Submitted:
09 May 2023
Posted:
09 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. In-house production of multi-enzyme system
2.2. Quantitative analysis of dried potato peels
2.3. Standardization of various thermal, chemical, and thermo-chemical strategies for efficient pre-treatment of potato peels
2.3.1. Thermal pre-treatments
2.3.2. Chemical pretreatments
2.3.3. Thermo-chemical pretreatments
2.4. Enzymatic hydrolysis of pretreated potato peel residues using in-house multi-enzyme production
2.5. Qualitative analysis of free sugars in the enzymatic hydrolysate of potato peels using thin layer chromatography (TLC)
2.6. Structural changes in untreated, thermo-acidic pretreated, and enzymatically hydrolyzed samples of potato peels
2.7. Fermentation of sugars released after enzymatic hydrolysis of thermo-chemically pretreated potato peels
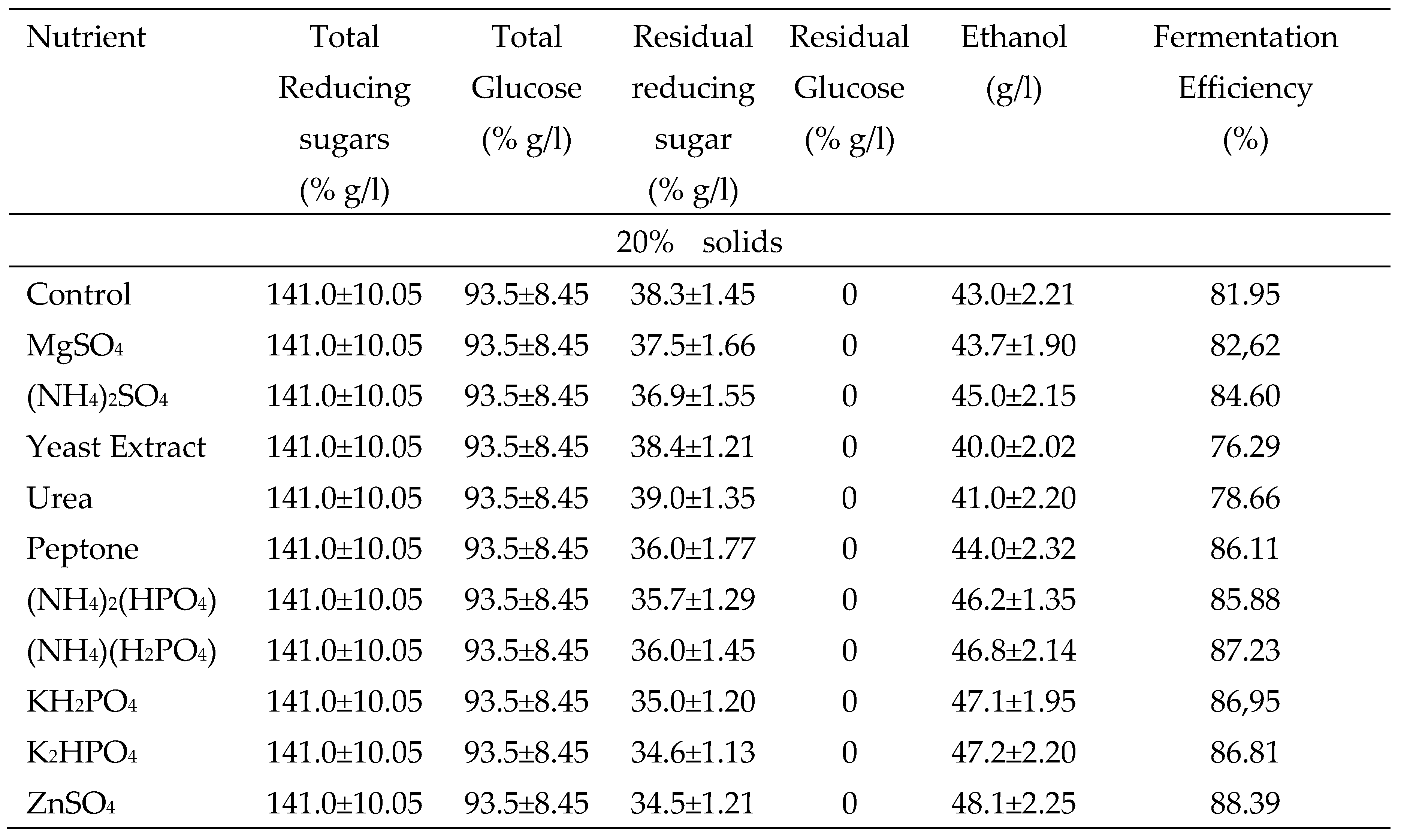
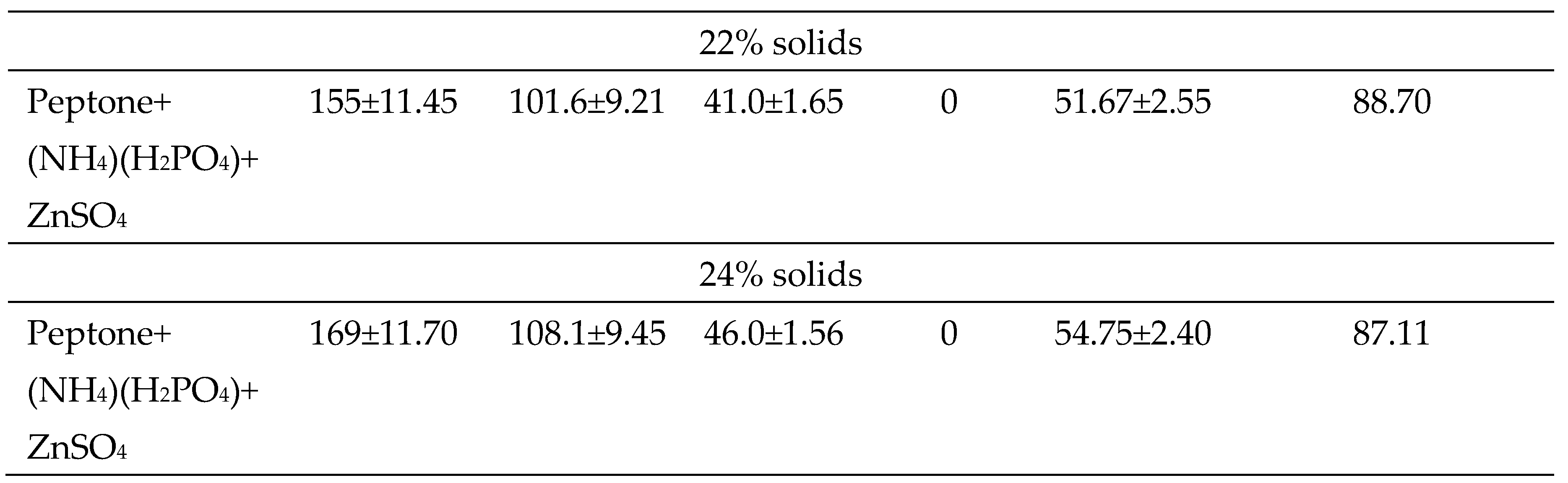
2.8. Effect of the supplementation of nutrients in the hydrolysate on fermentation and substrate loading
3. Results
3.1. Composition analysis of dried potato peels
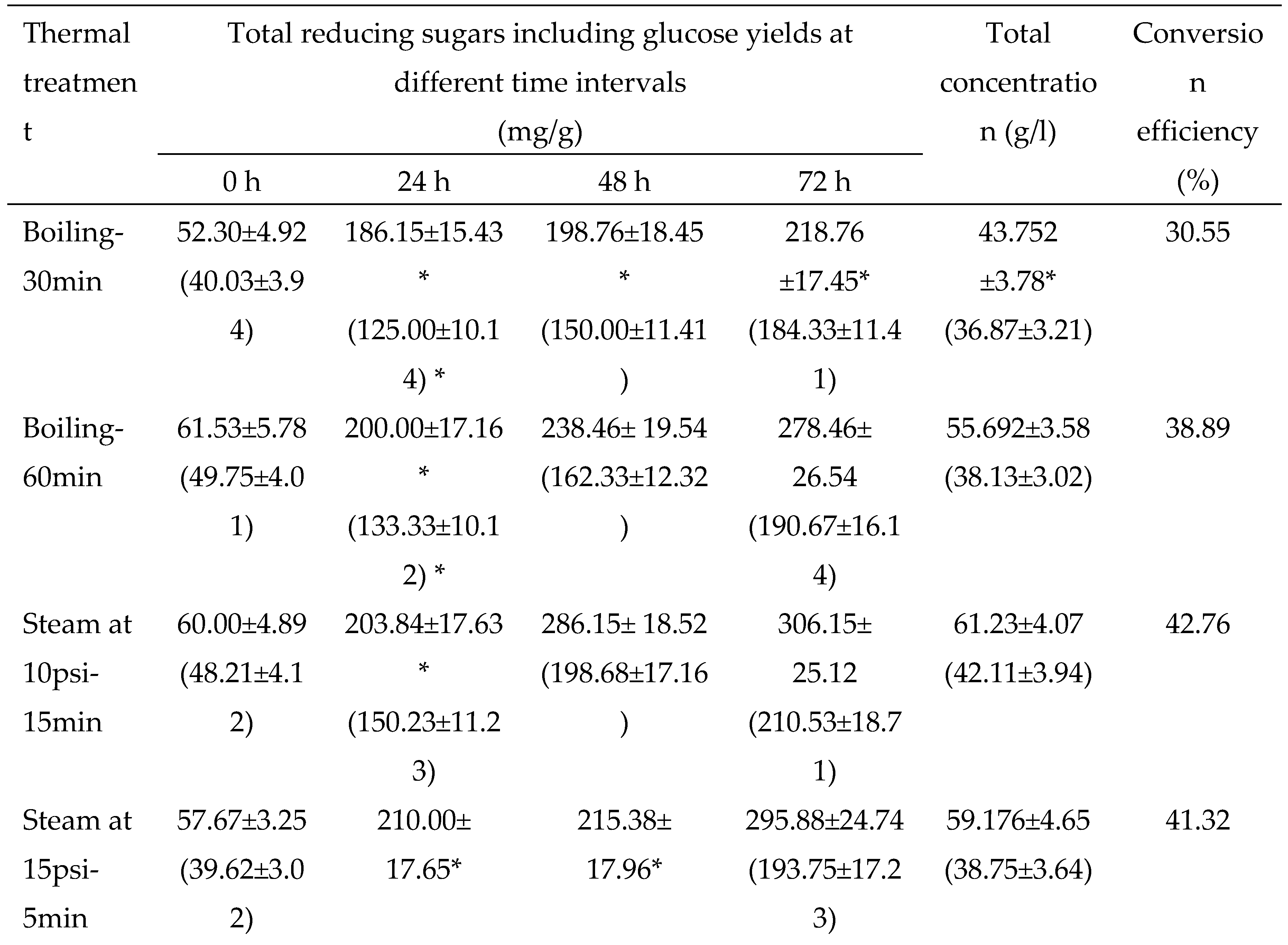
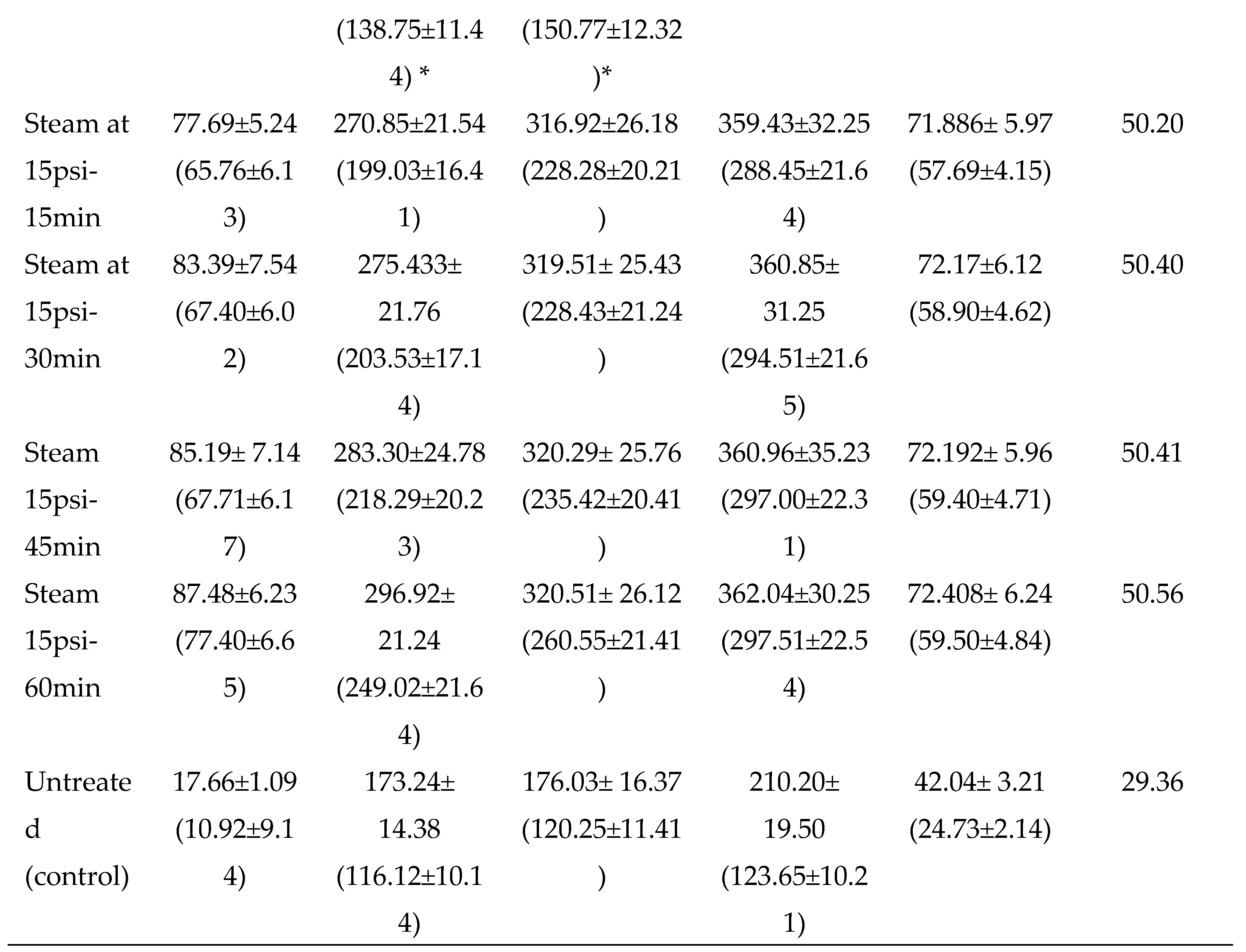
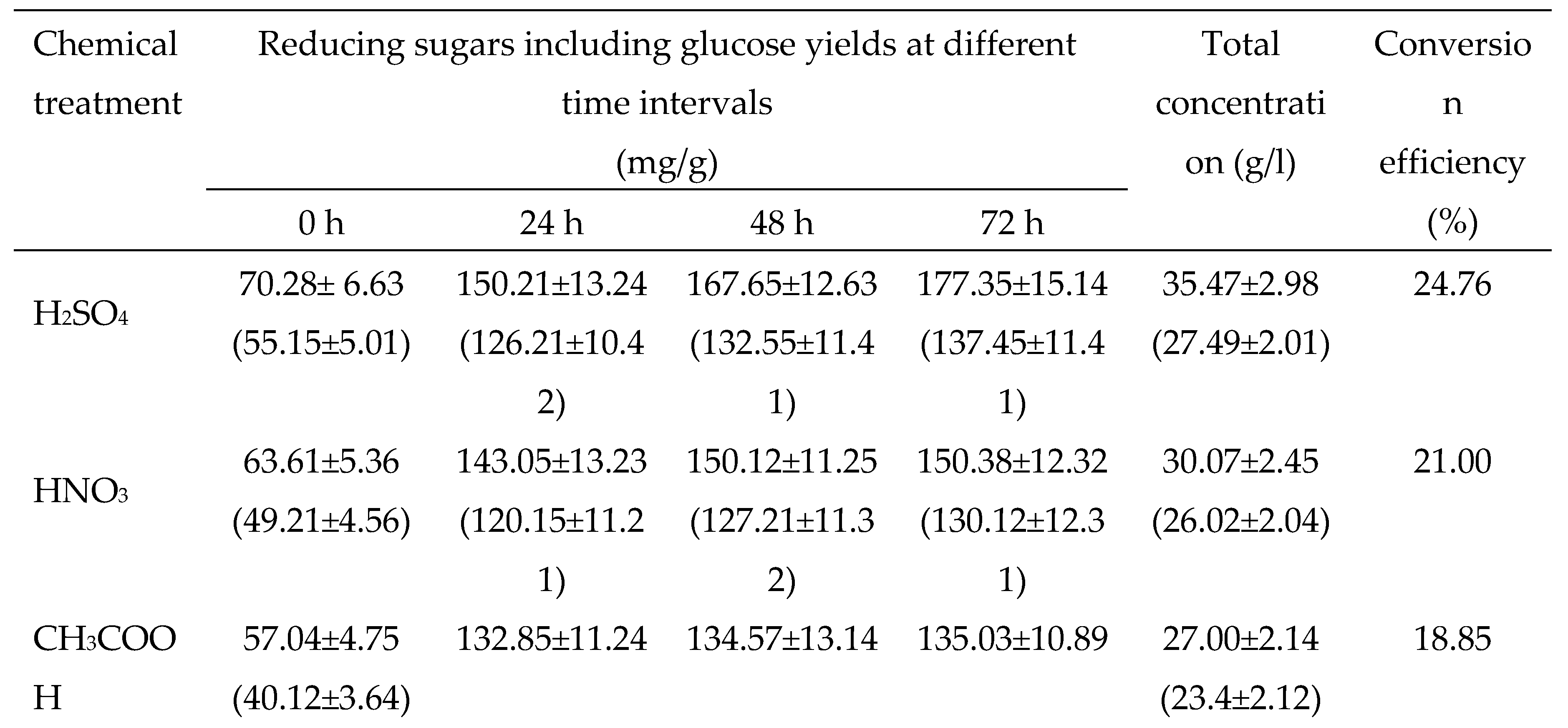
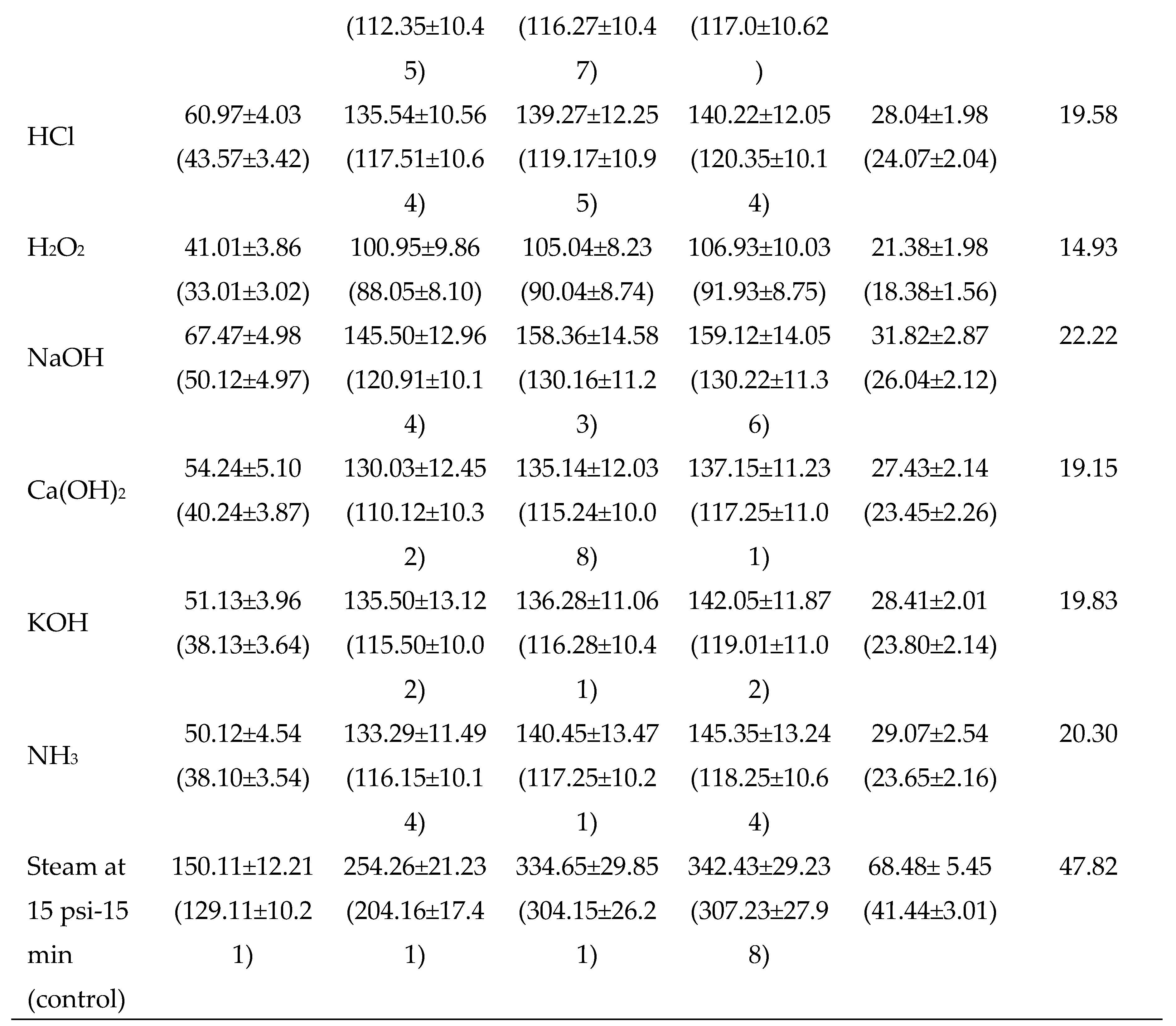
3.2. Evaluation and standardization of pretreatments of dried potato peels
3.2.1. Thermal pretreatments
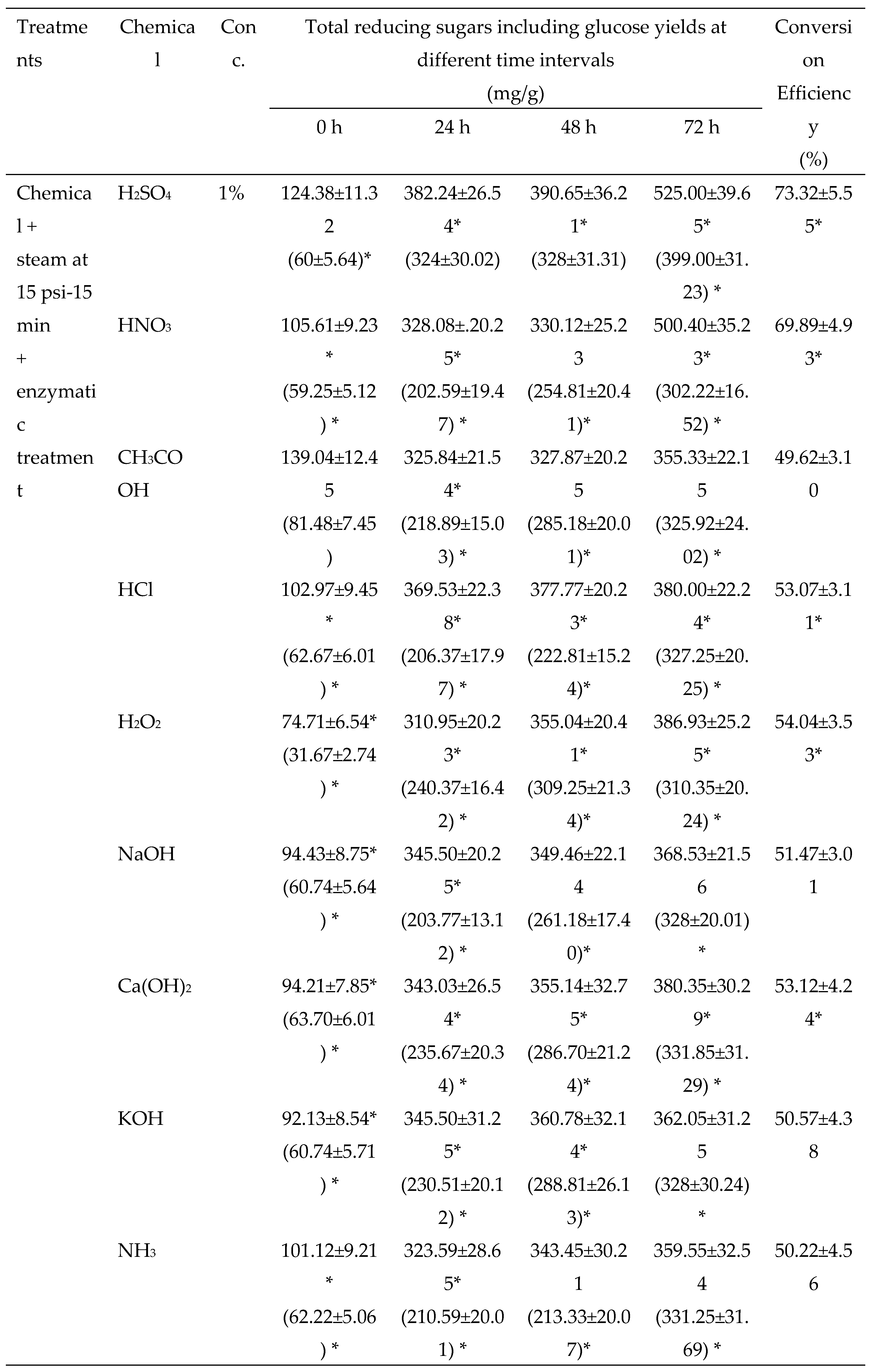
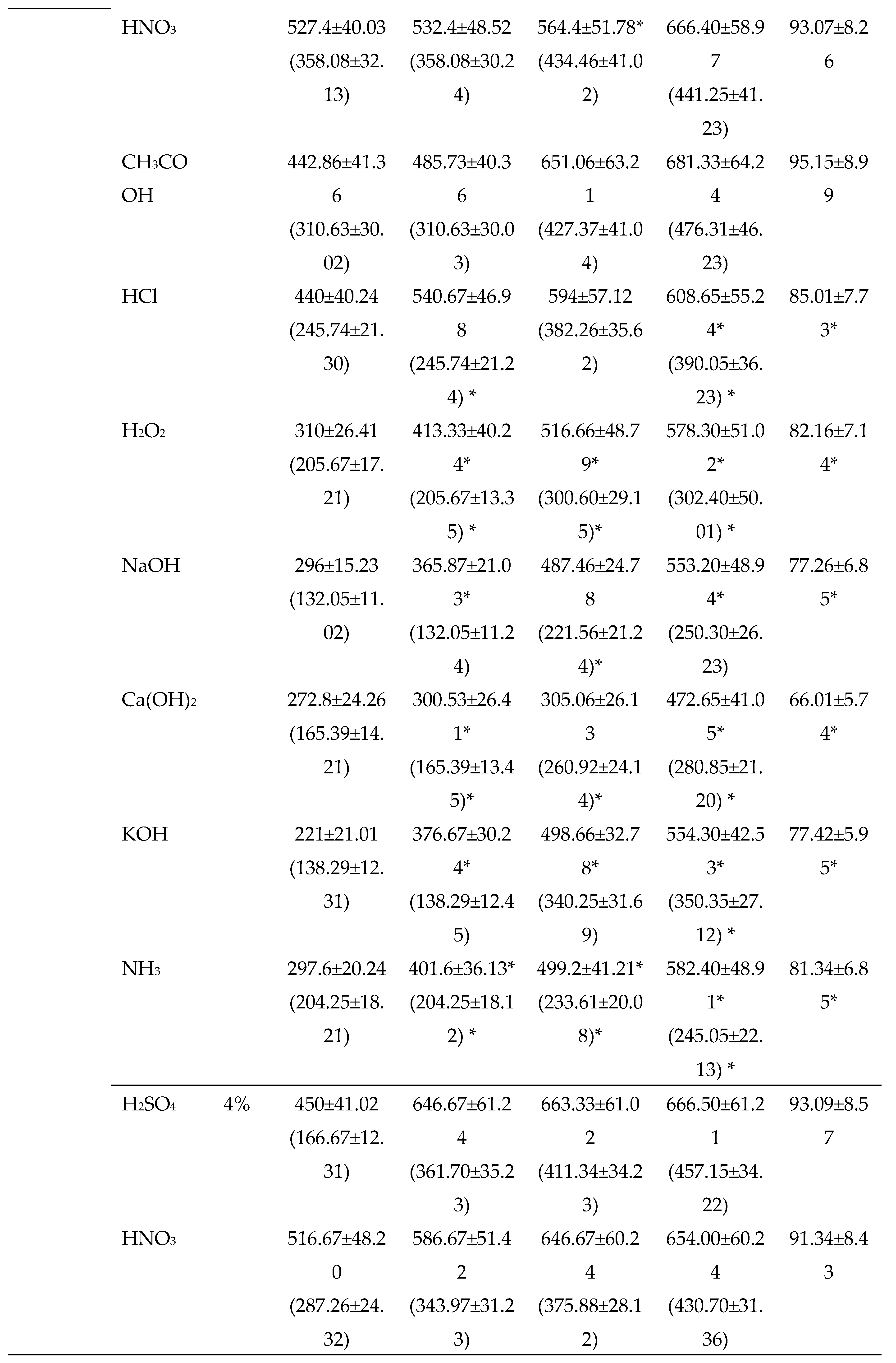
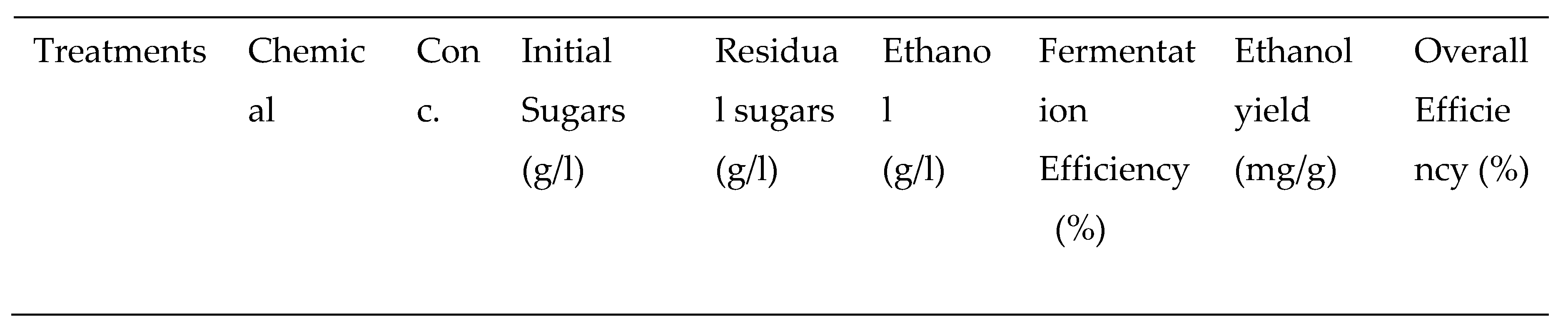
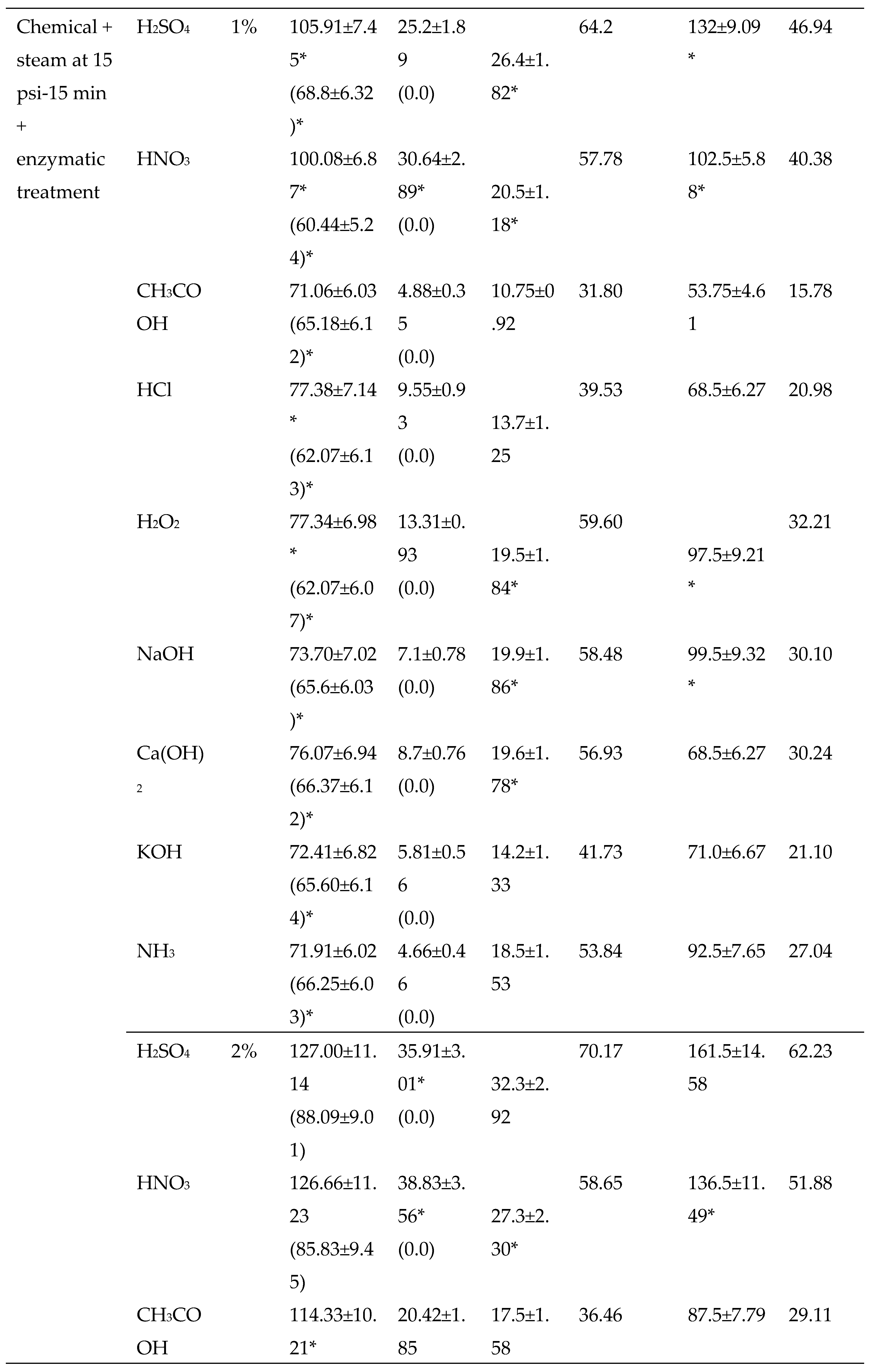
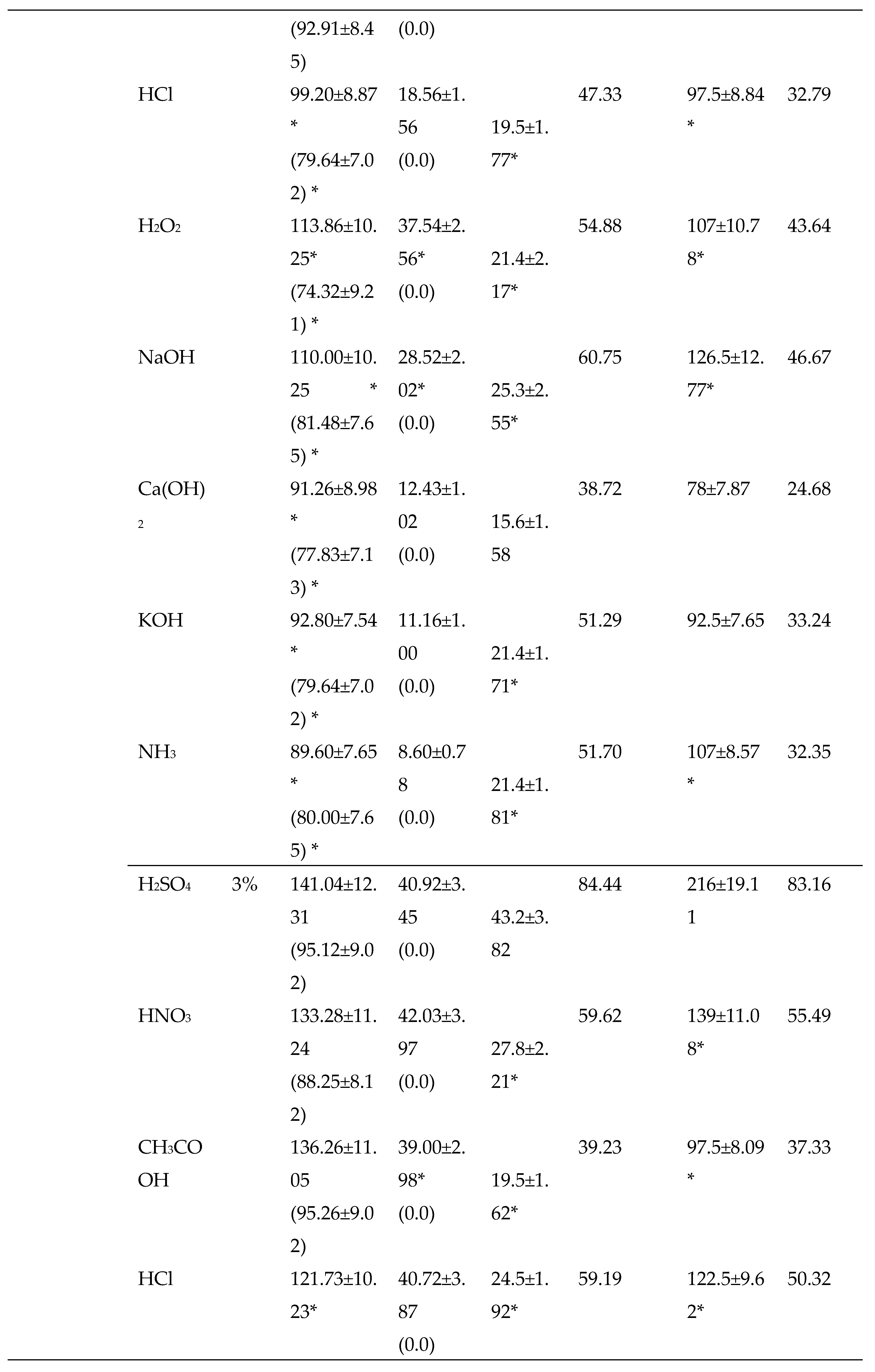
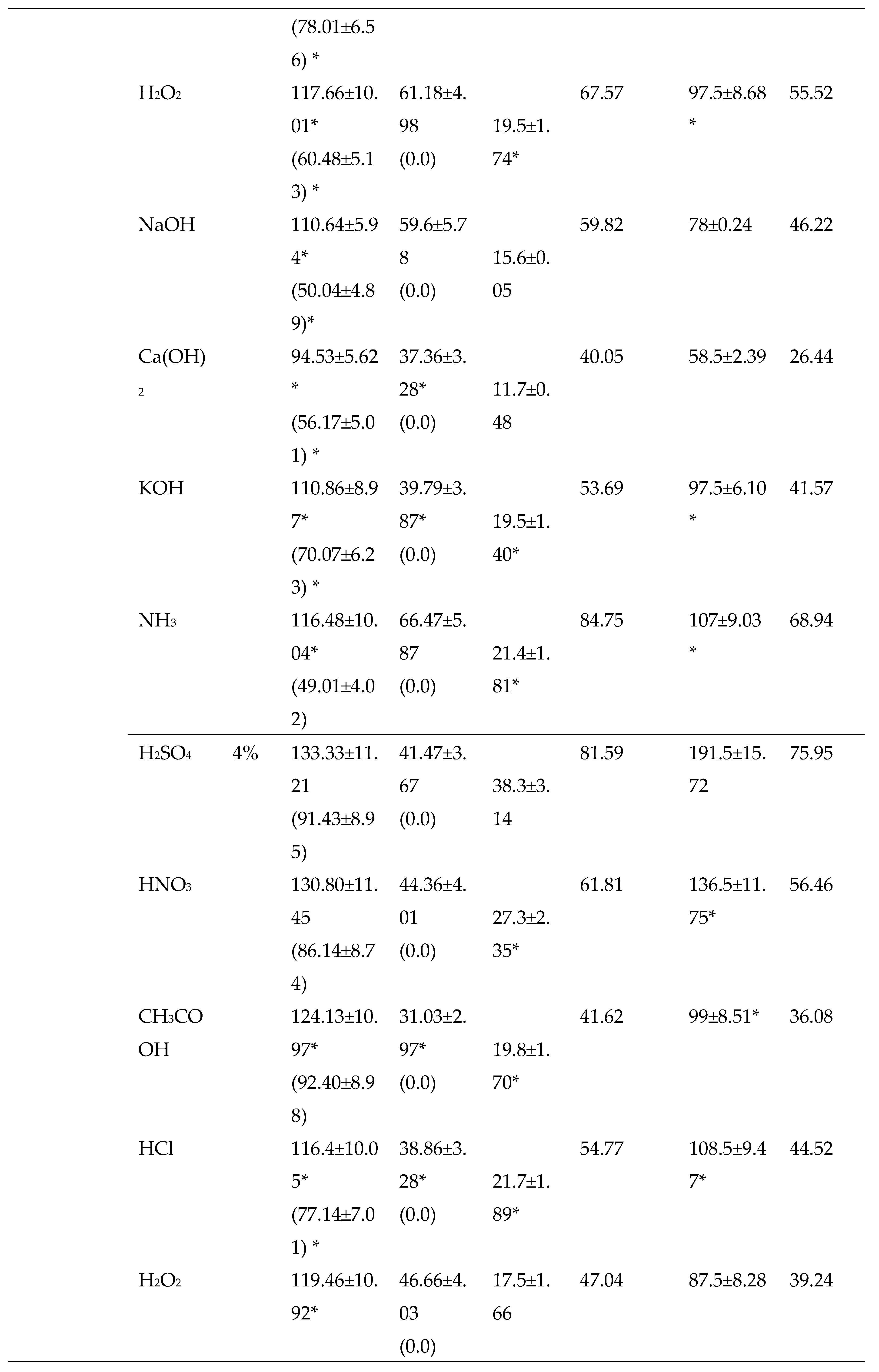
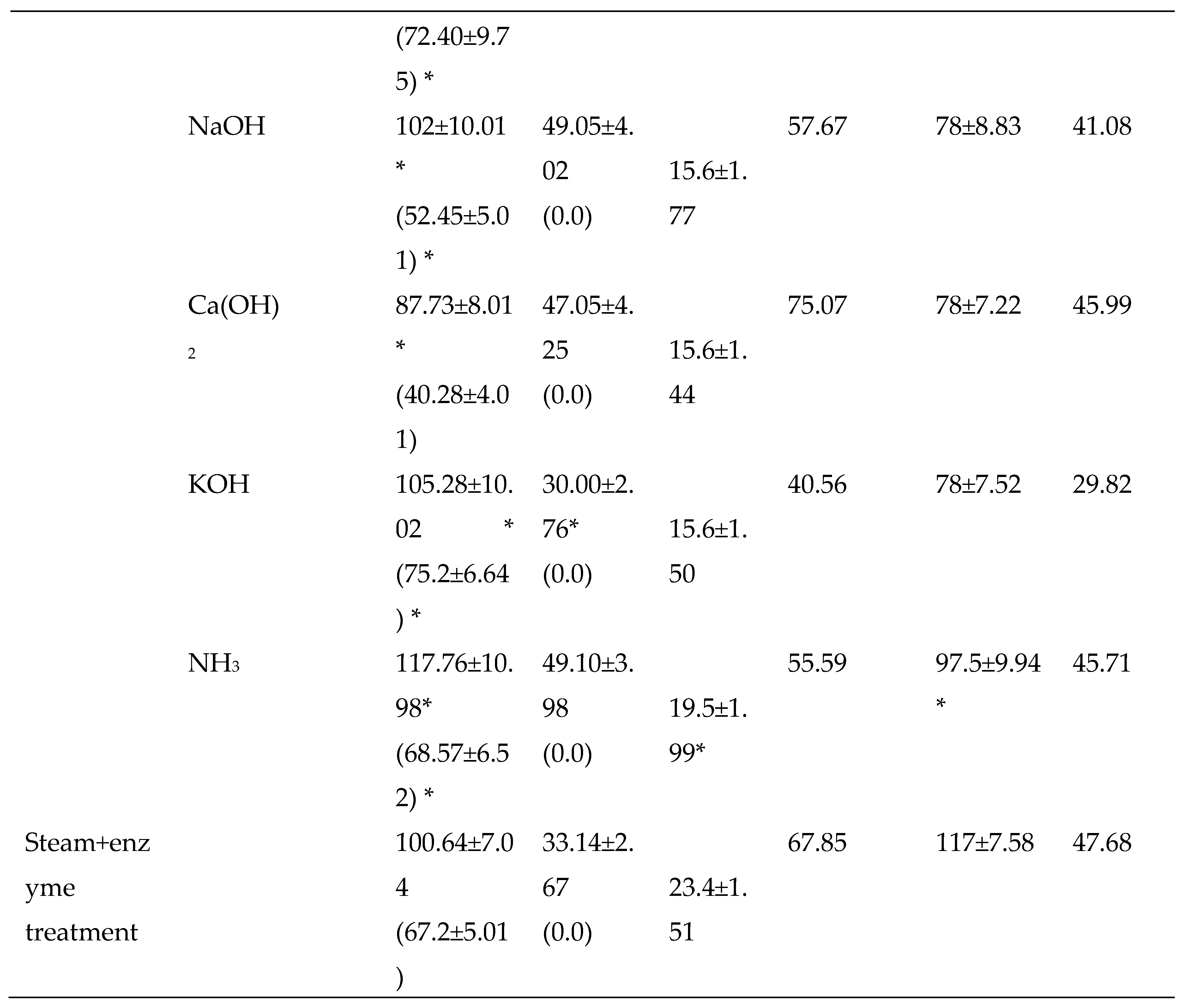
3.2.2. Chemical Pretreatments
3.2.3. Thermo-chemical pretreatments
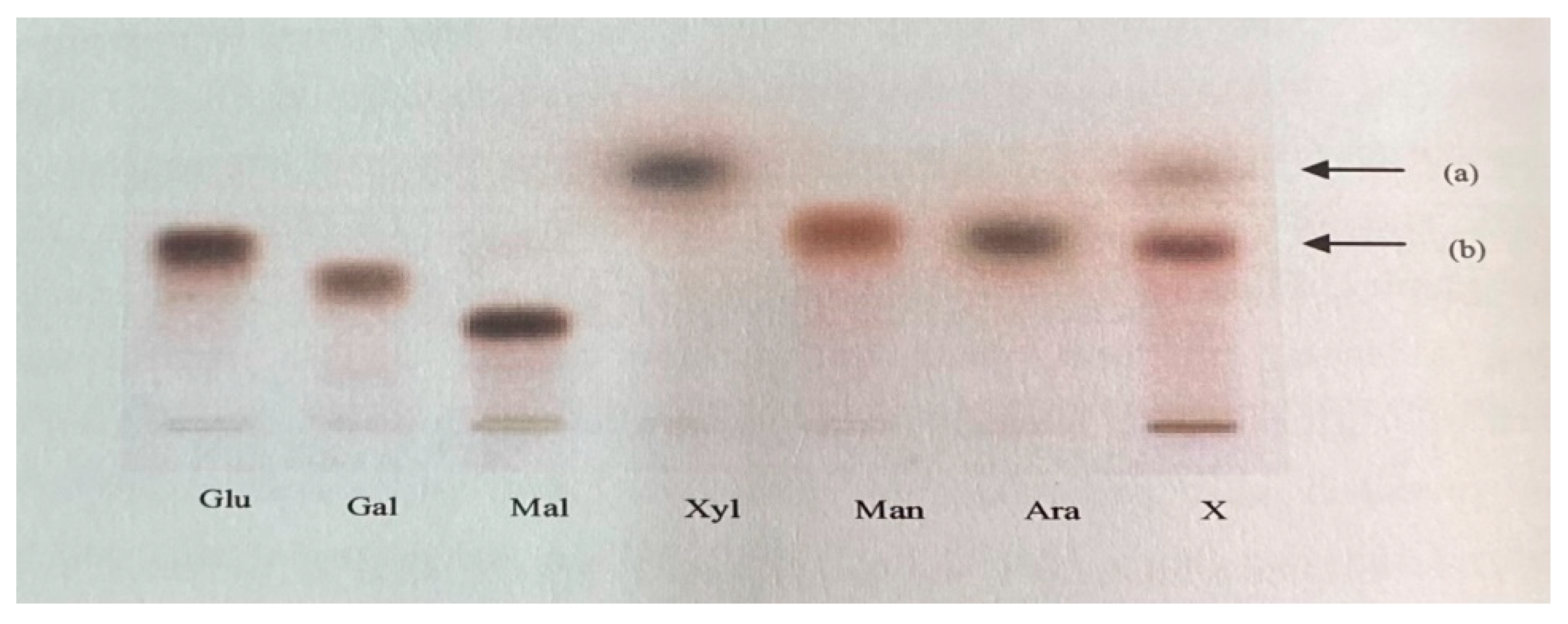
3.3. Qualitative detection of sugars in the enzymatic hydrolyzate of pretreated potato peels by TLC
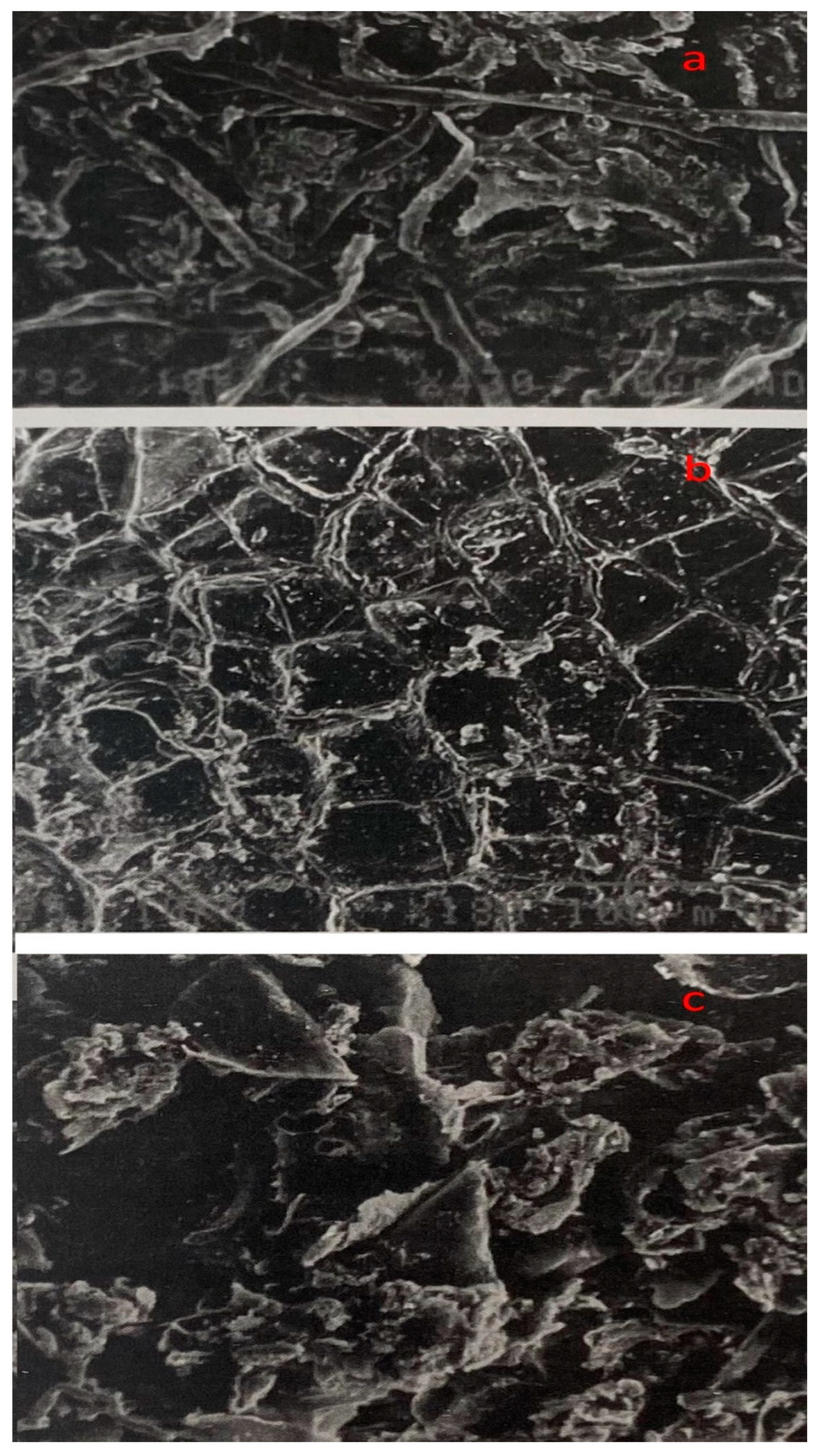
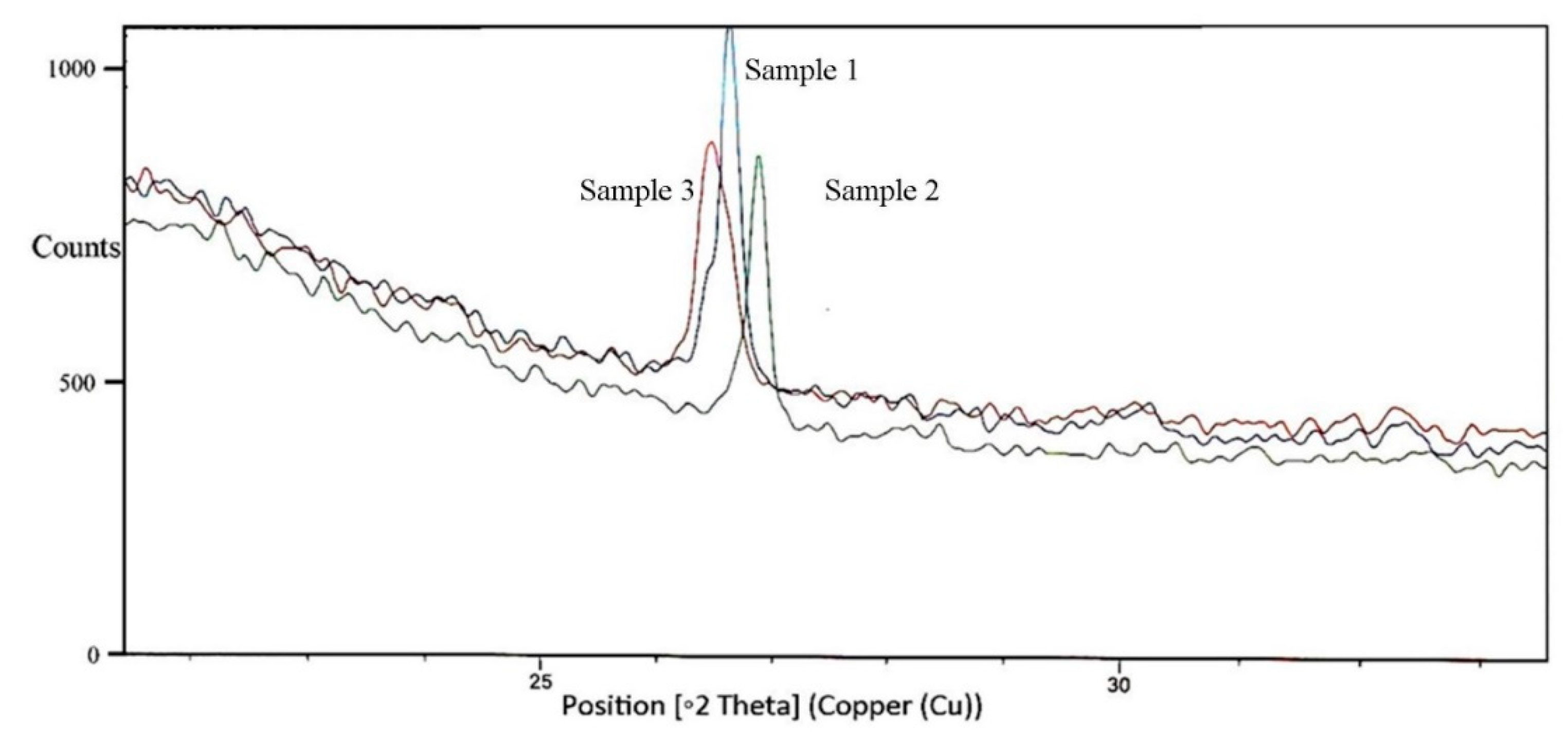
3.4. Structural changes in untreated, thermo-acidic pretreatment, thermo-acidic pretreatment followed by enzymatically hydrolyzed samples of potato peels
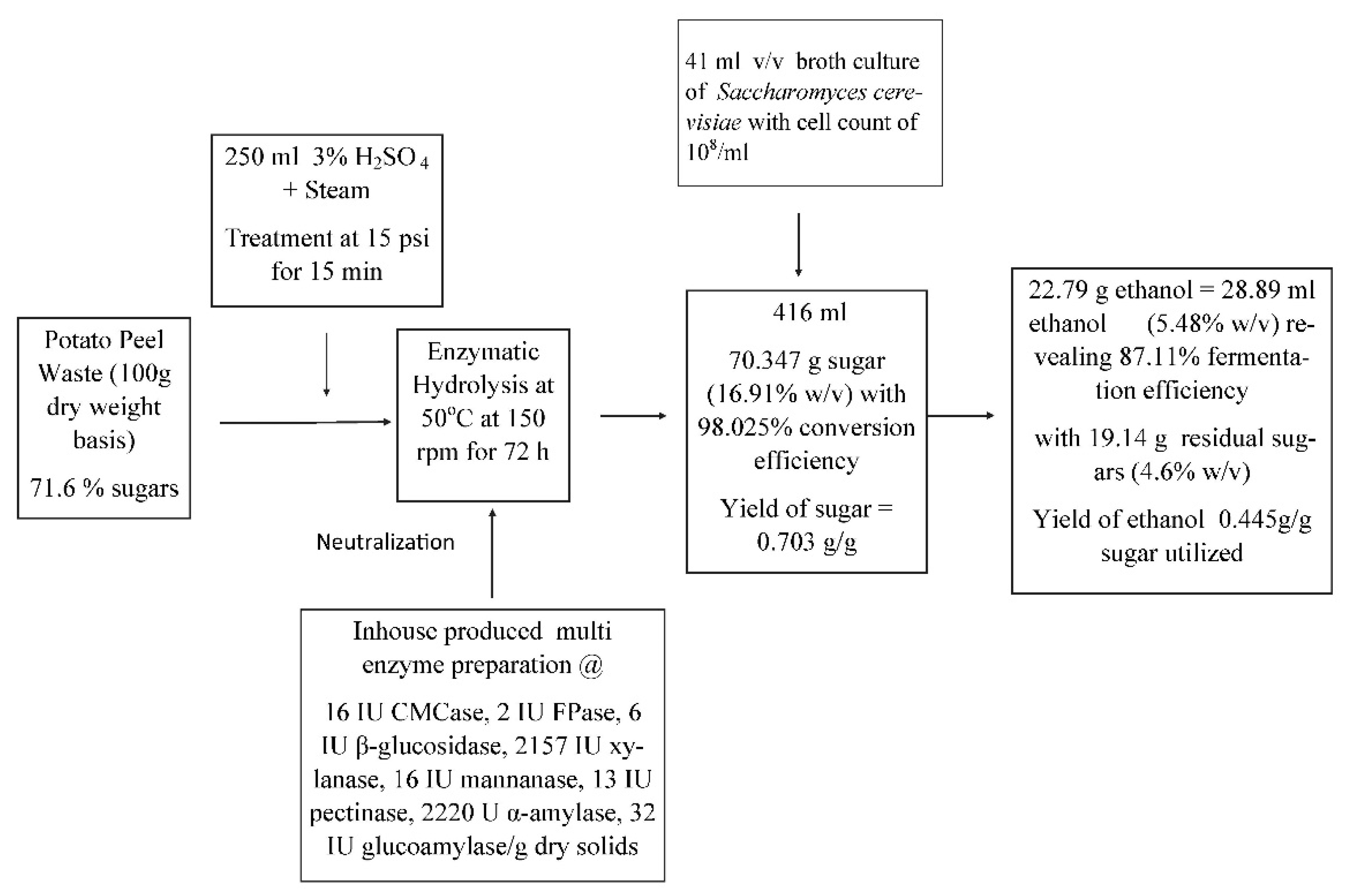
3.5. Fermentation of glucose released from potato peels
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sust. Energ. Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. Efficiency of dilute sulfuric acid pretreatment of distillery stillage in the production of cellulosic ethanol. Bioresour. Technol. 2018, 268, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Roberto, I.C.; Mussatto, S.I.; Rodrigues, R.C.L.B. Dilute-acid hydrolysis for optimization of xylose recovery from rice straw in a semi-pilot reactor. Ind. Crops Prod. 2003, 17, 171–176. [Google Scholar] [CrossRef]
- Roy, S.; Chowdhury, R.; Boro, H.; Sarma, B.K.; Kalita, S. Bioethanol Production from Lignocellulose Agricultural Waste Biomass. In: Agriculture Waste Management and Bioresource: The Circular Economy Perspective. John Wiley & Sons Ltd. West Sussex, United Kingdom. 2023, 218-237.
- Rezig, M.; Sahli, A.; Jeddi, F.B.; Harbaoui, Y. Adopting intercropping system for potatoes as practice ondrought mitigation under Tunisian conditions. Opt. Mediterr. A. 2010, 95, 329–334. [Google Scholar]
- Soltaninejad, A.; Jazini, M.; Karimi, K. Sustainable bioconversion of potato peel wastes into ethanol and biogas using organosolv pretreatment. Chemosphere 2022, 291, 133003. [Google Scholar] [CrossRef]
- Soltaninejad, A.; Jazini, M.; Karimi, K. Biorefinery for efficient xanthan gum, ethanol, and biogas production from potato crop residues. Biomass Bioenergy 2022, 158, 106354. [Google Scholar] [CrossRef]
- Felekis, V.; Stavraki, C.; Malamis, D.; Mai, S.; Barampouti, E.M. Optimisation of Bioethanol Production in a Potato Processing Industry. Fermentation 2023, 9, 103. [Google Scholar] [CrossRef]
- Lenihan, P.; Orozco, A.; O’Neill, E.; Ahmad, M.N.M.; Rooney, D.W.; Walker, G.M. Dilute acid hydrolysis of lignocellulosic biomass. Chem. Eng. J. 2010, 156, 395–403. [Google Scholar] [CrossRef]
- Schieber, A.; Saldaña, M.D. Potato peels: A source of nutritionally and pharmacologically interesting compounds - A review. Food 2009, 2, 23–29. [Google Scholar]
- Sujeeta, K.M.; Mehta, S.; Sihag, K. Optimization of conditions for bioethanol production from potato peel waste. Int. J. Chem. Stud. 2018, 6, 2021–2024. [Google Scholar]
- Liang, S.; McDonald, A.G. Chemical and thermal characterization of potato peel waste and its fermentation residue as potential resources for biofuel and bioproducts production. J. Agric. Food Chem. 2014, 62, 8421–8429. [Google Scholar] [CrossRef]
- Arapoglou, D.; Varzaka, T.; Vlysside, A.; Israilides, C. Ethanol production from potato peel waste. Waste Manage. 2010, 30, 1898–1902. [Google Scholar] [CrossRef]
- Hashem, M.; Darwish, S.M.I. Production of bioethanol and associated by-products from potato starch residue stream by Saccharomyces cerevisiae. Biomass. Bioenerg. 2010, 34, 953–959. [Google Scholar] [CrossRef]
- Izmirlioglu, G.; Demirci, A. Ethanol production from waste potato mash byusing Saccharomyces cerevisiae. J. Appl. Sci. 2012, 2, 738–753. [Google Scholar] [CrossRef]
- Khawla, B.J.; Sameh, M.; Imen, G.; Donyes, F.; Dhouh, G.; Raoudha, E.G.; Oumema, N.E. Potato peel as feedstock for bioethanol production: A comparison of acidic and enzymatic hydrolysis. Ind. Crops Prod. 2014, 52, 144–149. [Google Scholar] [CrossRef]
- Malakar, B.; Das, D.; Mohanty, K. Optimization of glucose yield from potato and sweet lime peel waste through different pre-treatment techniques along with enzyme assisted hydrolysis towards liquid biofuel. Renew. Energy. 2020, 145, 2723–2732. [Google Scholar] [CrossRef]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energ. Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Aditya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sust. Energy. Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Youjie, X.; Wang, D. Integrating starchy substrate into cellulosic ethanol production to boost ethanol titers and yields. Appl. Energy. 2017, 195, 196–203. [Google Scholar]
- Chugh, P.; Kaur, J.; Soni, R.; Sharma, A.; Soni, S.K. A low-cost process for efficient hydrolysis of deoiled rice bran and ethanol production using an inhouse produced multi-enzyme preparation from Aspergillus niger P-19. J. Mater. Cycles Waste Manag. 2023, 25, 359–375. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Verma, P. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sust. Energ. Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Yuan, T.; Li, X.; Xiao, S.; Guo, Y.; Zhou, W.; Xu, J.; Yuan, Z. Microalgae pretreatment with liquid hot water to enhance enzymatic hydrolysis efficiency. Bioresour. Technol. 2016, 220, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Chavez, A.Y.; Morales, R.; Gonzalez, C.; Moya, F.V. Production of ethanol from two varieties of potato peel waste through cellulolytic and amylolytic enzymes. Int. J. Energy Clean Environ. 2020, 21, 41–58. [Google Scholar] [CrossRef]
- Soni, S.K.; Sharma, A.; Soni, R. Microbial Enzyme Systems in the Production of Second Generation Bioethanol. Sustainability, 2023, 15, 3590. [Google Scholar] [CrossRef]
- Chugh, P.; Soni, R.; Soni, S.K. Deoiled rice bran: A substrate for co-production of a consortium of hydrolytic enzymes by Aspergillus niger P-19. Waste Biomass Valori. 2016, 7, 513–525. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitro-salicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Hedge, J.E.; Hofreiter, (1962). Carbohydrate chemistry 17. Whistler, RL and Be Miller, JN, Eds. Academic Press, New York. Ind. J. Plant Physiol. 1962, 21, 477–488.
- Updegraff, D.M. Semi-micro determination of cellulose in biological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]
- Hansen, J.; Moller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Gao, X.; Kumar, R.; Wyman, C.E. Fast hemicellulose quantification via a simple one-step acid hydrolysis. Biotechnol. Bioeng. 2014, 111, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Morin, L.G.; Prox, J. Single glucose oxidase- peroxidase reagent for two-minute determination of serum glucose. Clin. Chem. 1974, 19, 959–962. [Google Scholar] [CrossRef]
- Caputi, A.; Veda, M.; Brown, T. Spectrophotometric determination of ethanol in wine. Am. J. Enol. Vitic. 1968, 19, 160–165. [Google Scholar] [CrossRef]
- Kaur, J.; Chugh, P.; Soni, R.; Soni, S.K. A low-cost approach for the generation of enhanced sugars and ethanol from rice straw using in-house produced cellulase-hemicellulase consortium from A. niger P-19. Bioresour. Technol. Rep. 2020, 11, 100469. [Google Scholar] [CrossRef]
- Varga, E.; Réczey, K.; Zacchi, G. Optimization of steam pretreatment of corn stover to enhance enzymatic digestibility. Appl. Biochem. Biotechnol. 2004, 114, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.; Gnansounou, E. Use of Empty Fruit Bunches from the oil palm for bioethanol production: a thorough comparison between dilute acid and dilute alkali pretreatment. Bioresour. Technol. 2014, 159, 355–364. [Google Scholar] [CrossRef]
- Wu, S.; Lan, Y.; Wu, Z.; Peng, Y.; Chen, S.; Huang, Z.; Xu, L.; Gelbič, I.; Guan, X.; Zhang, L.; et al. Pretreatment of spent mushroom substrate for enhancing the conversion of fermentable sugar. Bioresour. Technol. 2012, 148, 596–600. [Google Scholar] [CrossRef]
- Ajandouz, E.H.; Tchiakpe, L.S.; Ore, F.D.; Benajiba, A.; Puigserver, A. Maillard reaction kinetics in fructose-lysine model systems. J. Food Sci. 2001, 66, 926–931. [Google Scholar] [CrossRef]
- Kim, J.Y.; Shin, E.J.; Eom, I.Y.; Won, K.; Kim, Y.H.; Choi, D.; Choi, I.G.; Choi, J.W. Structural features of lignin macromolecules extracted with ionic liquid from poplar wood. Bioresour. Technol. 2011, 102, 9020–9025. [Google Scholar] [CrossRef]
- Weingarten, R.; Conner, W.C.; Huber, G.W. Production of levulinic acid from cellulose by hydrothermal decomposition combined with aqueous phase dehydration with a solid acid catalyst. Energy Environ. Sci. 2012, 5, 7559–7574. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Kim, D.H.; Jung, S.; Ragauskas, A. Pseudo-lignin and pretreatment chemistry. Energ. Environ. Sci. 2011, 4, 1306–1310. [Google Scholar] [CrossRef]
- Rajan, K.; Carrier, D.J. Effect of dilute acid pretreatment conditions and washing on the production of inhibitors and on recovery of sugars during wheat straw enzymatic hydrolysis. Biomass Bioenerg. 2014, 62, 222–227. [Google Scholar] [CrossRef]
- Toquero, C.; Bolado, S. Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour. Technol. 2014, 157, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Barampouti, E.M.; Christofi, A.; Malamis, D.; Mai, S.A. Sustainable approach to valorize potato peel waste towards biofuel production. Biomass Convers. Biorefin. 2021, 1–12. [Google Scholar] [CrossRef]
- Bellido, C.; Bolado, S.; Coca, M.; Lucas, S.; González-Benito, G.; García-Cubero, M.T. Effect of inhibitors formed during wheat straw pretreatment on ethanol fermentation by Pichia stipites. Bioresour. Technol. 2011, 102, 10868–10874. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Reimann, A.; Nilvebrant, N.O.; Jönsson, L.J. Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl. Biochem. Biotechnol. 1999, 77, 91–103. [Google Scholar] [CrossRef]
- Procentese, A.; Raganati, F.; Olivieri, G.; Russo, M.E.; De La Feld, M.; Marzocchella, A. Agro food wastes and innovative pretreatments to meet biofuel demand in Europe. Chem. Eng. Technol. 2019, 42, 954–961. [Google Scholar] [CrossRef]
- Achinas, S.; Li, Y.; Achinas, V.; Euverink, G.J.W. Biogas Potential from the Anaerobic Digestion of Potato Peels: Process Performance and Kinetics Evaluation. Energies. 2019, 12, 2311. [Google Scholar] [CrossRef]
- Sivasakthivelan, P.; Saranraj, P.; Sivasakthi, S. Production of Ethanol by Zymomonas mobilis and Saccharomyces cerevisiae using sunflower head wastes-A comparative study. Int. J. Microbiol. Res. 2014, 5, 208–216. [Google Scholar]
- Atitallah, I.B.; Antonopoulou, G.; Ntaikou, I.; Alexandropoulou, M.; Nasri, M.; Mechichi, T.; Lyberatos, G. On the evaluation of different saccharification schemes for enhanced bioethanol production from potato peels waste via a newly isolated yeast strain of Wickerhamomyces anomalus. Bioresour. Technol. 2019, 289, 121614. [Google Scholar] [CrossRef]
- Sanusi, I.A.; Faloye, F.D.; Kana, E.B.G. Impact of various metallic oxide nanoparticles on ethanol production by Saccharomyces cerevisiae BY4743: screening, kinetic study and validation on potato waste. Catal. Lett. 2019, 149, 2015–2031. [Google Scholar] [CrossRef]
- Chohan, N.A.; Aruwajoye, G.S.; Sewsynker-Sukai, Y.; Kana, E.G. Valorisation of potato peel wastes for bioethanol production using simultaneous saccharification and fermentation: Process optimization and kinetic assessment. Renew. Energ. 2020, 146, 1031–1040. [Google Scholar] [CrossRef]
- Suriyachai, N.; Weerasia, K.; Laosiripojana, N.; Champreda, V.; Unrean, P. Optimized simultaneous saccharification and co-fermentation of rice straw for ethanol production by Saccharomyces cerevisiae and Scheffersomyces stipites using design of experiments. Bioresour. Technol. 2013, 142, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Vaid, S.; Nargotra, P.; Bajaj, B.K. Consolidated bioprocessing for biofuel-ethanol production from pine needle biomass. Environ. Prog. Sustain. 2018, 37, 546–552. [Google Scholar] [CrossRef]
- Hossain, T.; Miah, A.B.; Mahmud, S.A. Enhanced bioethanol production from potato pel waste via consolidated bioprocessing with statistically optimized medium. Appl. Biochem. Biotechnol. 2018, 186, 425–442. [Google Scholar] [CrossRef]
- Ntaikou, I.; Menis, N.; Alexandropoulu, M.; Antonopoulou, G.; Lyberatos, G. Valorization of kitchen biowaste for ethanol production via simultaneous saccharification and fermentation using co-cultures of the yeasts Saccharomyces cerevisiae and Pichia stipitis Bioresour. Technol. 2013, 263, 75–83. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, F.; Zhang, S.; Gong, Q.; Chen, G. Ethanol production by simultaneous saccharification and cofermentation of pretreated corn stalk. J. Basic Microbiol. 2019, 5, 744–753. [Google Scholar] [CrossRef]
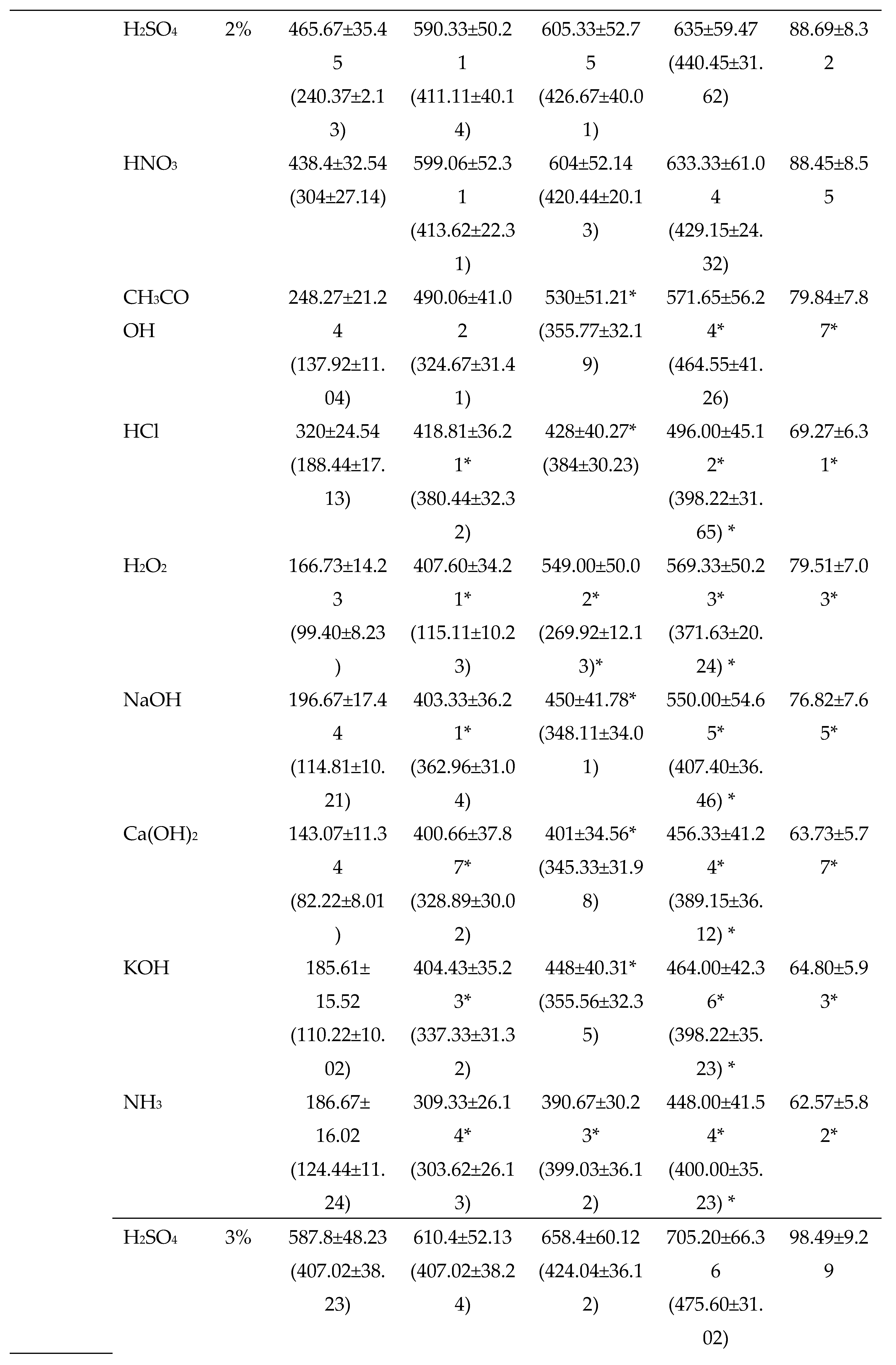
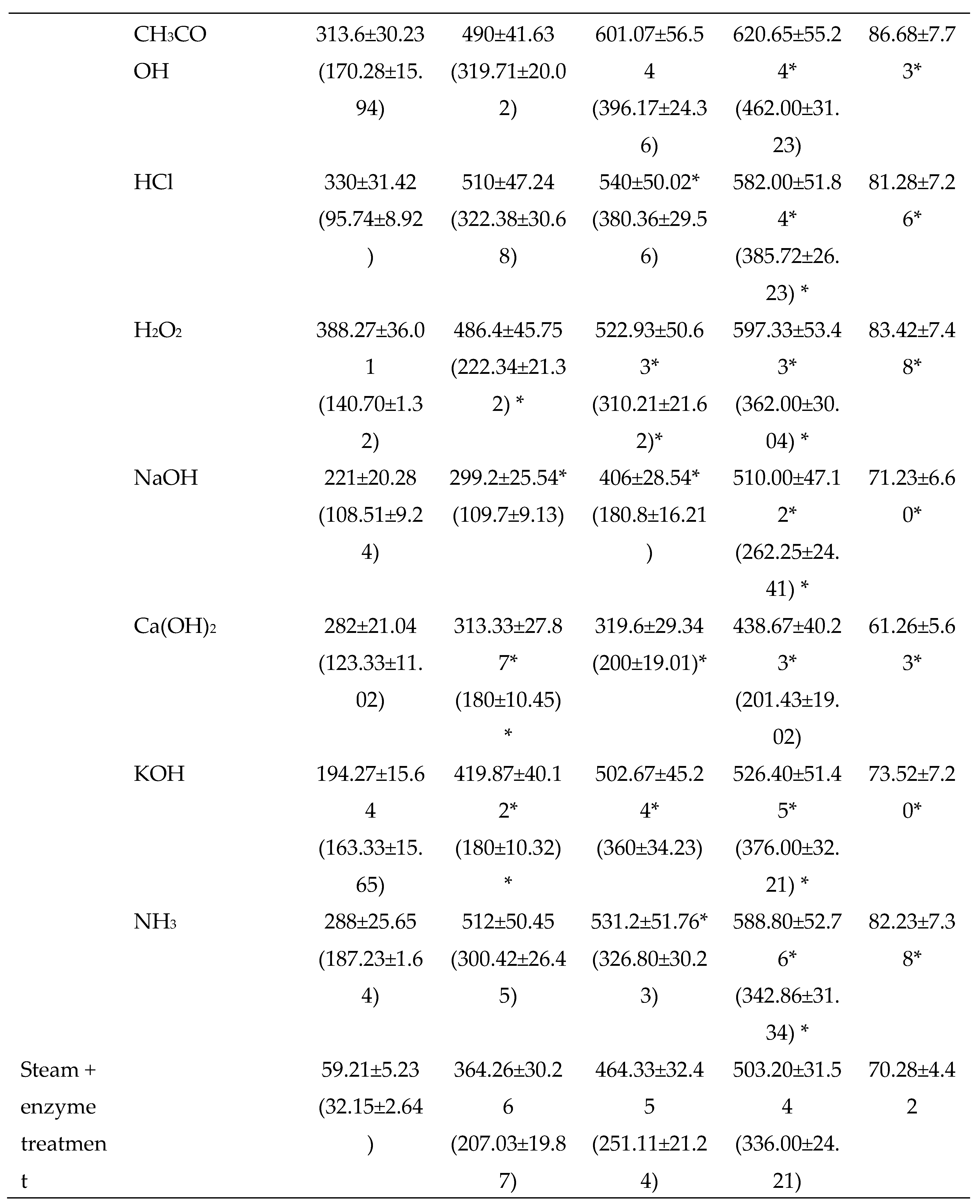
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





