1. Introduction
It is now more than two hundred years since Charles Badham used the term bronchitis to describe
“an inflammatory affection of the mucus membrane which lines the bronchial tubes” [
1,
2,
3] This was not a new disease but inventing a new name was a signal that the thinking about pulmonary disease was starting to better understand the implications of acute and chronic inflammation of the airways that accompanied most diseases of the ‘air sacs’ (alveoli) and pleura as well as being common in its own right. Well into the C21st chronic inflammation of the airway remains the cause of a substantial burden of morbidity and is amongst the highest causes of deaths worldwide [
4,
5]. Despite the intervening two centuries, progress in treating and preventing the consequences of a ‘chronic bronchitis’ has been, at best, disappointing. In the middle of the C20th the current situation was summed up by
Southwell who noted ‘
No one can pretend that the treatment of chronic bronchitis is anything but profoundly unsatisfactory’ [
6] and
Goodman who noted that
‘For many years this crippling disease (chronic bronchitis
) has been complacently accepted’. [
7] Seventy years later a recent editorial in the Lancet (2022) noted
‘Chronic obstructive pulmonary disease (COPD) has for too long been seen as a self-inflicted progressive disorder of smokers towards the end of life with few treatment options beyond symptom control. There has been no major progress in treatment or prevention for decades’ [
8].
The author of that editorial also noted that the current situation in which a
‘diagnosis of COPD is often accompanied by a sense of futility and a degree of stigma’ [
8] should not be acceptable and that our goal in the C21st should be lifelong respiratory health for all. We believe, and argue in this paper, that the diseases of the airways currently described as distinct and unrelated entities (e.g. ‘COPD’, ‘emphysema’, ‘chronic bronchitis’, ‘bronchiectasis’) are in fact a continuum resulting from chronic inflammation of the lower airways, the two great drivers of chronic inflammation being inhaled toxins and a chronic bacterial bronchitis (dysbiosis or the respiratory microbiome). The current siloed approach has effectively sidelined the role that chronic microbial induced neutrophilic inflammation plays in the symptoms and progressive pathology experienced by so many patients. It is unfortunate that, a time when we are developing a much better understanding of what constitutes a chronic bacterial infection and the associated dysbiosis of the pulmonary microbiome, many are looking to deal with the problem through ‘anti-inflammatory’ approaches that do not address the underlying drivers of inflammation. In order to achieve improved respiratory health we would argue that, in addition to reducing the burden of toxic agents inhaled by individuals through smoking cessation and addressing the many forms of atmospheric pollutants that challenge airways homeostasis, it is vital to develop effective treatment strategies that will deal with the highly developed defence mechanisms utilised by bacteria such as non-typable haemophilus influenzae (NTHi) to persist in the face of threats such as antibiotics. Closer cooperation between respiratory clinicians and microbiologists who have an interest in bacteria strategies such as the formation of biofilms, intracellular bacterial communities, persister bacteria, and disturbed microbiota offers the potential to take real strides towards prevention and mitigation of chronic bacterial infections of the airways.
Two hundred years ago Andral, in his preface to the 4th Edition of Laennec’s remarkable text [
9], noted ‘Medical history is replete with the mistakes of those who do not wish to learn for themselves; those who ignore the past; and those who accept doctrines without critical examination’. In this review we aim to highlight the important lessons of the past and challenge currently accepted doctrines while drawing on lessons learnt about chronic bacterial bronchitis in children during the past couples of decades.
2. Lessons from history
This section will contain a brief summary of the evolution of our thinking regarding airways disease over the past 200 years.
2.1. Most of the pathology had been described by the mid C19th and this included an understanding of the impact of inflammation on different regions of the conducting airways and respiratory zone
The late C18th and early C19th century was a time of great progress in medicine led by some outstanding clinicians undertaking postmortems on patients who had died in their care. Shortly after Badham coined the word bronchitis Laennec invented the stethoscope (1816) and then, in 1819, published his great work which included descriptions of bronchiectasis, obliteration of small airways (bronchioles) and emphysema amongst other pathologies [
9]. He noted that these three types of pathologies commonly coexisted and that bronchial catarrh (mucoid/purulent secretions) were almost universally present. The term chronic bronchitis was well accepted by the time Stokes published his textbook on disease of the chest in the early 1840’s [
10]. He considered bronchitis to be the most important disease of lungs noting
‘we find that bronchitis is present and has a most important share in almost all diseases of the lungs, whether acute or chronic.’ He, as did other authors, noted that acute bronchitis was generally most severe in the young and that chronic bronchitis generally resulted from an unresolved acute bronchiti. In adults, an acute bronchitis was most problematic in those with chronic respiratory symptoms being manifest as episodes of more severe symptoms that could be fatal on a background of chronic cough and sputum production. He noted that a patient’s secretions (catarrh) could range from transparent mucous to puriform and could change over time. Many appeared relatively well despite cough and expectoration which frequently largely abated in the summer becoming more troublesome again in the winter. With time the relative remissions diminish and the cough and sputum become permanent. He noted that such a natural progression generally ended in ‘dilation of the tubes’.
Crucially Stokes clearly understood the different susceptibilities of the conducting airways with mucous gland hypertrophy and dilation of larger cartilaginous bronchi (bronchiectaisis) with obliteration of the smaller ‘non-cartilage baring bronchi’ or bronchioles (an obliterative bronchiolitis) and associated dilation of ‘air sacs’ (emphysema), again noting that these finding were not exclusive but commonly found together. Obliteration of larger airways was also noted on occasions but far less commonly than bronchiectasis. These observations were again made in the 1950’s by L. Reid and others in their studies on patients with chronic bronchitis and ‘bronchiectasis’ [
10,
11,
12,
13,
14,
15,
16,
17] but are all too often forgotten. As noted by Walsh in his 1851 textbook [
18] the term ‘exacerbation’ was already in use for a severe ‘acute attack’ and he noted the link between disease activity and general wellbeing noting that he has known
‘as much weight lost during the first three weeks of an annual recurrence of chronic bronchitis as in the same period of cases of consumption in active progress’ It should be noted that these observations were made long before the era during which cigarette smoking became common. Infections and pollution (indoor and outdoor) largely due to burning coal were the dominant drivers of these ubiquitous airways diseases [
3]. The link between loss of airways smooth muscle homeostasis and asthma [
10,
11,
12,
13,
14,
15,
16,
17] had also been made by this time accurately marking out asthma as separate disease to those being considered in this review (though poorly controlled asthma certainly predisposes to the development of a persistent bacterialbronchitis). Walshe also highlighted the link between inhaled allergens such as grass pollen with hayfever and seasonal asthma [
18].
2.2. Germ theory, pneumonia and outcomes
While morbid anatomy and histology had greatly advanced our understanding of the consequences of airways disease clinicians were still struggling to understand the causes of these diseases and how to treat them.
The idea that ‘germs’ could cause disease was not invented in the second half of the C18th but gained strength and eventually became irresistible during this period through the works of many scientists including Pasteur and Koch [
21,
22]. Streptococcus pneumonia (Str Pn) was identified in1881 and had conclusively been linked with lobar (alveolar) pneumonia within a few years [
23].
It is of note that studies from the early C20th, that is in the pre-antibiotic period, ‘bronchopneumonia’ was significantly more common than alveolar pneumonia and had a significantly greater mortality. In a large paediatric study, the mortality from bronchopneumonia was 54% vs 7% in those with an ‘alveolar’ pneumonia [
24] (alveolar pneumonia was used rather than lobar as it frequently did not affect the whole of a lobe [
24]). Mortality in those with empyema was 40.5%. Most deaths in this era were in the very young and those in later life [
3,
24]. In another study, considering the impact of weather on deaths under 5 years of age it was noted that death rates due to ‘bronchitis’ were significantly higher than that for pneumonia [
25]. Presumably both studies included many infants with what we would now describe as having acute bronchiolitis [
26] while the high rate of deaths in the older population was attributed to exacerbations of chronic bronchitis [
3]. However, it is important to remember that Str Pn infections are a particular problem in infants [
23] while a study published in the pre-antibiotic found H Inf was isolated from the lower airways of 95% of children dying from bronchopneumonia [
27].
Currently the vast majority of children with pneumonia who are treated with antibiotics (usually orally) are expected to make a full recovery. However, a systematic review found high levels morbidity following a diagnosis of ‘pneumonia’ when studies published between 1970 and 2011 were considered [
28]. Of those hospitalised without a pathogen being identified 17.6% had evidence of restrictive lung disease and 4.2% developed chronic bronchitis. Few follow studies have considered chronic respiratory symptoms but in one that did the incidence of chronic cough in children with radiologically proven pneumonia was three times that in the control population [
29].
In the pre-antibiotic era, levels of respiratory morbidity following lower respiratory tract infections were much higher [
30,
31]. A follow up study of children admitted with an illness consistent will pneumonia found 25% were still experiencing chronic respiratory symptoms consistent with ‘chronic bronchitis’ some years later [
30]. In a Canadian study of army personnel with radiologically proven pneumonic illness Andrus [
111] noted that patients generally recovered rapidly with CXR changes resolving by three weeks
or they developed a cough and other symptoms which might take some time to resolve or fail to resolve at all. Respiratory illnesses were common in this population due to their confined living conditions in barracks. Those with on-going symptoms usually had residual had CXR changes and he noted that
‘Such changes which are variously named chronic infectious basal disease, chronic nontuberculous disease, chronic post-pneumonic disease or injury, chronic pneumonitis, pulmonectasis, etc.
etc., may
be of any intensity, from slight to gross’. As well as again highlighting the numerous terms used to describe a given condition, he also noted that bronchiectasis may or may not be present if sought in such patients and that its present was not predictable by clinical or radiological changes. He also noted that the majority in whom symptoms persisted had localised CXR prior to the acute illness and that chronic respiratory symptoms was the most common reason for medical discharge from the army.
The historical evidence from these and other studies clearly indicates that a significant proportion of those who had a pneumonic illness in pre-antibiotic days had ongoing respiratory symptoms and this was an issue in both children and healthy adults. It was also known that whooping cough and measles were two other precursors to chronic symptoms (with some deeveloping radiological change of bronchiectasis). However, in most cases irrespective of age ‘chronic bronchitis’ (cough and sputum) was considered to be a consequence of an unresolved acute bronchitis [
6].
2.2. Germ theory and chronic bacterial bronchitis.
In the early years of the C20th the ‘usual suspects’ (Str Pn, haemohilus influenzae (HI) Moraxella catarrhalis (MC)) that are still primarily associated with chronic bacterial bronchitis had been identified in those with chronic airways diseases [
33,
34,
35]. (Pseudomonas aeruginosa was not linked to CF and other advanced respiratory disease until half a century later [
36]). Reports of using vaccines prepared from sputum containing these organisms to treat those with chronic bronchitis and asthma appeared before the first world war [
37,
38] and this approach has become a recurring theme in the subsequent 100 or so years [
32,
39,
40,
41,
42,
43]. The interest in vaccines developing so soon after the link between chronic bronchitis and bacteria had been established is understandable in that there were no effective treatments (the development of antibiotics were decades in the future) and there was a history of vaccines being able to prevent disease going back to smallpox vaccines. In the case of those with a chronic bronchitis it was hoped that the vaccines might also help treat those affected. A number of reports but not all suggested some efficacy. The hope that chronic bacterial respiratory disease can be treated with ‘vaccines’ persists to this day though with little success to date. A further flurry of studies in the 1950’s confirmed the importance of these pathogens in chronic bronchitis [
44,
45,
46,
47,
48,
49] and a regular procession of studies since [
50,
51] then have all confirmed the observations made more than a hundred years ago.
2.3. Bronchiectasis becomes a ‘disease’ but it is recognised that chronic bronchitis and bronchiectasis generally cannot be distinguished clinically
Many authorities in the late C19th and early C20th commented on the difficulty in identifying patients with bronchiectasis in life. They noted that in advanced ‘classical’ cases the patients appeared unwell, produced copious purulent secretions, were wasted and clubbing and that these patients had a very poor prognosis. These patients generally died of bronchopneumonia, ‘exhaustion’, toxaemia, massive haemoptisis or cerebral abscess. However, it was clear from post-mortem examinations that many had much milder disease with symptoms indistinguishable from the majority of patients with a chronic bronchitis and indeed could be present in the absence of any symptoms. While the duration between apparent onset and death could be relatively short, in others the development of bronchiectasis was considered to be associated with a chronic disease that might cause symptoms for decades [
52,
53,
54,
55,
56,
57,
58]. A 1920 report noted that 2% of admissions to the Brompton Hospitals were reported to have bronchiectasis but this was probably a gross underestimate as it was always a consequence of other disease processes [
53]. Chronic bronchitis, unresolved pneumonia, whooping cough, measles and airways obstruction by tumour or foreign body were reported as being by far the commonest causes in those who did not have tuberculosis. McNeil and colleagues reviewing what the field in 1929 noted
‘From all these data, it would seem that the majority (probably the great majority) of cases of bronchiectasis date back to early childhood and originate in broncho-pneumonia or bronchitis’ [
55].
The advent of bronchograms in the 1920’s led to a renewed interest in this form of pathological change [
55,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73,
74] as doctors feel much more comfortable when they have a test rather than having to rely on their clinical acumen. Having this test completed the term ‘bronchiectasis’’s move from a pathological description to becoming a ‘disease’ even though it was universally considered to be the visible consequence of another disease. Many bronchograms were carried out to produce a ‘definitive’ diagnosis but the results had real clinical consequences. In the absence of other effective treatments other than postural drainage and change of climate, surgical resection of affected lobes in those particularly problematic symptoms became the treatment of choice. Bronchograms were required to identify diseased areas of the lung that could be resected. Roles & Todd noted in 1933 [
57] that the textbooks of the time suggest that
‘it is a condition of almost hopeless prognosis in which the patient has fouls sputum, clubbed fingers and signs of basal cavitation. We find it rarely conceded that there is a possibility of an earlier stage in which the diagnosis can be made and in which curative treatment is practicable’. They went on to describe the clinical features and outcome in 106 patients with confirmed bronchiectasis. Twenty-two had symptomless or ‘dry’ bronchiectasis, ten ‘simple’ bronchiectasis with only occasional sputum and 74 had septic or fetid sputum. However, they noted the disease progressed in most if treated medically with ten of 14 ‘dry’ patients becoming infected within 6 years of whom 3 were dead and 2 incapacitated. Of the 49 in their series treated medically 23 died within five years nine were totally incapacitated and only four were ‘dry’. They concluded that
‘lobectomy has proved to the greatest advance in the treatment of this condition, but depends upon diagnosis with certainty at an early age and experience attention to pre and post-operative measures’.
As the use of bronchography increased a number of features of chronic respiratory disease were clarified and by the 1950’s it was clear that: -
- (a)
‘Dilation of the bronchi does not always produce signs or symptoms’ [
54] ‘Dry’ bronchiectasis was well recognised and often diagnosed in asymptomatic patients presenting with haemoptysis [
54,
57,
62]
- (b)
Clinical symptoms alone could not distinguish between those with and without bronchiectasis. Many believed to have bronchiectasis had negative bronchograms (see below) while identifying bronchiectasis in those with simple ‘chronic bronchitis’ was not uncommon. In a study of service personnel undertaken at military hospital in which bronchogram were performed on 214 service personnel referred with ‘symptoms and signs suggestive of chronic bronchitis’ 46 (21%) proved to have evidence of bronchiectasis when a bronchogram was undertaken [
68]. The authors noted that such patients had often been labelled as having ‘simple bronchiectasis’ by other authors and note that ‘Such cases are rarely seen in civilian hospitals, because under ordinary conditions they have no difficulty in carrying on their work, the annual bouts of winter bronchitis being regarded as inevitable and lightly dismissed.’
- (b)
Following a bronchogram a patient who regularly produced sputum may be labelled as having chronic bronchitis with bronchiectasis (usually abbreviated to ‘bronchiectasis’) or chronic bronchitis (without bronchiectasis).
- (c)
Studies demonstrated that children and adults with symptomatic chronic bronchitis and a negative bronchograms might develop unequivocal bronchiectasis when re-examined a few years later giving rise to the term pre-bronchiectasis as an alternative to chronic bronchitis [
70,
72,
76]. In one follow-up study undertaken prior to antibiotic therapy, 40% pf children with ‘pre-bronchiectasis’ based on having symptoms but were found to have developed bronchiectasis three years later while 37% were ‘cured’ or rather had become symptom free [
70]. However, while a valuable concept in that highlights the opportunity for prevention, pre-bronchiectasis can only be applied accurately in retrospect.
- (d)
Bronchiectasis was commonly localised to certain lobes or segments of a lobe especially in early disease. Changes were not static and over time other lobes in both the ipsilateral and contralateral lung frequently became involved. This suggests that atmospheric pollution alone was unlikely to be responsible given that deposition would be widespread. Rather it strongly favours bacterial infection as the primary driver of the damaging inflammation though persistent and/or recurrent infection in a given area was likely to have been secondary to causes of impaired mucociliary clearance.
- (e)
Bronchiectasis was not necessarily ‘irreversible’ with reports of it resolving in adults and children at least in the early stages [
52,
70,
77,
78,
79] and such reports continue to appear [
80,
81].
- (f)
The majority of those affected appeared to have first developed symptoms in childhood most commonly in the first two years of life [
55,
66,
82,
83]. A community-based study noted 44% of those identified with bronchiectasis had been symptomatic before the age of 10 years but most had not come under medical until their 4th decade with a median delay between onset of symptoms and referral to secondary care of more than 17 years [
83]. Even in the post antibiotic era studies are producing similar results [
84].
- (g)
Typically, it followed chronic symptoms persisting after an acute lower respiratory tract infections such as acute bronchitis, pneumonia, whooping cough, measles and TB [
10,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73,
74,
75,
76,
77,
78,
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89,
90,
91]. It should be noted that in 1947 the most common cause for death amongst infants after congenital abnormalities and prematurity were ‘respiratory diseases’ and for those aged 1 -4 years the top 5 were pneumonia TB violence whooping cough and measles [
92]
- (h)
Surgical resection of affected lobes remained the treatment of choice for many well into the antibiotic era [
93].
- (i)
Follow up studies of children with significant bronchiectasis indicated identified that the majority improved clinically in their teens and twenties with deterioration being uncommon [
94,
95]. Longer term follow-up into middle age were not pursued but it is likely that poor recall of childhood events accounts for many cases of ‘idiopathic’ bronchiectasis presenting in middle age [
83]
- (j)
In terms of pathology there have been and are on-going debates about the processes leading to dilation of the medium to large bronchi and obliteration of bronchioles. From Laennec [
9] onwards inflammation associated with chronic pulmonary catarrh or chronic bronchitis was believed to be a key component though by the end of the C19th ‘traction’ due to adjacent collapse was also considered by some to be a factor in many cases. Bacterial infection driving inflammation was implicated in the early C20th and the idea of a vicious circle of impaired clearance, infection inflammation and damage was commonly held to be a key component by the mid C20th [
96,
97] recognising that the circle of infection, inflammation, damage predisposing to infection etc applied to chronic bronchitis and did not necessarily result in bronchiectasis. One publication from the 1950’s noted ‘Nowadays the divergence of opinion seems to be occupational, for, while many physicians and radiologists think of the bronchial inflammatory changes as mild and the dilatations of a mechanical nature, most surgeons and pathologists regard bronchiectasis as a destructive inflammatory process’ [
98]. The later, like Laennec, presumably had the advantage of seeing the condition in front of them while the physicians and radiologists could only speculate from afar. In earlier publications the existence of a vicious circle was assumed to be involved in the pathogenesis of chronic bacterial bronchitis and could lead to bronchiectasis in some. Following later iterations of the concept the ‘vicious circle’ became linked to ‘bronchiectasis’ [
99,
100] and the importance of this cycle prior to the development of bronchiectasis and ‘fibrosis’/obliteration of bronchioles came to be forgotten though this was never the intention of Cole.
The relatively large number of papers regarding bronchiectasis contrasted with the relatively lack of interest in chronic bronchitis illustrating the impact of a ‘test’ on priorities and medical thinking. Those with bronchiectasis might benefit from surgery, those without were offered cough medicine until such time they developed bronchiectasis. This seems to sum up current approaches to management and prevention if one changes antibiotics for surgery.
2.4. Cleaner air, smoking and improving living standards
By the 1950’s two of the key drivers of chronic airways disease, inhaled atmospheric pollutants and infections were being addressed. The indoor and outdoor pollution in urban areas had been falling for some time, partly due to poorly enforced legislation and partly due to the increasing use of electricity moving some coal burning to more rurally located power stations. Countering this to a significant degree was the rise of cigarette smoking which peaked at around 70% of men in 1960. The huge step change however was the advent of antibiotics.
2.4.1. Cleaner air
Atmospheric pollution in the industrialised cities of the UK peaked in the 1880’s and had fallen significantly over the subsequent 60 years [
3,
101,
102]. The great London smog of December 1952 which killed over 4,000 individuals in a four-day [
103] period and led to a long tail of excess deaths and increased morbidity was the last of the notorious London smogs. During these events a combination of burning cheap poor-quality coal in domestic properties (nuttyslack), industrial use of coal and climatic conditions combined to produce a toxic mix of particulates and gases such as sulphur dioxide. These had been particularly prevalent in Victorian London (London was commonly referred to as the ‘Big Smoke’ by the 1870’s) but their frequency and intensity had significantly decreased through the first half of the C20th paralleling the progressive fall in atmospheric levels of pollutants. This fall in pollution, mostly from the combustion of coal, was paralleled by a progressive fall in deaths from acute and chronic bronchitis, at least until the 1940’s [
3] which was in complete contrast to the rise of the in cigarette smoking and the parallel increase in lung cancer (which lagged approx. 20 years behind smoking rates). While the smogs of London received much attention the issue of atmospheric pollution was an issue in all major cities in the UK Europe and North America with a number of events similar to the London smogs being reported in Europe and Europe [
102].
The 1956 Clean air act in the U.K. mandating smokeless fuels in urban area and similar legislation in other industrial countries led to further improvements in air quality further reducing one of the great drivers of chronic airways disease [
102]. The number of vehicles burning fossil fuels was far lower than the case today though they churned out significantly more pollutants per vehicle than is the case today. More recently indoor and outdoor pollution has again received more attention both because of the burning of fossil fuels by vehicles in Cities and the use of fossil and biofuels for cooking and heating in much of the developing world.
2.4.2. Smoking
The 1950’s saw attention moving away from atmospheric pollution towards consideration of the respiratory effects of cigarette smoking and from the 1960’s on it appeared to become, for so many, the only show in town. However, it is prudent to reflect that lung cancer was a very rare disease before the widespread adoption of cigarette smoking chronic bronchitis, bronchiectasis and emphysema were reported to be very common throughout the C19th. A 1955 survey of more than 5,800 UK Civil Servants found, not surprisingly, that the majority smoked but the rates of chronic bronchitis, which was usually mild, in men aged 40-65 was 18.4% in the smokers and 15.8% in non-smokers [
104]. Importantly it is still the case that a significant proportion of those with COPD have not smoked while amongst those with COPD an associated chronic bronchitis is associated with more frequent exacerbations and a worse prognosis [
5]. Hence while smoking is very important in driving chronic respiratory ill health it is not the only factor.
The rates of smoking in Westernised countries rose progressively from the late 1880’s, when machine production of cheap cigarettes commenced [
105], peaking in the 1950’s. In the UK for example some 70-80% of the male population in the UK in in the 1950’s were smoking (not helped by the services issuing free cigarettes to servicemen in two world wars) [
3,
106]. The link between smoking and lung cancer had been made in the 1930’s by German physicians and indeed had been proposed by some much earlier [
107,
108]. The rates of lung cancer, which had been uncommon, rose in parallel with rates of smoking all be it with a 20 year or so delay. That there was a direct link between cigarette smoking and rates of lung cancer was established at the start of the 1950’s [
109,
110,
111,
112,
113]. A decade later the Royal College of Physicians in the UK [
114] and The Surgeon General in the USA [
115] produced statements that effectively put an end to debate about the validity of these studies.
Establishing this link spurred interest in the role of cigarette smoking in the causation of the most common chronic respiratory diseases. The decline in deaths due to chronic bronchitis in men noted in the early decades of the C20th which contrasted to rising rates of cigarette smoking and lung cancer was reversed from the late 1930’s and was rising. Interestingly the incidence of chronic bronchitis deaths is reported to have peaked in 1960 and then declined progressively. This decline was apparently in direct parallel with the decline in smoking rates which for men and women is now in the low to mid-teens as a percentage of the population. The increase in reported chronic bronchitis deaths in men reported from the late 1930’s to early 1960’s was apparently not seen in women, and this was attributed to both lower rates in women and lower consumption amongst those who smoked compared with men [
3]. The 1964 Surgeon General’s report was very clear that smoking was a major, but far from only cause of chronic bronchitis based on a large number of studies undertaken in the 1950’s [
115]. Over time the link made between smoking and chronic bronchitis [
115] was replaced by a focus on smoking and the development of irreversible impairment of airways function (COPD) to the exclusion of a chronic bronchitis.
Smoking prevention and cessation became to be viewed by many as the one intervention that could impact on both prevention and the progression of established respiratory disease. The fall in smoking rates in much of Europe and North America had a dramatic effect on lung cancer rates [
108], especially in middle age, and on deaths due to ischaemic hear disease [
118]. As with atmospheric pollution, those working in public health have had a far greater impact on respiratory health than physicians.
2.4.3. Improved housing, standards of living and vaccines
Studies in the 1950’s confirmed that the morbidity and mortality associated with chronic bronchitis were particularly high in the less affluent and more industrialised regions and inversely related to social class [
115,
116,
117,
118,
119,
120,
121,
122,
123,
124,
125,
126] thus appearing to represent important risk factors in their own right. In the UK, airways disease was even more prevalent in more Northern industrialised towns than in London and this appeared to relate to their greater prevalence of poverty, overcrowding and poor housing. The impact of social class, while still present, appeared to be less pronounced in the USA than the UK. Socio-economic factors that play such a big role in an individual’s health such as deprivation, overcrowding and sanitation all started to improve incrementally through the post war years.
Other key events in the UK leading to improvements in respiratory were the advent of a National Health service that permitted much wider access to medical care and the start of structured immunisation programmes following the effectiveness of vaccines in helping to deal with the polio epidemics of the time. The widespread introduction of vaccines over the following decades against whooping cough and measles [
127], amongst others, largely removed two of the well-recognised antecedents to developing a chronic bacterial bronchitis in childhood.
2.5. Antibiotics and chronic bronchitis’s moment in the sun
However, the greatest step change in respiratory health was the development of antibiotics [
128,
129]. At last, clinicians had an opportunity to effectively treat the bacterial pathogens that knew so well but about which they could do little. The extraordinary impact of these ‘magic bullets’ is often forgotten. Their impact treating TB and acute life-threatening infections including pneumonia was nothing less than miraculous. An illustration of the dramatic impact of sulphonomides, then penicillin and other antibiotics on the survival of children with pneumonia and the prevention of progression to bronchopneumonia and chronic symptoms was contained in a 1952 publication from Milwakee [
130]. The increasingly widespread use of antibiotics over the following decades had a dramatic effect on the incidence of chronic bronchitis in children, which in the 1940’s had been described as a very common condition [
131], to the point where few respiratory physicians believed it existed. Antibiotics also transformed the lives of children with bronchiectasis led to dramatic declines in the number of admissions to children’s hospitals, in some cases tenfold reductions in just a few years. As noted by Kasowitz et al [
130] as early as 1952, not only did the number of admissions with bronchiectasis fall dramatically so did its apparent incidence [
98,
130]. Withing a couple of decades the incidence had fallen so far that most paediatric and adult physicians considered it to be an ‘orphan disease’ encountered rarely and of little importance in the overall scheme of health-related problems [
132,
133]. This fall in incidence and prevalence was not because of the effect of antibiotics on those with bronchiectasis but almost certainly due to their effect on the health of individuals given antibiotics for an acute bronchitis – it is likely that the widespread use of antibiotics in primary care prevented many with a viral bronchitis and pneumonia developing a superadded persistent or chronic bacterial bronchitis which was the route into chronic poor respiratory health for so many.
The hope that antibiotics would transform the lives of those with chronic bronchitis, given the known ubiquitous presence of bacterial pathogens in sputum, suddenly provided clinicians with hope that they would have an effective therapy that went beyond a bottle of cough medicine. This led to a renewed interest in the microbiology associated with chronic and recurrent airways disease which confirmed earlier work regarding the importance of the ‘usual suspects’ and in particular that of Strep Pn and H Inf [
44,
45,
46,
47,
48,
49], That H Inf was identified in up to 90% of sputum samples from those with chronic airways disease led to further trials of H Inf vaccines but seemed to have little impact [
134]. Hers & Mulder demonstrated that H Inf, unlike viruses such as influenza and measles did not cause significant epithelial damage and were often identified between epithelial cells or even below the epithelial layer [
135]. They also noted that despite the lack of evident damage caused by the bacteria there was an associated inflammatory response. They speculated that viruses provided the opportunity for H Inf. to establish a chronic colonisation/infection.
Early studies noted the beneficial effects or short- and long-term antibiotics when used to treat the chronic symptoms of both bronchiectasis and chronic bronchitis and prevent and treat exacerbations [
136,
137,
138,
139,
140,
141,
142,
143,
144,
145,
146,
147,
148,
149,
150]. Investigators noted that it took two weeks or longer in most case for a significant improvement to be observed but that if there was no improvement by 4 weeks therapy should be discontinued, very similar to recent observations in children with so called PBB. However, it soon became apparent that recurrence of chronic symptoms and exacerbations were all too common on discontinuing the antibiotics, especially amongst those with non-typable haemophilus influenzae [
136,
137,
138,
142,
144,
145,
150] with the suggestion that the organism was able to persist despite antibiotic therapy [
150,
151] though investigators were uncertain of the mechanism. The idea of dormant ‘persister’ bacteria was well established by 1944 [
152], decades before the recognition of the role of biofilms and IBCs in human disease [
153,
154,
155,
156,
157,
158]. The recognition that acute illnesses generally involve more virulent rapidly dividing planktonic bacteria and that chronic infection with biofilms and occasional flare up with release of planktonic bacteria is still in its infancy in respiratory medicine.
Despite the potential for benefit, the push back against the use of antibiotics in chronic bronchitis commenced at an early stage. The realisation that in many/most cases the patient would not be cured led, understandably, to concerns regarding the potential impact on antibiotic resistance of repeated or long-term prescribing and suggestions that the cost of treating such a common chronic disease in this way would be too high for the fledgling health service [
159,
160]. It is perhaps worth noting that early studies found little resistance developing in the patients included in the studies, not too similar to the situation in PBB when the same antibiotic can often be used for prolonged periods without apparently losing its effectiveness. Studies from this period have subsequently been mis-interpreted as suggesting that antibiotics
, do not have a role in the treatment of most patients with chronic bronchitis/COPD other than brief exposure to antibiotics during ‘exacerbations’ re-reenforcing the perception that infection is not an important component. For example, the GOLD reports suggest that antibiotics for chronic bronchitis did not work based on two studies which wen published argued in favour of a positive effect [
148,
149]. For example, in one study said to show that antibiotics had no impact the study found a 50% reduction in days lost from work [
148]. A modern drug having such an impact would be hailed as a major breakthrough and promoted aggressively by the relevant pharma company.
These studies indicated that antibiotic therapy can indeed make a signficant impact but the extent of the benefit was limited by factors such as failing to understand the nature of biofilm diseases, including smokers in whom the benefit would be limited by the on-going inhalation of toxins (a greater effect is likely to be seen in ex-smokers with an on-going bacterial bronchitis) and the need to achieve high concentrations of the antibiotics in the diseased airways. Unfortunately, their results are still being misinterpreted as implying that antibiotics have no effect.
2.6. Emysema and dyspnoea
Laennec highlighted that emphysema was far more common in his post-mortem examinations than had been previously recognised and was common in patients with chronic bronchitis. To many prior to the mid C20th the difficulty breathing experienced by those with chronic bronchitis was simply a manifestation of the disease occurring at any age but particularly in the elderly –
‘Chronic bronchitis associated with emphysema, and in all respects like the disease common in old people, is by no means very uncommon in the young, that is to say, in patients under 20 years of age’ [
161]
. Now, if we admit that a man in a state of perfect health ought not to cough or expectorate, and also that expectoration implies catarrh, it follows that at no period in the progress of emphysema is that condition associated with catarrh’ [
162]. While most authorities in the UK, unlike North America, still considered the term emphysema a pathological description rather than a disease the move to becoming a disease came with increasing focus on the dyspnoea that developed over time in many with a chronic bronchitis. In the 1940’s it was noted that
“The diagnosis (emphysema) should be considered certain when dyspnea on exertion, of insidious onset, not due to bronchospasm, or left ventricular failure, appears in a patient who has some physical signs of emphysema together with chronic bronchitis and asthma” [
162]. Emphysema and obliteration or ‘fibrosis’ of bronchioles generally occur together but the gradual confabulating a pathological description of one aspect of the lungs with a disease characterised by chronic dyspnoea tend to obscure very important aspects of airways disease. Although the different pathologies had been described than 130 years earlier and which had again been highlighted and in studies through into the 1960’s the dichotomy of the breathless patient with ‘emphysema’ (pink puffers) and the patient with sputum production ‘chronic bronchitis’ who went on to develop right sided heart failure (blue bloaters) came to influence much of medical thinking for many decades.
The recognition that the airways obstruction most commonly seen in older individuals (the incidence rises significantly with age) was not an inevitable consequence of chronic bronchitis is highlighted by the evolution of definitions produced by Scaddding [
164]. In 1952 he had suggested.
"Chronic bronchitis refers to the condition of those patients suffering from chronic or recurrent cough, expectoration, and effort dyspnoea in whom these symptoms are not caused by diseases of the lungs, by localized disease of the bronchi, trachea, or upper respiratory tract, or by primary cardiovascular disease.". A decade later he considered chronic bronchitis to be
"a disease characterized by persistent cough and expectoration due to excessive mucus secretion in the bronchial mucosa’. (very similar to the current definition of PBB though without the implication that bacteria were driving the cough and mucous secretions). This reasonable minimalist definition, which does not aim to consider aetiology, is also notable for not having a minimum duration, was influenced by the histological studies of Reid and the recognition that dyspnoea was a late and not inevitable feature. He suggested this definition be used for the earlier
‘non-disabling phases’ with the term
‘chronic bronchitis with pulmonary insufficiency’ being used to label the disease of those in those with whom the chronic bronchitis who had, over time developed a significant functional impairment of the respirator system. In discussing the ‘jocular’ terms ‘pink puffer’ and ‘blue bloater’ that had crept into the UK medical terminology, he notes that the development of dyspnoea attributed to empysema without pre-existing chronic bronchitis was very uncommon [
164].
2.7. Clarifying disease definitions
As the 1950’s gave way to the 1960’s and hopes that antibiotics would rapidly cure those with chronic bronchitis were fading, the issue of diagnostic labels became more topical. The intention was to try and to improve diagnostic criteria and accuracy of diagnosis which would in turn help individual patients, improve the data generated in epidemiological studies and lead to clarity in improving therapy. A number of studies had demonstrated that then, as now, misdiagnosis of airways disease was rife such that a ‘doctor diagnosis’ of asthma or chronic bronchitis was commonly found to be incorrect when more detailed examination of a patient was undertaken. A doctor was expected to recognise a disease when he saw it but had little to guide his perception. Similarly, it was believed that one reason for the high prevalence of chronic bronchitits in the U.K. (the English Disease) when compared with other countries was differences in diagnostic labels fashionable at the time [
165,
166]. This was summed up in the introduction to the CIBA meeting attempting to improve the situation, a summary of which was published in 1959.
‘At present the diagnoses " chronic bronchitis," " asthma," and " emphysema " are used without any general agreement about the clinical conditions to which they refer. Anyone (or more) of these words may be used by different clinicians to describe the condition of the same patient. It appears that chronic bronchitis is often used in Great Britain to describe cases that would becalled asthma or emphysema in the United States’ [
166].
By the late 1950’s the field of respiratory medicine was changing rapidly with the advent of a number of effective therapies. The first pressurised meter dose inhaler (pMDI) containing adrenaline was replacing glass handheld single dose nebulisers administering adrenalin for the treatment of asthmatic bronchospasm [
167], the effectiveness of oral steroids in the treatment of asthma was being recognised [
168] and, as noted above, antibiotics had been shown to impact on the morbidity experienced by those with bronchiectasis and chronic bronchitis. The CIBA symposium aimed to produce robust clinical definitions that would help improve diagnostic accuracy and ensure patients would be prescribed the most appropriate treatment.
Their recommendations included the recommendation that the conditions being address formed a group of chronic non-specific lung diseases which manifest as 1) chronic or episodic excessive secretion of bronchial mucus (
chronic bronchitis) 2) intermittent obstruction to bronchial airflow (
asthma) 3) persistent obstruction of bronchial air flow
(irreversible or persistent obstructive lung disease) [
165].
Chronic bronchitis should be diagnosed clinically based on a history of a ‘chronic or recurrent cough with expectoration which is not attributable to conditions excluded from chronic non-specific lung disease. Infection of the bronchi is frequently but not necessarily present. Not infrequently subjects who produce sputum deny cough. Such subjects are included as having bronchitis. Subjects who habitually swallow sputum should also be included as having chronic bronchitis’. Thus, they were fully aware of the principle reasons that the condition frequently goes unrecognised and that there should not be artificial time criteria. Excluded conditions included the ‘localised lung diseases’ such as tuberculosis, pneumonia, ‘bronchiectasis’ and other conditions such as pneumoconiosis, collagen disease and psychoneurosis.
"Asthma refers to the condition of subjects with widespread narrowing of the bronchial airways, which changes its severity over short periods of time either spontaneously or under treatment and is not due to cardiovascular disease." This definition has stood the test of time and remains the basis of objective diagnosis. The lack of freely available spirometry in primary care has contributed to the high prevalence of over and underdiagnosis of asthma in many countries, a defect that is only slowly being addressed. Physicians would request a full blood count if concerned about anaemia but are happy to hand out an inhaler in the absence of objective confirmation of disease. Thus, asthma was defined as a disorder of function which may be exacerbated by factors such as allergies but that allergies were neither sufficient not necessary.
The CIBA symposium participants recognised that more than one feature could be present in an individual patient and the also recognised that emphysema was a pathological term and deliberately chose the term irreversible obstructive lung disease recognising this would include patients with emphysema when examined histologically and who had obliterated bronchioles.
Cognisant of the vagaries of clinical acumen, they also stated that lung function testing was essential and that his should include and assessment of bronchodilator responsiveness [
165]. Doctors like to have a test and feel on more certain ground when they have a test which will help make the diagnosis – few would make a firm diagnosis of anaemia (unless extreme) without having obtained a full blood count assessment. However, tests are not always enlightening. The adoption of bronchography provided doctors with confidence that a structural change had occurred in the airways of patients with a chronic bacterial bronchitis but the adoption of an artificial criteria for a new disease, ‘bronchiectasis’. masked the fact that this was a marker of damage not the underlying disease and did not necessarily correlate with disease activity or morbidity.
2.8. New ‘objective’ tests
Building on the work of others Hutchinson first described his spirometer in 1846 [
170]. By the 1930’s the bronchodilating effects of inhaled epinephrine had been demonstrated in asthmatics and the value of measuring FEV1 in addition to vital capacity was demonstrated a decade later [
170]. The rapid expansion of knowledge led to increased utilisation of lung function testing in respiratory centres providing more objective information on the identification of reversible airways obstruction (asthma) and irreversible obstructive lung disease (defined by CIBA as persisting for greater than a year). The term chronic constructive pulmonary disease (COPD) as an alternative for irreversible obstructive lung disease was coined in the USA in 1967 [
2] and slowly came to dominate the literature (COPD is American and rolls off the tongue better than IOLD) relating to chronic disease of the airways excluding ‘bronchiectasis’ and others listed in the CIBA symposium summary. Inevitably, given the criteria on which a diagnosis of COPD could be made was an arbitrary cut off defined by lung function testing, most of the therapeutic focus in this area has focused on trying to mitigate the effects of established impaired lung function. Treatment options were borrowed from ’reversible’ airways obstruction (asthma) and non-pharmaceutical interventions such as ‘pulmonary rehabilitation’ introduced but these have not been shown to have a significant impact on long term outcomes.
The 2021 GOLD report noted that ‘Spirometry is required to make the diagnosis and that a post-bronchodilator FEV1/FVC <0.7 confirms the presence of persistent airflow obstruction’ [
5]. Again, a test has hidden more than it has revealed. This focus on an arbitrary lung function defined cut-off meant that, as with ‘bronchiectasis’ symptomatic patients who had not demonstrated sufficient deterioration in lung function (or evidence of dilated bronchi on CT scans) are largely ignored despite symptoms that are frequently indistinguishable or worse that those with one of these two ‘diseases’ defined by an all or nothing test result. It is recognised that anaemia is a relatively late feature of iron deficiency anaemia but this does not prevent treatment of iron deficiency if identified and a search for the driver of that deficiency to prevent anaemia developing. This reliance on lung function and imaging to make an artificial clinical diagnosis has acted to obscure the disease leading to the structural damage identified in the ‘test’. Once sufficient damage has been caused and identified this ‘permits’ a clinician to become more proactive in their management as the patient then fits into a ‘guideline’. Unfortunately, this approach has impeded progress in preventing the airways disease from progressing to the point that patients meet these artificial thresholds.
2.9. ‘Chronic bronchitis’ becomes the Cinderella diagnosis in part because it did not have its own ‘test’ and a well-intentioned ATS initiative
As noted above the CIBA Foundation meeting generated a perfectly usable clinical definition of chronic bronchitis. A few years later the American Thoracic Society’s Committee on Diagnostic Standards for Nontuberculous Respiratory Diseases [
170] came up with definitions similar to that generated at the CIBA Symposium
but apparently introduced a time constraint on duration of symptoms prior to making a diagnosis of chronic bronchitis producing a definition that is widely quoted today – patients were required to have a
‘chronic productive cough for at least 3 months in two consecutive years’. The aim of this more stringent definition was presumably to try and limit inclusions to those who unequivocally had persistent symptoms for significant periods. Using this ‘definition’ a patient was only deemed to have developed ‘chronic bronchitis’ after a minimum of 15 months and indeed, in theory, they may have to wait 24 months for a diagnosis should they have two episodes separated by 18 months. A British Medical Research Council attempt to define chronic bronchitis in 1965 [
171] noted ‘
simple chronic bronchiti’s is defined as chronic or recurrent increase in the volume of mucoid bronchial secretion sufficient to cause expectoration’. Again they noted swallowing sputum habitually (a habit adopted by many, especially women) equated to expectoration. For the purposes of epidemiological studies, they noted for the purposes of comparing epidemiological studies the phrase chronic or recurrent should imply
‘expectoration has occurred in most days during at least three consecutive months for more than two successive years’. They noted two other manifestations frequently occur, bacterial infection and generalised airways obstruction which could occur separately or together.
Then along came COPD.
3. The continuous pulmonary airway from generation trachea to alveolus
Many make much of the ‘continuous airway’ by which they mean the overlap of disease in the upper and lower airways. It is certainly true that many with ‘hay fever’ and resultant allergic rhinitis will also have an allergic bronchitis with and associated cough though this is not asthma. If they happen to have asthma as well the allergic bronchitis may also destabilise the normal homeostatic control of ASM and trigger bronchospasm.
Biofilm diseases of the upper airways are common in the middle ear of children with chronic otitis media, which is a major cause of hearing impairment particularly in children, and older children and adults with chronic sinusitis [172.173]. Hence is not surprising that a similar condition can affect the lower airways [
173,
174,
175,
176]. The lungs, sinuses and middle ears all have mechanisms for trying to clear bacteria that do, on occasions, fail and certain bacteria are only too adept at taking advantage of these opportunities.
That a given insult can affect the whole lower airways from the trachea (generation 0) to the alveoli (generation 8 – 23 depending on the segment though most are closer pathways are closer to 23 generations [
177]) should not be matter for contention. Certainly, histological examination of the whole airway in those dying of these chronic respiratory diseases show changes across the whole airway. Nor should the fact that the impact on different zones within the contiguous lower airway differ depending on the structure of particular zones. The airways have two major zones: the tree like structure of the conducting airways providing the structure that permits the distal respiratory zone to have such a large surface area (equivalent to a tennis court in an adult) for gas exchange [
177,
178]. The former also protects the fragile respiratory zone (the distance between air and blood in an alveolus being only mm) by filtering out particulates and trapping pathogens which are subsequently removed by phagocytic cells or mucociliary clearance.
It is important to recognise that while the conducting airways are a continuum, their structure alters as one moves more peripherally and with this comes changes in the impact of damaging inflammation. The consequence is that the same driver of damage can cause bronchiectasis more centrally, loss of bronchioles distally and dilated alveoli. That there is overlap between those with ‘COPD’ and bronchiectasis for example should not be a surprise but should be expected when considering first principles.
Many of the histological studies referenced above noted that mucus gland and goblet cell hyperplasia with some inflammatory changes could exist without obvious damage to the epithelium though squamous metaplasia was more common than in healthy adults. With more advanced damage, commonly associated with mucopurulent secretions and bacteria, squamous metaplasia of the epithelium is more common together with destruction of elastic tissue and airways smooth muscle. In the most severe disease destruction of cartilage and dilation of the airways is observed.
Inflammation on the bronchioles (bronchiolitis) causes goblet cell metaplasia, increase mucus content, causing some obstruction and in time obliteration of the bronchioles occurs.
In the fragile respiratory zone beyond the terminal bronchioles, respiratory bronchioles and alveoli are frequently dilated with loss of alveolar walls (emphysema in its various forms) but can also be collapsed or filled with purulent secretions.
Currently a diagnosis of a ‘disease’ appears to be largely determined by the changes one can most readily demonstrate such as impaired lung function or dilated bronchi while ignoring the much larger group of symptomatic patients who have not yet demonstrated such gross changes.
3.1. Causes of chronic inflammation do not target particular airways generations but may affect certain regions more than others3.2. Particulates and gases –
That some drivers of inflammation target one area more than another is not surprising and again should inform our perceptual models. Tobacco smoke contains particulate and gaseous toxins as do pollutants derived from combustion of coal, petrol and the like. The gases and much of the submicronic/ultrafine particles (which make up a significant proportion of particulates from smoking) will predominantly deposit peripherally well beyond the bronchi [
178,
179,
180,
181,
182]. Because of the enormous cross-sectional area distally gas transfer beyond generation 16 is essentially through diffusion while sub-micronic particles deposit very effectively through Brownian motion. Those in the so called ‘respirable range’ will also deposit predominantly in the distal bronchioles and associated acinar [
179]. These particles, in the range 1-5mm, have a high chance of penetrating through the defences of the upper airways and depositing in the lungs. The ‘respirable range’ was originally identified by those working in occupational medicine dealing with disease such as pneumoconiosis, but the knowledge has been applied to therapy with inhaler devices generally generated the majority of their particulates in this range. While supposedly targeting the bronchi most of the particles in the respirable range deposits in the more distal airways. However, because the surface area of the more distal airways is so much greater than that of the early generations the concentration of particles per unit surface area is higher more centrally. Importantly mucociliary clearance of inert particles is biphasic with rapid clearance from the ciliated bronchi and bronchioles with very much slower clearance from the respiratory zone [
180,
181,
182].
Given that tobacco smoke and environmental pollutants are generally polydispersed with gases, submicronic particles and ‘respirable particles’ it is not surprising that toxins are deposited throughout the airways. Deposition patterns will also be influenced by innate anatomical differences, disease (deposition is more central and patchier in the presence of airways disease though total deposition increases), posture and indeed the technique used for inhalation with slower inhalation resulting in significantly greater peripheral deposition. That toxins from tobacco smoke deposit more centrally, particularly at airways bifurcations through inertial impaction, is only too evident in the distribution of primary lung cancers [
183].
In summary, smoking in particular and atmospheric pollution in general are likely to have effects throughout the airways even though the majority of particulates will be deposited distally. The deposition per unit surface area will vary with particle size and region of the lung. Importantly clearance from the periphery is much, much slower than that in the more central airways.
3.3. However inhaled toxins are neither necessary nor sufficient.
For the past 50 years the two dominant topics in chronic airways disease have been smoking and its role in causing COPD and asthma. More recently there has been a resurgence of interest in ‘bronchiectasis’ and the role of indoor and outdoor pollution in the causation of COPD in developing countries. Chronic bronchitis has remained the Cinderella diagnosis. However, it is important to note that the majority of smokers do not develop COPD or lung cancer. While still the major driver of disease worldwide, smoking appears to be factor in only about a third of cases and just over two thirds in developed countries [
184,
185,
186]. These estimates do of course not include chronic bacterial bronchitis as a driver of airways disease.
Moreover, it should also be noted that it is only a minority of smokers that develop COPD. Hence, as with smoking and ischaemic heart disease, smoking is neither necessary nor is it usually sufficient to cause COPD. A study that included healthy smokers found that neutrophil numbers were not elevated in their BAL samples when compared with healthy controls though the total number and % total of monocytes was [
187] consistent with the suggestion that smoking alone does not (necessarily) drive neutrophilic inflammation. Another study also found that a macrophage dominated bronchiolitis is an early marker of chronic inflammation associated with smoking. There was no evidence of a neutrophilic response in these patients nor evidence of any airways damage.
3.4. Bacteria as a driver of symptoms and damage
It is only recently that most respiratory physicians have finally given up the belief that the airways are sterile and the illusion that should bacteria stray below the vocal cords they are likely to cause disease [
125,
188,
189]. We now know that there is a very diverse pulmonary microbiome developing within hours of birth. The composition of the microbial community, as with that of the gut (from which the lungs develop embryologically) varies greatly between individuals and much is still to be learnt about how communities are maintained and evolve [
189,
190,
191,
192]. It has been known for many decades that ‘micro’ aspiration, especially during sleep, is normal and this probably provides a constant supply of organisms derived from the upper airways [
193,
194,
195,
196] in addition to those inhaled. It is also of note that mucociliary clearance almost ceases during sleep and that mucociliary clearance is not a linear process in that secretions can travel in a retrograde direction entering other lobes and the contralateral lung [
197]. Beyond this, little is certain with some believing that the microbiome is a stable self-sustaining community while others have providing evidence that the community is largely dependent on a dynamic wash-in, wash-out environment with organisms constantly being cleared (by host phagocytes and mucociliary clearance) and replenished by further micro aspiration [
185,
189,
194]. Amongst evidence produced to support this concept are studies showing similarity between upper airways flora and that in the conducting airways, and the demonstration that the density of organisms in the airways appears to decline the further one samples from the larynx.
Given that aspiration from the upper airways seems to account for the origin of most bacteria and that their density is greatest in the more central medium and large bronchi should chronic infection develop it is most likely to be most prominent in the earlier generation. However, such infections can also extend more peripherally through mechanisms described above and indeed in post-mortem studies on patients dying of CF biofilms were all too evident in alveoli in the pre very aggressive antibiotic therapy era [
199]. As Reid noted in the 1950s purulent secretions were not uncommon in alveoli of patients dying with chronic bronchitis in the absence of bronchiectasis
Hence damage due to inhaled toxins or due a chronic bacterial bronchitis (which is frequently secondary to either impaired mucociliary clearance or immune impairment) is likely to involve both bronchi and bronchioles and indeed alveoli but the maximal impact may vary and hence the predominant measurable effect (bronchiectasis or impaired FEV1/FVC ration) may differ.
4. ‘Cinderella you shall go to the ball’ – redefining ‘lung infections’
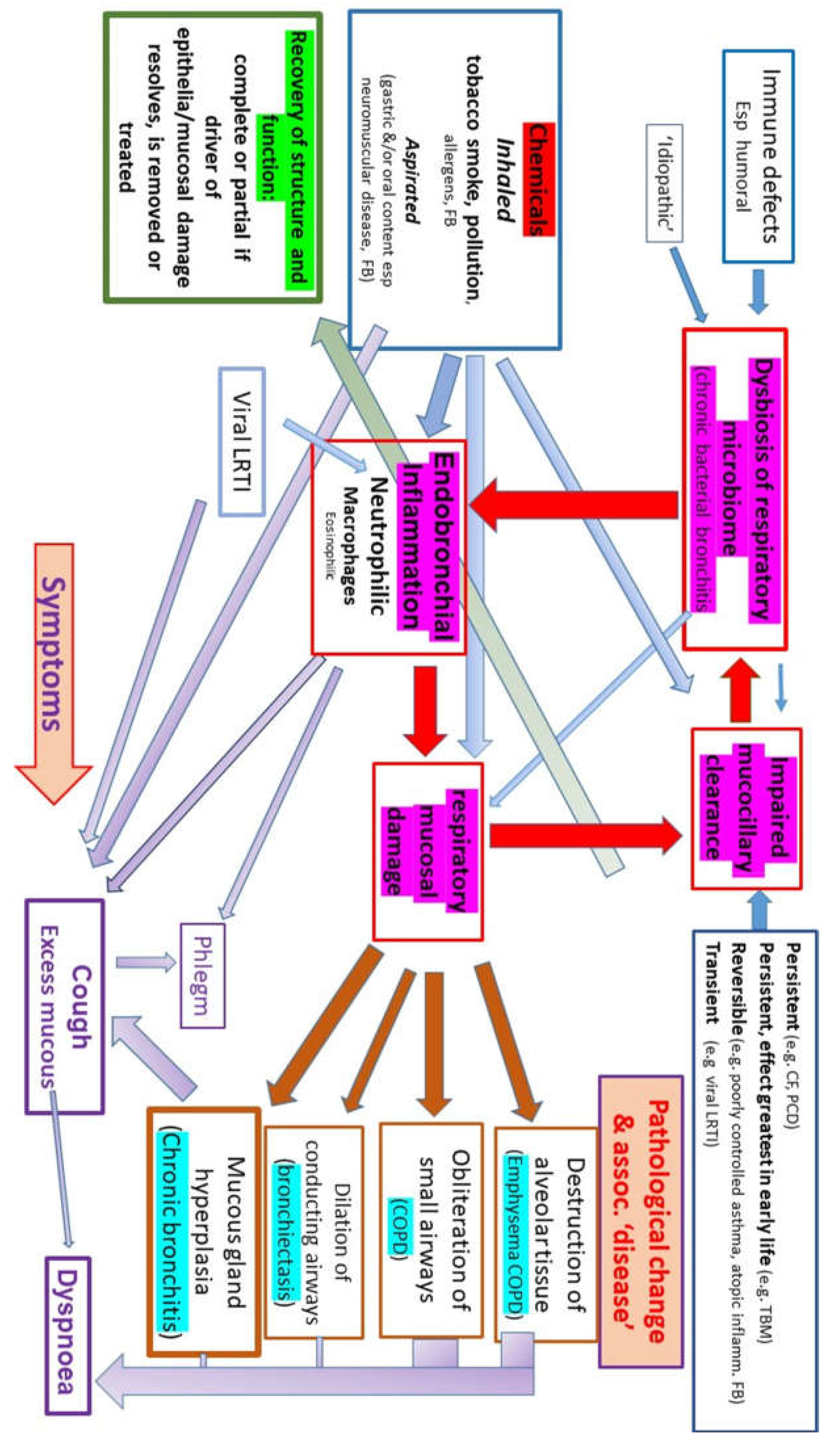
While a bacterial bronchitis does not cause smoking, impaired mucociliary clearance secondary to the effects of smoking and/or atmospheric pollutants will predispose to a bacterial bronchitis as will many other conditions such as poorly controlled asthma, viral and bacterial lower respiratory tract infections, CF, recurrent significant aspiration as seen in neuromuscular conditions (
Figure 1). Impaired clearance has long been known to be a major risk factor for chronic disease with aggressive physical therapy being recommended for areas of collapse long before antibiotics became available as such patients were believed to be particularly at risk of chronic infection and structural damage recognizable as bronchiectasis.
Interest in the role of a chronic bacterial bronchitis has increased to an extent driven both by 1) the recognition that a chronic bacterial bronchitis in young children (given the name PBB) is far more common in children than previously thought 2) the recognition that bacterial biofilms are responsible for many disease including a chronic bacterial bronchitis 3) interest in the role of a dysbiosis of the healthy respiratory ‘microbiome’ and chronic disease. The rapid progress in our understanding of bacterial behaviour has helped to explain many of the features of chronic bronchitis and opens the way to develop far more effective ways of dealing with these infections.
4.1. Lessons from ‘PBB’
Following the introduction of antibiotics into clinical practice in the mid C20th hospital admissions with bronchiectasis amongst both children and adults fell rapidly. Pror to the 1960’s clinician recognised that chronic bronchitis was a very common childhood condition but by the 1980s the idea that children might develop a chronic bronchitis (in the absence of a conditions such as CF or immunodeficiency) was denied by the majority of paediatric respiratory physicians even though a significant number of epidemiological studies from a range of countries reported a significant percentage (often >10%) of children had a chronic cough with phlegm [
200].
A decade later a number of reports started to appear suggesting that such patients were again becoming relatively common and in 2006 one group coined a new term protracted bacterial bronchitis ‘PBB’ to describe such patients [
175,
176,
201,
202,
203,
204,
205]. What distinguished this ‘new disease’ was that the label could be applied after only a few weeks (4 - 8wks though most were being referred much later) of on-going wet cough (young children do not expectorate – in an older child and adult producing sputum they might be labelled as chronic suppurative bronchitis! – all would be considered to have a chronic bacterial bronchitis) and the diagnosis was confirmed by the wet cough resolving with antibiotics. Initially, a two-week course of antibiotics was considered to be sufficient, but this was then stretched by some to resolution after four weeks of treatment and indeed in some intravenous antibiotics are required. This is in stark contrast to the need for the two or more years of symptoms currently required to qualify for a label of ‘chronic bronchitis’ which as noted above is a definition intended for epidemiological studies not clinical use. Of course, the definition is consistent with the original clinical definition of chronic bronchitis proposed by the CIBA symposium and MRC [
164,
171]. One consequence of the artificial time constraint for the diagnosis of chronic bronchitis is not so benign neglect rather than aggressive treatment. Initially the idea of PBB and hence chronic bacterial bronchitis in childhood was strongly resisted – if you do not know something exists you will not see it – but it is widely accepted at least in primary care. Reports of PBB (chronic bacterial bronchitis) like symptoms responding to antibiotics are starting to appear in the adult literature [
206,
207,
208] but again the idea that they will alter our treatment of chronic bronchitis is being strongly resisted. Interestingly in one bronchoscopic studies bacteria were identified in 33 of 52 samples. The other 19 samples were excluded as they were deemed no-diagnostic growing NTHi and Moraxella! [
207].
The need to respond to an antibiotic place bacterial pathogens clearly in the frame as the driver of symptoms. However, a chronic bacterial bronchitis must start somewhere, and patients will have had a wet cough for at least 4- 8 weeks (though in reality patients are usually symptomatic for much longer before a diagnosis is made) and thus will have qualified for a diagnosis of PBB long before eligible for a diagnosis of ‘chronic bronchitis’. An arbitrary cut off of 4 or 8 weeks of coughing is simply to minimise the use over use of antibiotics for viral infections during which a wet cough is not uncommon. Delayed presentation is common amongst children with an on-going cough with many being told it is ‘asthma – give her this inhlaer’ or ‘it is just another virus’. It is common to find children who would meet the supurious current criteria for a diagnosis of ‘chronic bronchitis’ (>3 mths of coughing in two yrs) yet respond to antibiotics and are thus labelled as having PBB because that is the in-vogue diagnosis. As the ERS task force noted the criteria are to be considered as useful for day-to-day practice but it goes no further than that. Thus, a patient with chronic bronchitis will, by definition, almost certainly have qualify for a diagnosis of PBB at some point in the evolution of the condition but the reverse is not true due to the artificial qualification criteria.
It is also of note that most, but far from all cases, commence in infancy and pre-school years [
201] consistent with historical reports from the pre-antibiotic era. Those studies linking early childhood respiratory illness and respiratory morbidity and mortality later in life note that it is respiratory infections in this period that link with that in late life [
209,
210,
211,
212,
213].
The incidence of ‘PBB’ in westernised countries also appears to be increasing given the rapid rise in publications in this area and the observations of those who have worked through this period. In part this may be diagnostic transfer, that is the focus on asthma resulted in considerable misdiagnosis (which is still ongoing). If you do not believe a condition exists, you will never see it. If you are taught that all chronic cough and other childhood symptoms in childhood are asthma and that chronic bronchitis does not exist you will not diagnose it. Other potential factors are the progressive decline in antibiotic prescribing due to antibiotic governance having some effect in primary care [
214] thus many patients who would have inadvertently been treated at an early stage in the development of a chronic bacterial bronchitis will have missed out on the
‘prophylaxis against chronic bronchitis in the next generation’. Another factor may be the effect of conjugate pneumococcal vaccines and HiB vaccines which while suppressing the acute life-threatening illness associated with virulent strains have predisposed to replacement with less virulent biofilm favouring strains and NTHi [
215].
4.1.1. PBB is curable and early bronchiectasis is reversible
If treated aggressively with high dose antibiotics the vast majority can and should be be cured though this may, in the minority, take years of treatment dealing with relapses and involving long courses of antibiotics [
175,
176,
201]. The vicious cycle hypothesis does suggest that most cases in which bronchiectasis is evident on a CT scan (in adults and children) represent medical mismanagement through failure to intervene at an appropriate time. In adult medicine bronchiectasis is irreversible. Experience in children indicates that the bronchiectasis is reversible, at least in the relatively early stages. As noted above reversal of even fairly advanced bronchiectasis had also been observed in adults in the pre-antibiotic era. The lack of recent reports in adults may reflect the erroneous but almost universally held view that bronchiectasis is irreversible and a failure to be aggressive in cases in which bronchial dilatation appears to be relatively mild. Alternatively, adults genuinely do not have the same capacity to repair damaged airways as children though studies from 70 years ago suggest that this is not the case.
To summarise, PBB is relatively common chronic disease (though in a proportion appears to resolve without treatment) and that it is driven by a bacterial dysbiosis that can be addressed with the use of antibiotics with cure being the aim of therapy. It also seems to be becoming more common again, but our lack of good epidemiology means this cannot be proven though what little evidence that exists suggests it maybe as common as asthma in young children. It has a spectrum of severity and in some the inflammation appears to lead to bronchiectasis, but this is frequently reversible with aggressive treatment. The concept was resisted forcefully by many paediatric respiratory authorities for a number of years but is now widely accepted in tertiary care. It has yet to be embarrassed in primary and secondary care and certainly not in the adult realm.
4.1.2. We still lack a test beyond a wet cough that gets better with antibiotics
The great challenge is once again the lack of diagnostic test. The ‘definition’ widely used for the diagnosis of ‘PBB’, which is in fact simply a description of symptoms as for example type 1 diabetes is a condition characterised by polyuria and weight loss that resolves with insulin (rather than DM is a condition resulting from autoimmune destruction of islet cells resulting in loss of homeostatic control of blood glucose leading to excessive high levels of blood glucose …). The key symptom is a ‘wet cough’ rather than sputum production, but this is purely pragmatic as the majority of paediatric cases appear to commence in the first few years of life when children swallow rather than expectorate sputum. The ‘wet’ cough implies secretions in the airways and is not specific to PBB/Chr Br. For example, an asthmatic may have a ’wet cough’ when the disease is poorly controlled, during an intercurrent viral infection or post an acute exacerbation. As with any symptom elicited by history the parental reporting of wet and dry coughs is far from robust not least because a child with PBB may have a wet cough in the morning and a dry cough later in the day and hence, as always, considerable care is required when taking a history [
216].
A further weakness in the ‘definition’ of PBB is that in some patients the cough does not resolve after two or even four weeks of oral antibiotics and require a change of dose or antibiotic and occasionally intravenous antibiotics are required. Poor adherence appears to be a relatively uncommon problem, presumably because of the dramatic difference mothers observe in their children when on treatment. The ‘definition’ of PBB also suggests there should be an
‘absence of symptoms or signs (i.e. specific cough pointers) suggestive of other causes of wet or productive cough’. The specific pointers they refer to are ones that might suggest a specific aetiology (e.g., cystic fibrosis, inhaled FB, recurrent aspiration etc) [
205]. However, this fails to recognise that the pathology in that there is a significant chronic disturbance in the respiratory bacterial microbiome driving inflammation (a persistent bacterial bronchitis) is common across the conditions and indeed they cannot be distinguished on the basis of conventional microbiology or 16SRNA studies [
217,
218].
4.1.3. Bacterial biofilms in the respiratory tract and the respiratory microbiome
Two key concepts have developed in the 56 years since the term COPD was devised and the idea that antibiotics had little to offer in the treatment of airways diseases (other than for the treatment of bronchiectasis and exacerbations of COPD) took hold despite the available evidence. This was in large part because the antibiotics were not curing patients with long established chronic bronchitis. Antibiotics were supposed to kill bacteria and produce a cure and there was no obvious mechanism for bacteria to cause a chronic infection.
As respiratory medicine headed in this direction the role of ‘slime’ associated with bacteria started to become evident and over the past 50 years we have come to understand the role of biofilms in many chronic disease and airways disease in particular [
153,
154,
155,
156,
157,
158,
219,
220,
221,
222]. Indeed, bacterial biofilm communities are the default for most bacteria. In pulmonary disease the first pathogen recognised to produce biofilms was PsA but later NTHI Strep Pn and the usual suspects were all shown to produce extra cellular and in some cases such as NTHi, intracellular biofilms. Importantly, although organisms such as NTHi and Str Pn compete when in the planktonic phase they often co-exist within biofilms [
223,
224,
225]. In the presence of biofilms neutrophilic inflammation is often chronic while neutrophil nets contribute biofilm formation particularly in those produced by NTHi [
226,
227,
228,
229,
230].
More recently the massive advances in sequencing have allowed investigators to start exploring the resident microbiome of the conducting airways in health and disease. The field is fraught with challenges, not least avoiding contamination from everything from the sampling process to the reagents but they have highlighted that in disease there typically a dysbiosis but that there is no signature finding. The common characteristics im chronic bronchitis, be it associated with COPD, bronchiectasis or PBB, is reduced biodiversity and increase in prevalence of some OTUs. Interestingly, but not surprisingly, the dominant OUT determined by 16SRNA is frequently a different species from the ‘pathogen’ identified on conventional culture [
191]. The inter-subject variations with the same disease is considerable and, not surprisingly, disease seems to be on a continuum with health.
What is surprising is the relative lack of discussion about the role of biofilms in permitting organisms to establish a strong hold from which they can start to occupy a disproportionate fraction of the total community. More surprising is how few respiratory physicians and indeed microbiologists appear to be exploring the implications of these fields in chronic airways disease.
4.1.4. Why we need tests beyond conventional culture
What is desperately required for clinicians to improve their care of patients who may have a chronic bacterial bronchitis (a neutrophilic dominated bronchitis driven by a bacterial dysbiosis) is a test that will identify both the inflammation (this may be using metabolomic on exhaled breath for examples) and, if possible, a robust way of identifying biofilm disease. Conventional culture provides at best a guide and is not an adequate mechanism for identifying biofilms disease.
Conventional microbiology is beset with many challenges from sampling to processing. Sputum when available can be helpful but vagaries in processing can lead to differences results in different hands. The results are often criticised for being contaminated by upper airways bacteria though and some argue that bronchoscopy samples (bronchial aspirates or bronchoalvolar lavage) is the gold standard. Rarely undertaken in adults for sample collection, it is used in paediatrics, usually when a patient relapses a number of time, but requires a general anaesthetic and hence is not a minor undertaken. It is well known that in symptomatic patients the chances of growing a ‘pathogen’ increases with the number of lobes sampled. It is also clear that the aliquots of the same sample can be sent to two different laboratories [
231] or indeed to the same laboratory and generate significantly different samples. Similarly quantitative results do not appear to have a role in diagnosing a chronic bacterial bronchitis [
231,
232] which is not surprising in that original concept was to try and distinguish planktonic bacterial disease from contamination and was never intended for use with biofilm related disease. Hence studies suggesting sputum or cough swabs are inaccurate are flawed because the ‘gold standard’ they compare it to is certainly not reproducible and reliable. A negative result may simply be due to failure to sample an affected lobe or maybe related to processing. Similarly sampling and interpretation issues largely explain studies in which biofilms are not recovered from all patients with a chronic bacterial bronchitis [
233] and of course BAL will not recover intracellular bacterial communities.
4.1.5. Treatment is largely an evidence free zone
The typical child with PBB will become cough free after two weeks of treatment with high dose of an antibiotic such as co-amoxiclav or azithromycin (this is for its antibacterial effects not its often-over-hyped anti-inflammatory effects!). Typically, the improvement in the cough is slow and often with little improvement by the end of first week (by which time most courses in primary care and for adults have ceased) though there may be improvements such as the child appearing to have more energy, be sleeping better and is less fractious. Of course, like many conditions not all patients behave in the same way – some will see the cough resolve by 7 days while others will require intravenous antibiotics to get on top of the cough. This is a common experience in the CF clinic whereby many episodes of a ‘wet cough’ will resolve with oral antibiotics though some. even in those patients with few respiratory problems previously, may require intravenous antibiotics to treat the chronic bacterial bronchitis.
Relapse is common presumably due in large part to the resilience of bacteria in biofilms and the associated persister cells [
234,
235] and there is now increasing data to support our practice of using a longer course of antibiotics should the cough resolve (the improvement has to be dramatic and unequivocal to make the ‘diagnosis’) with typically a total of 6 - 8 weeks in the first instance [
175,
179,
201,
236] (others have reported using 3 or even 6 months initially). The rational being that this provides time for the mucosa and epithelium to recover and regenerate cilia before being re-colonised.
Though not explored, it is possible that early intervention is associated with less relapses before cure is achieved but there are all too many exceptions to these expectations with some who have been symptomatic for a long time resolving rapidly and those seen within a few months taking years to resolve.
Adults developing Chr Br must go through an early stage when the cough has only been present for a few weeks and early intervention would again be appropriate. Once present for two years or more, a cure may be much more difficult. Failure to intervene until there is significant impairment of function and a label of COPD has been applied should be unacceptable.
It is also worth noting that studies suggest that, in the vast majority of cases, a persistent dry cough not attributable to asthma will resolve by 6 months but occasionally a patient with PBB will have a dry irritating cough. As with so many symptoms and signs in respiratory medicine, no one sign or symptom is unequivocally linked with a particular condition which is not surprising given the limited range of responses of the airways.
5. Recent revisions to the concept of ‘COPD’ have missed the elephant in the room
The lack of progress in the care of, and, more importantly, the prevention of irreversible lung function impairment has raised concerns regarding the direction of travel and these concerns have been reflected in the 2023 revision of the GOLD guidelines [
237] and a recent Lancet Commission [
238]. However, both seemed to have again missed the elephant in the room. Infection is mentioned in both but neither gives any real credence to the importance of a chronic bacterial bronchitis. Both mention TB and HIV are factors in the causation ‘COPD’ worldwide and that early childhood respiratory illness are linked to increased morbidity in later life implying that they were an acute hit type event. Chronic infection receives a single mention in the Lancet publication. Gold recommends short courses of antibiotics only for those with ‘
increase purulence and volume of sputum and those being ventilated’! This is despite the fact that the same document notes that a chronic bronchitis is associated with more rapid deterioration and mortality in those with ‘COPD’ and that exacerbations (acute on chronic infections) are also associated with more rapid deterioration and death. It has also been reported that the airways inflammation and associated rate of decline in lung function does not resolve in all those who give up smoking and it has been suggested that this is due to the smoking having established its own self-sustaining inflammation whereas a much more plausible explanation is that they have an associated bacteria bronchitis that would greatly benefit from treatment. Meek et al. noted that those with chronic bronchitis commonly had worse symptoms and quality of life than those chronic airflow obstruction [
239] yet these important patient reported outcomes are rarely discussed.
A similar cognitive dissonance occurs in the ‘field’ of ‘bronchiectasis’ in that the vicious cycle hypothesis places a chronic bacterial bronchitis clearly in the cycle that drives the damage that is manifest as bronchiectasis but will only treat it aggressively once the damage has been established. Where is the holistic approach to prevention? There are many with ‘bronchiectasis’ who experience no or only mild symptoms and experience less morbidity than many with ‘chronic bronchitis’ who are not even offered a bottle of cough medicine. That the current definition of chronic bronchitis is still in clinical use is a further example of all too prevalent disconnect in thinking. The disease process and symptoms in someone who qualifies for a diagnosis by coughing continuously for 15 months and 1 days are not different to that present 12 months before.
A suggested alternative approach for developing a coherent and comprehensive nomenclature is presented in
Table 1.
A chronic bacterial bronchitis occurs when the normal microbiome free from chronic inflammation is disrupted by biofilm forming organisms that drive a chronic neutrophilic inflammation. Any disruption to normal clearance of microbes (most commonly impaired mucociliary clearance) provides bacteria with opportunity to establish a permanent presence.
Inhaled toxins drive inflammation that may have little or no effect or may lead to severe airways obstruction and/or secondary bacterial bronchitis.
Accelerated decline in lung function should replace COPD in that intervention is required before advanced disease is established. This involves sequential lung function which should be part of an annual health review every bit as important as measuring blood pressure.
6. Prevention not palliation - the need for alternative or supplemental approaches
The purpose to moving bacterial infection/dysbiosis back into centre stage is to prevent structural abnormalities developing as far as possible rather than, as is generally the case today, waiting until a predetermined level of damage has been reached before intervening. As noted above, one of the drivers for placing chronic bacterial bronchitis in the ‘too hard box’ is the need to use long courses of antibiotics to achieve clinical improvement which makes ‘responsible’ clinicians uncomfortable in respect of antibiotic governance – limiting its use to those with ‘bronchiectasis’ helps keep the numbers down and provides a ‘justification’. Even though macrolides appear to drive greater levels of antibiotic resistance in the community and individuals they are the default for many clinicians. Evidence from ENT practice suggests that high dose amoxicillin (90mg/kg/day) in three divided doses are required for effective treatment of acute flare ups of chronic secretory otitis media yet the majority of paediatricians will use twice daily co-amoxiclav at much lower doses for the treatment of PBB. The higher dose of amoxicillin with appropriate clavulanic acid is the norm for example in Italy (A Kantar – personal communication) where it is believed they achieve higher cure rates. Such issues should clearly be the focus of research in both children and adults.
Such high doses and long courses are required to address the challenges posed by bacterial biofilms. An acute exacerbation, often triggered by a viral infection, appears to be associated with the release of planktonic bacteria which are much more susceptible to antibiotics and hence symptoms will start to regress towards base line but to eliminate infection and symptoms is a much greater challenge.
To both improve outcomes for patients and to try and minimise antibiotic use supplemental or alternative approaches to biofilms need to be developed. There is considerable interest in developing safe and effective biofilm disruptors [
240] while others have focused on developing phages to target the bacteria. Others believe that ignoring the infection all together and developing anti-inflammatory approaches, particularly anti-neutrophil agents will be the way forward. Resistance to phages is likely to be a problem [
241] while suppression of neutrophilic activity will have to be very tightly controlled to prevent severe side effects suggesting that a biofilm disruptor may be a better option.
However, until we have developed a position where we recognise the elephant in the room and work to place a chronic bronchitis amongst the main characters airways pathology, we are unlikely to make progress.
7. Summary
The airways communicate with the outside world and are vulnerable to the effects of chronic inflammation caused by chronic bacterial infections, the repeated inhalation of toxins or the repeated aspiration of material from the upper airway. The lower airways is a continuum from generation 0 to generation 23 and the different insults do not localise to discrete zones but produce effects from 0 - 23 all be it the region of maximal impact varies from patient to patient. Hence all the conditions noted above are part of a continuum of airways disease. The last 70 years have been characterised by a focus on cigarette smoking and to a lesser extent indoor and outdoor pollution. Clearly addressing these are essential in promoting respiratory health but unless we deal with the third arm progress will be limited. The role of a chronic bacterial bronchitis has been largely side-lined in large part because antibiotics did not appear to produce a cure combined with a concern about the consequences of aggressive antibiotic therapy.
The resurgence of awareness of chronic bacterial bronchitis in children provides a wake-up call to the respiratory community that the whole area of respiratory health needs to be revisited with a view to developing objective tests and improving therapy. Those interested in micro-organisms need to be encouraged to see this as an exciting an potentially very fruitful area in which to work given the huge burden of chronic airways disease world-wide. Bringing knowledge about bacterial behaviour in chronic disease, biofilms, micro-organism interactions and their effect on the normal homeostatic process that maintain a healthy microbiome free from chronic inflammation is essential. Developing improved diagnostics and therapies (which will involve non-antibiotic approaches either to enhance antibiotic effectiveness or ideally effective in their own right) will go a long way to dealing with this neglected, pernicious cause of morbidity and mortality.
Clinical scenario
65 yr old paediatrician smoked daily for 47 years
On open questioning about effects of smoking on him he reports he has a bit of a smokers cough but does not have chronic bronchitis and does not produce sputum. Is worried he may develop ‘COPD’ at some point.
On specific questioning reports coughing first thing every morning and on going to bed. He swallows sputum regularly and only occasionally expectorates it. Sputum is green when it is spat out. Gets worse in winter and with colds but avoids going to see his GP and it generally settles. Never had lung function or sputum culture. Not unduly out of breath but rarely participates in any physical activity beyond walking.
|
This is likely to be a very common scenario. Appears to have a bronchitis attributable to inhaling toxins in the form of cigarette smoke and to have developed a chronic bacterial bronchitis but despite medical background denies he has a ‘chronic bronchitis’ presumably because his mental image is of chronic bronchitis is of someone with copious sputum production and he ‘rarely produces any’ (though he, like so many swallow it and hence can ignore it). Most smokers will not reach the current threshold to be given a diagnosis of COPD but since this individual has never had lung function testing it is impossible to tell whether his lung function decline is accelerating ahead of that expected. The individual would question whether he needs antibiotics but since we do not know whether the smoking or the infection is likely to have the greatest impact on his respiratory status in the future we cannot determine whether addressing the bacterial bronchitis is warranted. In a non-smoker the argument for treatment would be clearer but given current thinking they would still not be treated.
Conflicts of Interest
None
References
- Badham, C. Observations on the Inflammatory Affections of the Mucus Membrane of the Bronchiae. J. Callow;1808.
- Petty, TL. The history of COPD. Int J Chron Obstruct Pulmon Dis. 2006, 1, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Widdicombe, JH. A Brief History of Bronchitis in England and Wales. Chronic Obstr Pulm Dis. 2020, 7, 303–314. [Google Scholar] [CrossRef]
- https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- https://goldcopd.org/2021-gold-reports/.
- Southwell, N. The aetiology, prevention and treatment of chronic bronchitis. Br J Industr Med. 1946, 3, 75–77. [Google Scholar] [CrossRef]
- Goodman N, Lane RE, Rampling SB. Chronic bronchitis: an introductory examination of existing data. Br Med J. 1953, 2, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Editorial. COPD: from an end-stage disease to lifelong lung health Lancet. 2022, 400, 863. [CrossRef]
- Laënnec RTH. De l’ausculatation médiate, un Traité du diagnostic des maladies des poumons et du coeur, fonde, principalement sur ce naveau moyen d’exploration. Paris: Brosson et Chande 1819.
- Stokes, W. A Treatise on the Diagnosis and Treatment of Diseases of the Chest. Diseases of the Lung and Windpipe. Haswell, Barrington, and Haswell;1837.
- Reid, LM. Reduction in bronchial subdivision in bronchiectasis. Thorax. 1950, 5, 233–247. [Google Scholar] [CrossRef]
- Hayward J, Reid LM. Observations on the anatomy of the intrasegmental bronchial tree. Thorax. 1952, 7, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Duprez A, Mampuys R. Cystic enlargement of the mucous glands of the bronchus associated with chronic bronchitis. Thorax. 1953, 8, 141–147. [Google Scholar] [CrossRef]
- Reid, LM. Pathology of chronic bronchitis. Lancet. 1954, 266, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Reid, LM. Correlation of certain bronchographic abnormalities seen in chronic bronchitis with the pathological changes. Thorax. 1955, 10, 199–204. [Google Scholar] [CrossRef]
- Reid, LM. Pathology of chronic bronchitis. Proc R Soc Med. 1956, 49, 771–773. [Google Scholar]
- Reid, LM. Measurement of human bronchial glands in chronic bronchitis: experimental study of the response of the bronchial tree to irritation; types of emphysema in chronic bronchitis. Am Rev Respir Dis. 1961, 83, 416–417. [Google Scholar] [PubMed]
- Walshe, WH. A practical treatise on the diseases of the lungs : including the principles of physical diagnosis. 1851 Blanchard, Philadelphia: https://archive.org/details/apracticaltreat03walsgoog.
- Williams, CT. The pathology and treatment of spasmodic asthma. Br Med J. 1874, 1, 769–7. [Google Scholar] [CrossRef] [PubMed]
- Anthracopoulos M, Everard ML. Asthma: - a loss of post-natal homeostatic control of airways smooth muscle with regression towards a pre-natal state. Frontiers Ped Pulmonol 2020, 8, 95. [CrossRef]
- Crowfoot, WM. An Address on the Germ-Theory of Disease. Br Med J. 1882, 2, 551–554. [Google Scholar] [CrossRef] [PubMed]
- The history of germ theory. The British Medical Journal 1888, 1, 312.
- Austrian, R. The pneumococcus at the millennium: Not down, not out. J Inf Dis. 1999, 179, S338–S341. [CrossRef]
- McNeil C, Macgregor AR, Alexander WA. Studies of Pneumonia in childhood: 1.Statistical analysis of pneumonia and bronchitis. Arch Dis Child. 1929, 4, 12–3289. [Google Scholar] [CrossRef] [PubMed]
- Young, M. The Influence of Weather Conditions on the Mortality from Bronchitis and Pneumonia in Children. J Hyg (Lond). 1924, 23, 151–175. [Google Scholar] [CrossRef]
- Everard, ML. Acute bronchiolitis and croup. In Common Respiratory Symptoms and Illness: a graded evidence based approach. Ed Chang Pediatr Clin North Am, Sauders, Philidelphia 2009.
- Liston, WG. Note on the Bacteriology of Broncho-Pneumonia of Children. Arch Dis Child. 1929, 4, 283–290. [Google Scholar] [CrossRef]
- Edmond K, Scott S, Korczak V, Ward C, Sanderson C, Theodoratou E, Clark A, Griffiths U, Rudan I, Campbell H. Long term sequelae from childhood pneumonia; systematic review and meta-analysis. PLoS One. 2012, 7, e31239. [Google Scholar]
- Eastham KM, Hammal DM, Parker L, Spencer DA. A follow-up study of children hospitalised with community-acquired pneumonia. Arch Dis Child 2008, 93, 755–759.
- Jameson, HP. Remote Prognosis of Pneumonia. Arch Dis Child. 1929, 4, 365–376. [Google Scholar] [CrossRef]
- Finke, W. Childhood pneumonia, a common cause of bronchopulmonary disease; importance of prophylaxis and adequate therapy. AMA Am J Dis Child 1952, 83, 755–762. [Google Scholar] [CrossRef]
- Andrus, PM. Emphysema under the Age of Forty: Clinical and Pathological Significance. Can Med Assoc J. 1941, 44, 344–347. [Google Scholar]
- Hastings TW, Niles WL. The bacteriology of sputum in common non-tuberculous infections if the upper and lower respiratory tracts. J Exp Med 1911, 13, 638–651. [CrossRef]
- Wollstein M, Meltzer SJ. Experimental bronchopneumonia by intrabronchial insufflation. J Exp Med 1912, 16, 126–138. [CrossRef]
- Cecil RL, Blake FG. Studies on experimental pneumonia. Pathological experimental influenza and of bacillus influenza pneumonia in monkeys. J Exp Med 1920, 32, 719–744. [CrossRef]
- Doggett, RG. Incidence of mucoid Pseudomonas aeruginosa from clinical sources. Appl Microbiol. 1969, 18, 936–937. [Google Scholar] [CrossRef]
- Gillett, HT. Vaccine therapy in chronic bronchitis. Br Med J. 1913, 1, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Pirie, JH. Vaccines in the treatment of chronic bronchitis and asthma. Br Med J. 1913, 1, 1268–1271. [Google Scholar] [CrossRef]
- Mackey, L. Some Observations on the bacteriology and vaccine treatment of chronic bronchitis: Based on 300 Cases, with Special Reference to Associated Nasal Infections. Br Med J. 1922, 2, 715–717. [Google Scholar] [CrossRef]
- Teo E, Lockhart K, Purchuri SN, Pushparajah J, Cripps AW, van Driel ML. Haemophilus influenzae oral vaccination for preventing acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017, 6, CD010010, CD010010.pub3. [Google Scholar] [CrossRef]
- Bailey MT, Lauber CL, Novotny LA, Goodman SD, Bakaletz LO. Immunization with a Biofilm-Disrupting Nontypeable Haemophilus influenzae Vaccine Antigen Did Not Alter the Gut Microbiome in Chinchillas, Unlike Oral Delivery of a Broad-Spectrum Antibiotic Commonly Used for Otitis Media. mSphere. 2020, 5, e00296–20. [Google Scholar] [CrossRef]
- Rodrigues A, Gualdi LP, de Souza RG, Vargas MH, Nuñez NK, da Cunha AA, Jones MH, Pinto LA, Stein RT, Pitrez PM. Bacterial extract (OM-85) with human-equivalent doses does not inhibit the development of asthma in a murine model. Allergol Immunopathol (Madr). 2016, 44, 504–511. [Google Scholar] [CrossRef]
- Andreas S, Testa M, Boyer L, Brusselle G, Janssens W, Kerwin E, Papi A, Pek B, Puente-Maestu L, Saralaya D, Watz H, Wilkinson TMA, Casula D, Di Maro G, Lattanzi M, Moraschini L, Schoonbroodt S, Tasciotti A, Arora AK, Maltais F; NTHi-Mcat-002 study group. Non-typeable Haemophilus influenzae-Moraxella catarrhalis vaccine for the prevention of exacerbations in chronic obstructive pulmonary disease: a multicentre, randomised, placebo-controlled, observer-blinded, proof-of-concept, phase 2b trial. Lancet Respir Med. 2022, 10, 435–446. [Google Scholar] [CrossRef]
- Mulder, J. The bacteriology of chronic bronchitis. Proc R Soc Med. 1956, 49, 773–775. [Google Scholar]
- May, JR. The bacteriology of chronic bronchitis. Lancet. 1953, 265, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Stuart-Harris CH, Pownall M, Scothorne CM, Franks Z. The factor of infection in chronic bronchitis. Q J Med. 1953, 22, 121–132.
- May, JR. Pathogenic bacteria in chronic bronchitis. Lancet. 1954, 267, 839–842. [Google Scholar] [CrossRef]
- Oswald, NC. Chronic bronchitis; factors in pathogenesis and their clinical application. Lancet. 1954, 266, 271–274. [Google Scholar] [CrossRef]
- Brumfitt W, Willoughby ML, Bromley LL. An evaluation of sputum examination in chronic bronchitis. Lancet 1957, 273, 1306–1309. [Google Scholar] [CrossRef]
- Armitage MN, Spittle DA, Turner AM. A Systematic Review and Meta-Analysis of the Prevalence and Impact of Pulmonary Bacterial Colonisation in Stable State Chronic Obstructive Pulmonary Disease (COPD). Biomedicines. 2021, 10, 81. [Google Scholar] [CrossRef]
- Weeks JR, Staples KJ, Spalluto CM, Watson A, Wilkinson TMA. The Role of Non-Typeable Haemophilus influenzae Biofilms in Chronic Obstructive Pulmonary Disease. Front Cell Infect Microbiol. 2021, 11, 720742. [Google Scholar] [CrossRef]
- Fowler JK, Godlee RJ. The diseases of the lungs. 1898 Longmans, Green and Co. London.
- Jex-Blake, AJ. A lecture on bronchiectasis. Br Med J. 1920;1: 591-4. [CrossRef]
- Findlay L, Graham S. Bronchiectasis in Childhood: Its Symptomatology, Course and Cause. Arch Dis Child. 1927, 2, 71–96. [Google Scholar] [CrossRef] [PubMed]
- McNeil C, Macgregor AR, Alexander WA. McNeil C, Macgregor AR, Alexander WA. Studies of Pneumonia in childhood: IV. Bronchiectasis and Fibrosis of the Lung. Arch Dis Child. 1929, 4, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Findlay L, Graham S. Prognosis in Bronchiectasis. Arch Dis Child. 1931, 6, 1–10. [Google Scholar] [CrossRef]
- Roles FC, Todd GS. Bronchiectasis: diagnosis and prognosis in relation to treatment. Br Med J. 1933, 2, 639–643. [Google Scholar] [CrossRef]
- Cookson HA, Mason GA. Bronchiectasis-A Fatal Disease. Edinb Med J. 1938, 45, 844–854. [Google Scholar]
- Banyai, AL. Golden anniversary of bronchography by Sicard and Forestier. Chest. 1973, 63, 164. [Google Scholar] [CrossRef]
- Miller JA, Eglee EP. Bronchograms in the Study of Pulmonary Disease. Trans Am Climatol Clin Assoc. 1926, 42, 29–44. [Google Scholar]
- Bethune, N. The technique of bronchography for the General Practitioner. Can Med Assoc J. 1929, 21, 662–667. [Google Scholar]
- C Wall, J C Hoyle. Observations on dry bronchiectasis Br Med J. 1933, 1, 597–620. [CrossRef]
- Findlay, L. Atelectatic or compensatory bronchiectasis. Arch Dis Child. 1935, 10, 61–84. [Google Scholar] [CrossRef]
- Van Loon EL, Coonel P. Bronchoscopy in the child with chronic cough. J Am Med Womens Assoc. 1947, 2, 444. [Google Scholar]
- Wishart, DE. Bronchoscopy in the diagnosis and treatment of bronchiectasis in children. Arch Otolaryngol. 1947, 46, 571. [Google Scholar] [CrossRef]
- Badger, TL. Bronchiectasis; treatment and prevention. N Engl J Med 1947, 237, 937–941. [Google Scholar] [CrossRef]
- Gann, EL. Bronchography in bronchiectasis in children. Ann Otol Rhinol Laryngol. 1948, 57, 153–162. [Google Scholar] [CrossRef]
- Wearing JDH. Bronchiectasis simulating chronic bronchitis; a study of 46 cases. Lancet. 1948;1: 822-4. .
- Field, CE. Bronchiectasis in childhood; clinical survey of 160 cases. Pediatr. 1949, 4, 21–46. [Google Scholar] [CrossRef]
- Field, CE. Bronchiectasis in childhood; aetiology and pathogenesis, including a survey of 272 cases of doubtful irreversible bronchiectasis. Pediatr 1949, 4, 231–248. [Google Scholar] [CrossRef]
- Field, CE. Bronchiectasis in childhood; prophylaxis, treatment and progress with a follow-up study of 202 cases of established bronchiectasis. Pediatrics. 1949, 4, 355–372. [Google Scholar]
- Bates M, English IC, Foreman HM, Wilson TM. Bronchography in children. Thorax 1951, 6, 408–416. [Google Scholar] [CrossRef]
- Harwood, HB. Pre-bronchiectasis. Med J Aust. 1951, 2, 258–264. [Google Scholar] [CrossRef]
- Wynn-Williams, N. Observations on the aetiology of bronchiectasis. Edinb Med J. 1952, 59, 427–442. [Google Scholar]
- Lees, AW. Chronic localized catarrhal bronchitis. Glasgow Med J. 1953, 34, 160–169. [Google Scholar]
- Eastham KM, Fall AJ, Mitchell L, Spencer DA. The need to redefine non-cystic fibrosis bronchiectasis in childhood. Thorax. 2004, 59, 324–327. [Google Scholar] [CrossRef]
- Fleischner, FG. Reversible Bronchiectasis. Am. J. Roentgenol 1941, 46, 166–172. [Google Scholar]
- Finke, W. Reversibility of early bronchiectasis: Its implications for therapy and prevention. N Y State J Med. 1951, 51, 1163–1166. [Google Scholar]
- Pontius JR, Jacobs LG. The reversal of advanced bronchiectasis. Radiol 1957, 68, 204–208.
- Gaillard EA, Carty H, Heaf D, Smith R. et al. Reversible bronchial dilatation in children: comparison of serial high-resolution computer tomography scans of the lungs. Eur J Radiol 2003, 47, 215–220.
- Kucuk C, Turkkani MH, Arda K. A case report of reversible bronchiectasis in an adult: Pseudobronchiectasis. Respir Med Case Rep. 2019, 26, 315–316. [Google Scholar] [CrossRef]
- Strang, C. The fate of children with bronchiectasis. Ann Intern Med. 1956, 44, 630–656. [Google Scholar] [CrossRef]
- Wynn-Williams, N. Bronchiectasis: a study centred on Bedford and its environs. Br Med J. 1953, 1, 1194–1199. [Google Scholar] [CrossRef]
- Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA, Flower CD, Bilton D, Keogan MT. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000, 162, 1277–1284. [Google Scholar] [CrossRef]
- Lees, AW. Atelectasis and bronchiectasis in pertussis. Br Med J 1950, 2, 1138–1141. [Google Scholar] [CrossRef]
- Boyd, GL. Bronchiectasis in children. Can Med Assoc J. 1931, 25, 174–182. [Google Scholar]
- Warner, WP. Bronchiectasis: aetiology, diagnosis and treatment. Can Med Assoc J. 1932, 27, 583–593. [Google Scholar]
- Warner WP. Some Factors Causing Bronchial Dilatation in Bronchiectasis. Trans Am Clin Climatol Assoc. 1934;50:172-82.
- Ukil AC, De KN. Bronchiectasis: Its aetiology, Pathology, Diagnosis, Prognosis and Treatment. Ind Med Gaz. 1939, 74, 529–537. [Google Scholar]
- Mallory, TB. The pathogenesis of bronchiectasis. N Engl J Med. 1947, 237, 795–798. [Google Scholar] [CrossRef]
- Wynn-Williams, N. Observations on the aetiology of bronchiectasis. Edinb Med J. 1952, 59, 427–442. [Google Scholar]
- Daley A. The health of the nation. Br Med J. 1951;1: 1279-85. [CrossRef]
- Sanderson JM, Kennedy MC, Johnson MF, Manley DC. Bronchiectasis: results of surgical and conservative management. A review of 393 cases. Thorax. 1974, 29, 407–416. [CrossRef]
- Glauser EM, Cook CD, Harris GB. Bronchiectasis: a review of 187 cases in children with follow-up pulmonary function studies in 58. Acta Paediatr Scand. 1966, Suppl 165, 1+. [CrossRef]
- Field, CE. Bronchiectasis. Third report on a follow-up study of medical and surgical cases from childhood. Arch Dis Child 1969, 44, 551–561. [CrossRef]
- Young, RA. Chronic bronchitis and emphysema. Br Med J. 1935, 1, 261–263. [Google Scholar] [CrossRef]
- Engel, S. The pathogenesis of bronchial catarrh and of acute and chronic bronchitis. J Clin Pathol 1958, 11, 302–305. [Google Scholar] [CrossRef]
- Whitwell, F. A study of the pathology and pathogenesis of bronchiectasis. Thorax. 1952, 7, 213–239. [Google Scholar] [CrossRef]
- Cole, PJ. Inflammation: a two-edged sword – the model of bronchiectasis. Eur J Respir Dis Suppl 1986, 147, 6–15. [Google Scholar]
- Cole, P. The damaging role of bacteria in chronic lung infection. J AntimicrobChemother 1997, 40 (Suppl A), 5–10. [Google Scholar] [CrossRef]
- Brimblecombe, P. London air pollution, 1500-1900. Atmos Environ. 1977, 11, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Fowler D, Brimblecombe P, Burrows J, Heal MR, Grennfelt P, Stevenson DS, Jowett A, Nemitz E, Coyle M, Lui X, Chang Y, Fuller GW, Sutton MA, Klimont Z, Unsworth MH, Vieno M. A chronology of global air quality. Philos Trans A Math Phys Eng Sci. 2020, 378, 20190314. [CrossRef]
- Logan, WP. Mortality in the London fog incident, 1952. Lancet 1953, 1, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Oswald NC, Medvei VC. Chronic bronchitis; the effect of cigarette-smoking. Lancet. 1955, 269, 843–844. [Google Scholar] [CrossRef]
- https://web.archive.org/web/20110921054816/http://www.time.com/time/magazine/article/0,9171,1905530,00.html.
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 5, 321, 323-9. [CrossRef]
- Doll, R. Uncovering the effects of smoking: historical perspective. Stat Methods Med Res 1998, 7, 87117. [Google Scholar] [CrossRef] [PubMed]
- Proctor, RN. The history of the discovery of the cigarette-lung cancer link: evidentiary traditions, corporate denial, global toll. Tob Control. 2012, 21, 87–91. [Google Scholar] [CrossRef]
- Doll R, Hill AB. Smoking and carcinoma of the lung. Preliminary report. BMJ 1950, ii, 73948.
- Wynder EL, Graham EA. Tobacco smoking as a possible etiologic factor in bronchogenic carcinoma. JAMA 1950;143:32936. Levin ML, Goldstein H.
- Gerhardt, PR. Cancer and tobacco smoking. JAMA 1950; 143: 3368. Mills CA, Porter MM. Tobacco smoking habits and cancer of the mouth and respiratory system. Cancer Res 1950, 10, 53942. [Google Scholar]
- Schrek R, Baker LA, Ballard GP, Dolgoff S. Tobacco smoking as an etiologic factor in disease. Cancer Res 1950, 10, 4958.
- Doll R, Hill AB. A study of the aetiology of carcinoma of the lung. BMJ 1952;ii:127186.
- Royal College of Physicians of London. Smoking and Health. London: Pitman Medical Publishing; 1962.
- Advisory Committee to the Surgeon General of the US Public Health Service. Smoking and Health. Washington, DC: US Government Printing Office; 1964. Public Health Service Publication no. 1103.
- Unal B, Critchley J, Capewell S. Impact of smoking reduction on coronary heart disease mortality trends during 1981-2000 in England and Wales. Tob Induc Dis. 2003, 1, 185. [Google Scholar] [CrossRef]
- Goodman N, Lane RE, Rampling SB. Chronic bronchitis: an introductory examination of existing data. Br Med J. 1953, 2, 237–243. [Google Scholar] [CrossRef]
- Fry, J. Chronic bronchitis in general practice. Br Med J. 1954, 1, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Reid, DD. General epidemiology of chronic bronchitis. Proc R Soc Med. 1956, 49, 767–771. [Google Scholar] [PubMed]
- Stuart-Harris, CH. Chronic bronchitis and emphysema: a symposium. I. The epidemiology of chronic bronchitis. Br J Radiol. 1959, 32, 286–289. [Google Scholar] [CrossRef]
- Reid, D. Diagnostic standardization in geographic comparisons of morbidity. Am Rev Respir Dis. 1962, 86, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Colley JR, Reid DD. Urban and social origins of childhood bronchitis in England and Wales. Br Med J. 1970, 2, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Reid, DD. The beginnings of bronchitis. Proc R Soc Med. 1969, 62, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Higgins, IT. The epidemiology of chronic respiratory disease. Prev Med. 1973, 2, 14–33. [Google Scholar] [CrossRef]
- Higgins, IT. The epidemiology of chronic respiratory disease: a literature review. 1974: EPA-650/1-74-007.
- Speizer FE, Tager IB. Epidemiology of chronic mucus hypersecretion and obstructive airways disease. Epidemiol Rev. 1979, 1, 124–142. [Google Scholar] [CrossRef]
- Karzon, DT. Immunization practice in the United Sttes and Great Britain: a comparative study. Postgrad Med J. 1969, 45, 147–160. [Google Scholar] [CrossRef]
- Williams, KJ. The introduction of 'chemotherapy' using arsphenamine - the first magic bullet. J R Soc Med. 2009, 102, 343–8. [Google Scholar] [CrossRef]
- Hoel D, Williams DN. Antibiotics: past, present, and future. Unearthing nature's magic bullets. Postgrad Med. 1997, 101, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Kassowitz KE, Muscato GH Jr The long range effect of antibacterial therapy on pneumonia, empyema, bronchiectasis and pulmonary abscess; an analysis of incidence and mortality in 74,489 admissions to a Children's Hospital in twenty years. Dis Chest. 1952, 21, 161–173. [CrossRef]
- Patterson D, Moncrieff A. Diseases of Children. 4th ed. (Vol. 1). London:Edward Arnold & Co (1947). p. 648–62.
- Barker AF, Bardana EJ Jr. Bronchiectasis: update of an orphan disease. Am Rev Respir Dis. 1988;137: 969-78. [CrossRef]
- Callahan CW, Redding GJ. Bronchiectasis in children: orphan disease or persistent problem? Pediatr Pulmonol. 2002, 33, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Brown HM, Wilson RN. Chronic bronchitis in industry; an account of a trial of H. influenzae vaccine. Br Med J. 1959, 1, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Hers JF, Mulder J. The mucosal epithelium of the respiratory tract in muco-purulent bronchitis caused by Haemophilus influenzae. J Pathol Bacteriol. 1953, 66, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Finke, W. Childhood pneumonia, a common cause of bronchopulmonary disease; importance of prophylaxis and adequate therapy. Am J Dis Child. 1952, 83, 755–762. [Google Scholar] [CrossRef]
- Mulder J, Goslings WR, Vabn der Plas MC, Cardozo PL. Studies on the treatment with antibacterial drugs of acute and chronic mucopurulent bronchitis caused by Hemophilus influenzae. Acta Med Scand. 1952, 143, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Franklin AW, Garrod LP. Chloramphenicol treatment of bronchiectasis in children. Br Med J. 1953, 2, 1067–1069. [Google Scholar] [CrossRef]
- Barach AL, Bickerman HA, Beck GI. Antibiotic therapy in infections of the respiratory tract. AMA Arch Intern Med. 1952, 90, 808–849. [Google Scholar] [CrossRef]
- Finke, W. Prospects for prevention of chronic bronchitis and bronchiectasis; rational management of bronchopulmonary infections by penicillin aerosol therapy. J Pediatr. 1948, 33, 29–42. [Google Scholar] [CrossRef]
- May, JR. Antibiotics in chronic bronchitis. Lancet. 1953, 265, 899–902. [Google Scholar] [CrossRef] [PubMed]
- May JR, Oswald NC. Effect of antibiotics on purulent sputum. Lancet. 1957, 273, 345–346. [Google Scholar] [CrossRef]
- May JR, Oswald NC. Long-term chemotherapy in chronic bronchitis. Lancet. 1956, 271, 814–818, Stop if not better after 4 weeks. [Google Scholar] [CrossRef]
- Allibone EC, Allison PR, Zinnemann K. The Significance of Haemophilus influenzae in Chronic Bronchiectasis of Children. Proc R Soc Med. 1955, 48, 1102 2 + weeks to clear relapse frequent NYHi keeps inflammation going. [Google Scholar]
- Knox K, Elmes PC, Fletcher CM. Chronic bronchitis; an attempt to control chronic infection with Haemophilus influenzae by aerosol therapy. Lancet 1955, 268, 120–122. [CrossRef]
- Finke, W. Long-term treatment with antimicrobials and anti-inflammatory steroids in chronic bronchitis and infectious asthma. Its potential value for rehabilitation and prevention. Am Rev Respir Dis. 1960, 82, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Wynn-Williams, N. Control of respiratory infections in children by tetracycline. Br Med J. 1961, 1, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Francis RS, May JR, Spicer CC. Chemotherapy of bronchitis. Influence of penicillin and tetracycline administered daily, or intermittently for exacerbations. A report to the Research Committee of the British Tuberculosis Association by its Bronchitis Subcommittee. Br Med J. 1961, 2, 979–985. [Google Scholar] [CrossRef]
- Francis RS, May JR, Spicer CC. Influence of daily penicillin, tetracycline, erythromycin, and sulphamethoxypyridazine on exacerbations of bronchitis. A report to the research committee of the british tuberculosis association. Br Med J. 1964, 1, 728–732. [Google Scholar] [CrossRef]
- May, JR. The bacteriology and chemotherapy of chronic bronchitis. Br J Dis Chest. 1965, 59, 57–65. [Google Scholar] [CrossRef]
- Relapse or reinfection in chronic bronchitis? Lancet. 1955, 269, 601–602. [CrossRef]
- Bigger, JW. Treatment of staphylococcal infections with penicllin by intermittent sterilisation. Lancet 1944; 497-500.
- Flemming HC, Baveye P, Neu TR, Stoodley P, Szewzyk U, Wingender J, Wuertz S. NPJ Who put the film in biofilm? The migration of a term from wastewater engineering to medicine and beyond. Biofilms Microbiomes. 2021, 7, 10. [Google Scholar] [CrossRef]
- Vestby LK, Grønseth T, Simm R, Nesse LL. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics (Basel). 2020, 9, 59. [Google Scholar] [CrossRef]
- Fux CA, Stoodley P, Hall-Stoodley L, Costerton JW. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Rev Anti Infect Ther. 2003, 1, 667–683. [Google Scholar] [CrossRef]
- Høiby, N. A short history of microbial biofilms and biofilm infections. APMIS 2017, 125, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Read RC, Rutman AA, Jeffery PK, Lund VJ, Brain AP, Moxon ER, Cole PJ, Wilson R. Interaction of capsulate Haemophilus influenzae with human airway mucosa in vitro. Infect Immun. 1992, 60, 3244–3252. [Google Scholar] [CrossRef] [PubMed]
- Clementi CF, Murphy TF. Non-typeable Haemophilus influenzae invasion and persistence in the human respiratory tract. Front Cell Infect Microbiol. 2011, 1, 1, eCollection 2011. [Google Scholar] [CrossRef]
- Antibiotics in chronic respiratory infections. Lancet. 1953, 1, 529–530.
- Antibiotics in chronic bronchial infection. Br Med J. 1957, 2, 459–460.
- Gee S. The Lumleian Lectures on Bronchitis, Pulmonary Emphysema, and Asthma: Delivered before the Royal College of Physicians of London. Lecture I. Br Med J. 1899: 1: 645-9. [CrossRef]
- Gee, S. The Lumleian Lectures on Bronchitis, Pulmonary Emphysema, and Asthma: Delivered before the Royal College of Physicians of London. Lecture II Br Med J. 1899, 1, 715–724. [Google Scholar] [CrossRef]
- Christie, RV. 1944. Emphysema of the lungs (part II). BMJ, 1–145.
- Scadding, JG. Meaning of diagnostic terms in broncho-pulmonary disease. Br Med J. 1963, 2, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Ciba Guest Symposium. Terminology, definitions, and classification of chronic pulmonary emphysema and related conditions. Thorax 1959, 14, 286–299.
- Reid, D. Diagnostic standardization in geographic comparisons of morbidity. Am Rev Respir Dis. 1962, 86, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M. Inhalation therapy: an historical review. Prim Care Respir J. 2007, 16, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Brown, HM. Treatment of chronic asthma with prednisolone; significance of eosinophils in the sputum. Lancet. 1958, 2, 1245–1247. [Google Scholar] [CrossRef]
- Kouri A, Dandurand RJ, Usmani OS, Chow CW. Exploring the 175-year history of spirometry and the vital lessons it can teach us today. Eur Respir Rev. 2021, 30, 210081. [Google Scholar] [CrossRef]
- Committee on Diagnostic Standards for Nontuberculous Respiratory Diseases, American Thoracic Society. Definitions and classification of chronic bronchitis, asthma, and pulmonary emphysema. Am Rev Respir Dis 1962, 85, 762–769.
- Definition and classification of chronic bronchitis for clinical and epidemiological purposes. A report to the Medical Research Council by their Committee on the Aetiology of Chronic Bronchitis. Lancet. 1965, 1, 775–779. [Google Scholar]
- Bakaletz, LO. Bacterial biofilms in the upper airway - evidence for role in pathology and implications for treatment of otitis media. Paediatr Respir Rev. 2012, 13, 154–159. [Google Scholar] [CrossRef]
- Hamilos, DL. Biofilm Formations in Pediatric Respiratory Tract Infection : Part 1: Biofilm Structure, Role of Innate Immunity in Protection Against and Response to Biofilm, Methods of Biofilm Detection, Pediatric Respiratory Tract Diseases Associated with Mucosal Biofilm Formation. Curr Infect Dis Rep. 2019, 21, 6. [Google Scholar] [CrossRef]
- Everard ML. The unified airway – a bug’s eye view. Pediatr Resp Rev 2012; 13; 133-4.
- Craven V, Everard ML. Protracted bacterial bronchitis: reinventing an old disease. Arch Dis Child. 2013, 98, 72–76.
- Ishak A, Everard ML. Persistent and recurrent bacterial bronchitis - a paradigm shift in our understanding of chronic respiratory disease. Frontiers in Pediatric Pulmonol 2017, 5, 19. [CrossRef]
- Stocks J, Hislop AA. Structure and function of the respiratory system. In Drug Delivery to the Lung. Eds Bisgaard H, O’Calagan C, Smaldone GC. Lung Biology in Health & Disease Vol. 162; pp47-104 2002 Marcel Dekker, New York.
- Devadason S, Everard ML. Pediatric Inhalation Therapy. International Society of Aerosols in Medicine Textbook of Inhaled Therapy 2015.
- Heyder J, Svartengren M. Basic principles of particle behaviour in the human respiratory tract. Eds Bisgaard H, O’Calagan C, Smaldone GC. Lung Biology in Health & Disease Vol. 162; pp47-104 2002 Marcel Dekker, New York.
- Anderson M, Philipson K, Svartengren M, Camner P. Human deposition and clearance of 6-micron particles inhaled with an extremely low flow rate. Exp Lung Res. 1995, 21, 187–195. [Google Scholar] [CrossRef]
- Möller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, Häussinger K, Kreyling WG. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med. 2008, 177, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Bennett, WD. Design of In Vivo Deposition and Clearance Experiments. J Aerosol Med Pulm Drug Deliv. 2022, 35, 286–290. [Google Scholar] [CrossRef]
- Balashazy I, Hofmann W, Heistracher T. Local particle deposition patterns may play a key role in the development of lung cancer. J Appl Physiol (1985). 2003, 94, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009, 374, 733–743.
- Holm M, Toren K, Andersson E. Incidence of chronic bronchitis: a prospective study in a large general population. Int J Tuberc Lung Dis 2014, 18, 871–876.
- Collaborators GCRD. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015. Lancet Respir Med. 2017, 5, 691–706.
- Heron M, Grutters JC, ten Dam-Molenkamp KM, Hijdra D, van Heugten-Roeling A, Claessen AM, Ruven HJ, van den Bosch JM, van Velzen-Blad H. Bronchoalveolar lavage cell pattern from healthy human lung. Clin Exp Immunol. 2012, 167, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Cotran, RS.; Kumar, V.; Collins, T.; Robbins, SL. Robbins Pathologic Basis of Disease. Philadelphia: Saunders; 1999.
- Dickson RP, Erb-Downward JR, Martinez FJ, Huffnagle GB. The Microbiome and the Respiratory Tract. Annu Rev Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Durack J, Christophersen CT. Human Respiratory and Gut Microbiomes-Do They Really Contribute to Respiratory Health? Front Pediatr. 2020, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson L, Craven V, Bingle L, Cookson WOCM, Everard ML, Moffatt MF. The impact of persistent bacterial bronchitis on the pulmonary microbiome of children. PLoS One. 2017, 12, e0190075. [Google Scholar] [CrossRef]
- Dickson RP, Erb-Downward JR, Huffnagle GB. Homeostasis and its disruption in the lung microbiome. Am J Physiol Lung Cell Mol Physiol. 2015, 309, L1047–L1055. [Google Scholar] [CrossRef]
- Quinn LH, Meyer OO. The relationship of sinusitis and bronchiectasis. Arch Otolaryngol. 1929, 10, 152–165.
- Amberson, JB. A clinical consideration of abscesses and cavities of the lung. Bull Johns Hopkins Hosp. 1954, 94, 227–237. [Google Scholar]
- Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest. 1997, 111, 1266–1272.
- Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978, 64, 564–568.
- Hasani A, Agnew JE, Pavia D, Vora H, Clarke SW. Effect of oral bronchodilators on lung mucociliary clearance during sleep in patients with asthma. Thorax. 1993, 48, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Falkowski NR, Huffnagle GB, Curtis JL. Bacterial Topography of the Healthy Human Lower Respiratory Tract. mBio. 2017, 8, e02287–16. [Google Scholar] [CrossRef]
- Bjarnsholt T, Jensen PØ, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB, Pressler T, Givskov M, Høiby N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol. 2009, 44, 547–58. [Google Scholar] [CrossRef] [PubMed]
- Cook DG, Strachan DP. Health effects of passive smoking. 3. Parental smoking and prevalence of respiratory symptoms and asthma in school age children. Thorax 1997, 52, 1081–1094. [CrossRef]
- Donnelly D, Critchlow A, Everard ML. Outcomes in children treated for persistent bacterial bronchitis. Thorax 2007, 61, 80–84.
- Marchant JM, Masters IB, Taylor SM, et al. Evaluation and outcome of young children with chronic cough. Chest 2006, 129, 1132–1141.
- Chang AB, Byrnes CA, Everard ML. Diagnosing and preventing chronic suppurative lung disease (CSLD) and bronchiectasis. Pediatr Resp Rev 2011, 12, 97–103.
- Cuthbertson L, Craven V, Bingle L, , Cookson WO, Everard ML, Moffat M. The lung microbiome in healthy children and those with persistent bacterial bronchitis – a change in diversity and density. PLOSone 2017, 12, e0190075.
- A Kantar A, Chang AB, Shields MS, Marchant J, Grimwood K, Grigg J, Priftis KN, Cutrera R, Midulla F, Brand P, Everard ML. ERS Task Force: Protracted bacterial bronchitis in children. Eur Respir J 2017, 50, p. 1602139–1602139.
- Martin MJ, Lee H, Clayton C, Pointon K, Soomro I, Shaw DE, Harrison TW. Idiopathic chronic productive cough and response to open-label macrolide therapy: An observational study. Respirology. 2019, 24, 558–565. [Google Scholar] [CrossRef]
- Heching M, Rosengarten D, Shitenberg D, Shtraichman O, Abdel-Rahman N, Unterman A, Kramer MR. Bronchoscopy for Chronic Unexplained Cough: Use of Biopsies and Cultures Increase Diagnostic Yield. J Bronchology Interv Pulmonol. 2020, 27, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Huang L, Lai K, Zhan C, Long L, Yi F, Zhou J, Zhan W, Lu H, Jiang Z, Chen Y, Jiang M, Chen R, Xie J, Luo W. Heliyon. Clinical characteristics of protracted bacterial bronchitis in adults. 2022, 9, e12299. [CrossRef]
- Barker DJ, Osmond C. Childhood respiratory infection and adult chronic bronchitis in England and Wales. Br Med J 1986, 293, 1271–1275.
- Shaheen SO, Barker DJ, Holgate ST. Do lower respiratory tract infections in early childhood cause chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 1995, 151, 1649–1651. [Google Scholar] [CrossRef] [PubMed]
- Svanes C, Sunyer J, Plana E, Dharmage S, Heinrich J, Jarvis D, de Marco R, Norbäck D, Raherison C, Villani S, Wjst M, Svanes K, Antó JM. Early life origins of chronic obstructive pulmonary disease. Thorax 2010, 65, 14–20.
- Allinson JP, Chaturvedi N, Wong A, Shah I, Donaldson GC, Wedzicha JA, Hardy R. Early childhood lower respiratory tract infection and premature adult death from respiratory disease in Great Britain: a national birth cohort study. Lancet. 2023: S0140-6736(23)00131-9. [CrossRef]
- Perret JL, Wurzel D, Walters EH, Lowe AJ, Lodge CJ, Bui DS, Erbas B, Bowatte G, Russell MA, Thompson BR, Gurrin L, Thomas PS, Hamilton G, Hopper JL, Abramson MJ, Chang AB, Dharmage SC. Childhood 'bronchitis' and respiratory outcomes in middle-age: a prospective cohort study from age 7 to 53 years. BMJ Open Respir Res. 2022, 9, e001212. [Google Scholar] [CrossRef] [PubMed]
- Unsworth L, Walley T. Trends in primary care antibiotic prescribing in England 1994-1998. Pharmacoepidemiol Drug Saf. 2001, 10, 309–314. [Google Scholar] [CrossRef]
- Priftis KN, Litt D, Manglani S, Anthracopoulos MB, Thickett K, Tzanakaki G, Fenton P, Syrogiannopoulos GA, Vogiatzi A, Douros K, Slack M, Everard ML. Bacterial bronchitis caused by Streptococcus pneumoniae and nontypable Haemophilus influenzae in children: the impact of vaccination. Chest. 2013, 143, 152–157.
- Donnelly DE, Everard ML. ‘Dry’ and ‘wet’ cough – how reliable is parental reporting? BMJ Open Respir Res 2019, 6, e000375. [CrossRef]
- Broderick DTJ, Waite DW, Marsh RL, Camargo CA Jr, Cardenas P, Chang AB, Cookson WOC, Cuthbertson L, Dai W, Everard ML, Gervaix A, Harris JK, Hasegawa K, Hoffman LR, Hong SJ, Josset L, Kelly MS, Kim BS, Kong Y, Li SC, Mansbach JM, Mejias A, O'Toole GA, Paalanen L, Pérez-Losada M, Pettigrew MM, Pichon M, Ramilo O, Ruokolainen L, Sakwinska O, Seed PC, van der Gast CJ, Wagner BD, Yi H, Zemanick ET, Zheng Y, Pillarisetti N, Taylor MW. Bacterial Signatures of Paediatric Respiratory Disease: An Individual Participant Data Meta-Analysis. Front Microbiol. 2021, 12, 711134, eCollection 2021. [Google Scholar] [CrossRef]
- van der Gast CJ, Cuthbertson L, Rogers GB, et al. Three clinically distinct chronic pediatric airway infections share a common core microbiota. Ann Am Thorac Soc. 2014, 11, 1039–1048. [Google Scholar] [CrossRef]
- Hall-Stoodley L, McCoy KS. Biofilm aggregates and the host airway-microbial interface. Front Cell Infect Microbiol. 2022;12: 969326. [CrossRef]
- Swords, WE. Nontypeable Haemophilus influenzae biofilms: role in chronic airway infections. Front Cell Infect Microbiol 2012, 2, 97. [Google Scholar] [CrossRef] [PubMed]
- Poh WP, Lester SE, Nguyen PT, Bakaletz LO, Reynolds PN, Hodge S, Roscioli E. COPD-Related Modification to the Airway Epithelium Permits Intracellular Residence of Nontypeable Haemophilus influenzae and May Be Potentiated by Macrolide Arrest of Autophagy. Int J Chron Obstruct Pulmon Dis. 2020, 15, 1253–1260. [Google Scholar] [CrossRef]
- Weeks JR, Staples KJ, Spalluto CM, Watson A, Wilkinson TMA. The Role of Non-Typeable Haemophilus influenzae Biofilms in Chronic Obstructive Pulmonary Disease. Front Cell Infect Microbiol. 2021, 11, 720742. [Google Scholar] [CrossRef]
- Murphy TF, Bakaletz LO, Smeesters PR. Microbial interactions in the respiratory tract. Pediatr Infect Dis J. 2009, 28 (Suppl. 10), S121–S126. [Google Scholar] [CrossRef]
- Everard, ML. ‘Reccurent lower respiratory tract infections’ – going around in circles respiratory medicine style. Pediatr Respir Rev 2012, 13, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Dagan R, Leibovitz E, Greenberg D, Bakaletz L, Givon-Lavi N. Mixed pneumococcal nontypeable Haemophilus influenzae otitis media is a distinct clinical entity with unique epidemiologic characteristics and pneumococcal serotype distribution. J Infect Dis. 2013, 208, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Porto, B.N.; Stein, R.T. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front. Immunol. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Mincham KT, Bruno N, Singanayagam A, Snelgrove RJ. Our evolving view of neutrophils in defining the pathology of chronic lung disease. Immunology. 2021, 164, 701–721. [Google Scholar] [CrossRef]
- Jo A, Kim DW. Neutrophil Extracellular Traps in Airway Diseases: Pathological Roles and Therapeutic Implications. Int J Mol Sci. 2023, 24, 5034. [Google Scholar] [CrossRef]
- Juneau RA, Pang B, Weimer KE, Armbruster CE, Swords WE. Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect Immun. 2011, 79, 431–438. [Google Scholar] [CrossRef]
- Huang YJ, Erb-Downward JR, Dickson RP, Curtis JL, Huffnagle GB, Han MK. Understanding the role of the microbiome in chronic obstructive pulmonary disease: principles, challenges, and future directions. Transl Res. 2017, 179, 71–83. [Google Scholar] [CrossRef]
- Craven V, Hausdorff WP, Everard ML. High levels of inherent variability in microbiological assessment of bronchoalveolar lavage samples from children with persistent bacterial bronchitis and healthy controls. Pediatr Pulmonol. 2020, 55, 3209–3214. [Google Scholar] [CrossRef] [PubMed]
- Escribano Montaner A, García de Lomas J, Villa Asensi JR, et al.Bacteria from bronchoalveolar lavage fluid from children with sus-pected chronic lower respiratory tract infection: results from a multi-center, cross-sectional study in Spain. Eur J Pediatr. 2018, 177, 181-192.
- Everard, ML. Everard ML. We should not underestimate role of biofilms in a persistent/chronic bronchitis. Lancet Microbe 2022: S2666-5247(22)00188-4. [CrossRef]
- Fisher RA, Gollan B, Helaine S. Persistent bacterial infections and persister cells. Nat Rev Microbiol. 2017, 15, 453–464. [CrossRef]
- Berkvens A, Chauhan P, Bruggeman FJ. Integrative biology of persister cell formation: molecular circuitry, phenotypic diversification and fitness effects. J R Soc Interface. 2022;19: 20220129. [CrossRef]
- Gross-Hodge E, Carroll WD, Rainford N, Gamble C, Gilchrist FJ. Duration of initial antibiotic course is associated with recurrent relapse in protracted bacterial bronchitis. Arch Dis Child. 2020, 105, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, Bourbeau J, Han MK, Martinez FJ, Montes de Oca M, Mortimer K, Papi A, Pavord I, Roche N, Salvi S, Sin DD, Singh D, Stockley R, López Varela MV, Wedzicha JA, Vogelmeier CF. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur Respir J. 2023, 61, 2300239. [Google Scholar] [CrossRef] [PubMed]
- Stolz D, Mkorombindo T, Schumann DM, Agusti A, Ash SY, Bafadhel M, Bai C, Chalmers JD, Criner GJ, Dharmage SC, Franssen FME, Frey U, Han M, Hansel NN, Hawkins NM, Kalhan R, Konigshoff M, Ko FW, Parekh TM, Powell P, Rutten-van Mölken M, Simpson J, Sin DD, Song Y, Suki B, Troosters T, Washko GR, Welte T, Dransfield MT. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet. 2022, 400, 921–972. [Google Scholar] [CrossRef]
- Meek PM, Petersen H, Washko GR, Diaz AA, Klm V, Sood A, Tesfaigzi Y. Chronic Bronchitis Is Associated With Worse Symptoms and Quality of Life Than Chronic Airflow Obstruction. Chest. 2015, 148, 408–416. [Google Scholar] [CrossRef]
- Phelan PD, Landau LI, Robertson CF. Suppurative lung disease. In: Respiratory illness in children, 4th edn. Oxford, UK: Blackwell Scientific, 1994:295–06.
- Chang AB, Bell SC, Byrnes CA, Dawkins P, Holland AE, Kennedy E, King PT, Laird P, Mooney S, Morgan L, Parsons M, Poot B, Toombs M, Torzillo PJ, Grimwood K. Respirology. Thoracic Society of Australia and New Zealand (TSANZ) position statement on chronic suppurative lung disease and bronchiectasis in children, adolescents and adults in Australia and New Zealand. 2023, 28, 339–349. [CrossRef]
- Ding L, Wang J, Cai S, Smyth H, Cui Z. Pulmonary biofilm-based chronic infections and inhaled treatment strategies. Int J Pharm. 2021, 604, 120768. [CrossRef]
- Abedon, ST. Ecology and Evolutionary Biology of Hindering Phage Therapy: The Phage Tolerance vs. Phage Resistance of Bacterial Biofilms. Antibiotics (Basel). 2023, 12, 245. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).