Submitted:
08 May 2023
Posted:
10 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
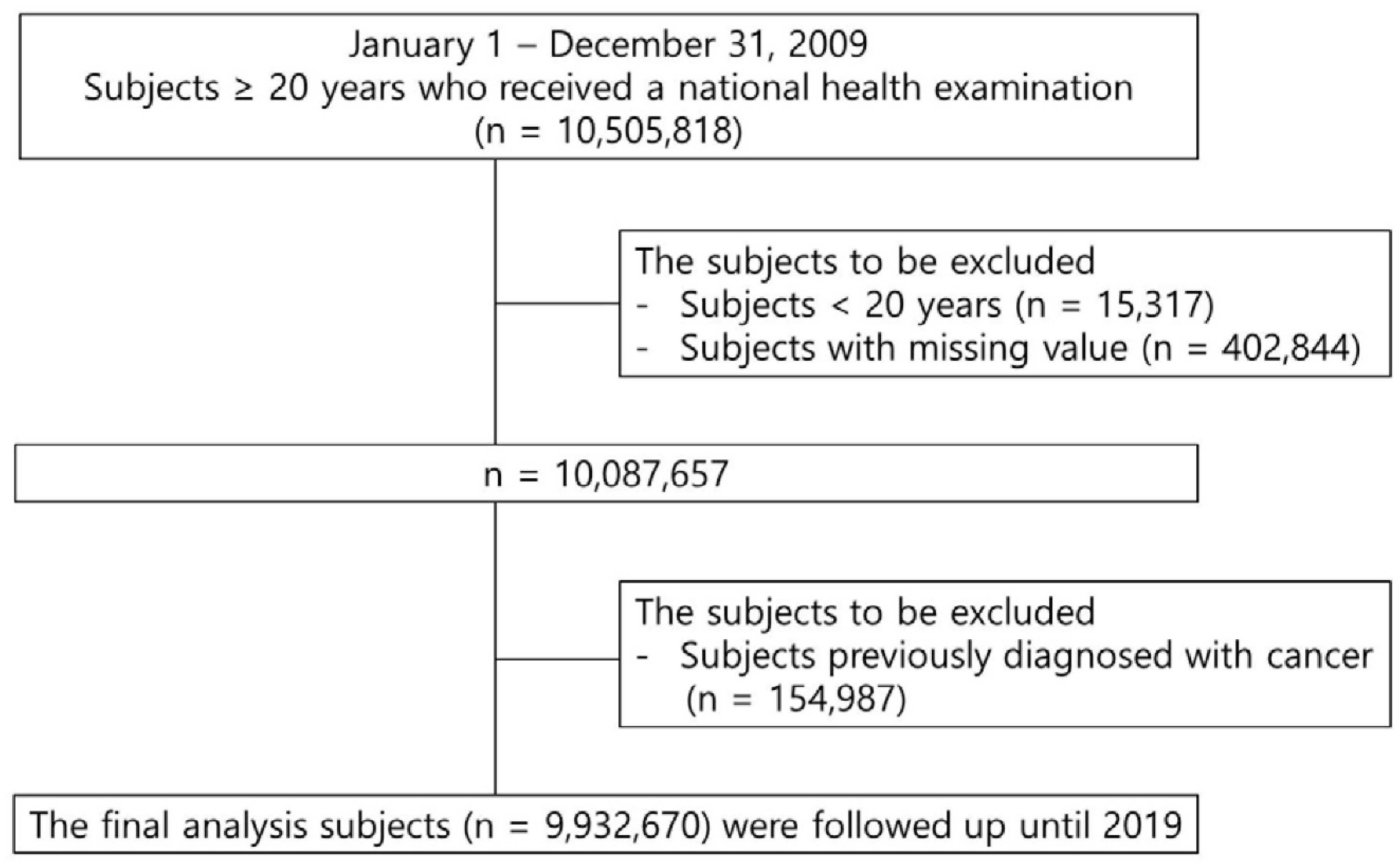
2.1. Data Source
2.2. Study Population
2.3. Definition
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Study Population
3.2. Association of Metabolic Syndrome with Kidney Cancer According to Age
3.3. Gender Difference in the Association of Kidney Cancer with Metabolic Syndrome
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Park, J.H.; Choi, I.S.; Han, K.D.; Park, H.; Kim, K.H.; Kim, J.S. Association Between Fatty Liver Index and Risk of Breast Cancer: A Nationwide Population-Based Study. Clin. Breast Cancer 2020, 20, e450-e457. [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394-424. [CrossRef]
- Safiri, S.; Kolahi, A.A.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Sullman, M.J.M.; Bettampadi, D.; Qorbani, M.; Moradi-Lakeh, M.; Ardalan, M.; et al. The burden of kidney cancer and its attributable risk factors in 195 countries and territories, 1990-2017. Sci. Rep. 2020, 10, 13862. [CrossRef]
- Adams, K.F.; Leitzmann, M.F.; Albanes, D.; Kipnis, V.; Moore, S.C.; Schatzkin, A.; Chow, W.H. Body size and renal cell cancer incidence in a large US cohort study. Am. J. Epidemiol. 2008, 168, 268-277. [CrossRef]
- Hidayat, K.; Du, X.; Zou, S.Y.; Shi, B.M. Blood pressure and kidney cancer risk: Meta-analysis of prospective studies. J. Hypertens. 2017, 35, 1333-1344. [CrossRef]
- Neovius, M.; Sundström, J.; Rasmussen, F. Combined effects of overweight and smoking in late adolescence on subsequent mortality: Nationwide cohort study. BMJ 2009, 338, b496. [CrossRef]
- Scelo, G.; Larose, T.L. Epidemiology and Risk Factors for Kidney Cancer. J. Clin. Oncol. 2018, 36, Jco2018791905. [CrossRef]
- Ko, S.H.; Han, K.D.; Yun, J.S.; Chung, S.; Koh, E.S. Impact of obesity and diabetes on the incidence of kidney and bladder cancers: A nationwide cohort study. Eur. J. Endocrinol. 2019, 181, 489-498. [CrossRef]
- Kim, S.Y.; Han, K.D.; Joo, Y.H. Metabolic Syndrome and Incidence of Laryngeal Cancer: A Nationwide Cohort Study. Sci. Rep. 2019, 9, 667. [CrossRef]
- Sin, S.; Lee, C.H.; Choi, S.M.; Han, K.D.; Lee, J. Metabolic Syndrome and Risk of Lung Cancer: An Analysis of Korean National Health Insurance Corporation Database. J. Clin. Endocrinol. Metab. 2020, 105. [CrossRef]
- Oh, T.R.; Han, K.D.; Choi, H.S.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Metabolic Syndrome Resolved within Two Years is Still a Risk Factor for Kidney Cancer. J. Clin. Med. 2019, 8. [CrossRef]
- Lee, Y.H.; Han, K.; Ko, S.H.; Ko, K.S.; Lee, K.U. Data Analytic Process of a Nationwide Population-Based Study Using National Health Information Database Established by National Health Insurance Service. Diabetes Metab. J. 2016, 40, 79-82. [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640-1645. [CrossRef]
- Shen, T.; Shu, X.O.; Xiang, Y.B.; Li, H.L.; Cai, H.; Gao, Y.T.; Zheng, W.; Lipworth, L. Association of hypertension and obesity with renal cell carcinoma risk: A report from the Shanghai Men’s and Women’s Health Studies. Cancer Causes Control 2015, 26, 1173-1180. [CrossRef]
- Kim, C.S.; Han, K.D.; Choi, H.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Association of Hypertension and Blood Pressure With Kidney Cancer Risk: A Nationwide Population-Based Cohort Study. Hypertension 2020, 75, 1439-1446. [CrossRef]
- Bao, C.; Yang, X.; Xu, W.; Luo, H.; Xu, Z.; Su, C.; Qi, X. Diabetes mellitus and incidence and mortality of kidney cancer: A meta-analysis. J. Diabetes Complications 2013, 27, 357-364. [CrossRef]
- Tseng, C.H. Type 2 Diabetes Mellitus and Kidney Cancer Risk: A Retrospective Cohort Analysis of the National Health Insurance. PLoS ONE 2015, 10, e0142480. [CrossRef]
- Zhu, J.; Tu, H.; Matin, S.F.; Tannir, N.M.; Wood, C.G.; Wu, X. Glycemic index, glycemic load and carbohydrate intake in association with risk of renal cell carcinoma. Carcinogenesis 2017, 38, 1129-1135. [CrossRef]
- Landberg, A.; Fält, A.; Montgomery, S.; Sundqvist, P.; Fall, K. Overweight and obesity during adolescence increases the risk of renal cell carcinoma. Int. J. Cancer 2019, 145, 1232-1237. [CrossRef]
- di Meo, N.A.; Lasorsa, F.; Rutigliano, M.; Loizzo, D.; Ferro, M.; Stella, A.; Bizzoca, C.; Vincenti, L.; Pandolfo, S.D.; Autorino, R.; Crocetto, F.; Montanari, E.; Spilotros, M.; Battaglia, M.; Ditonno, P.; Lucarelli, G. Renal Cell Carcinoma as a Metabolic Disease: An Update on Main Pathways, Potential Biomarkers, and Therapeutic Targets. Int. J. Mol. Sci. 2023, 23, 22. [CrossRef]
- Yu, C.P.; Ho, J.Y.; Huang, Y.T.; Cha, T.L.; Sun, G.H.; Yu, D.S.; Chang, F.W.; Chen, S.P.; Hsu, R.J. Estrogen inhibits renal cell carcinoma cell progression through estrogen receptor-β activation. PLoS ONE 2013, 8, e56667. [CrossRef]
- Czarnecka, A.M.; Niedzwiedzka, M.; Porta, C.; Szczylik, C. Hormone signaling pathways as treatment targets in renal cell cancer (Review). Int. J. Oncol. 2016, 48, 2221-2235. [CrossRef]
- Song, W.; He, D.; Chen, Y.; Yeh, C.R.; Hsu, I.; Huang, Q.; Zhang, X.; Chang, L.S.; Zuo, L.; Chen, J.; et al. Targeting newly identified ERβ/TGF-β1/SMAD3 signals with the FDA-approved anti-estrogen Faslodex or an ERβ selective antagonist in renal cell carcinoma. Mol. Oncol. 2018, 12, 2055-2071. [CrossRef]
| Age (years) | Total | 20-39 | 40-64 | 65- | ||||||||
| Kidney cancer | (-) | (+) | p | (-) | (+) | p | (-) | (+) | p | (-) | (+) | p |
| n | 9919912 | 12758 | 3153202 | 1297 | 5500258 | 7829 | 1266452 | 3632 | ||||
| BMI (kg/m2) | 23.71±3.2 | 24.68±3.09 | <.0001 | 23.13±3.5 | 24.89±3.68 | <.0001 | 24±2.99 | 24.84±2.96 | <.0001 | 23.9±3.15 | 24.24±3.11 | <.0001 |
| WC (cm) | 80.24±9.06 | 84.61±8.4 | <.0001 | 78.08±9.7 | 83.41±9.83 | <.0001 | 80.8±8.54 | 84.47±8.2 | <.0001 | 83.21±8.38 | 85.35±8.21 | <.0001 |
| Glucose (mg/dl) | 97.08±22.83 | 102.27±26.45 | <.0001 | 90.99±16.01 | 93.66±18.08 | <.0001 | 99.12±24.34 | 102.79±27.5 | <.0001 | 103.37±26.82 | 104.24±26.06 | 0.0507 |
| SBP (mmHg) | 122.41±14.92 | 127.64±15.4 | <.0001 | 118.48±12.96 | 123.15±14.38 | <.0001 | 122.87±14.9 | 126.77±15.06 | <.0001 | 130.16±16.2 | 131.11±15.82 | 0.0004 |
| DBP (mmHg) | 76.3±9.97 | 78.9±10.17 | <.0001 | 74.37±9.28 | 77.46±10.33 | <.0001 | 76.96±10.15 | 79.33±10.08 | <.0001 | 78.25±10.05 | 78.48±10.24 | 0.1789 |
| HDL (mg/dl) | 55.56±20.98 | 52.65±22.33 | <.0001 | 56.45±18.09 | 53.07±18.86 | <.0001 | 55.34±20.88 | 52.74±21.73 | <.0001 | 54.25±27.1 | 52.31±24.64 | <.0001 |
| TG (mg/dl) | 134.13±92.77 | 151.31±97.92 | <.0001 | 123.63±92.77 | 154.21±109 | <.0001 | 138.67±94.47 | 154.07±99.57 | <.0001 | 140.51±82.57 | 144.32±89.46 | 0.0054 |
| Renal duration | 8.26±0.79 | 4.49±2.48 | <.0001 | 8.31±0.37 | 5.01±2.32 | <.0001 | 8.3±0.65 | 4.48±2.49 | <.0001 | 7.92±1.62 | 4.3±2.47 | <.0001 |
| Age (years) | Total | 20-39 | 40-64 | 65- | ||||||||
| Mets Var. | Incidence | Model 1 | Model 2 | Incidence | Model 1 | Model 2 | Incidence | Model1 | Model2 | Incidence | Model1 | Model2 |
| Body mass index (kg/m2) | ||||||||||||
| <18.5 | 0.07 | 0.67 (0.58,0.76) |
0.78 (0.68,0.9) |
0.02 | 0.73 (0.53,1.02) |
1.03 (0.74,1.43) |
0.09 | 0.77 (0.62,0.96) |
0.78 (0.62,0.97) |
0.29 | 0.9 (0.73,1.1) |
0.86 (0.7,1.06) |
| 18.5-22.9 | 0.11 | 1 (Ref.) | 1 (Ref.) | 0.03 | 1 (Ref.) | 1 (Ref.) | 0.12 | 1 (Ref.) | 1 (Ref.) | 0.33 | 1 (Ref.) | 1 (Ref.) |
| 23-24.9 | 0.17 | 1.55 (1.48,1.62) |
1.31 (1.25,1.38) |
0.05 | 1.72 (1.47,2.02) |
1.34 (1.14,1.58) |
0.17 | 1.44 (1.36,1.53) |
1.28 (1.2,1.36) |
0.38 | 1.17 (1.07,1.27) |
1.22 (1.1,1.32) |
| 25-29.9 | 0.21 | 1.94 (1.86,2.03) |
1.61 (1.54,1.68) |
0.09 | 2.85 (2.49,3.25) |
2.07 (1.8,2.39) |
0.23 | 1.88 (1.78,2) |
1.6 (1.52,1.7) |
0.39 | 1.19 (1.1,1.29) |
1.34 (1.24,1.46) |
| ≥30 | 0.22 | 2.07 (1.9,2.25) |
2.06 (1.89,2.24) |
0.10 | 3.25 (2.6,4.06) |
2.56 (2.05,3.21) |
0.28 | 2.31 (2.072,2.57 |
2.25 (2.02,2.5) |
0.39 | 1.21 (1.0,1.45) |
1.66 (1.38,1.99) |
| Waist circumference (cm, M/F) | ||||||||||||
| <70/ <65 | 0.05 | 0.52 (0.45,0.6) |
0.7 (0.61,0.81) |
0.02 | 0.58 (0.43,0.8) |
0.8 (0.59,1.1) |
0.08 | 0.74 (0.61,0.9) |
0.81 (0.67,0.99) |
0.19 | 0.6 (0.44,0.82) |
0.54 (0.39,0.74) |
| 70-79/ 65-74 | 0.09 | 1 (Ref.) | 1 (Ref.) | 0.03 | 1 (Ref.) | 1 (Ref.) | 0.10 | 1 (Ref.) | 1 (Ref.) | 0.32 | 1 (Ref.) | 1 (Ref.) |
| 80-89/ 75-84 | 0.17 | 1.95 (1.86,2.05) |
1.44 (1.37,1.51) |
0.06 | 2.07 (1.81,2.37) |
1.58 (1.38,1.82) |
0.18 | 1.73 (1.63,1.84) |
1.39 (1.31,1.48) |
0.36 | 1.13 (1.03,1.25) |
1.2 (1.09,1.32) |
| 90-99/ 85-94 | 0.25 | 2.76 (2.62,2.91) |
1.81 (1.72,1.91) |
0.09 | 3.14 (2.67,3.69) |
2.28 (1.93,2.7) |
0.25 | 2.41 (2.25,2.58) |
1.8 (1.68,1.93) |
0.38 | 1.2 (1.08,1.33) |
1.37 (1.23,1.51) |
| ≥100/ ≥95 | 0.29 | 3.21 (2.94,3.5) |
2.27 (2.08,2.47) |
0.13 | 4.2 (3.25,5.43) |
3.35 (2.59,4.34) |
0.28 | 2.72 2.41,3.06) |
2.16 (1.92,2.44) |
0.45 | 1.42 (1.22,1.65) |
1.82 (1.57,2.12) |
| Age (years) | All | 20-39 | 40-64 | 65- | ||||
| HR (95% CI) | HR (95% CI) | HR (95% Cl) | HR (95% CI) | |||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model1 | Model2 | Model1 | Model2 | |
| Obesity | 1.65 (1.6,1.71) | 1.48 (1.43,1.53) | 2.47 (2.21,2.75) | 1.89 (1.69,2.12) | 1.63 (1.56,1.71) | 1.48 (1.421.55) | 1.12 (1.05,1.2) | 1.26 (1.18,1.35) |
| Central obesity | 1.93 (1.83,1.98) | 1.48 (1.43,1.54) | 2.38 (2.1,2.69) | 1.88 (1.65,2.13) | 1.72(1.64,1.8) | 1.47 (1.4,1.54) | 1.14 (1.07,1.22) | 1.28 (1.2,1.37) |
| DM | 1.7 (1.64,1.76) | 1.21 (1.17,1.26) | 1.43 (1.26,1.62) | 1.12 (0.98,1.27) | 1.44 (1.37,1.5) | 1.16 (1.11,1.21) | 1.16 (1.09,1.24) | 1.12 (1.04,1.19) |
| HTN | 2.47 (2.38,2.56) | 1.58 (1.52,1.64) | 1.97 (1.77,2.2) | 1.49 (1.33,1.68) | 1.97 (1.88,2.06) | 1.54 (1.47,1.61) | 1.41 (1.3,1.53) | 1.44 (1.33,1.56) |
| low HDL | 1.57 (1.51,1.63) | 1.38 (1.33,1.44) | 1.44 (1.27,1.64) | 1.38 (1.21,1.57) | 1.29 (1.24,1.35) | 1.34 (1.28,1.4) | 1.02 (0.96,1.1) | 1.32 (1.24,1.41) |
| High TG | 1.7 (1.64,1.76) | 1.29 (1.25,1.34) | 1.87 (1.67,2.09) | 1.29 (1.15,1.45) | 1.54 (1.47,1.61) | 1.25 (1.19,1.3) | 1.12 (1.05,1.19) | 1.22 (1.14,1.3) |
| MetS | 2.28 (2.2,2.36) | 1.56 (1.51,1.62) | 2.55 (2.25,2.89) | 1.82 (1.6,2.07) | 1.87 (1.78,1.95) | 1.5 (1.43,1.57) | 1.2 (1.13,1.28) | 1.37 (1.29,1.47) |
| Number of MetS components | ||||||||
| 1 | 1.67 (1.56,1.77) | 1.23 (1.16,1.31) | 1.48 (1.28,1.72) | 1.21 (1.04,1.41) | 1.36 (1.26,1.47) | 1.16 (1.07,1.26) | 1.09 (0.94,1.27) | 1.13 (0.97,1.32) |
| 2 | 2.5 (2.35,2.66) | 1.52 (1.43,1.61) | 2 (1.7,2.35) | 1.39 (1.18,1.64) | 1.88 (1.74,2.03) | 1.43 (1.33,1.55) | 1.22 (1.06,1.42) | 1.34 (1.16,1.55) |
| 3 | 3.21 (3.02,3.42) | 1.77 (1.66,1.89) | 3.11 (2.61,3.7) | 1.98 (1.65,2.37) | 2.26 (2.09,2.44) | 1.61 (1.49,1.75) | 1.27 (1.1,1.47) | 1.5 (1.29,1.73) |
| 4 | 4.29 (4.01,4.58) | 2.18 (2.04,2.33) | 4 (3.19,5.01) | 2.43 (1.93,3.06) | 2.97 (2.73,3.23) | 2.01 (1.85,2.19) | 1.44 (1.24,1.67) | 1.74 (1.51,2.04) |
| 5 | 5.25 (4.83,5.71) | 2.53 (2.33,2.76) | 4.98 (3.32,7.48) | 2.92 (1.94,4.41) | 3.67 (3.29,4.09) | 2.41 (2.16,2.69) | 1.53 (1.29,1.81) | 1.97 (1.66,2.33) |
| Obesity/ MetS | ||||||||
| (-/+) | 2.32 (2.2,2.44) | 1.44 (1.37,1.52) | 2.3 (1.75,3) | 1.57 (1.2,2.07) | 1.71 (1.6,1.83) | 1.33 (1.24,1.42) | 1.14 (1.05,1.24) | 1.29 (1.19,1.41) |
| (+/-) | 1.42 (1.35,1.49) | 1.33 (1.26,1.4) | 2.18 (1.92,2.49) | 1.74 (1.52,1.99) | 1.41 (1.32,1.5) | 1.33 (1.24,1.41) | 0.99 (0.88,1.12) | 1.12 (0.99,1.26) |
| (+/+) | 2.61 (2.5,2.73) | 1.87 (1.79,1.95) | 3.47 (3.01,4.01) | 2.41 (2.08,2.8) | 2.27 (2.15,2.4) | 1.82 (1.72,1.92) | 1.26 (1.16,1.37) | 1.53 (1.4,1.66) |
| Gender | Age (years) | BMI cutoff (kg/m2) | Youden’s index | Sensitivity | Specificity | WC cutoff (cm) | Youden’s index | Sensitivity | Specificity |
| Total | 20-39 | 23.9 | 0.229 | 0.608 | 0.621 | 81 | 0.244 | 0.645 | 0.598 |
| 40-64 | 23.9 | 0.133 | 0.626 | 0.507 | 82 | 0.191 | 0.652 | 0.539 | |
| ≥65 | 23.5 | 0.058 | 0.609 | 0.449 | 84 | 0.106 | 0.592 | 0.514 | |
| Male | 20-39 | 24.1 | 0.182 | 0.655 | 0.527 | 81 | 0.178 | 0.746 | 0.432 |
| 40-64 | 24 | 0.106 | 0.643 | 0.463 | 85 | 0.121 | 0.601 | 0.521 | |
| ≥65 | 23.5 | 0.071 | 0.574 | 0.497 | 84 | 0.074 | 0.639 | 0.435 | |
| Female | 20-39 | 21.6 | 0.117 | 0.496 | 0.621 | 72 | 0.097 | 0.462 | 0.635 |
| 40-64 | 23.1 | 0.124 | 0.666 | 0.458 | 79 | 0.133 | 0.534 | 0.600 | |
| ≥65 | 24.4 | 0.095 | 0.567 | 0.528 | 84 | 0.076 | 0.496 | 0.580 |
| Age (years) | All | 20-39 | 40-64 | 65- | |||||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||||
| Gender | Male | Female | Male | Female | Male | Female | Male | Female | |||
| Body mass index (kg/m2) | |||||||||||
| <18.5 | 0.75 (0.64,0.89) | 0.7 (0.56,0.89) | 0.86 (0.49,1.5) | 1.01 (0.66,1.53) | 0.78 (0.59,1.03) | 0.76 (0.53,1.11) | 0.92 (0.73,1.16) | 0.69 (0.44,1.09) | |||
| 18.5-22.9 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| 23-24.9 | 1.35 (1.28,1.43) | 1.29 (1.18,1.41) | 1.52 (1.27,1.82) | 0.74 (0.46,1.19) | 1.33 (1.24,1.44) | 1.2 (1.08,1.34) | 1.3 (1.08,1.32) | 1.27 (1.08,1.47) | |||
| 25-29.9 | 1.7 (1.61,1.79) | 1.53 (1.41,1.66) | 2.3 (1.96,2.69) | 1.27 (0.83,1.92) | 1.7 (1.59,1.82) | 1.45 (1.3,1.6) | 1.32 (1.2,1.46) | 1.39 (1.2,1.61) | |||
| ≥30 | 2.22 (2,2.46) | 2 (1.73,2.31) | 2.84 (2.23,3.61) | 1.77 (0.86,3.61) | 2.41 (2.11,2.75) | 2.04 (1.7,2.44) | 1.71 (1.31,2.24) | 1.64 (1.27,2.12) | |||
| Waist circumference (cm) | |||||||||||
| <70/ <65 | 0.63 (0.52,0.78) | 0.74 (0.6,0.92) | 0.65 (0.38,1.1) | 0.82 (0.55,1.22) | 0.71 (0.54,0.95) | 0.91 (0.69,1.21) | 0.49 (0.34,0.71) | 0.74 (0.4,1.37) | |||
| 70-79/65-74 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| 80-89/75-84 | 1.52 (1.43,1.61) | 1.42 (1.31,1.54) | 1.73 (1.48,2.03) | 1.15 (0.82,1.61) | 1.43 (1.32,1.54) | 1.37 (1.24,1.52) | 1.2 (1.08,1.34) | 1.19 (1,1.43) | |||
| 90-99/85-94 | 1.99 (1.87,2.12) | 1.62 (1.46,1.78) | 2.55 (2.13,3.06) | 1.06 (0.57,1.96) | 1.91 (1.75,2.08) | 1.62 (1.43,1.83) | 1.4 (1.24,1.57) | 1.32 (1.091.59) | |||
| ≥100/≥95 | 2.61 (2.33,2.9) | 1.91 (1.64,2.22) | 3.79 (2.88,5) | 1.78 (0.73,4.36) | 2.51 (2.17,2.89) | 1.62 (1.3,2.04) | 1.83 (1.5,2.23) | 1.82 (1.44,2.31) | |||
| MetS | 1.64 (1.57,1.71) | 1.44 (1.34,1.54) | 1.82 (1.6,2.09) | 1.61 (0.91,2.83) | 1.55 (1.47,1.63) | 1.39 (1.27,1.52) | 1.42 (1.31,1.53) | 1.3 (1.15,1.46) | |||
| Obesity | 1.52 (1.46,1.58) | 1.45 (1.36,1.56) | 1.96 (1.74,2.21) | 1.41 (0.98,2.03) | 1.52 (1.44,1.6) | 1.41 (1.3,1.54) | 1.25 (1.15,1.36) | 1.29 (1.15,1.45) | |||
| Central obesity | 1.56 (1.49,1.63) | 1.35 (1.25,1.45) | 1.94 (1.7,2.21) | 1.21 (0.72,2.01) | 1.53 (1.44,1.62) | 1.33 (1.21,1.47) | 1.3 (1.2,1.41) | 1.25 (1.11,1.4) | |||
| DM | 1.21 (1.16,1.26) | 1.24 (1.15,1.32) | 1.08 (0.95,1.24) | 1.38 (0.96,1.98) | 1.14 (1.08,1.2) | 1.21 (1.11,1.32) | 1.11 (1.03,1.21) | 1.13 (1,1.26) | |||
| HTN | 1.61 (1.54,1.68) | 1.57 (1.46,1.69) | 1.52 (1.34,1.71) | 1.29 (0.87,1.91) | 1.55 (1.47,1.64) | 1.52 (1.39,1.65) | 1.45 (1.31,1.6) | 1.43 (1.24,1.65) | |||
| Low HDL | 1.43 (1.37,1.49) | 1.29 (1.21,1.38) | 1.39 (1.2,1.6) | 1.35 (0.99,1.83) | 1.4 (1.32,1.48) | 1.22 (1.12,1.32) | 1.36 (1.26,1.48) | 1.24 (1.1,1.39) | |||
| High TG | 1.33 (1.27,1.38) | 1.25 (1.17,1.34) | 1.27 (1.13,1.44) | 1.52 (1.01,2.3) | 1.29 (1.23,1.36) | 1.15 (1.05,1.25) | 1.25 (1.15,1.35) | 1.17 (1.05,1.32) | |||
| Obesity and Metabolic syndrome | |||||||||||
| MetS only | 1.49 (1.4,1.59) | 1.39 (1.26,1.53) | 1.53 (1.15,2.04) | 2.38 (1.05,5.39) | 1.34 (1.23,1.45) | 1.32 (1.16,1.51) | 1.33 (1.3,1.47) | 1.23 (1.05,1.44) | |||
| Obesity only | 1.34 (1.26,1.42) | 1.43 (1.3,1.57) | 1.79 (1.55,2.06) | 1.47 (0.98,2.19) | 1.32 (1.23,1.43) | 1.36 (1.22,1.53) | 1.07 (0.92,1.23) | 1.22 (1,1.5) | |||
| Both | 1.97 (1.88,2.08) | 1.73 (1.59,1.89) | 2.49 (2.13,2.91) | 1.36 (0.63,2.89) | 1.9 (1.78,2.02) | 1.66 (1.48,1.85) | 1.54 (1.4,1.71) | 1.49 (1.29,1.73) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





