Introduction
There is increased evidence that COVID-19 infection spreads regardless of gender, age, comorbidities, or other transient health adversities. However, after the infection establishment, the disease's progress and severity look clearly influenced by all these conditions [
1]. These insights have become available because of the cumulative number of studies rising from cohort studies that evaluate such combinatory events in patients admitted to hospitals worldwide [
2,
3,
4].
Tabagism has been proposed as an adverse risk factor for COVID-19-infected people, and the latest literature has discussed the subject [
5]. The rationality of this belief is consensual since active (still smoking) or non-active smokers (former smokers) are expected to have their respiratory capacity reduced to some extent due to the uptake of toxic substances accompanying the smoking practice [
5]. In earlier stages, this effect includes asymptomatic illnesses such as bronchiolitis, cancer, or emphysema.
In other words, the previous smoking-derived damage to the lungs is not counted for smokers suffering COVID-19 during hospitalization and intensive care period. However, inside this group, the time of smoking and the time passed after smoking abstinence are internal variances not registered [
5]. The combinatory effect of these conditions and risk factors (obesity, hypertension, age, among others) makes it difficult to understand the contribution of tabagism as an influential adverse factor for individuals affected by COVID-19 complications.
Some studies plead for adversities associated with active smokers due to their habits, including sharing cigarettes, using cigarette accessories, and other devices (narguile) without adequate cleanliness [
6]. Different observational studies have also investigated correlations between smoking practice and the severity of COVID-19 in patients admitted to hospitals [
5,
6,
7,
8]. Although some studies were inconclusive, others suggested a positive correlation between tabagism and COVID-severity and death [
5,
6,
8]. A study based on meta-analyses revealed that, in some cases, smokers are less susceptible to COVID-19. However, once the infection is established, smokers present a worse prognosis than non-smokers [
9]. In this context, Bernardis and Busà [
10] raised the hypothesis that tobacco smoking could provide protective status, at least in the initial phase of infection, against SARS-CoV-2. Based on that, many studies have sought insights and information to understand this puzzle.
In the present study, it is proposed that the tabagism practice, exposure, repeatedly (daily) and for a long time (years), the smoker to the tobacco mosaic virus (TMV), a non-infectious to humans but immunogenic virus present in the tobacco, a component of cigarettes. TMV is an RNA virus with a protein coat (CP). TMV-CP is the protein with the highest contact with humans during the consumption of cigarettes. So, whether TMV proteins might share possible common antigenic epitopes with the SARS-CoV-2 proteins is an exciting challenge. Through bioinformatics analysis, it was possible to identify epitopes of type-B lymphocytes and major histocompatibility complexes (MHC) I and II, which could trigger an immune response against TMV-CP. Additionally, we investigated the overall performance of one hundred individuals (50 smokers and 50 non-smokers) diagnosed with COVID-19. We were admitted to a specialized Hospital in Fortaleza, Ceara, Brazil, to get insights into this proposal.
Methodology
Ethical Concerns
The access and all the procedures for data collection and analysis were carried out after due approval of the Institutional Ethical Committee and registered in the Plataforma Brazil (Register code N. 5.132.180), fulfilling all current Brazilian Legislation concerned.
Study Design, Setting, and Participants
Two independent groups of data, composed of fifty patients each, were included in the study. These separated smoking (n=50) and non-smoking ones (n=50). All were clinically diagnosed with COVID-19 infection and hospitalized (March-July, 2021) at public Hospital São José de Doenças Infecciosas (Fortaleza, Brazil). All individuals included in the study were older than 18 years. Gender and age were not further considered in groups.
Patients have not assessed themselves and were not identified in the text. Only their medical records, deposited in the Hospital documental repository, were recorded for data collection. The subjects were classified into the smoking and non-smoking groups according to the following parameters: death or recovery; time of hospital care (days) and classified as non-severe (without the need for ventilation support); severe (with use of non-invasive oxygen support) and critical (with use of invasive oxygen supplier) COVID-19 infection. Two additional laboratorial parameters, C-reactive protein (inflammatory marker) and Lactate dehydrogenase (biochemical marker) levels, were annotated to better characterize the clinical status of the patients included in the study. These data were informed in their medical records.
Statistical Analysis
For continuous variables, the mean (SD) and median (IQR) were used for normally and abnormally distributed data. Categorical variables were expressed as counts (%). For laboratory test results recorded, it was determined whether the measurement results were outside the normal range.
Results
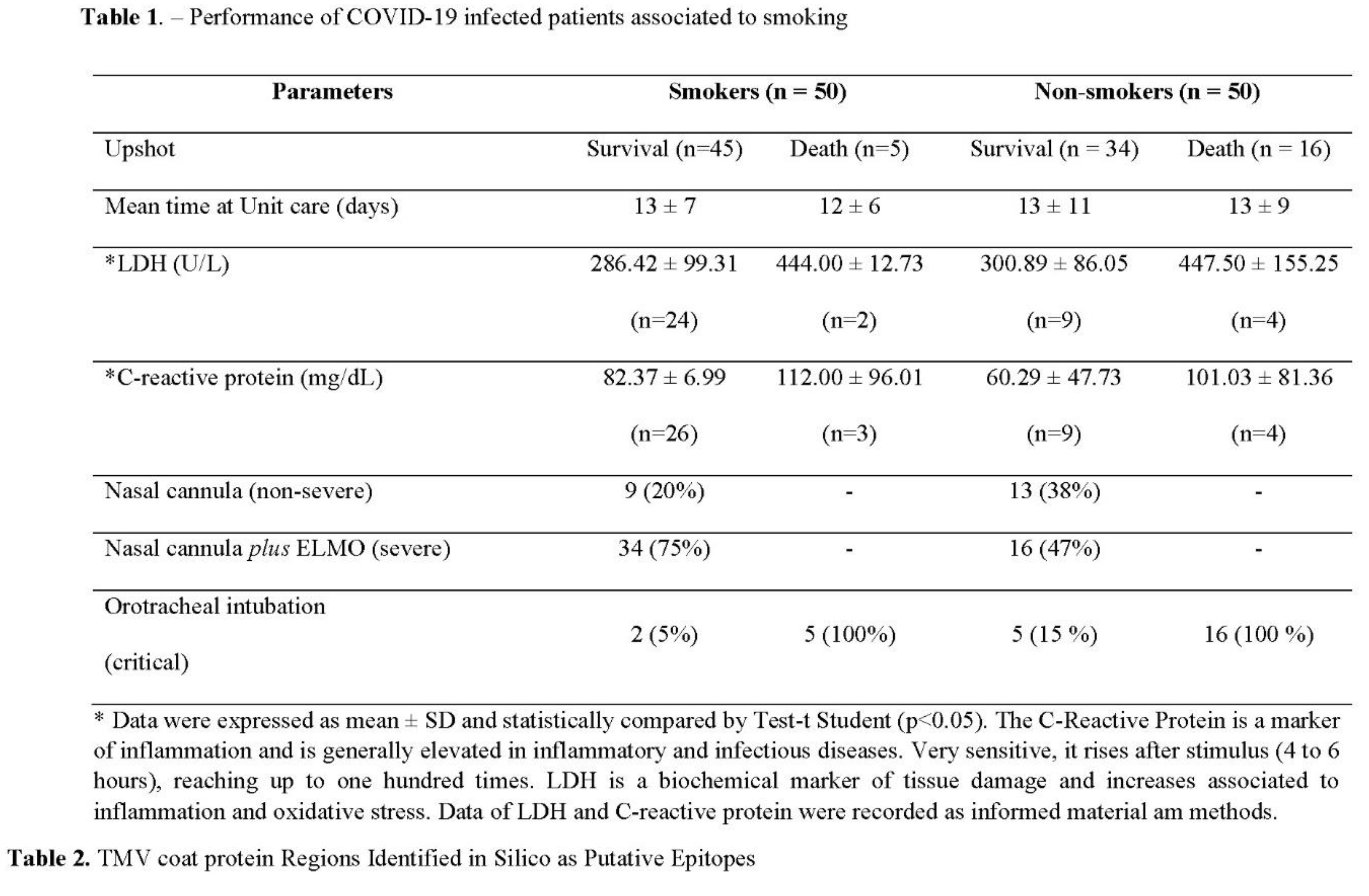
As observed, the prevalence of death documented among non-smoking individuals infected by COVID-19 was more than three times higher than among individuals with historic smoking practices (Table 1). Worth noting that the better survival performance of smoking patients did not correlate to a reduced time of hospital internment. Two acute or chronic inflammatory markers, C-reactive Protein (CRP) and Lactate Dehydrogenase (LDH) levels, have been accepted as immediate criteria for COVID-19 progression. Although the determined levels of CRP and LDH were highly elevated in all infected COVID patients, both markers were similarly higher in those with death or survival upshot, whether smoking or non-smoking (Table 1). Therefore, both markers were suitable for confirming the acute inflammation status of all patients, but their increased detection did not correlate with smoking and non-smoking individuals. Stratifying patients' performance according to their need for supplementary O2 support did not produce a clear picture among the groups (Table 1). Therefore, the only apparent difference among the groups was higher severity and survival among smokers.
Based on Table 1, the TMV-CP was analyzed through bioinformatics tools to evaluate the possibility of generating epitopes for B-cells. Raw data are in Table 2. Three tools were employed as described in the methods. Fifteen potential epitopes were predicted by the three tools employed in the sequence of TMV-CP (Table 2). Five were identified by ElliPro (Figure 1A), three by Epitopia (Figure 1B), and seven by DiscoTope (Figure 1C).
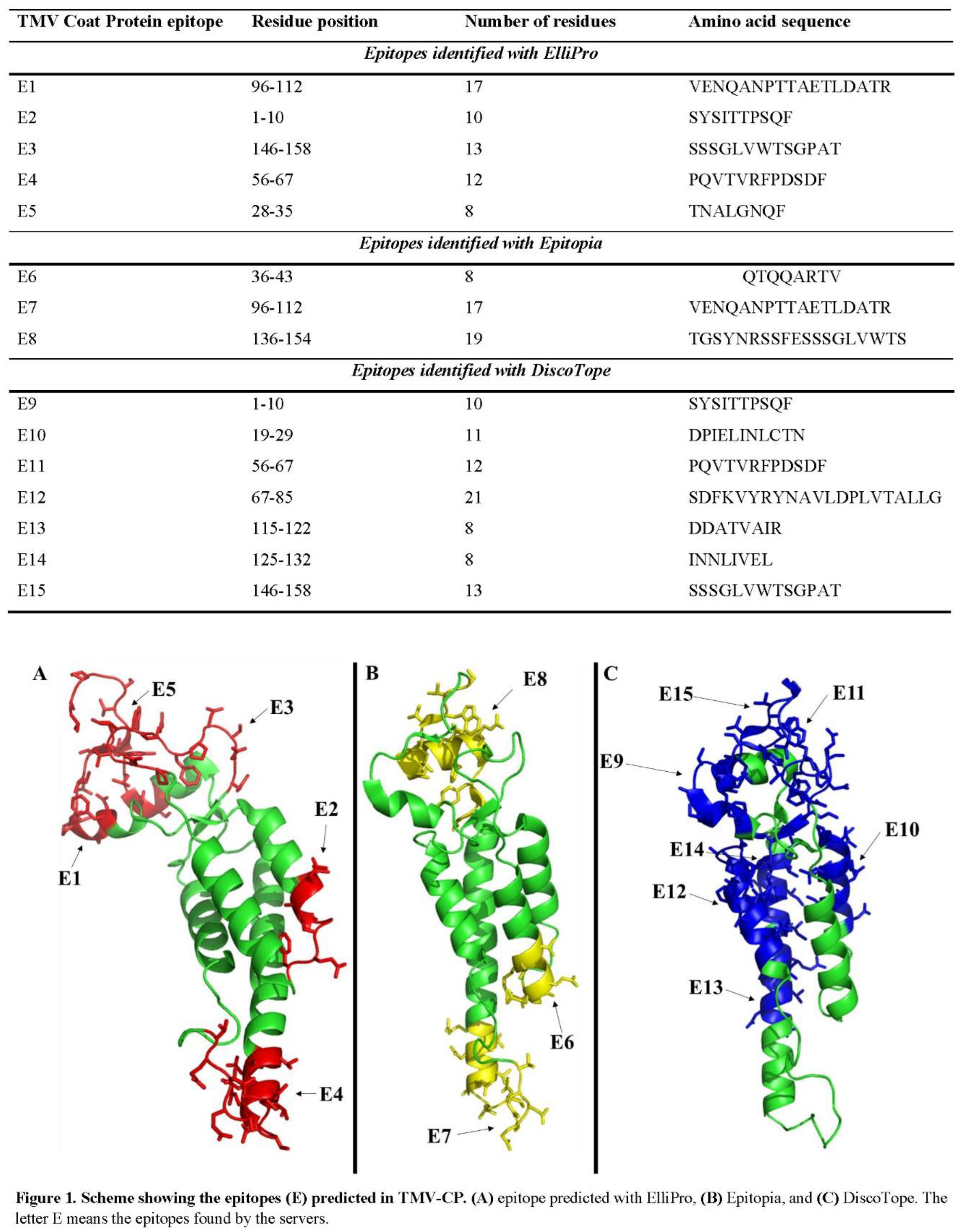
Interestingly, there were some epitopes identified independently by two tools. For example, the epitope VENQANPTTAETLDATR was identified by both, ElliPro and Epitopia. The epitopes SYSITTPSQF, SSSGLVWTSGPAT, and PQVTVRFPDSDF were identified by ElliPro and Discotope (Table 2). Epitopia and Discotope predicted no common epitopes.
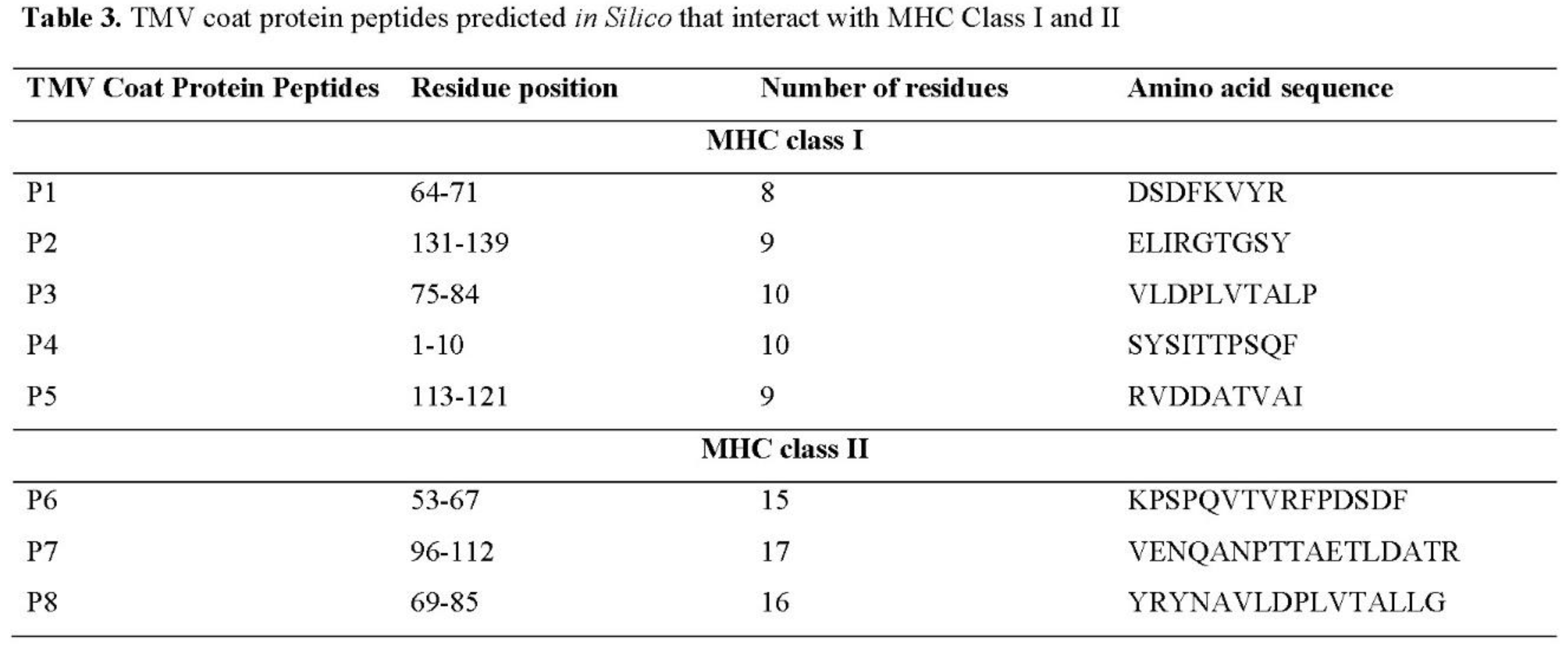
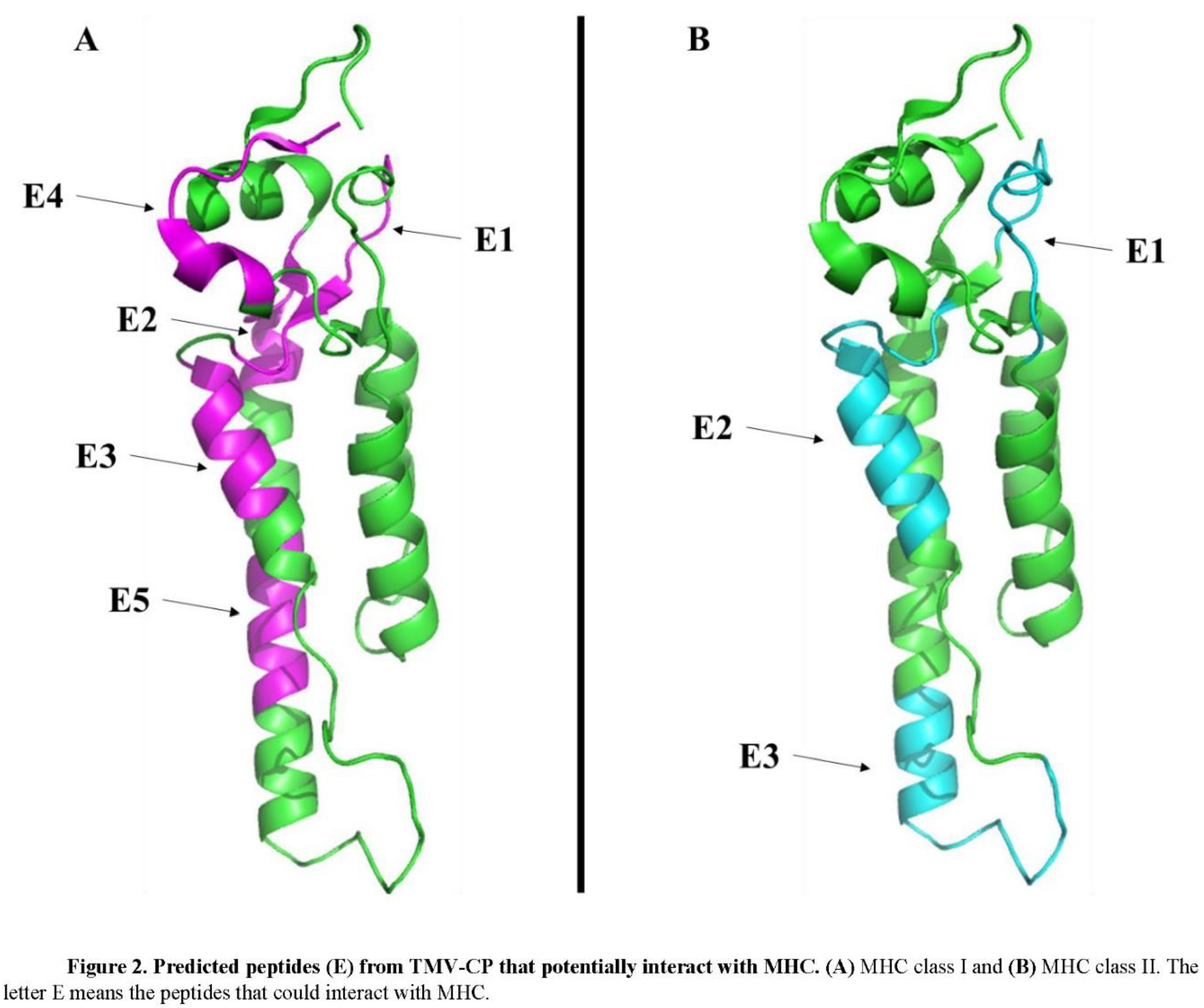
In epitope prediction, it was also performed prediction about the presence of peptides in the TMV-CP that could interact with either MHC-I or MHC-II (Table 3). The analysis, which ran out on TepiTool, predicted eight peptides, five potentially interacting with MHC-I (Figure 2A) and three with MHC-II (Figure 2B). Interestingly, the sequence SYSITTPSQF predicted by ElliPro and Discotope (Table 2) as epitope also was predicted with the ability to interact with MHC-I. The epitope VENQANPTTAETLDATR identified by ElliPro and Epitopia was predicted to interact with MHC-II (Table 3). These predictions suggest antibodies against TMV-CP can be found in smokers’ patients.
Discussion
Innate and acquired immunity differentiate themselves in numerous biochemical, physiological, and immunological aspects. Although both biological processes are responses triggered by the broadest abnormal circumstances faced by the body (infections, tissue damage, trauma, edema, etc.), each pathway performs a complex, partially independent, and highly orchestrated multi-mediated action [
10]. The time course of innate and acquired immunity seemingly have profound implications in the rapid course of COVID-19 infection.
First-time infected COVID-19 individuals are expected not to possess acquired immunity against the virus. Therefore, the innate system mediates the only driving physiological response that triggers an immediate unfocused inflammatory response since the virus is widespread in the organism, despite its preferable binding site (ACR-2). This agrees with the cytokine storm always observed in severe COVID-19 patients. Accordingly, both non-smokers and smoking individuals included in the present analysis were shown to have highly increased levels of two key inflammatory markers. The acquired immunity needs the help of innate immunity to initiate its role in the protective process that will culminate with the synthesis and release of specific antibodies capable of inactivating or down-regulating the viral activity [
15,
16,
17].
The observation that smoking individuals perform better in COVID-19 infection than non-smokers was previously commented on by Landoni et al. [
9]. Other studies have discussed the COVID-19 establishment in three groups: 1) current smokers, 2) former smokers, and 3) non-smokers [
18]. The data presented by Constantine and Nikitara [
18] revealed that smokers had 1.4 and 2.4 more times, respectively, to develop severe symptoms of COVID-19 and need for mechanical ventilation, or die, compared to non-smokers. These results contrast with those shown here (Table 1). In the present data set, from 50 smokers and 50 non-smokers, 5 and 16 died of COVID-19 (Table 1).
These data support the hypothesis that smokers could have a differential response to COVID-19 infection, posted by Bernardisa and Busàb [
10]. However, this belief does not involve the downregulation of acute inflammatory reaction or cytokine storm reduction, primarily established in the curse of the innate response.
Many other studies indirectly also support this hypothesis. During the infection by a new pathogen (i.e., SARS-CoV-2), the immune system requires time to respond appropriately. Sometimes, pathogens are faster in causing infection than the immune system to produce a response, leading to death.
Regarding respiratory viruses such as SARS-CoV-2, the type I and III interferons compose the essential anti-viral response [
19,
20]. For example, a study revealed that pretreatment with type I and III interferon reduced SARS-CoV-2 replication in vitro [
21]. However, the timing of interferon release is critical for the host, and the dysregulation in the interferon release could lead to a cytokine storm leading to severe COVID-19 [
21]. The interferons are also crucial to the body's defense to plant viruses, such as TMV [
22]. Even though TMV cannot cause infection in humans, it induces an increase in interferon levels but could be important in the first contact with SARS-CoV-2. In the case of smokers, the daily exposition to TMV, a non-pathogenic but immunogenic virus particle, by oral use of tobacco could chronically, over the years, stimulate a natural antibody-mediated antiviral response in the respiratory mucosa conferring initial resistance to initial steps of SARS-CoV-2 infection.
TMV RNA is present in sputum and saliva specimens of smokers and absent in non-smokers, as expected [
23,
24]. In an exciting study, Li et al. [
25] reported that the transfection of TMV RNA in HeLa cells leads to the production of TMV-CP and, thus, induction of autophagy activated by Toll-like receptors initiating antiviral responses. Additionally, a study of antibody production revealed that both mice and humans produced anti-TMV antibodies after exposure to TMV [
26,
27]. In the human case, the authors identified the increase in levels of IgG (subclasses IgG1, IgG2, IgG3, and IgG4), IgM, and IgA [
27]. The induction of IgA is exciting, and the IgA antibody is abundant and essential for mucosal defense against respiratory infections [
28]. So, it is feasible to suggest those IgA antibodies produced by TMV in smokers could help them not develop a mild version of COVID-19, whether a cross-reaction takes place.
These results and arguments agree with our bioinformatics results. We have shown that TMV-CP protein has many sequences that might act as epitopes for B-cells and thus could induce the production of antibodies (Table 2 and Fig. 1). Additionally, bioinformatics analysis predicted TMV-CP also could generate peptides that trigger the MHC I and II to induce an immune response (Table 3 and Fig. 2).
Conclusions
Altogether, the results presented here and from the literature strengthen the hypothesis that somehow and in some patients' previous smoke history might help them to fight against SARS-CoV-2, at least in the initial stage of infection. The prediction of antibodies (IgA) production induced by TMV-CP might be acting by immune cross-reaction to neutralize the SARS-CoV-2. Although these results gave more robustness about this paradoxical effect of smoke on COVID-19 establishment, more profound studies even, including the genetics of the population and more biochemical markers, are required to understand better the correlation between smoking and COVID-19 outcome.
Data Availability Statement
Data are available under reasonable requirements.
Acknowledgments and Funding
The authors are grateful to the São José Hospital administration for providing access to the medical records. Pedro F. N. Souza has a grant for senior researchers from CNPq at process number 305003/2022-4.
Conflict of Interest
The authors have declared no conflict of interest, including financial, personal, or other relationships with other people or organizations.
References
- E.A. Oliveira, E.A. E.A. Oliveira, E.A. Colosimo, A.C. Simões e Silva, R.H. Mak, D.B. Martelli, L.R. Silva, H. Martelli-Júnior, M.C.L. Oliveira, Clinical characteristics and risk factors for death among hospitalised children and adolescents with COVID-19 in Brazil: an analysis of a nationwide database, Lancet Child Adolesc. Heal. 5 (2021) 559–568. [CrossRef]
- R. Zakeri, R. R. Zakeri, R. Bendayan, M. Ashworth, D.M. Bean, H. Dodhia, S. Durbaba, K. O’Gallagher, C. Palmer, V. Curcin, E. Aitken, W. Bernal, R.D. Barker, S. Norton, M. Gulliford, J.T.H. Teo, J. Galloway, R.J.B. Dobson, A.M. Shah, A case-control and cohort study to determine the relationship between ethnic background and severe COVID-19, EClinicalMedicine. 28 (2020). [CrossRef]
- L. Huang, Q. L. Huang, Q. Yao, X. Gu, Q. Wang, L. Ren, Y. Wang, P. Hu, L. Guo, M. Liu, J. Xu, X. Zhang, Y. Qu, Y. Fan, X. Li, C. Li, T. Yu, J. Xia, M. Wei, L. Chen, Y. Li, F. Xiao, D. Liu, J. Wang, X. Wang, B. Cao, 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study, Lancet. 398 (2021) 747–758. [CrossRef]
- S. Søvik, P.M. S. Søvik, P.M. Bådstøløkken, V. Sørensen, P. Langeland Myhre, C. Prebensen, T. Omland, J.E. Berdal, A single-centre, prospective cohort study of COVID-19 patients admitted to ICU for mechanical ventilatory support, Acta Anaesthesiol. Scand. 65 (2021) 351–359. [CrossRef]
- C.A. Jiménez-Ruiz, D. C.A. Jiménez-Ruiz, D. López-Padilla, A. Alonso-Arroyo, R. Aleixandre-Benavent, S. Solano-Reina, J.I. de Granda-Orive, COVID-19 and Smoking: A Systematic Review and Meta-Analysis of the Evidence, Arch. Bronconeumol. 57 (2021) 21–34. [CrossRef]
- A.L.O. Da Silva, J.C. A.L.O. Da Silva, J.C. Moreira, S.R. Martins, COVID-19 and smoking: a high-risk association, Cad. Saude Publica. 36 (2020). [CrossRef]
- L. Arcavi, N.L. L. Arcavi, N.L. Benowitz, Cigarette smoking and infection, Arch. Intern. Med. 164 (2004) 2206–2216. [CrossRef]
- W. Engl. J. Med. 382 (2020) 1708–1720. [CrossRef]
- Recent exposure to smoking and COVID-19. – ScienceOpen, (n.d.). https://www.scienceopen.com/document?vid=5bc165ae-6d8e-4a5b-bba8-01079266c817 (accessed , 2022). 11 March.
- E. de Bernardis, L. E. de Bernardis, L. Busà, A putative role for the tobacco mosaic virus in smokers’ resistance to COVID-19, Med. Hypotheses. 143 (2020) 110153. [CrossRef]
- J. Ponomarenko, H.H. J. Ponomarenko, H.H. Bui, W. Li, N. Fusseder, P.E. Bourne, A. Sette, B. Peters, ElliPro: a new structure-based tool for the prediction of antibody epitopes, BMC Bioinformatics. 9 (2008). [CrossRef]
- N.D. Rubinstein, I. N.D. Rubinstein, I. Mayrose, E. Martz, T. Pupko, Epitopia: a web-server for predicting B-cell epitopes, BMC Bioinformatics. 10 (2009) 287. [CrossRef]
- P. Haste Andersen, M. P. Haste Andersen, M. Nielsen, O. Lund, Prediction of residues in discontinuous B-cell epitopes using protein 3D structures, Protein Sci. 15 (2006) 2558–2567. [CrossRef]
- S. Paul, J. S. Paul, J. Sidney, A. Sette, B. Peters, TepiTool: A Pipeline for Computational Prediction of T Cell Epitope Candidates, Curr. Protoc. Immunol. 114 (2016) 18.19.1-18.19.24. [CrossRef]
- V.K. Shah, P. V.K. Shah, P. Firmal, A. Alam, D. Ganguly, S. Chattopadhyay, Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past, Front. Immunol. 11 (2020) 1949. [CrossRef]
- A. Sette, S. A. Sette, S. Crotty, Adaptive immunity to SARS-CoV-2 and COVID-19, Cell. 184 (2021) 861. [CrossRef]
- S.C. Jordan, Innate and adaptive immune responses to SARS-CoV-2 in humans: relevance to acquired immunity and vaccine responses, Clin. Exp. Immunol. 204 (2021) 310–320. [CrossRef]
- C.I. Vardavas, K. C.I. Vardavas, K. Nikitara, COVID-19 and smoking: A systematic review of the evidence, Tob. Induc. Dis. 18 (2020). [CrossRef]
- C. Cheroni, L. C. Cheroni, L. Manganaro, L. Donnici, V. Bevilacqua, R.J.P. Bonnal, R.L. Rossi, R. De Francesco, Novel interferon-sensitive genes unveiled by correlation-driven gene selection and systems biology, Sci. Reports 2021 111. 11 (2021) 1–15. [CrossRef]
- M. Immunol. 11 ( 2020. [CrossRef]
- N. Vabret, G.J. N. Vabret, G.J. Britton, C. Gruber, S. Hegde, J. Kim, M. Kuksin, R. Levantovsky, L. Malle, A. Moreira, M.D. Park, L. Pia, E. Risson, M. Saffern, B. Salomé, M. Esai Selvan, M.P. Spindler, J. Tan, V. van der Heide, J.K. Gregory, K. Alexandropoulos, N. Bhardwaj, B.D. Brown, B. Greenbaum, Z.H. Gümüş, D. Homann, A. Horowitz, A.O. Kamphorst, M.A. Curotto de Lafaille, S. Mehandru, M. Merad, R.M. Samstein, M. Agrawal, M. Aleynick, M. Belabed, M. Brown, M. Casanova-Acebes, J. Catalan, M. Centa, A. Charap, A. Chan, S.T. Chen, J. Chung, C.C. Bozkus, E. Cody, F. Cossarini, E. Dalla, N. Fernandez, J. Grout, D.F. Ruan, P. Hamon, E. Humblin, D. Jha, J. Kodysh, A. Leader, M. Lin, K. Lindblad, D. Lozano-Ojalvo, G. Lubitz, A. Magen, Z. Mahmood, G. Martinez-Delgado, J. Mateus-Tique, E. Meritt, C. Moon, J. Noel, T. O’Donnell, M. Ota, T. Plitt, V. Pothula, J. Redes, I. Reyes Torres, M. Roberto, A.R. Sanchez-Paulete, J. Shang, A.S. Schanoski, M. Suprun, M. Tran, N. Vaninov, C.M. Wilk, J. Aguirre-Ghiso, D. Bogunovic, J. Cho, J. Faith, E. Grasset, P. Heeger, E. Kenigsberg, F. Krammer, U. Laserson, Immunology of COVID-19: Current State of the Science, Immunity. 52 (2020) 910–941. [CrossRef]
- P. Orchansky, M. P. Orchansky, M. Rubinstein, I. Sela, Human interferons protect plants from virus infection, Proc. Natl. Acad. Sci. U. S. A. 79 (1982) 2278–2280. [CrossRef]
- F. Balique, P. F. Balique, P. Colson, D. Raoult, Tobacco mosaic virus in cigarettes and saliva of smokers, J. Clin. Virol. 55 (2012) 374–376. [CrossRef]
- R.A. LeClair, Recovery of culturable tobacco mosaic virus from sputum and thoracentesis fluids obtained from cigarette smokers with a history of pulmonary disease, Am. Rev. Respir. Dis. 95 (1967) 510–511. [CrossRef]
- L. Li, L. L. Li, L. Wang, R. Xiao, G. Zhu, Y. Li, C. Liu, R. Yang, Z. Tang, J. Li, W. Huang, L. Chen, X. Zheng, Y. He, J. Tan, The invasion of tobacco mosaic virus RNA induces endoplasmic reticulum stress-related autophagy in HeLa cells, Biosci. Rep. 32 (2012) 171–184. [CrossRef]
- F. Balique, P. F. Balique, P. Colson, A.O. Barry, C. Nappez, A. Ferretti, K. Al Moussawi, T. Ngounga, H. Lepidi, E. Ghigo, J.L. Mege, H. Lecoq, D. Raoult, Tobacco mosaic virus in the lungs of mice following intra-tracheal inoculation, PLoS One. 8 (2013). [CrossRef]
- R. Liu, R.A. R. Liu, R.A. Vaishnav, A.M. Roberts, R.P. Friedland, Humans have antibodies against a plant virus: evidence from tobacco mosaic virus, PLoS One. 8 (2013). [CrossRef]
- A. Bonner, A. A. Bonner, A. Almogren, P.B. Furtado, M.A. Kerr, S.J. Perkins, Location of secretory component on the Fc edge of dimeric IgA1 reveals insight into the role of secretory IgA1 in mucosal immunity, Mucosal Immunol. 2009 21. 2 (2008) 74–84. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).








