J.W.; Edwards, J.D.; Bai, Y. The Tomato Yellow Leaf Curl Virus Resistance Genes Ty-1 and Ty-3 Are Allelic and Code for DFDGD-Class RNA – Dependent RNA Polymerases. PLoS Genet. 2013, 9, 1–11. [Google Scholar] [CrossRef]
- Yan, Z.H.E.; Elink, R.K. Ty-1, a Universal Resistance Gene against Geminiviruses That Is Compromised by Co-Replication of a Betasatellite. Mol. Plant Pathol. 2018, 1, 160–172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




