1. Introduction
After the cessation of radiotherapy for brain tumors, over 50% of cancer survivors report impaired cognition. Many studies have reported the nature and extent of various impairments that are inherently difficult to quantify, and often underestimate the severity of patient complaints that are detected in the clinic [
1,
2,
3,
4]. However, treatment-associated effects on mood, memory, concentration and executive functions reported, whether mild or severe, are persistent and have a major negative impact on quality of life. The use of more recent treatment modalities, such as hypofractionation or ultra-high dose rate (UHDR) irradiation, with a
dose rate larger than 40Gy/s, increases the need for research with pre-clinical models to quantify these risks to patients.
Following promising results in mouse model of gastrointestinal (GI) injury using high dose-rates in the 1960’s and 1970s [
5,
6], a reemergence of interest in ultra-high dose rate (UHDR) therapy has occurred in the last few years due to experiments showing reduced skin, GI, and brain tissue normal tissue damage with similar tumor control as found with conventional irradiation [
7,
8,
9,
10,
11,
12] and has been termed the UHDR effect. This led to the development of a few facilities using electron, X-ray or proton irradiation to study UHDR irradiation in mouse models, including the Dartmouth facility using 10 MeV electrons with dose-rates as high as 300 Gy/s used in the current study. Some studies show reduced cognitive injury using UHDR irradiation. Montay-Gruel et al. [
11] used whole brain irradiation (WBI) of female C57BL/6J (age 2 month) mice with dose of 10 Gy of 4.5 MeV electrons, while varying the dose-rate from 0.1 to 500 Gy/s to show that novel object recognition (NOR) was not affected for dose-rates >60 Gy/s. A study involving the same mouse model [
13] considered synchrotron X-rays with an average dose-rate of 37 Gy/s showed no NOR detriments for UHDR irradiation which do occur for conventional X-rays at 2- and 6-months post irradiation (IR). In addition, UHDR irradiation reduced hippocampal cell-division impairment and induced less reactive astrogliosis [
14] compared to conventional IR. A study involving female C57BL/6J mice (age 2 month) [
15] using WBI with 6 MeV electrons (dose-rate>100 Gy/s) showed reduced impacts on measures of anxiety and depression and on performance in cognitive tests related to extinction memory at 10 or 12 Gy, but similar measures at 14 Gy compared to conventional IR. A similar study [
11] involving 3-week-old C57BL/6J mice undergoing whole WBI with 8 Gy of UHDR (6 MeV electrons with a single pulse at 4.4x10
6 Gy/s) or conventional IR. Simmons et al. [
9] used 3-month-old male C57BL/6J mice and WBI at 30 Gy with 16 (200 Gy/s) or 20 MeV (300 Gy/s) electrons. At 10 weeks post-IR, UHDR irradiation did not impair NOR or novel location recognition, and reduced hippocampal dendritic spine loss and neuroinflammation occurred compared to convention IR. While these studies show benefits of UDHR irradiation, they are limited in scope of cognitive tests, with regard to comparisons of age, sex and dose and dose fractionation, and time at assessment. Only one study involving mouse models of glioblastoma showed a UHDR sparring effect in female mice relying strictly on the novel object recognition test [
16]. Therefore, in the current study we assessed the effects of conventional and UHDR irradiation on behavioral and cognitive performance one month following exposure and assessed whether these effects were associated with alterations in the number of immune cells in the hippocampus using flow cytometry.
2. Materials and Methods
Mice and irradiation. Two-month-old female and male C57BL/6J mice (
n = 9 mice/sex or 18 mice in total) purchased from JAX labs received whole brain conventional or UHDR irradiation (
Table 1,
Figure 1). One UHDR-irradiated female mouse died, so there were five mice in that group. UHDR dose mice were irradiated with a target dose of 18 Gy delivered by the Linac-based/modified beam control. The delivered UHDR dose was verified by GAF chromatic film after irradiation. The conventional dose rate was delivered to match the UHDR. Twenty four hours is typically allowed between UHDR and conventional irradiations to allow the film to stabilize, however, scheduling constraints prevented this stabilization time between irradiations (in these mice). Early estimates from the film (within 1 hour of irradiation) indicated a delivered dose of 18 Gy UHDR and so this dose was delivered to the conventional-irradiated group. Conventional mice were given 18 Gy to match the UHDR mice. After 24 hours passed, the films were reanalyzed and it was found that the early estimates were high, only 16 Gy was delivered in the UHDR group. There were six mice per treatment group, with three mice per sex per group. The mice were irradiated or sham-irradiated at Dartmouth and the following week shipped to OHSU after irradiation or sham-irradiation. Following three weeks of quarantine, the mice were behaviorally and cognitively tested between 27 and 41 days after exposure, as described below. Subsequently, the mice were tested for circadian home cage activity over two weeks. Mice were group housed at OHSU on a ventilated Thoren rack throughout the experimental period, except when they were singly housed for home cage activity monitoring on a conventional Metro rack using an MLog (BioBServe, Germany) home cage sensor system following the week following fear conditioning, as described [
17].
All mice were kept under a constant 12-h light: 12-h dark cycle, and water and food (PicoLab Rodent Diet 20, no. 5053; PMI Nutrition International, St. Louis, MO, USA) were provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committees at Dartmouth and OHSU and were in compliance with all Federal regulations. Ten weeks following radiation or sham-irradiation, the mice were euthanized by cervical dislocation and their hippocampi analyzed for alterations in numbers of immune cells by flow cytometry.
Spontaneous alternation in the Y-maze. Hippocampus-dependent spontaneous alterations were assessed in a Y-shaped maze with raised sides (3.8 cm bottom width, 12.55 cm, top width, 12.55 cm height) and made of non-reflective opaque gray plastic (37.98 cm length) (O’ Hara & Co., Ltd., Tokyo, Japan) as described [
18]. Mice were placed into the center of the maze at the beginning of each 5-minute trial. The mazes were cleaned with 0.5% acetic acid between trials. The mazes were surrounded with a white curtain in order to isolate the mice from the surrounding room as well as the experimenter. Spontaneous alternations and activity levels were analyzed in the Y-maze. Videos were analyzed to measure the number of arm entries and to calculate the percent spontaneous alternations. The criteria for an arm entry was when all four limbs were within the arm. The spontaneous alternation percentage was calculated by dividing the number of 3-arm alternations by the number of possible 3-arm alternations and multiplying the value by 100.
Novel object recognition. The mice were exposed to the open field containing two identical objects (orange wooden blocks in the shape of hexagonal prisms) for a 15-minute trial. The objects were placed 10 cm apart and 15 cm from the adjacent walls of the arena. The following day, one object was replaced with a novel object (green wooden block in the shape of a triangular prism) and mice were allowed to explore for 15 minutes. During object recognition trials, objects were affixed to the floor of the arena using masking tape. The arenas and objects were cleaned with 0.5% acetic acid between trials. Physical interaction with the object in the form of sniffing within a 2 cm proximity was coded as object exploration by hand scoring videos acquired with Noldus Ethovision software (version 17, Wageningen, The Netherlands) . A discrimination index was calculated as previously described 50. The time spent exploring the familiar object was subtracted from the time exploring the novel object, and the resulting number was divided by the total time spent exploring both objects.
Elevated zero maze. Measures of anxiety were assessed in the elevated zero maze as described [
19]. The elevated zero maze consists of two open and two closed areas (each 35.5 cm in length; Hamilton-Kinder, Poway, CA). The closed areas are surrounded by opaque walls (15 cm tall). Mice were placed into the maze into one of the open areas and allowed to explore for a single 10-min trial. The time spent in the open areas was analyzed with Noldus 17 Ethovision video tracking software.
Light-dark test. In the light-dark test, mice were placed in an open-field enclosure (40.64 x 40.64 cm) containing black plastic inserts covering from the sides and the top 50% of the open field (Kinder Scientific, Poway, CA), as described [
20]. A single opening in the wall of the insert adjacent to the open area allowed the mice to enter or exit the more anxiety-provoking light area of the maze. Active times, distance moved and rest time were recorded for a single 10-min session. Breaks in the photo beams were used to calculate path length, active times and rest time in the open and closed compartments of the enclosure. Mice with increased levels of anxiety in this enclosure spend less time in the lighted compartment of the maze.
Spatial Y maze. The spatial Y-maze test was conducted in a maze had raised sides and made of non-reflective opaque gray plastic (30 cm x 6 cm x 15 cm) (Harvard Apparatus, Panlab, Holliston, MA, United States), as described [
21]. This task was conducted over 2 consecutive days. On day 1, one arm was blocked off and mice were allowed to explore the maze for 15 min. Extra-maze spatial cues were taped on all three walls of the biosafety cabinet in which the mice were being tested. On day 2, all of the arms were accessible, and mice were allowed to explore for a 5-min trial. Performance of the mice was tracked using Ethovision software. Digital videos of day 2 were later analyzed to measure the number of entries into and the percent time spent in the novel arm (the arm that was blocked off during day 1). The criteria for an arm entry was when all four limbs were within the arm.
Fear learning and cued fear memory. Fear conditioning was assessed over the course of 2 consecutive days using a Med Associates mouse fear conditioning system (PMED-VFC-NIR-M, Med Associates, St. Albans, Vermont) and Med Associates VideoFreeze automated scoring system, as described [
22]. Mice were placed inside the fear conditioning chamber, where chamber lights (100 lux) were turned on at the beginning of the trial. Following a 90-second habituation period, there was a 30-second (2800 hz, 80 dB) tone (cue). A 2-second 0.35 mA foot shock was administered at 28 seconds, co-terminating with the tone at 30 seconds. After a 90-second inter-stimulus-interval, there was another tone-shock pairing. On day 2, cued fear memory was assessed by placing mice in a new environment (scented with vanilla extract, cleaned with 10% isopropanol instead of 0.5% glacial acetic acid, novel floor texture covering the shock-grid, and rounded walls). The mice were habituated to the new environment for 90 s (pre-tone), and exposed to the tone (cue) for 180 s.
Flow cytometry. Flow cytometry was performed using hippocampi from one hemibrain as described as described [
23], with the following modifications. Following cervical dislocation, the brains were quickly removed and the hippocampi dissected in RPMI medium (Gibco, ThermoFischer Scientific, Pittsburgh, PA). The hippocampus of one hemibrain was collected in 300 μl of RPMI. To work out the conditions and as control, spleen tissue of one mouse was used and collected in 600 μl of RPMI. The tissues were homogenized using a pestle and Dounce homogenizer on ice (30 strokes) and filtered using 40 μm Falcon Nylon Cell Strainer filters (Corning Incorporated, Corning, NY) over 50-ml tubes. The Dounce tubes were washed with 5 x 1 ml of RPMI (10 x 1 ml RPMI for the spleen tissue) and the filtrates were transferred to 15-ml tubes and centrifuged at 400 rcf for 5 min. The supernatants were aspirated and the pellets were resuspended in 1 ml of Lysis buffer (BD Pharm Lyse, BD Biosciences, San Jose, CA) and incubated for 5 min at room temperature. After adding 5 ml of FACS buffer (Hanks without calcium and magnesium, and with 2% goat serum), the tubes were centrifuged at 400 rcf for 5 min. The supernatants were aspirated, washed with 5 ml PBS (with calcium and magnesium), and centrifuged at 400 rcf for 3 min. The supernatants were aspirated and the pellets incubated in the dark with ghost dye (anti human/mouse CD11b-APC-Cy7 (Tonbo, San Diego, CA), diluted 1 : 1,000) for 30 min on ice. After adding 5 ml of FACS buffer, the tubes were centrifuged at 400 rcf for 3 min. The supernatants were aspirated and the pellets resuspended in 100 μl of blocking antibody (anti-mouse CD16/32, diluted 1 : 100 in FACS buffer, eBioscience, Pittsburgh, PA) and incubated on ice for 20 min. After adding 5 ml of FACS buffer, the tubes were centrifuged at 400 rcf for 3 min. The supernatants were aspirated and the pellets resuspended in 100 μl of neutrophil panel (CD11b-FITC, 1:100, eBioscience, Pittsburgh, PA; Ly6G-BV570 (Biolegend, San Diego, CA), 1:100; Ly6G-PerCP-Cy5.5, 1:100 (Biolegend); CD45-Violet Fluor 450, 1:100 (Tonbo, San Diego, CA); and CD206-MMR-Alexa Fluor 647, 1:100 (Biolegend) and incubated on ice for 30 min. After adding 5 ml of FACS buffer, the tubes were centrifuged at 400 rcf for 3 min. The supernatants were aspirated and the pellets resuspended in 500 μl of FACS buffer.
Spectral compensation was achieved by using compensation beads (ABC Anti-Mouse Bead Kit, Invitrogen, Life Technologies Corporation, Eugene, OR) conjugated to the above mentioned antibodies in 100 μl of FACS buffer and following a vendor recommended protocol. Following vortexing the comp beads, one drop of each bottle of com beads was added and the tubes was incubated for 20 min at room temperature. After adding 3 ml of FACS buffer, the tubes were centrifugated at 400 rcf for 5 min. The supernatants were aspirated and the pellets resuspended in 500 μl of FACS buffer.
Data for flow cytometry were acquired on a BD FAC Symphony A5 (BD Immunocytometry Systems) equipped with 405nm (CD45, BF450; Ly6G, BV570), 488nm (CD11b, FITC; Ly6G, PerCP), and 628 nm (CD206, AF647) excitation lasers. Data were collected and analyzed using BD FACS Diva Software (BD Biosciences) and FlowJo software (FLOWJO, Ashland, OR). As female and male samples were analyzed on separate days, the percent subpopulation of CD45+ Ly6G+ cells was normalized to the sex-matched sham-irradiated samples analyzed on the same day.
Data analyses. Data are expressed as mean ± SEM. Graphs were generated using GraphPad software v.8.2.0 (La Jolla, CA, USA). Data were analyzed using GraphPad and SPSS software (Version 25, Armonk, NY: IBM Corp.). The data were analyzed using ANOVAs with exposure conditions as between-group factors, followed up by post hoc tests when appropriate. Performance over multiple trials was analyzed by repeated-measures ANOVA. For assessing relationships between flow cytometry and behavioral measures Spearman correlational analyses were used.
3. Results
Y maze. There was no effect of radiation on spontaneous alternation or entries, an activity measure, in the Y maze (Suppl.
Figure 1).
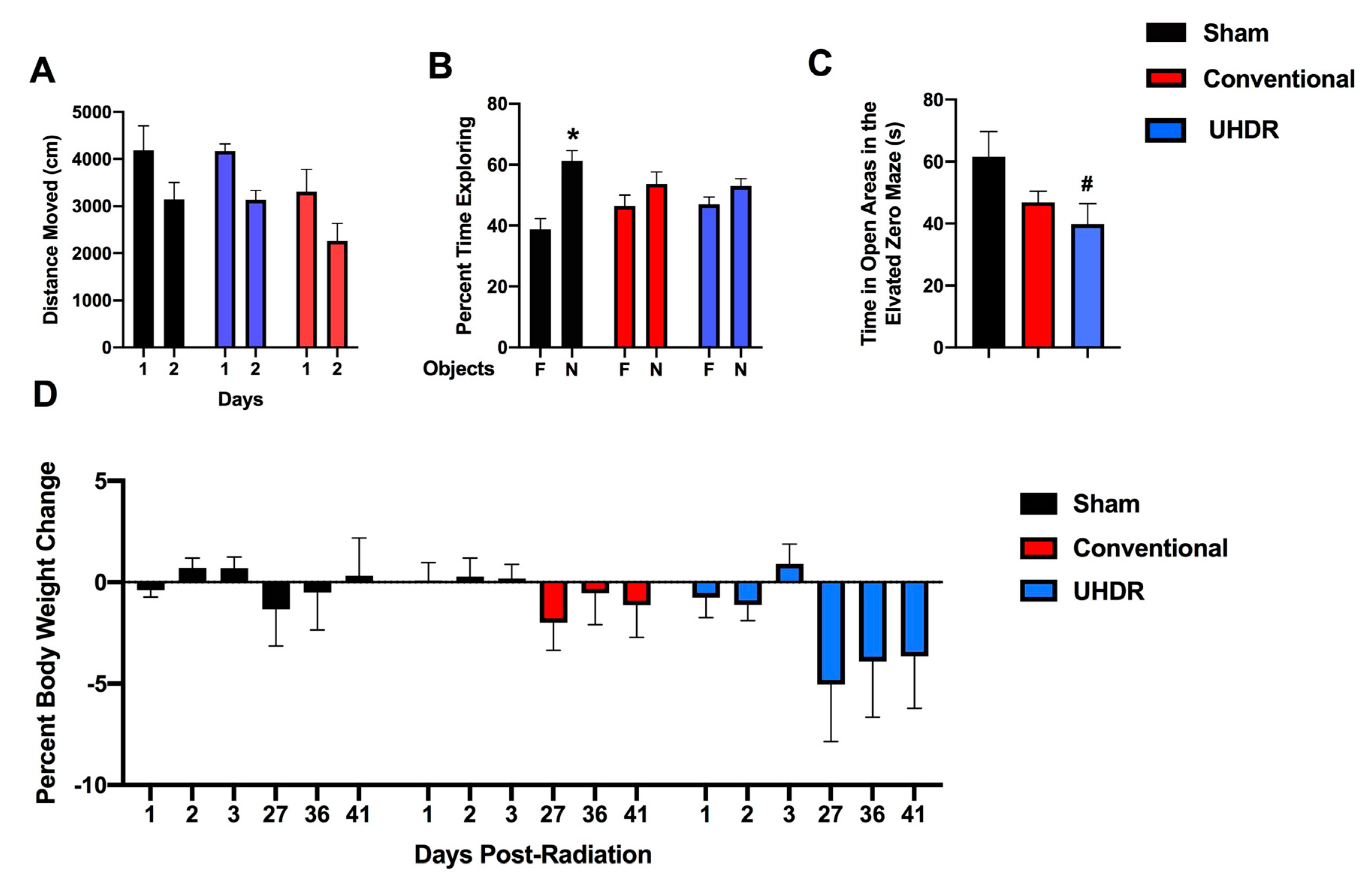
Novel object recognition. In the open field containing the objects, all groups moved less on day 2 than day 1 (F(1,14) = 37.85, p < 0.001) but there was no effect of treatment on activity levels over the two days of testing (Fig. 2A). However, while sham-irradiated mice showed novel object recognition and spent more time exploring the novel than the familiar object (t = 3.185, p = 0.0244, paired t-test), conventional- (t = 0.9447, p =0.3882) and UHDR-irradiated (t = 1.266, p = 0.2743) mice did not (Fig. 2B).
Elevated zero maze. Next measures of anxiety were assessed in the elevated zero maze. There was a trend towards an effect of irradiation on measures of anxiety in the elevated zero maze (F(2,14) = 1.255, p = 0.0820) and a trend towards higher measures of anxiety in UHDR- than sham-irradiated mice (p = 0.0592, Dunnett’s) (Fig. 2C).
Body weights. There was no effect of irradiation on percent body weight change (calculated based on the pre-exposure body weight) in the mice (
Figure 2D). Visual inspection suggests that there might be body weight loss in UHDR-irradiated mice starting at 27 days post exposure but that did not reach significance.
Light-dark test. In the light-dark test, the mice spent more time in the dark than the light compartment (F(1,14) = 65.02, p < 0.0001) but there was no effect of radiation on the percent time spent in the light or dark compartment (Suppl. Figure 2A) or the number of entries into the more anxiety-provoking light compartment (Suppl. Figure 2B).
Spatial Y maze. There was a trend towards an effect of radiation on the percent entries into the novel arm of the spatial Y maze (F(2,14) = 2.947, p = 0.0885), with a trend towards a higher percent entries in UHDR- than sham-irradiated mice (p =0.0830) (Suppl. Fig. 3A). There was no effect of radiation on the percent time spent in the novel arm (Suppl. Fig. 3B). When the number of arm entries was analyzed, there was a radiation x arm interaction (F(4,28) = 2.812, p = 0.0443) (Suppl. Fig. 3C), with a trend towards less arm 2 entries in UHDR- than sham-irradiated mice (p = 0.08, Dunnett’s).
Fear learning and cued fear memory. During the baseline period of fear learning (prior to the first tone), there was an effect of radiation on activity levels (F(2, 14) = 4.200, p = 0.0372) (
Figure 3A). UHDR-irradiated mice moved less than sham-irradiated mice (p = 0.0436, Dunnett’s) and there was a trend towards conventional-irradiated mice to move less than sham-irradiated mice (p = 0.0514, Dunnett’s). There was no effect of radiation on percent freezing during the tones (Fig. 3B). When activity levels during the tones were analyzed, there was an effect of tone (F(1, 14) = 8.385, p = 0.0117) and a trend towards lower activity levels in conventional-irradiated than sham-irradiated mice (p = 0.09) (Fig. 3C). Performance during the inter-stimulus interval (ISI) is considered the best measure of fear learning in the fear conditioning test. There was a trend towards an effect of irradiation on freezing levels during the ISI (F(2, 14) = 2.967, p = 0.0843) and a trend towards higher freezing levels in conventional- than sham-irradiated mice (p = 0.0757, Dunnett’s) (Fig. 3D). There was an effect of radiation on activity levels during the ISI (F(2, 14) = 5.861, p = 0.0142) with lower activity levels in conventional-irradiated (p = 0.0139, Dunnett’s) and UHDR-irradiated (p = 0.0307) than sham-irradiated mice (Fig. 3E). On day 2, cued fear memory was assessed by placing mice in a new environment. The mice were habituated to the new environment for 90 s (pre-tone), and exposed to the tone (cue) for 180 s. There was an effect of period on freezing (F(1, 14) = 54.62, p < 0.0001) with higher freezing levels during the tone than during the pre-tone period (Fig. 3F). There was also an effect of period on activity levels (F(1, 14) = 19.56, p = 0.0006) with lower activity levels during the tone than pre-tone but there was no effect of radiation on cued fear memory.
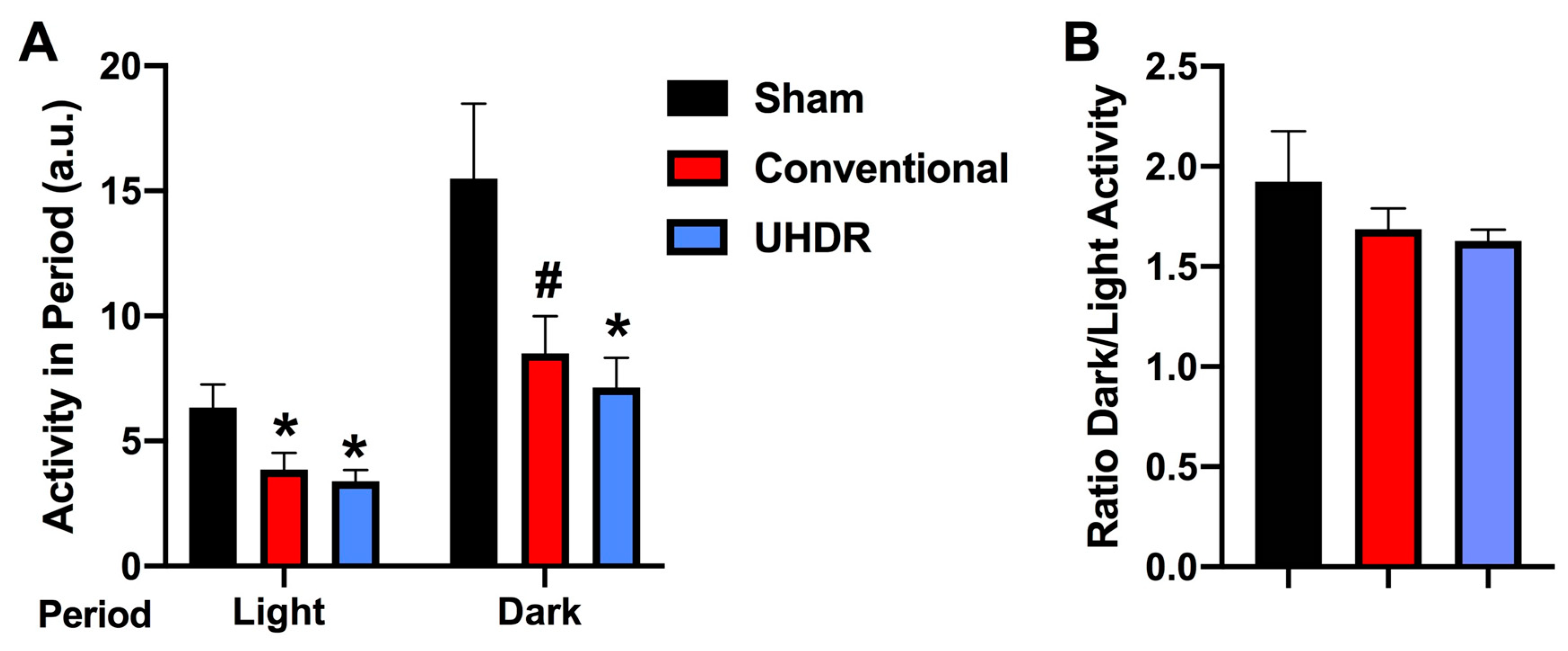
Circadian home cage activity. Circadian activity levels were assessed over two weeks using infrared sensors in individually housed mice. During the light period, there was an effect of radiation (F(2, 14) = 4.804, p = 0.0258) with lower activity levels in conventional- (p =0.0475, Dunnett’s) and UHDR-irradiated (p =0.0256, Dunnett’s) mice than sham-irradiated mice (Fig. 4A). There was also an effect of radiation during the active dark period (F(2, 14) = 4.418, p = 0.0258), with lower activity levels in UHDR- than sham-irradiated mice (p =0.0315, Dunnett’s) and a trend towards lower activity levels in conventional- than UHDR-irradiated mice (p =0.0589, Dunnett’s) (Fig. 3A). Consistent with this pattern, there was no effect of radiation on the ratio dark/light activity (
Figure 4B).
Flow cytometry. There was an effect of radiation on the percent CD45+ Ly6G+ cells in the hippocampus (F(2, 12) = 10.82, p = 0.0005, Kruskall-Wallis) with a lower percent in conventional- (p = 0.0066, Dunn’s) and UHDR- irradiated p = 0.0190, Dunn’s) than sham-irradiated (Fig. 5A) mice. As there were three samples for sham- and conventional-irradiated mice, we also analyzed these data without normalization. When sham- and conventional-irradiated female samples were analyzed without normalization, the percent Ly6G+ CD45+ cells in the hippocampus was also lower in conventional- (33.6 ± 2.7 %) than sham-irradiated mice (47.1 ± 1.7 %) (t = 4.327, p = 0.0133, 2-tailed t-test). There was no effect of radiation on the percent of CD11b+ CD45+ cells, CD45+ cells expressing high levels of CD45, CD45+ cells expressing low levels of CD45, or 206+ CD45+ cells.
The percent CD45+ Ly6G+ cells in the hippocampus correlated positively with peformance in the second trial of the gripstrength test (r = 0.6398, p = 0.02, 2-tailed Spearman, Fig. 5B). The percent CD45+ Ly6G+ cells in the hippocampus also correlated positively with the time spent exploring the novel object in the object recognition test (r = 0.5604, p = 0.049, 2-tailed Spearman, Fig. 5C).
We next analyzed effects of irradiation on the percent CD45+ CD11b+ Ly6G+ and CD45+ CD11b+ Ly6G- cells in the hippocampus. There was an effect of irradiation on the percent CD45+ CD11b+ Ly6G+ in the hippocampus F(2, 12) = 5.348, p = 0.0218, ANOVA), with a lower percent CD45+ CD11b+ Ly6G+ cells in conventional than sham-irradiated mice (p = 0.0130, Fig. 5D). The percent CD45+ CD11b+ Ly6G+ cells in the hippocampus corrrelated negatively with the percent entries in the novel arm of spatial Y maze (r = -0.7153, p = 0.0163, Spearman, Fig. 5E). Irradiation also affected the percent CD45+ CD11b+ Ly6G- cells in the hippocampus (F(2, 12) = 5.384, p = 0.0218, ANOVA), with a higher percent CD45+ CD11b+ Ly6G+ cells in conventional than sham-irradiated mice (p = 0.0130, Fig. 5F). The percent CD45+ CD11b+ Ly6G- cells in the hippocampus corrrelated positively with the percent entries in the novel arm of spatial Y maze (r = 0.7153, p = 0.0163, Spearman,
Figure 5G).
4. Discussion
In the current study, conventional- and UHDR -irradiated mice showed impaired novel object recognition. Consistent with these findings, the only study that involved NOR testing at one-month post exposure, comparable to the time line of our study, NOR was impaired in both conventional- and UHDR-irradiated female nude ((NU
(Ico)-Foxn1
nu) mice at 14 Gy, a dose similar to the 16 Gy in our study. Preservation of NOR in UHDR but not conventional-irradiated mice was seen following exposure to 10 Gy [
11]. During fear learning, conventional- and UHDR -irradiated mice moved less during the ISI and UHDR-irradiated mice also moved less during the baseline period (prior to the first tone). In irradiated mice, reduced activity levels were also seen in the home cage; conventional- and UHDR-irradiated mice moved less during the light period and UHDR-irradiated mice moved less during the dark period. Ly6G is a marker of neutrophils. The percent Ly6G+ CD45+ cells in the hippocampus was lower in conventional- and UHDR -irradiated than sham-irradiated mice, suggesting that neutrophils might be particularly sensitive to radiation. As the Ly6G+ CD45+ cells in the hippocampus was positively correlated with the time spent exploring the novel object in the object recognition test, the reduced percent Ly6G+ CD45+ cells in the hippocampus might mediate some of the detrimental radiation-induced cognitive effects. The percent CD45+ CD11b+ Ly6G+ in the hippocampus was lower in conventional than sham-irradiated mice and corrrelated negatively with the percent entries in the novel arm of spatial Y maze, while the percent CD45+ CD11b+ Ly6G- cells in the hippocampus was higher in conventional than sham-irradiated mice and correlated positively with the percent entries in the novel arm of spatial Y maze.
In the context of cancer, reduced neutrophil levels are likely also beneficial for tumor control as neutrophils were shown to promote tumor resistance to radiotherapy, while a lower neutrophil count following chemoradiotherapy was associated with higher rates of local tumor control, metastasis-free survival, and overall survival [
24] [
25] [
26]. Consistent with these data, an increased neutrophil to lymphocyte ratio following neoadjuvant radiotherapy is associated with poor survival outcome in patients with rectal cancer [
27].. In addition, in a mouse model of pancreatic ductal adenocarcinoma in which anorexia and muscle catabolism are seen, brain infiltrating immune cells were mostly neutrophils that expressed the chemokine receptor C-C chemokine receptor type 2 (CCR2) and blocking CCR2 decreased the brain infiltration of the immune cells and cachexia [
28]. CCR2 deficiency also protected cognitive function and hippocampal neurogenesis in mice receiving traumatic brain injury, irradiation, or receiving both [
29]. The age at irradiation and activational state of the neutrophils might be important to consider. Neutrophil-specific deletion of a kinase critical in the activational state of neutrophils improved cognitive function following traumatic injury to the developing brain [
23]. Conventional, but not UHDR, irradiated reduced the percent of CD45+ CD11b+ Ly6G+ in the hippocampus. This differential response and more desired response after UHDR than conventional irradiation might be beneficial for tumor control, as CD11+ Ly6G+ cells were shown to inhibit tumor growth [
10]. This conventional radiation effect might be beneficial for cognitive function though, as a lower percent of CD45+ CD11b+ Ly6G+ in the hippocampus was associated with enhanced cognitive performance in the spatial Y maze.
In the current study, there was a trend towards increased measures of anxiety in the elevated zero maze. Consistent with this trend, when activity levels in the center of the open field were assessed by beam brakes in neonatally irradiated rats, they were lower in conventional- and UHDR-irradiated rats that received 8 Gy and conventional-irradiated rats that received 5 Gy and they seemed lower in 5 Gy UHDR-irradiated rats as well although it did not reach significance [
30]. These data highlight the importance of including measures of anxiety in these brain radiation studies. There was no effect of irradiation on percent body weight change in the mice but visual inspection suggested that there might be body weight loss in UHDR-irradiated mice starting at 27 days post exposure. Consistent with this pattern, in neonatal irradiated rats, there was no change in body weight up to 24 days after exposure but starting at 31 days following exposure, body weights were lower in conventional- and UHDR-irradiated rats that received 8 Gy [
30]. There was no effect of radiation on cued fear memory, consistent with the lack of an effect of conventional and UHDR irradiation in neonatal rats exposed to 5 or 8 Gy [
10].
In the current study, we did not see reduced cognitive injury following UHDR as compared to conventional cranial irradiation. Together with other studies, these data indicate that the protective cognitive effects of UHDR irradiation are very dose-dependent and might be seen at lower and higher doses than used in the current study. In addition, there is likely a dependence on cognitive test used to compared conventional to UHDR irradiation. Protective effects of UHDR irradiation for novel object recognition memory were seen at doses of 8, 10, or 12 Gy in female mice but not anymore when the dose was 14 Gy [4, 11, 31]. Protective effects in the novel object recognition test were also seen in male mice receiving 30 Gy of UHDR irradiations [
9]. Together, these data suggest that the protective effects of UHDR on cognitive performance might involve a bell-shaped curve but additional studies involving mice of both sexes and involving a battery of behavioral measures would be required to fully model the response to UHDR and conventional irradiation
Infiltration of immune cells into the brain might play a role in detrimental effects of activated microglia and seems a central theme in cognitive injury. For example, in mice with tauopathy and in AD brains, microglia-mediated T cell infiltration induced tauopathy, a marker of AD neuropathology [
32]. The number of T cells, especially cytotoxic T cells, was increased in areas with tau pathology and correlated with neuronal loss, and the cells dynamically transformed their cellular characteristics from activated to exhausted states. When tauopathy mice were given drug or an antibody known to result in the death of microglia or T cells, both decreases brain atrophy. Depletion of microglia reduced the number of T-cells in the brain and T-cell depletion reverted microglia to a state more like that seen in a healthy brain.
While cognitive injury was comparable in conventional and UHDR mice, the percent CD45+ CD11b+ Ly6+ and CD45+ CD11b+ Ly6G- cells in the hippocampus cells in the hippocampus was altered in conventional, but not UHDR, irradiated mice. As the percent CD45+ CD11b+ Ly6+ and CD45+ CD11b+ Ly6G- cells correlated negatively and positively, respectively, with the percent entries in the novel arm of the spatial Y maze, a cognitive measure not affected by conventional irradiation, and CD11+ Ly6G+ cells were shown to inhibit tumor growth [
10], these data suggest that some immune measures in the hippocampus might have opposite effects on cognition versus tumor control. Future efforts are warranted to increase our understanding of the role of immune cells infiltrating the brain in the detrimental effects of cranial irradiation on cognition in the absence and presence of tumors.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1: There was no effect of radiation on spontaneous alternation (A) or entries (B) in the Y maze; Figure S2: In the light-dark test, there was no effect of radiation on the percent time spent in the two compartments (A) or the number of entries into the light compartment (B); Figure S3: A. In the spatial Y maze, there was a trend towards a higher percent entries in UHDR- than sham-irradiated mice. #p =0.0830). B. There was no effect of radiation on the percent time spent in the novel arm. C. For the number of arm entries, there was a radiation x arm interaction (F (4,28) = 2.812, p = 0.0443, with a trend towards less arm 2 entries in UHDR- than sham-irradiated mice. #p = 0.08, Dunnett’s).
Author Contributions
Conceptualization, J.H., F.C., and J.R.; methodology, J.H., F.C. P.C., A.C., A.S., J.S., A.K., A.O., A.S., D.S., B.P., R.Z., C.R.T., and J.R.; formal analysis, A.C., P.C., and J.R.; investigation, A.C., J.H., P.C., A.S., D.S., B.P., R.Z., and J.R.; resources, P.C., J.H., and J.R.; data curation, A.C., P.C., J.R.; writing—original draft preparation, J.R.; writing—review and editing, A.C., P.C., F.C., J.H., A.O., A.S., J.S., A.K., and J.R.; visualization, A.C., P.C., A.S., R.Z. and J.R.; supervision, J.H., P.C., and J.R.; project administration, J.H., and J.R.. All authors have read and agreed to the published version of the manuscript.
Funding
The irradiations were performed using the Dartmouth Cancer Center Irradiation and Imaging Shared Resource (5P30CA023108-40) as well as technology developed by the NIH NCI U01CA260446 grant. The mice were purchased, housed irradiated and shipped using the U01 funds. The OHSU Flow Cytometry Shared Resource is supported, in part, by the OHSU Knight Cancer Institute NCI Cancer Center Support Grant P30CA069533. This research was partially funded by NASA 80NSSC19K0498 –P00001 and the development account of Dr. Raber.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Review Committees of Dartmouth and OHSU.
Data Availability Statement
Data are available upon request.
Acknowledgments
We thank Christina Metea of the OHSU Flow Cytometry Shared Resource for all her help and support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Abayomi, O. K. "Pathogenesis of Irradiation-Induced Cognitive Dysfunction." Acta Oncol 35, no. 6 (1996): 659-63. [CrossRef]
- Roman, D.D., and P.W. Sperduto. "Neuropsychological Effects of Cranial Radiation: Current Knowledge and Future Directions." Int. J. Radiat. Oncol. Biol. Phys. 31, no. 4 (1995): 983-98. [CrossRef]
- Surmaho, O., M. Niemela, J. Vilkki, M. Kouri, A. Brander, O. Salonen, A. Pateau, M. Kallio, J. Pyykkonen, and J. Jaaskelainen. "Adverse Long-Term Effects of Brain Radiotherapy in Adult Low-Grade Glioma Patients." Neurology 56 (2001): 1285-90. [CrossRef]
- Blomstrand, M, and et al. "Estimated Clinical Benefit of Protecting Neurogenesis in the Developing Brain During Radiation Therapy for Pediatric Medulloblastoma." Neuro-Onc 14 (2012): 882-89. [CrossRef]
- Horney, S, and T. Alper. "Unexpected Dose-Rate Effect in the Killing or Mouse by Radiation." Nature 210 (1966): 212. [CrossRef]
- Hornsey, S, and DK Bewley. "Hypoxia in Mouse Intestine Induced by Electron Irradiation at High Dose-Rates." Int J Radiat Biol Rel Studies Phys Chem Med 19 (1971): 479-83. [CrossRef]
- Favaudon, V, and et al. "Ultrahigh Dose-Rate Uhdr Irradiation Increases the Differential Response between Normal and Tumor Tissue in Mice." Sci Trans Med 6 (2014): 245ra93. [CrossRef]
- Montay-Gruel, P, and et al. "Long-Term Neurocognitive Benefits of Uhdr Radiotherapy Driven by Reduced Reactive Oxygen Species." Proc Nath Acad Sci USA 117 (2020): 25947. [CrossRef]
- Simmons, DA, and et al. "Reduced Cognitive Deficits after Uhdr Irradiation of Whole Brain Are Associated with Less Hippocampal Dendritic Spine Loss and Neuroinflammation." Radiother Oncol 139 (2019): 4-10. [CrossRef]
- Williams, MT, and et al. "Cognitive and Behavioral Effects of Whole Brain Conventional or High Dose Rate (Uhdr) Proton Irradiation in a Neonatal Sprague Dawley Rat Model." PLOSOne 024007 (2022). [CrossRef]
- Alaghband, Y, and et al. "Neuroprotection of Radiosensitive Juvenile Mice by Ultra-High Dose Rate Uhdr Irradiation." Cancers 12 (2020): 1671. [CrossRef]
- Ruan, J, and et al. "Irradiation at Ultra-High (Uhdr) Dose Rates Reduces Normal Tissue Toxicity in the Mouse Gastrointestinal System." Int J Radiat Oncol Biol Phys 111 (2021): 1251-61. [CrossRef]
- Montay-Gruel, P, and et al. "X-Rays Can Trigger the Uhdr Effect: Ultra-High Dose-Rate Synchrotron Light Source Prevents Normal Brain Injury after Whole Brain Irradiation in Mice." Radiother Oncol 129 (2018): 582-88. [CrossRef]
- ———. "Ultra-High-Dose-Rate Uhdr Irradiation Limits Reactive Gliosis in the Brain." Radiat Res 194 (2020): 636-45. [CrossRef]
- ———. "Long-Term Neurocognitive Benefits of Uhdr Radiotherapy Driven by Reduced Reactive Oxygen Species." Proc Nath Acad Sci USA 116 (2019): 10943-51. [CrossRef]
- ———. "Hypofractionated Uhdr-Rt as an Effective Treatment against Glioblastoma That Reduces Neurocognitive Side Effects in Mice." Clin Cancer Res 27 (2021): 775-84. [CrossRef]
- Johnson, LA, DG Zuloaga, E Bidiman, T Marzulla, S Weber, H Wahbeh, and J Raber. "Apoe2 Exaggerates Ptsd-Related Behavioral, Cognitive, and Neuroendocrine Alterations." Neuropsychopharmacology 40 (2015): 2443-53. [CrossRef]
- Kundu, P, ERS Torres, K Stagaman, KD Kasschau, M Okhovat, S Holden, S Ward, KA Nevonen, BA Davis, T Saito, TC Saido, L Carbone, TJ Sharpton, and J Raber. "Integrated Analysis of Behavioral, Epigenetic, and Gut Microbiome Analyses in Appnl-G-F , Appnl-F, and Wild Type Mice." Scientific Rep 11 (2021): 4678. [CrossRef]
- Siegel, JA, GE Haley, and J Raber. "Apolipoprotein E Isoform-Dependent Effects of Anxiety and Cognition in Female Tr Mice." Neurobiol Aging 33 (2012): 345-58. [CrossRef]
- Eastwood, E, CN Allen, and J Raber. "Effects of Neonatal Methamphetamine and Thioperamide Exposure on Spatial Memory Retention and Circadian Activity Later in Life." Beh Brain Res 230 (2012): 229-36. [CrossRef]
- Kundu, P, K Stagaman, K Kasschau, S Holden, N Shulzhenko, TJ Sharpton, and J Raber. "Fecal Implants from Appnl-G-F and Appnl-G-F/E4 Donor Mice Sufficient to Induce Behavioral Phenotypes in Germ-Free Mice." Front Behav Neurosci in press (2022). [CrossRef]
- Minnier, J, MR Emmett, R Perez, H-L Ding, BL Barnette, RE Larios, C-S Hong, TH Hwang, Y. Yu, CM Fallgren, MD Story, MM Weil, and J Raber. "Associations between Lipids in Selected Brain Regions, Plasma Mirna, and Behavioral and Cognitive Measures Following 28Si Ion Irradiation." Sci Rep 11 (2021): 14899. [CrossRef]
- Trivedi, A, KG Tercovich, AJ Casbon, J Raber, C. A. Lowell, and LJ Noble-Haesskein. "Neutrophil-Specific Deletion of Syk Results in Recruitment-Independent Stabilization of the Barrier and a Long-Term Improvement in Cognitive Function after Traumatic Injury to the Developing Brain." Neurobiol Dis 157 (2021): 105430. [CrossRef]
- Wisdom, AJ, CS Hong, AJ Lin, and D. G. Kirsch. "Neutrophils Promote Tumor Resistance to Radiation Therapy." Proc Nath Acad Sci USA 116 (2019): 18584-89. [CrossRef]
- Schernberg, A, P Blanchard, C Chargari, and E Deutch. "Neutrophils, a Candidate Biomarker and Target for Radiation Therapy?" Acta Oncol 56 (2017): 1522-30. [CrossRef]
- Nolan, E, and et al. "Radiation Exposure Elicits a Neutrophil-Driven Response in Healthy Lung Tissue That Enhances Metastatic Colonization." Nat Cancer 3 (2022): 173-87. [CrossRef]
- Yang, G., and et al. "Association of Neutrophil-to-Lymphocyte Ratio, Radiotherapy Fractionation/Technique, and Risk of Development of Distant Metastasis among Patients with Locally Advanced Rectal Cancer." Radiat Oncol 17 (2022): 100. [CrossRef]
- Burfeind, KG, X Zhu, MA Norgard, PR Levasseur, C Huisman, AC Buenafe, B Olson, KA Michaelis, ERS Torres, S Jeng, S McWheeny, J Raber, and DL Marks. "Circulating Myeloid Cells Invade the Central Nervous System to Mediate Cachexia During Pancreatic Cancer." Elife 9 (2020): e54095. [CrossRef]
- Allen, AR, K Eilertson, S Sharma, D Schneider, J Baure, B Allen, S Rosi, J Raber, and J. R. Fike. "Effects of Radiation Combined Injury on Hippocampalfunction Are Modulated in Mice Deficient in Chemokine Receptor 2 (Ccr2)." Radiat Res 180 (2013): 78-88. [CrossRef]
- Vorhees, C. V., RE Vatner, and MT Williams. "Review of Conventional and High Dose Rate Brain Radiation (Uhdr): Neurobehavioural, Neurocognitive and Assessment Issues in Rodent Models." Clin Oncol (R Coll Radiol) 33 (2021): e482-e91. [CrossRef]
- Montay-Gruel, P, and et al. "Irradiation in a Uhdr: Unique Sparing of Memory in Mice after Brain Irradiation with Dose-Rates above 100 Gy/S." Radiother Oncol 124 (2017): 365-936. [CrossRef]
- Chen, X-F, and et al. "Microglia-Mediated T Cell Infiltration Drives Neurodegeneration in Tauopathy." Nature 615 (2023): 668-77. [CrossRef]
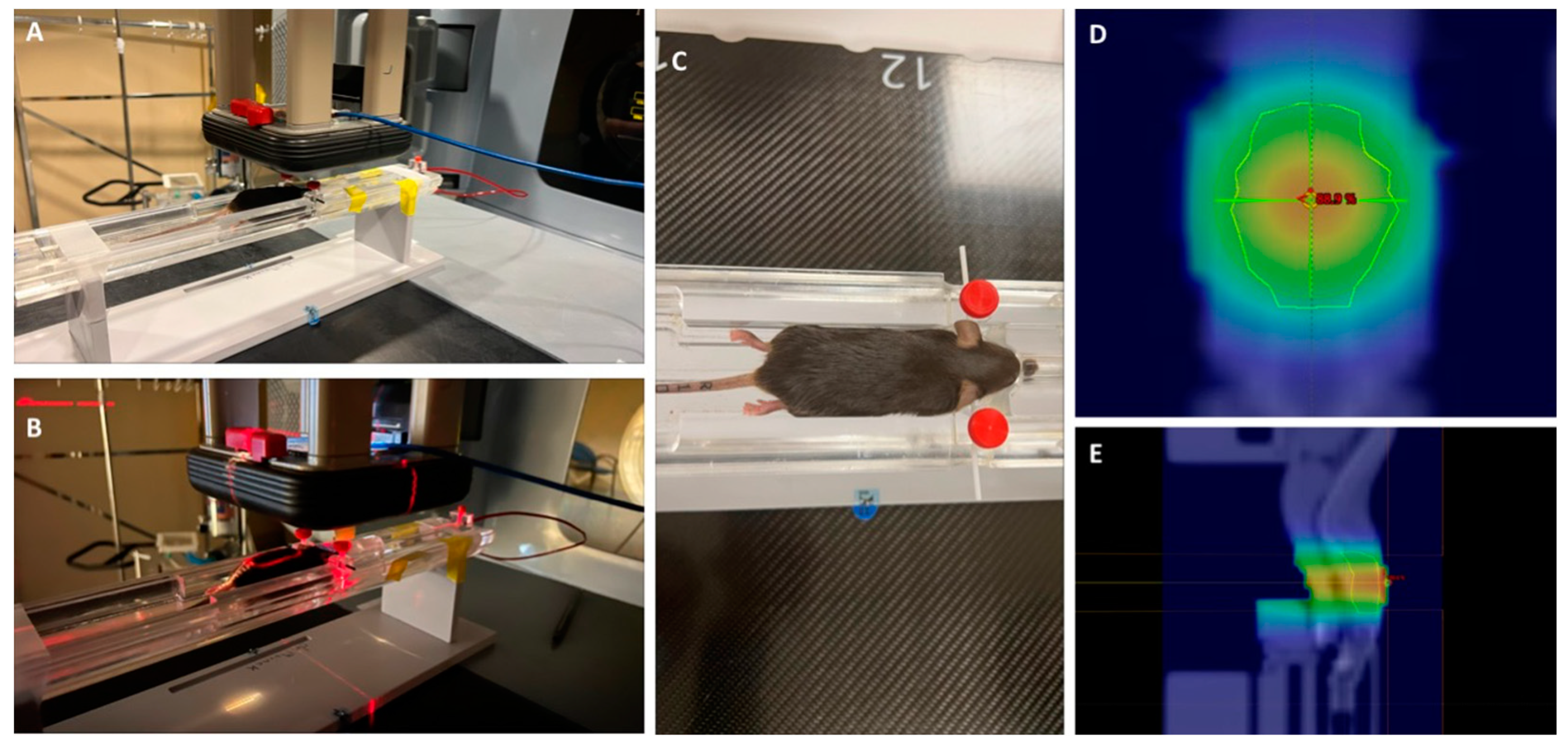
Figure 1.
A-C. Images of the radiation set-up. D-E. two dose wash maps, normalized to 100%. D = 5 mm. The sagittal view is at the midplane of the brain. the AP is 5mm below the top of the mouse.
Figure 1.
A-C. Images of the radiation set-up. D-E. two dose wash maps, normalized to 100%. D = 5 mm. The sagittal view is at the midplane of the brain. the AP is 5mm below the top of the mouse.
Figure 2.
A. There was no effect of radiation on activity levels in the open field containing objects. B. Sham-irradiated mice spent more time exploring the novel than familiar object but conventional- and UHDR-irradiated mice did not. *p < 0.05, paired t-test. C. There was a trend towards an effect of radiation on measures of anxiety in the elevated zero maze with higher measures of anxiety in UHDR- than sham-irradiated mice. #p = 0.0592, Dunnett’s. D. There was no effect of irradiation on percent body weight change in the mice.
Figure 2.
A. There was no effect of radiation on activity levels in the open field containing objects. B. Sham-irradiated mice spent more time exploring the novel than familiar object but conventional- and UHDR-irradiated mice did not. *p < 0.05, paired t-test. C. There was a trend towards an effect of radiation on measures of anxiety in the elevated zero maze with higher measures of anxiety in UHDR- than sham-irradiated mice. #p = 0.0592, Dunnett’s. D. There was no effect of irradiation on percent body weight change in the mice.
Figure 3.
A. During the baseline period of fear learning (prior to the first tone), UHDR-irradiated mice moved less than sham-irradiated mice. *p = 0.0436, Dunnett’s. There also was a trend towards conventional-irradiated mice to move less than sham-irradiated mice. #p = 0.0514, Dunnett’s). B. There was no effect of radiation on percent freezing during the tones. C. When activity levels during the tones were analyzed, there was a trend towards lower activity levels in conventional-irradiated than sham-irradiated mice. #p = 0.09). D. There was a trend towards higher freezing levels in conventional- than sham-irradiated mice. #p = 0.0757, Dunnett’s. E. There was an effect of radiation on activity levels during the ISI (F(2, 14) = 5.861, p = 0.0142) with lower activity levels in conventional-irradiated and UHDR-irradiated than sham-irradiated mice. *p < 0.05, Dunnett’s. F. In the cued fear memory test, there was an effect of period on freezing (F(1, 14) = 54.62, p < 0.0001) with higher freezing levels during the tone than during the pre-tone period but there was no effect of radiation on cued fear memory.
Figure 3.
A. During the baseline period of fear learning (prior to the first tone), UHDR-irradiated mice moved less than sham-irradiated mice. *p = 0.0436, Dunnett’s. There also was a trend towards conventional-irradiated mice to move less than sham-irradiated mice. #p = 0.0514, Dunnett’s). B. There was no effect of radiation on percent freezing during the tones. C. When activity levels during the tones were analyzed, there was a trend towards lower activity levels in conventional-irradiated than sham-irradiated mice. #p = 0.09). D. There was a trend towards higher freezing levels in conventional- than sham-irradiated mice. #p = 0.0757, Dunnett’s. E. There was an effect of radiation on activity levels during the ISI (F(2, 14) = 5.861, p = 0.0142) with lower activity levels in conventional-irradiated and UHDR-irradiated than sham-irradiated mice. *p < 0.05, Dunnett’s. F. In the cued fear memory test, there was an effect of period on freezing (F(1, 14) = 54.62, p < 0.0001) with higher freezing levels during the tone than during the pre-tone period but there was no effect of radiation on cued fear memory.
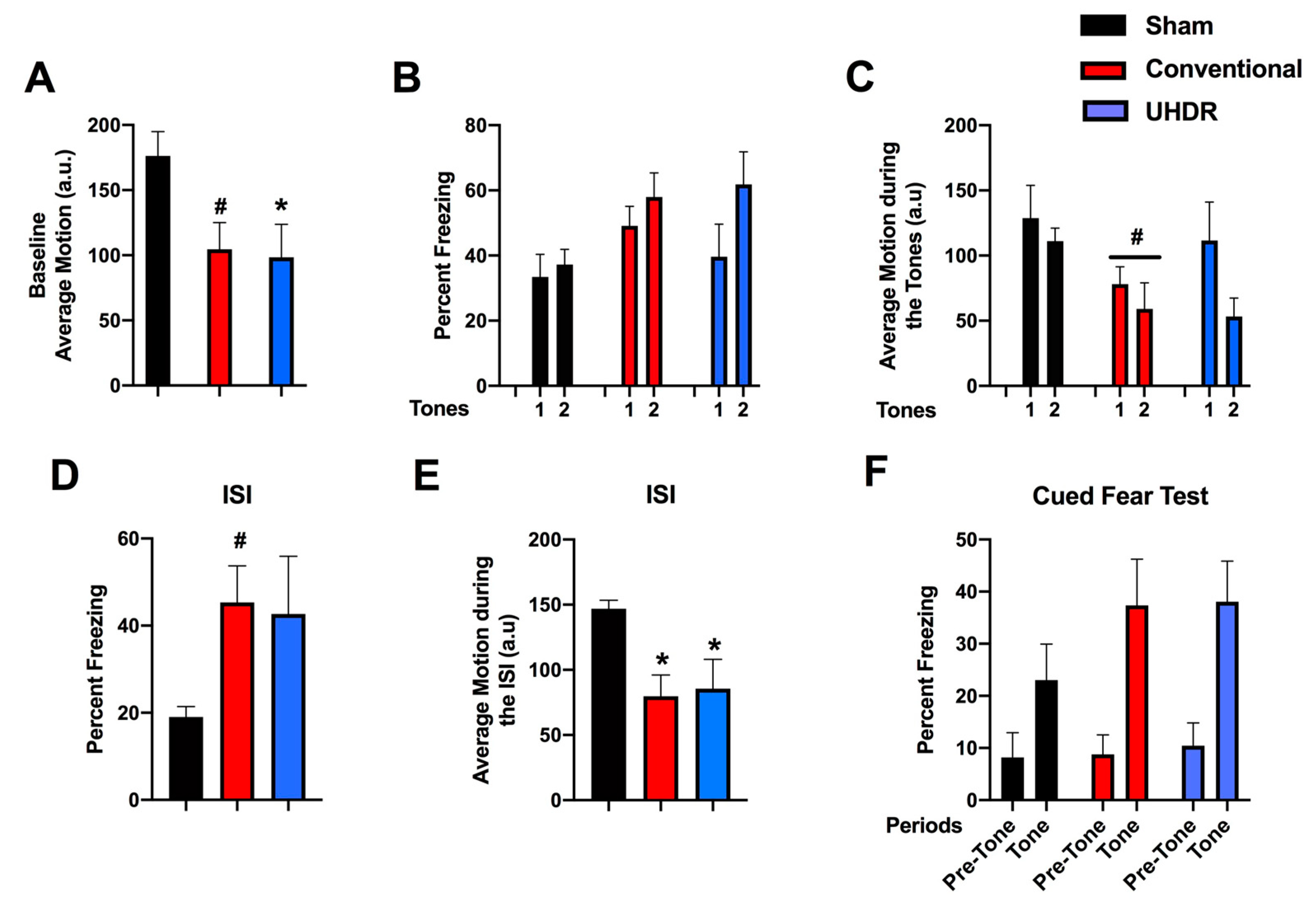
Figure 4.
A. During the light period, there was an effect of radiation (F(2, 14) = 4.804, p = 0.0258) with lower activity levels in conventional- and UHDR-irradiated mice than sham-irradiated mice. There was also an effect of radiation during the active dark period (F(2, 14) = 4.418, p = 0.0258), with lower activity levels in UHDR- than sham-irradiated mice and a trend towards lower activity levels in conventional- than UHDR-irradiated mice. *p < 0.05, Dunnett’s, #p =0.0589, Dunnett’s. B. There was no effect of radiation on the ratio dark/light activity.
Figure 4.
A. During the light period, there was an effect of radiation (F(2, 14) = 4.804, p = 0.0258) with lower activity levels in conventional- and UHDR-irradiated mice than sham-irradiated mice. There was also an effect of radiation during the active dark period (F(2, 14) = 4.418, p = 0.0258), with lower activity levels in UHDR- than sham-irradiated mice and a trend towards lower activity levels in conventional- than UHDR-irradiated mice. *p < 0.05, Dunnett’s, #p =0.0589, Dunnett’s. B. There was no effect of radiation on the ratio dark/light activity.
Figure 5.
A. There was an effect of radiation on the percent Ly6G+ CD45+ cells in the hippocampus (F(2, 12) = 10.82, p = 0.0005, Kruskall-Wallis). The percent Ly6G+ CD45+ cells in the hippocampus was in conventional- and UHDR- irradiated than sham-irradiated. **p < 0.01, *p < 0.05. B. The percent Ly6G+ CD45+ cells in the hippocampus correlated positively with peformance in the second trial of the gripstrength test (r = 0.6398, p = 0.02, 2-tailed Spearman). Best fit linear regression with 95% confidence intervals are shown. C. The percent Ly6G+ CD45+ cells in the hippocampus correlated positively with the time spent exploring the novel object in the object recognition test (r = 0.5604, p = 0.049, 2-tailed Spearman). D. The percent CD45+ CD11b+ Ly6G+ cells in the hippocampus was lower in conventional than sham-irradiated mice. *p < 0.05 versus sham-irradiation. E. The percent CD45+ CD11b+ Ly6G+ cells in the hippocampus corrrelated negatively with the percent entries in the novel arm of spatial Y maze. r = -0.7153, p = 0.0163, Spearman. F. The percent CD45+ CD11b+ Ly6G- cells in the hippocampus was higher in conventional than sham-irradiated mice. *p < 0.05 versus sham-irradiation. G. The percent CD45+ CD11b+ Ly6G- cells in the hippocampus corrrelated positively with the percent entries in the novel arm of spatial Y maze (r = -0.7153, p = 0.0163, Spearman). Best fit linear regression with 95% confidence intervals are shown.
Figure 5.
A. There was an effect of radiation on the percent Ly6G+ CD45+ cells in the hippocampus (F(2, 12) = 10.82, p = 0.0005, Kruskall-Wallis). The percent Ly6G+ CD45+ cells in the hippocampus was in conventional- and UHDR- irradiated than sham-irradiated. **p < 0.01, *p < 0.05. B. The percent Ly6G+ CD45+ cells in the hippocampus correlated positively with peformance in the second trial of the gripstrength test (r = 0.6398, p = 0.02, 2-tailed Spearman). Best fit linear regression with 95% confidence intervals are shown. C. The percent Ly6G+ CD45+ cells in the hippocampus correlated positively with the time spent exploring the novel object in the object recognition test (r = 0.5604, p = 0.049, 2-tailed Spearman). D. The percent CD45+ CD11b+ Ly6G+ cells in the hippocampus was lower in conventional than sham-irradiated mice. *p < 0.05 versus sham-irradiation. E. The percent CD45+ CD11b+ Ly6G+ cells in the hippocampus corrrelated negatively with the percent entries in the novel arm of spatial Y maze. r = -0.7153, p = 0.0163, Spearman. F. The percent CD45+ CD11b+ Ly6G- cells in the hippocampus was higher in conventional than sham-irradiated mice. *p < 0.05 versus sham-irradiation. G. The percent CD45+ CD11b+ Ly6G- cells in the hippocampus corrrelated positively with the percent entries in the novel arm of spatial Y maze (r = -0.7153, p = 0.0163, Spearman). Best fit linear regression with 95% confidence intervals are shown.
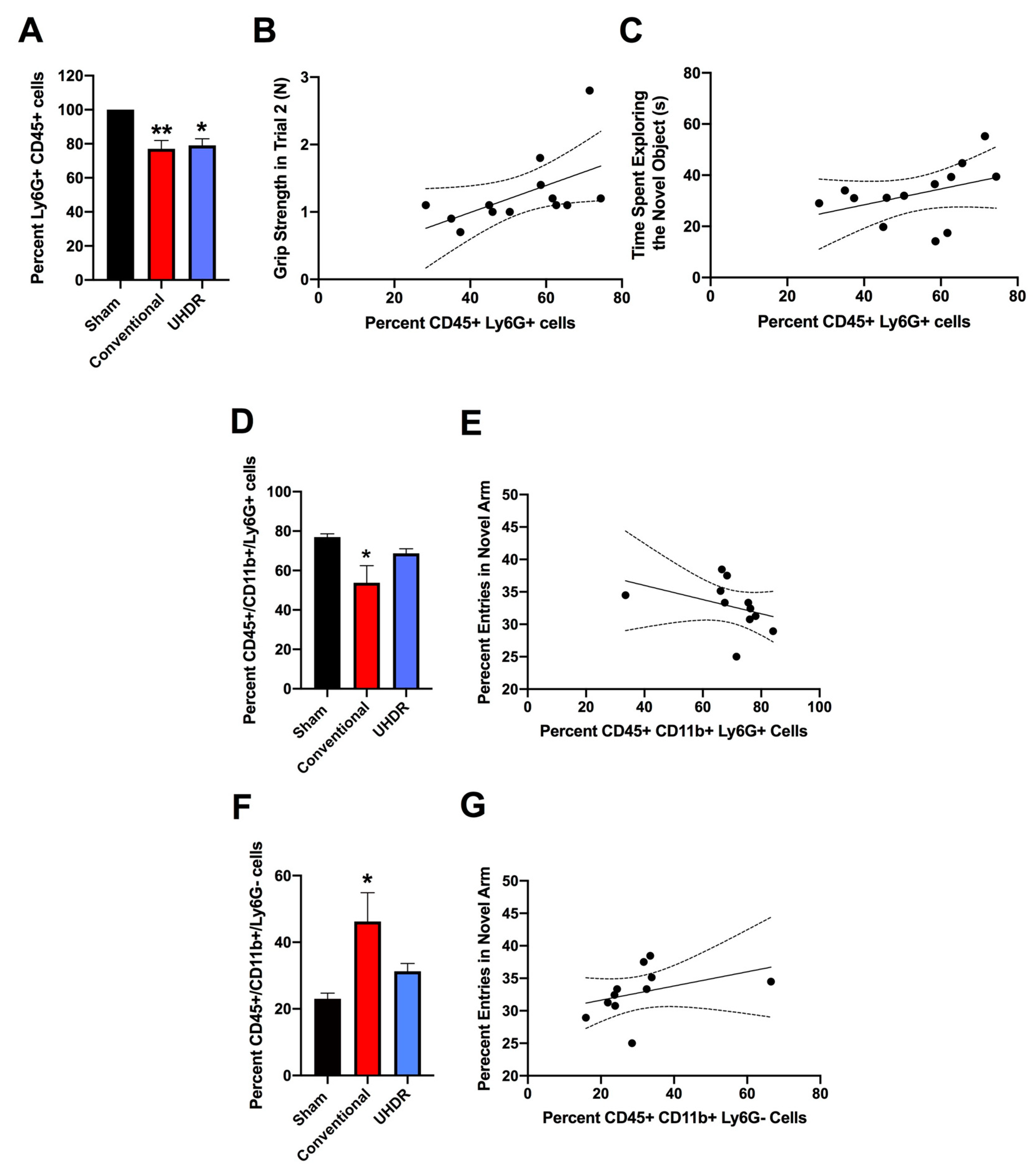
Table 1.
UHDR irradiation details.
Table 1.
UHDR irradiation details.
| Pulsed Prescribed |
Pulses Delivered |
Average Pulse Delivered |
Standard deviation |
| 15 |
4x15, 4x16 |
17.3 Gy |
0.55 Gy |
| 15 |
4x15 |
16.8 |
0.13 Gy |
| 4x16 |
17.7 |
0.21 Gy |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).