Submitted:
09 May 2023
Posted:
10 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Patient Characteristics and Clinical Outcomes
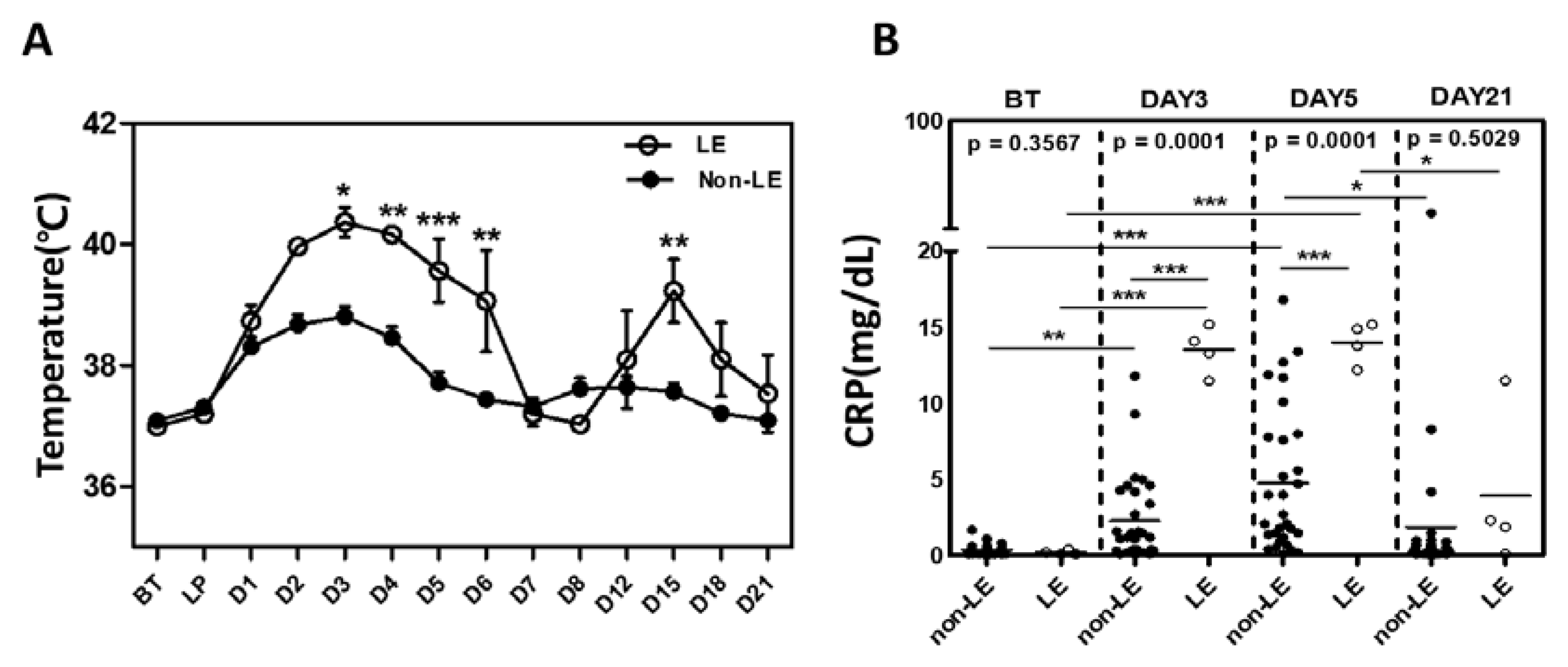
2.2. Pro-Inflammatory Cytokine Levels between LE and Non-LE Patients
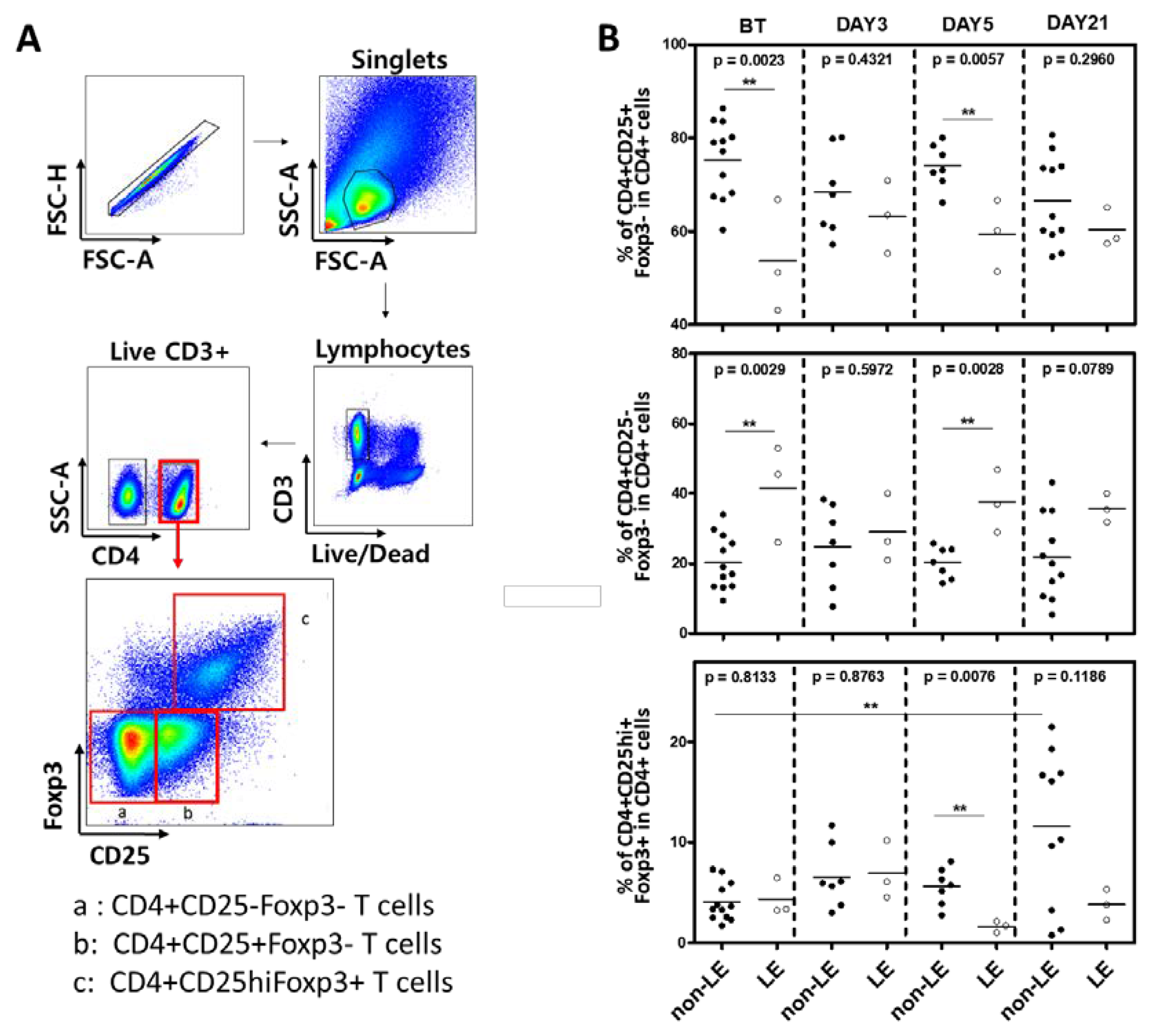
2.3. Flow Cytometry of Conventional and Regulatory CD4+ T Cells
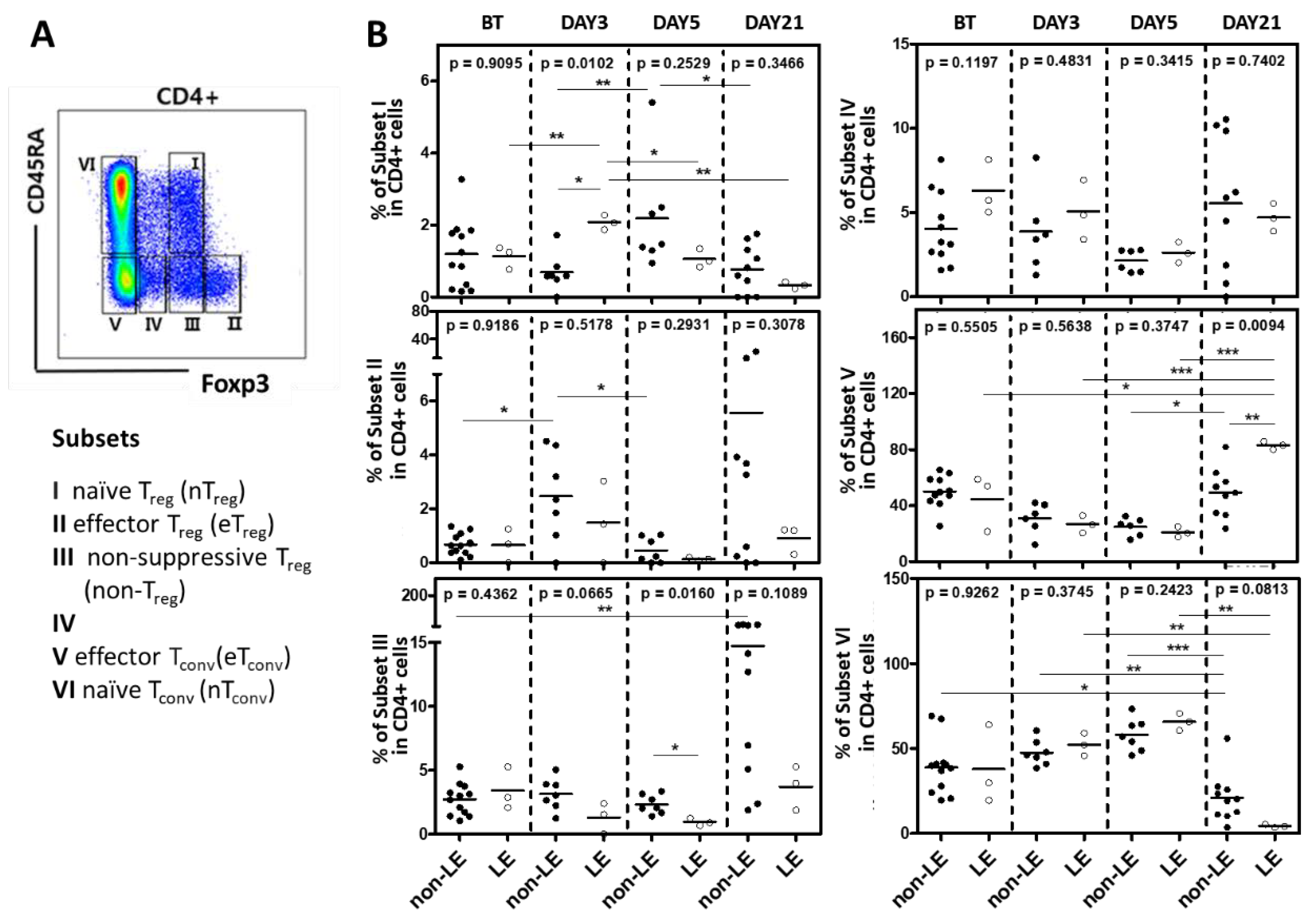
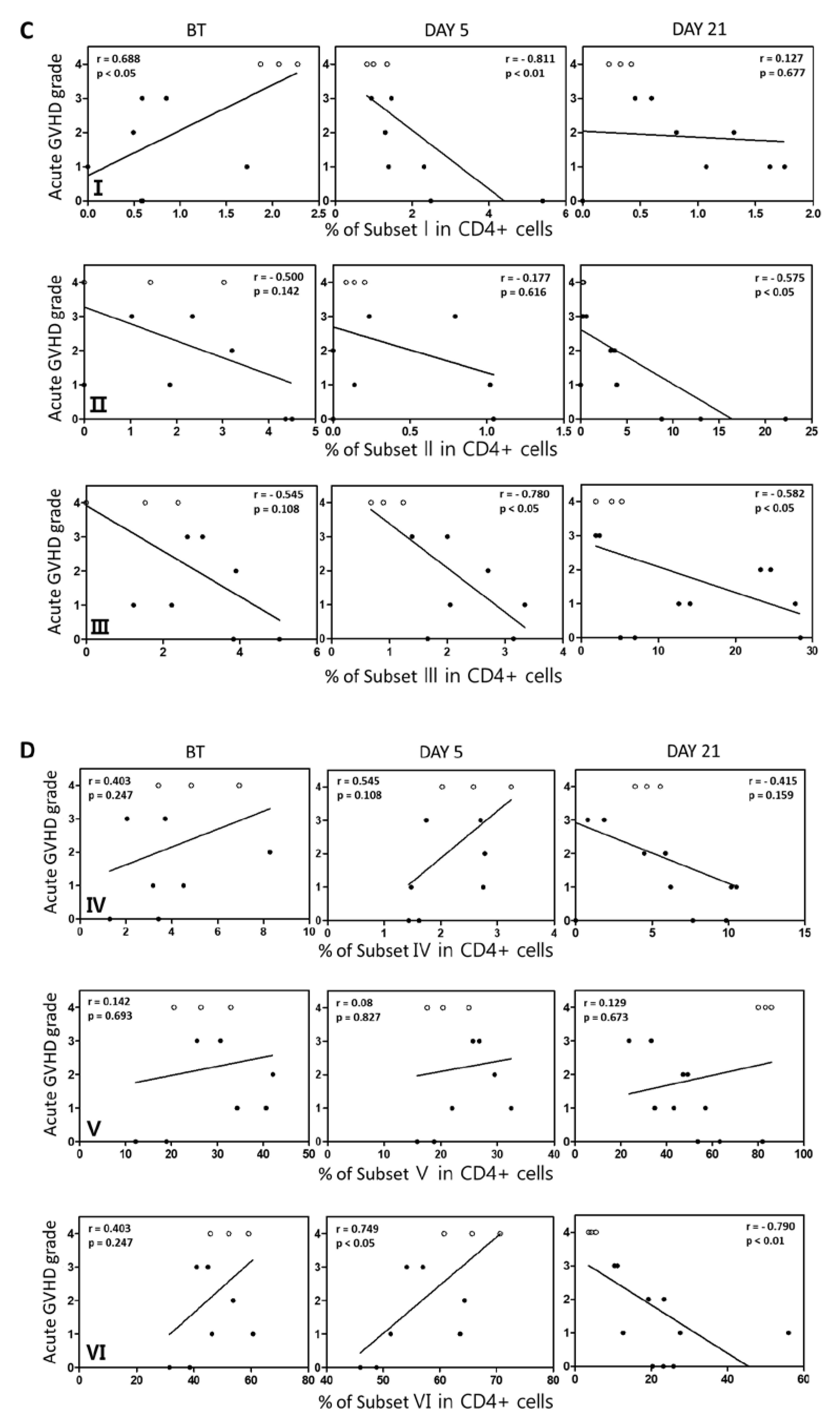
2.4. Subsets of Conventional and Regulatory CD4+ T Cell Regulatory
3. Discussion
4. Materials and Methods
4.1. Patients and Diagnosis of Autoimmune Limbic Encephalitis
4.2. Clinical Assessment
4.3. Analysis of Cytokines and Treg Cells
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gooley, T.A.; Chein, J.W.; Pergam, S.A.; Hingorani, S.; Sorror, M.L.; Boeckh, M.; Martin, P.H.; Sandmaier, B.M.; Marr, K.A.; Appelbaum, F.R.; Storb, R.; McDonald, G.B. Reduced mortality after allogeneic hematopoietic cell transplantation. N. Engl. J. Med. 2010, 363, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- ML, S.; MB, M.; Storb R; Baron F; BM, S.; DG, M., Storer B Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [CrossRef] [PubMed]
- Bashey, A.; Zhang, X.; Sizemore, C.A. , et al. T cell replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of comtemporaneous HLA-matched related and unrelated donor transplantation. J. Clin. Oncol. 2013, 31, 1310–1316. [Google Scholar] [PubMed]
- Luznik, L.; Fuchs, E.J. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol. Res. 2010, 47, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, A.; Y sun; Labopin, M. ; Bacigalupo, A.; Lorentino, F.; Arcese, W., et al. Post-transplant cyclophosphamide versus anti-thymocyte globulin as graft-versus-host disease prophylaxis in haploidentical transplant. Haematologica 2017, 102, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef]
- Budhram, A.; Leung, A.; Nicolle, M.W.; Burneo, J.G. Diagnosing autoimmune limbic encephalitis. CMAJ 2019, 191, E529–E534. [Google Scholar] [CrossRef]
- Giordano, A.; Fazio, R.; Gelibter, S.; Minicucci, F.; Vabanesi, M.; Anzalone, N. , et al. Diagnosing autoimmune encephalitis in a real-world single-center setting. J. Neurol. 2020, 267, 449–460. [Google Scholar] [CrossRef]
- Kyritsis, A.P.; Markoula, S.; Alexiou, G.; Asimakopoulos, A.; Jabbour, P.; Fotopoulos, A. , et al. Diagnosis and treatment of limbic encephalitis in the cancer patient. Future Oncol. 2020, 16, 1647–1655. [Google Scholar] [CrossRef]
- Imus, P.H.; Blackford, A.L.; Bettinotti, M. , et al. Severe cytokine release syndrome after Haploidentical peripheral blood stem cell transplantation. Biol. Blood Marrow Transplant. 2019, 25, 2431–2437. [Google Scholar] [CrossRef]
- Abboud, R.; Keller, J.; Slade, M. , et al. Severe cytokine-release syndrome after T cell-replete peripheral blood haploidentical donor transplantation is associated with poor survival and anti-IL-6 therapy is safe and well tolerated. Biol. Blood Marrow Transplant. 2016, 22, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.D.; Tambaro, F.P.; Kohorst, M. , et al. Immune effector cell associated neurotoxicity (ICANS) in pediatric and young adults patients following chimeric antigen receptor (CAR) T-cell therapy: Can we optimize early diagnosis? Front Oncol 2021, 11, 634445. [Google Scholar]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L. , et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A. , AY Rudensky. Foxpe programs the development and function of CD4+CD25+ T regulatory cells. Nat. Immunol. 2003, 4, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Ikegawa, S. , KI Matsuoka. Harnessing Treg homeostasis to optimize posttransplant immunity: Current concepts and future perspectives. Front. Immunol. 2021, 12, 713358. [Google Scholar] [CrossRef] [PubMed]
- JM, M.; M?nch N; Peter K; Freund D; Oelschl?gel U; H?lig K, et al. Automated Clinical Grade Expansion of Regulatory T Cells in a Fully Closed System. Front. Immunol. 2019, 10, 38. [Google Scholar]
- Ikegawa; S. ; Matsuoka, K.I. Harnessing Treg Homeostasis to Optimize Posttransplant Immunity: Current Concepts and Future Perspectives. Front. Immunol. 2021, 12, 713358. [Google Scholar] [CrossRef]
- Graus, F.; Titulaer, M.J.; Balu, R. , et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef]
- Przepiorka, D.; Wisdorf, D.; Martin, P. , et al. 1994 Consensus Conference on Acute GVHD grading. Bone Marrow Transplant. 1995, 15, 825–828. [Google Scholar]
- Lee, S.J.; Vogelsan, G.; Flowers, M.E.D. Chronic graft-versus-host disease. Biol. Blood Marrow Transpl. 2003, 9, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Malard, F.; Abecassis, M. , et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016, 51, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Graus F; MJ, T. ; Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Grosso; D. ; Carabasi; M.; Filicko-O'Hara; J.; Kasner; M.; Wagner; J.L.; Colombe; Farley, C.; P.; O'Hara; W.; Flomenberg; P.; Werner-Wasik; M.; Brunner; J.; Mookerjee; B.; Hyslop; T.; Weiss; M.; Flomenberg, N. A 2-step approach to myeloablative haploidentical stem cell transplantation: a phase 1/2 trial performed with optimized T-cell dosing. Blood. 2011, 118, 4732–9. [Google Scholar]
- Solomon; S. R.; Sizemore; C.A.; Sanacore; M.; Zhang; X.; Brown; S.; Holland; H.K.; Morris; L.E.; Bashey, A. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol. Blood Marrow Transplant. 2012, 18, 1859–66. [Google Scholar]
- Mancusi; A. ; Ruggeri; L.; Velardi, A. Haploidentical hematopoietic transplantation for the cure of leukemia: from its biology to clinical translation. Blood 2016, 128, 2616–23. [Google Scholar] [CrossRef]
- Kanakry; C. G.; Fuchs; E.J.; Luznik, L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat. Rev. Clin. Oncol. 2016, 13, 132. [Google Scholar] [CrossRef]
- Wing; J. B.; Tanaka; A.; Sakaguchi, S. Human FOXP3+ Regulatory T Cell Heterogeneity and Function in Autoimmunity and Cancer. Immunity. 2019, 50, 302–316. [Google Scholar] [CrossRef]
- Abboud; Wan; Mariotti et al. Cytokine release syndrome after haploidentical hematopoietic cell transplantation: an international multicenter analysis. Bone Marrow Transplant. 2021, 56, 2763–2770. [Google Scholar] [CrossRef]
- Lee, D.W.; Gardner, R.; Porter, D.L. , et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Devine, S.M. Haploidentical hematopoietic cell transplantation using post-transplantation cyclophosphamide: Does graft source matter? J. Clin. Oncol. 2017, 35, 2984–2986. [Google Scholar] [CrossRef] [PubMed]
- Abboud, R.; Wan, F.; Mariotti, J. , et al. Cytokine release syndrome after haploidentical hematopoietic cell transplantation: an international multicenter analysis. Bone Marrow Transplant. 2021, 56, 2763–2770. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Godel, R.; Subklewe, M. , et al. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Sheth, V.S. , J Gauthier. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. 2021, 56, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, T.; Shoji, H.; Takahashi, Y. , et al. Cerebrospinal fluid levels of cytokines in non-herpetic acute limbic encephalitis: Comparison with herpes simplex encephalitis. Cytokine 2008, 44, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Norelli; M.; Camisa; B.; Barbiera; G.; Falcone; L.; Purevdorj; A.; Genua; M.; Sanvito; F.; Ponzoni; M.; Doglioni; C.; Cristofori; P.; Traversari; C.; Bordignon; C.; Ciceri; F.; Ostuni; R.; Bonini; C.; Casucci; M.; Bondanza, A. 37. Norelli; M.; Camisa; B.; Barbiera; G.; Falcone; L.; Purevdorj; A.; Genua; M.; Sanvito; F.; Ponzoni; M.; Doglioni; C.; Cristofori; P.; Traversari; C.; Bordignon; C.; Ciceri; F.; Ostuni; R.; Bonini; C.; Casucci; M.; Bondanza, A. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018; 739–748. [Google Scholar]
- Greco; R. ; Lorentino; F.; Nitti; Stanghellini, L.; M.T.; Giglio; F.; Clerici; D.; Xue; E.; Lazzari; L.; Piemontese; S.; Mastaglio; S.; Assanelli; A.; Marktel; S.; Corti; C.; Bernardi; M.; Ciceri; F.; Peccatori, J. Interleukin-6 as Biomarker for Acute GvHD and Survival After Allogeneic Transplant with Post-transplant Cyclophosphamide. Front. Immunol. 2019, 10, 2319. [Google Scholar]
- Andrea S Henden, Geoffrey R Hill. Cytokines in Graft-versus-Host Disease. J. Immunol. 2015, 194, 4604–4612. [Google Scholar] [CrossRef]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; Traversari, C.; Bordignon, C.; Ciceri, F.; Ostuni, R.; Bonini, C.; Casucci, M.; Bondanza, A. , Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef]
- Tadamitsu, K. Interleukin-6, from basic science to medicine--40 years in immunology. Annu. Rev. Immunol. 23, 1–21.
- Ogura, H.; Murakami, M.; Okuyama, Y.; Tsuruoka, M.; Kitabayashi, C.; Kanamoto, M.; Nishihara, M.; Iwakura, Y.; Hirano, T. , Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 2008, 29, 4. [Google Scholar] [CrossRef]
- Tanaka, T.; Kishimoto, T. The Biology and Medical Implications of Interleukin-6. Cancer Immunol Res 2014, 2, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Gust; J. ; Ponce; R.; Liles; W.C.; Garden; G.A.; Turtle, C.J. Cytokines in CAR T Cell-Associated Neurotoxicity. Front. Immunol. 2020, 11, 577027. [Google Scholar] [CrossRef] [PubMed]
- Abboud, R.; Keller, J.; Slade, M. , et al. Severe cytokine-release syndrome after T cell-replete peripheral blood haploidentical donor transplantation is associated with poor survival and anti-IL-6 therapy is safe and well tolerated. Biol. Blood Marrow Transplant. 2016, 22, 1851–1860. [Google Scholar] [CrossRef]
- Khadka, R.H.; Sakemura, R.; Kenderian, S.S. , et al. Management of cytokine release syndrome: an update on emerging antigen-specific T cell engaging immunotherapies. Immunotherapy 2019, 11, L851–L857. [Google Scholar] [CrossRef]
- Neelapu, S.S. Managing the toxicities of CAR T-cell therapy. Hematol. Oncol. 2019, 37, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Hayden, P.J.; Roddie, C.; Bader, P. , et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann. Oncol. 2022, 33, 259–275. [Google Scholar] [PubMed]
- Korn, T. , M Hiltensperger. Role of IL-6 in the commitment of T cell subsets. Cytokine 2021, 146, 155654. [Google Scholar] [CrossRef]
- van Loosdregt J; Fleskens V; Fu J; AB, B. ; CP, B.; CE, P.; Meerding J; CR, B.; Barbi J; Gr?ne A; AJ, S.; MM, M.; Kalkhoven E; BJ, P.; Ovaa H; Pan F; DM, Z., Coffer PJ. Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity. 2013, 39, 259–71. [Google Scholar] [CrossRef]
- Dominguez-Villar; M. ; Hafler, D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018, 19, 665–673. [Google Scholar] [CrossRef]
- VH, N.; Zeiser R; DL, D. ; DS, C.; Beilhack A; CH, C., et al. In Vivo Dynamics of Regulatory T-cell Trafficking and Survival Predict Effective Strategies to Control Graft-Versus-Host Disease Following Allogeneic Transplantation. Blood 2007, 109, 2649–56. [Google Scholar]
- Copsel S; Wolf D; Kale B; Barreras H; CO, L. ; CS, B., et al. Very Low Numbers of CD4(+)?FoxP3(+)?Tregs Expanded in Donors Via TL1A-Ig and Low-Dose Il-2 Exhibit a Distinct Activation/Functional Profile and Suppress GVHD in a Preclinical Model. Biol. Blood Marrow Transplant. 2018, 24, 1788–94. [Google Scholar] [CrossRef] [PubMed]
- Bolton; H. A.; Zhu; E.; Terry; A.M.; Guy; T.V.; Koh; W.P.; Tan; S.Y.; Power; C.A.; Bertolino; P.; Lahl; K.; Sparwasser; T.; Shklovskaya, E.; Fazekas de St Groth, B. Selective Treg reconstitution during lymphopenia normalizes DC costimulation and prevents graft-versus-host disease. J. Clin. Invest. 2015, 125, 3627–41. [Google Scholar]
- Wolf; D. ; Bader; C.S.; Barreras; H.; Copsel; S.; Pfeiffer; B.J.; Lightbourn; C.O.; Altman; N.H.; Komanduri; K.V.; Levy, R.B. Superior immune reconstitution using Treg-expanded donor cells versus PTCy treatment in preclinical HSCT models. JCI Insight. 2018, 3, e121717. [Google Scholar] [CrossRef] [PubMed]
- Ganguly; S. ; Ross; D.B.; Panoskaltsis-Mortari; A.; Kanakry; C.G.; Blazar; B.R.; Levy; R.B.; Luznik, L. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood. 2014, 124, 2131–41. [Google Scholar] [CrossRef] [PubMed]
- Alho; A. C.; Kim; H.T.; Chammas; M.J.; Reynolds; C.G.; Matos; T.R.; Forcade; E.; Whangbo; J.; Nikiforow; S.; Cutler; C.S.; Koreth; J.; Ho; V.T.; Armand; P.; Antin; J.H.; Alyea; E.P.; Lacerda; J.F.; Soiffer; R.J.; Ritz, J. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. 2016, 127, 646–57. [Google Scholar]
- X. ; Wang; Q.; Guo; W.; Pei; X.; Niu; Q.; Liu; M.; Liu; Y.; Chen; S.; Feng; S.; He; Y.; Yang; D.; Zhang; R.; Ma; Q.; Zhai; W.; Pang; A.; Wei; J.; Huang; Y.; Luo; Y.; Han; M.; Feng; X.; Jiang, E. Loss of Lkb1 impairs Treg function and stability to aggravate graft-versus-host disease after bone marrow transplantation. Cell Mol. Immunol. 2020, 17, 483–495. [Google Scholar]
- Zeiser, R.; Teshima, T. , Nonclassical manifestations of acute GVHD. Blood 2021, 138, 2165–2172. [Google Scholar] [CrossRef]
- Mariotti, J.; Penack, O.; Castagna, L. , Acute Graft-versus-Host-Disease Other Than Typical Targets: Between Myths and Facts. Transplant. Cell Ther. 2021, 27, 115–124. [Google Scholar] [CrossRef]
- Ruggiu, M.; Cuccuini, W.; Mokhtari, K.; Meignin, V. , Régis Peffault de Latour, Marie Robin, Flore Sicre de Fontbrune, Aliénor Xhaard, Gérard Socié, David Michonneau. Case report: Central nervous system involvement of human graft versus host disease: Report of 7 cases and a review of literature. Medicine 2017, 96. [Google Scholar] [CrossRef]
- Yamamoto, H.; Uchida, N.; Ishiwata, K.; Araoka, H.; Takagi, S.; Tsuji, M.; Kato, D.; Matsuhashi, Y.; Seo, S.; Matsuno, N.; Masuoka, K.; Wake, A.; Yoneyama, A.; Makino, S. , Shuichi Taniguchi. Possible graft-versus-host disease involving the central nervous system soon after cord blood transplantation. Am. J. Hematol. 2009, 84. [Google Scholar] [CrossRef]
- Hartrampf, S.; Dudakov, J.A.; Johnson, L.K.; Smith, O.M.; Tsai, J.; Singer, N.V.; West, M.L.; Hanash, A.M.; Albert, M.H.; Liu, B.; Toth, M. , Marcel R M van den Brink. The central nervous system is a target of acute graft versus host disease in mice. Blood 2013, 121. [Google Scholar] [CrossRef]
- Belle, L.; Zhou, V. , Kara L Stuhr, Margaret Beatka, Emily M Siebers, Jennifer M Knight, Michael W Lawlor, Casey Weaver, Misato Hashizume, Cecilia J Hillard, William R Drobyski, Host interleukin 6 production regulates inflammation but not tryptophan metabolism in the brain during murine GVHD. JCI Insight 2017, 2. [Google Scholar]
- Kaliyaperumal, S.; Watkins, B.; Sharma, P.; Furlan, S.; Ramakrishnan, S.; Giver, C.; Garcia, A.; Courtney, C.; Knight, H.; Strobert, E.; Elder, E.; Crenshaw, T.; Blazar, B.R.; Waller, E.K.; Westmoreland, S. , Leslie S Kean. CD8-predominant T-cell CNS infiltration accompanies GVHD in primates and is improved with immunoprophylaxis. Blood. 2014, 123, 1967–9. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.G. , Edwin P Alyea 3rd, Patrick Y Wen, Umberto Degirolami, Santosh Kesari. Graft-versus-host disease of the CNS after allogeneic bone marrow transplantation, J. Clin. Oncol. 2009, 27. [Google Scholar]
- Goddard, C.A.; Butts, D.A. , Carla J Shatz. Regulation of CNS synapses by neuronal MHC class I. Proc. Natl. Acad. Sci. USA 2007, 104. [Google Scholar] [CrossRef]
- Cebrián, C.; Zucca, F.A.; Mauri, P.; Steinbeck, J.A.; Studer, L.; Scherzer, C.R.; Kanter, E.; Budhu, S.; Mandelbaum, J.; Vonsattel, J.P.; Zecca, L.; Loike, J.D.; Sulzer, D. , MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat. Commun. 2014, 5, 3633. [Google Scholar] [CrossRef]
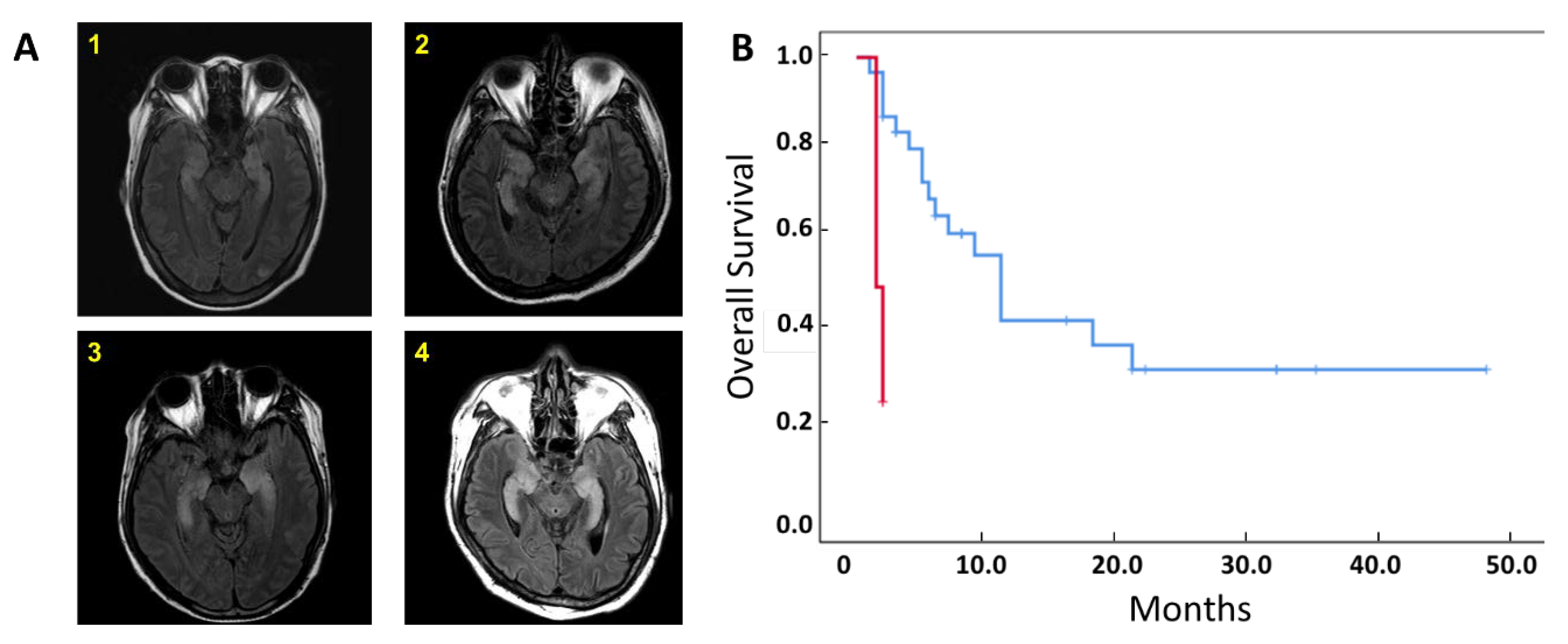


| Limbic Encephalitis | All patients (N=35) | P value | Patients with Treg analysis (N=13) | P value | ||
|---|---|---|---|---|---|---|
| Patients with LE (N=4) | Patients without LE (N=31) | Patients with LE (N=3) | Patients without LE (N=10) | |||
| Age, median (range) | 54 (28-60) | 58 (42-72) | 0.429* | 56 (52-60) | 60 (42-72) | 0.533* |
| Gender, M:F | 3, 1 | 14, 17 | 0.261§ | 2, 1 | 4, 6 | 0.453§ |
| Disease AML ALL MDS |
2 (50%) 2 (50%) 0 (0.0%) |
19 (61.3%) 5 (16.1%) 7 (22.6%) |
0.222§ |
2 (66.7%) 1 (33.3%) 0 (0.0%) |
6 (60%) 2 (20%) 2 (20%) |
0.562§ |
| Poor Risk status♣ | 4 (100%) | 18 (58.1%) | 0.102§ | 3 (100%) | 7 (70%) | 0.290§ |
| Disease status 1st CR 2nd CR MDS Refractory |
4 (100%) 0 (0.0%) 0 (0.0%) 0 (0.0%) |
19 (61.3%) 4 (12.9%) 7 (22.6%) 1 (3.2%) |
0.502§ |
3(100%) 0 (0.0%) 0 (0.0%) 0 (0.0%) |
5 (50%) 2 (20%) 2 (20%) 1 (10%) |
0.466§ |
| Conditioning MAC RIC |
2 (50%) 2 (50%) |
17 (57.8%) 14 (45.2%) |
0.855§ |
1 (33.3%) 2 (66.7%) |
6 (60%) 4 (40%) |
0.453§ |
| HCT-CI 0 1 2 3- |
4 (100%) 0 (0.0%) 0 (0.0%) 0 (0.0%) |
15 (48.4%) 10 (32.3%) 2 (6.5%) 4 (12.9%) |
0.433§ |
3 (100%) 0 (0.0%) 0 (0.0%) 0 (0.0%) |
6 (60%) 3 (30%) 0 (0.0%) 1 (10%) |
0.370§ |
| BMI | 28 (23.4 – 29.4) | 22.9 (18-27.4) | 0.070* | 26.9 (23.4-29.1) | 23.7 (18-32) | 0.197* |
| Donor Gender, M;F | 4, 0 | 24, 7 | 0.288§ | 3, 0 | 8, 2 | 0.377§ |
| Donor Age | 40 (21-60) | 37.5 (50-55) | 0.477* | 28 (21-52) | 38 (20-55) | 0.559* |
| TNC (x 108 cells/kg) | 12.5 (9.9-14.7) | 11.6 (5.9-20.4) | 0.858* | 12.4 (9.9-12.6) | 10.9 (5.9-20.4) | 0.897* |
| CD3+cell (x108 cells/kg) | 3.2 (1.7-5.1) | 2.4 (1.3-4.9) | 0.903* | 3.0 (1.7-3.3) | 2.2 (1.0-4.9) | 0.837* |
| CD34+cell (x106 cells/kg) | 5.2 (2.2-9.6) | 6.7 (4.8-17.7) | 0.081* | 5.9 (4.5-9.6) | 6.8 (4.8-17.7) | 0.612* |
| CRS grade, median (range) | 2 (2-3) | 1 (0-1) | 0.004§ | 2 (2-2) | 1 (0-1) | 0.004§ |
| VOD | 2 (50%) | 3 (9.7%) | 0.030§ | 1 (33.3%) | 0 (0%) | 0.026§ |
| CMV reactivation | 2 (50%) | 24 (77.4%) | 0.238§ | 2 (66.7%) | 9 (90%) | 0.201§ |
| F/U duration, mo Median (range) |
1.7 (1.5-2.0) | 5 (1 – 16) | 0.096* | 2.0 (1.5-2.0) | 5.5 (1.0-16.0) | 0.089* |
| Patients Number |
Age | Sex | Disease | Initial manifestation |
Day of LE diagnosis after HSCT |
HHV-6 status | Grade of CRS | CSF analysis | EEG | Hepatic VOD |
Grade of Acute GVHD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | F | AML | Seizure | 33 | Negative | 2 | Not done | Not done | No | 3 |
| 2 | 60 | M | AML | Seizure | 26 | Negative | 2 | Not done | Epileptic wave from right anterior temporal lobe | Yes | 4 |
| 3 | 28 | M | ALL | Seizure | 15 | Negative | 3 | WBC < 5/µL | Slow-wave activity with low to medium voltage | Yes | 4 |
| 4 | 56 | M | ALL | Seizure | 24 | Negative | 2 | WBC < 5/µL | Slow-wave activity with low to medium voltage | No | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





