1. Introduction
The perception of a sound that has not been originated in any source outside or inside the body is named tinnitus [
1]. This auditory perception is rather heterogeneous in its risk factors as well as mechanisms [
2], laterality, type of sound and frequency [
3], and the associated tinnitus-related distress [
4]. Despite its prevalence (it affects around 10-15 % and impact severely on the life of roughly 0.5-2 % of adult people), there is not any approved medicine for the cure of tinnitus [
5]. However, many treatments are available to relief tinnitus distress, including psychological (e.g. Cognitive Behavioral Therapy (CBT)) and sound stimulation (e.g. Tinnitus Retraining Therapy (TRT)) [
6].
Tinnitus arises in the neural auditory pathway due to aberrant compensation response to deafferentiation from the peripheral auditory system [
7]. This deafferentiation triggers plastic changes in the auditory pathway, including hyperactivity, hypersynchrony and/or reorganization of the tonotopic map, which lead to tinnitus [
7]. Deafferentation can be produced, for instance, by hearing loss (even hidden hearing loss [
8]).
Once the tinnitus is triggered, other neural systems, as the amygdala and the hippocampus, are involved in the strengthening of the tinnitus-related distress [
9]. The exacerbation of the network between the auditory and limbic systems is especially relevant in the growing and worsening of emotional symptoms of tinnitus, such as stress, anxiety and/or depression [
9]. Therefore, an enhanced understanding of the auditory-limbic network should contribute to the design of new strategies for tinnitus treatment [
10]. The TRT is the treatment applied in many audiological clinics that takes into account this auditory-limbic interrelation, which is also known as the neurophysiological model of tinnitus [
9]. The main goal of TRT is to achieve tinnitus habituation through counselling and sound therapy. Whilst counselling acts on the limbic system providing habituation to the negative reactions, sound therapy works more on the auditory system to decrease the aberrant neural activity related to tinnitus [
9].
Although standard TRT uses a broadband noise in the audible frequencies, many other sounds have been proposed for tinnitus treatment [
11,
12,
13]. According to Jasterboff and Jastreboff [
14], any type of sound that does not annoy, create discomfort, or damage hearing is better for tinnitus than silence. Nevertheless, customized sounds, personalized either to the tinnitus frequency [
15,
16,
17,
18] or the hearing thresholds [
19,
20,
21,
22], tend to provide more tinnitus relief.
Schaette and Kempter [
23,
24] established that a customized wideband noise matched to the hearing curve of the subject could reverse tinnitus. Noreña and Eggermont [
25,
26] established that a group of animals with altered cortical tonotopic maps (one of the mechanisms of tinnitus) due to a traumatizing noise, returned to their normal tonotopic map when they were exposed to an enriched acous tic environment (EAE) containing a random sequence of tone-pips. This sequence of tone-pips was applied mainly in animal models [
27,
28]. Noreña and Chery-Croze [
29], on the other hand, used another sequential EAE, in this case tone-bursts of random frequency and amplitude proportional to the hearing thresholds, as a sound therapy to decrease the hypersen sitivity suffered by a group of subjects with hyperacu sis. Cobo [
19] adapted this EAE to tinnitus sound therapy, adding a new sequence of gammatones.
Therefore, EAE therapy of tinnitus can be implemented either as a sequence of tones or as a continuous broadband sound matched to the Hearing Level (HL) curves. This article is aimed to compare the efficiency of EAE therapy, using these sequential and continuous types of sound stimuli, on the relief of tinnitus-related distress on a sample of subjects.
2. Materials and Methods
2.1. Subjects
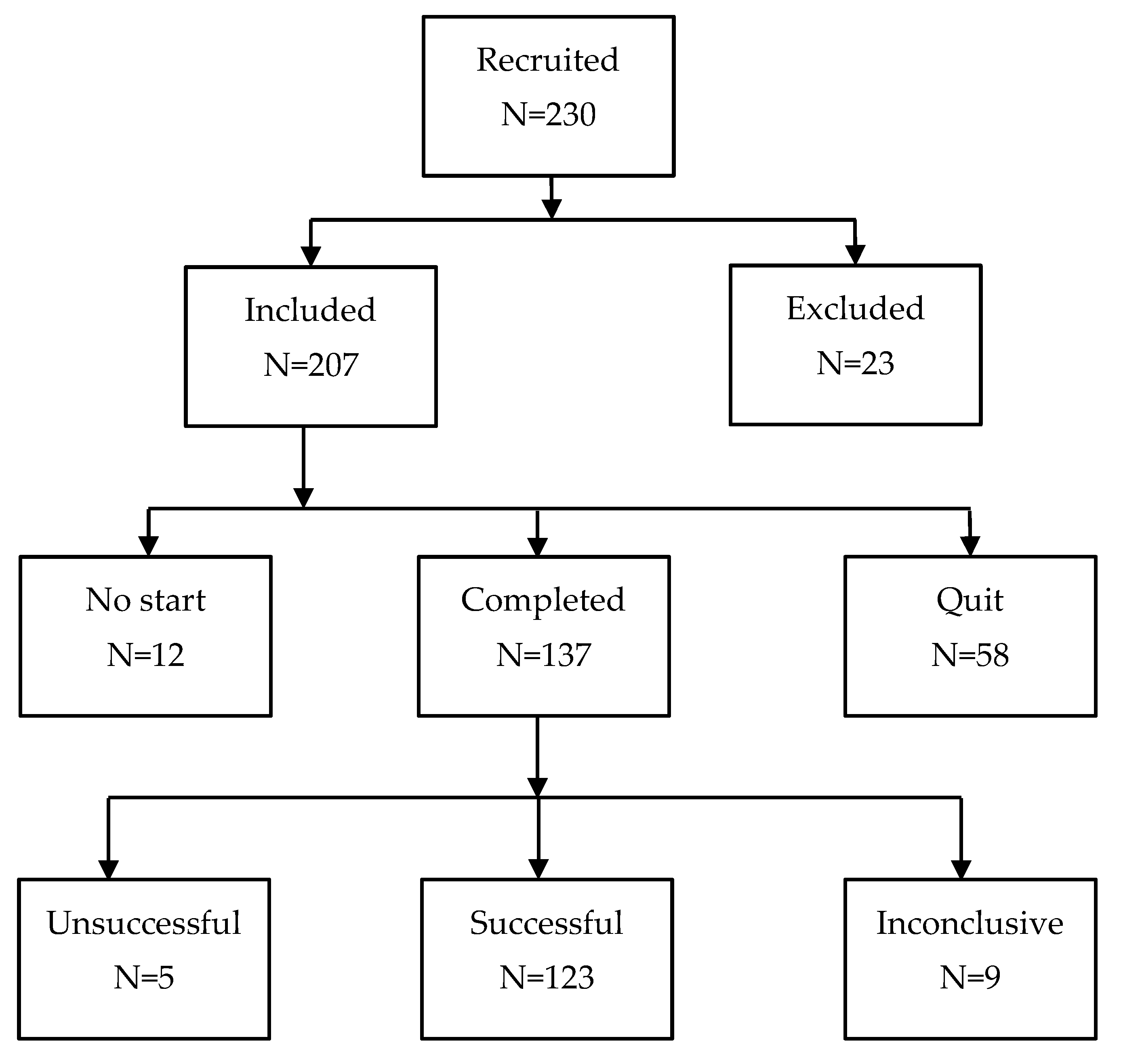
The study was approved by the Institutional CSIC Ethics Committee (internal code 004/2022) and was conducted in accordance with the Spanish Law of Data Protection (RD1720/2007). 230 volunteers with tinnitus (see
Figure 1), which gave their written informed consent, were recruited through a call in our institutional webpage (Estudio clínico sobre terapias sonoras del acúfeno | ITEFI.csic.es).
Patients enrolled in tinnitus clinical studies must comply with some selection requirements. Exclusion/inclusion criteria usually includes temporal (duration of the tinnitus), age (subjects within an age range), psychiatric (grade of psychiatric severity), audiological disorders (Meniere, severe hydrops) and tinnitus severity. This last requirement involves excluding patients with tinnitus outcome under some score. For instance, Bauer
et al. proposed a Tinnitus Handicap Inventory (THI) score of 36. In a previous pilot non-controlled observational study, Cuesta and Cobo [
31] excluded patients with THI≤32. However, a THI score reduction of 20 points is considered to be clinically relevant [
9]. Therefore, tinnitus subjects with THI≥20 were included in this study.
Thus, the criteria to be included in this study were as follows: subjects older than 18 and younger than 75 years, with non-pulsatile tinnitus, and with THI≥20 at baseline. Patients with recent ear surgery, severe Ménière syndrome, and hydrocephaly were excluded. After applying these criteria, 207 patients were primarily included and 23 were excluded.
From the 207 originally included subjects, 12 refused to start the treatment after explaining them its fundamentals and expectations. Other 58 subjects quit the study before finishing (they did not respond to the monthly follow-up controls), although it requires a short time period of 4 months. This leaves 137 patients completing the treatment (see
Figure 1). The treatment was successful for 123 of these subjects, unsuccessful for 5, and inconclusive for 9 others.
Table 1 summarizes the mean and standard deviation age and gender of the 123 successful participants. Female participants were, on average, 3 years younger.
2.2. Hearing levels
Since this sound therapy stimulates selectively the auditory system of participants according to their HL, an audiometry is needed for each patient. Before the COVID19 lockdown (March 14, 2020), the HL of each participant were measured in our Audiology Room by pure-tone audiometry using pure tones at eleven pre-specified frequencies between 125 and 8000 Hz. After the COVID 19 lockdown, subjects had to provide their hearing threshold curves measured in any external clinic.
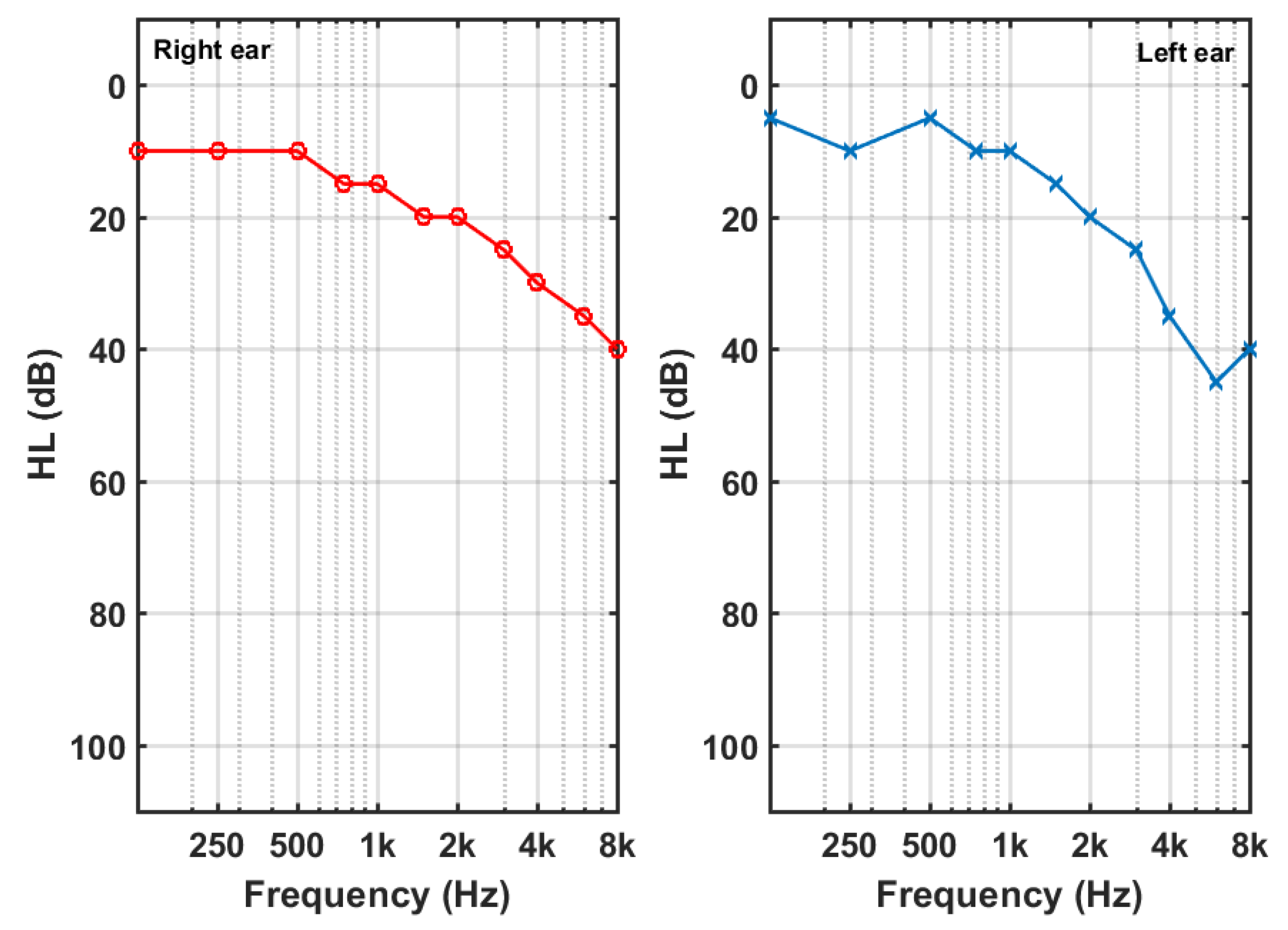
The average audiograms of all participants are shown in
Figure 2. Tinnitus participants displayed high-frequency hearing losses, a little larger at the left ear, and higher for male than female. The shaded area around the curves outlines the confidence intervals (±1.96 SD/sqrt(N)), being SD the standard deviation and N the number of subjects.
2.3. Tinnitus characteristics
Case histories of participants were assessed just after they signed the informed consent using a tinnitus characteristics form.
The gathered information of participants included tinnitus aspects such as temporal variability, frequency and type of sound, and lateralization (bilateral, left or right ear) as well as their clinic history and tinnitus-related severity through a validated Spanish version of the THI [
32]. Furthermore, participants were asked about the possible etiology of their tinnitus, previous tinnitus treatments, and relevant comorbidities.
A custom-designed Graphical User Interface (GUI) was used to match the tinnitus pitch and sound type [
21]. This GUI generates a band-pass filtered noise, where the central frequency (CF) and bandwidth (BW) can be tuned. These parameters are varied to generate tonal (BW<0.01 CF), ringing (0.02 CF < BW < 0.1 CF), or hissing (BW > 0.1 CF) sound types.
For the tinnitus pitch, participants might to match the CF of the sound type to their own tinnitus sound. Firstly, they were familiarized to the sound type (tonal, ringing and hissing) and the effect of the BW. Then they identified roughly the sound type more similar to their tinnitus. Finally, they tuned closely the generated sound to their own tinnitus.
The mean and SD of the tinnitus duration, tinnitus pitch and THI at baseline of all successful participants, as well as for male/female, are summarized in
Table 2.
Females had significantly lesser tinnitus duration (2.8 years), lower tinnitus pitch (1905 Hz) and higher tinnitus severity (THI) at baseline (4.6). These differences were statistically significant at p<0.05.
2.4. Counselling
Participants were subjected to a unique counselling session of roughly 60 minutes just after they accepted to be enrolled in the study. A PowerPoint presentation was used to explain to the participants the fundamentals of the auditory system functioning, as well as the mechanisms, epidemiology, and possible treatments of tinnitus. After that, the bases of EAE were presented. Finally, the participants chose the EAE stimulus (either sequential or continuous) and they were instructed in its hearing protocol (volume of sound, type of headphones, time of hearing, etc.).
2.5. Sound therapy
Participants were exposed to sound therapy one hour per day along four months. The stimulus consisted of a stereo sound customized to their HL curves. The stimulus, an EAE, could be sequential or continues. The bases of both stimuli are presented in the following.
2.5.1. Sequential EAE
The equation for a sequential EAE is
where
fm is a random frequency within the audio frequency range (125 Hz-8 kHz),
Am is an amplitude factor given by
which contains the customization of the stimulus to the HL curves,
τm is the repetition time of tones (inverse of number of tones per second),
envm is the envelope of the tone
being (
α,γ) two parameters controlling the envelope of the tone-pips, w a time-window function (e.g. Hanning, Hamming, Blackman-Harris, etc. [
33]), and
bm is the Equivalent Rectangular Band (ERB), given by [
34,
35]
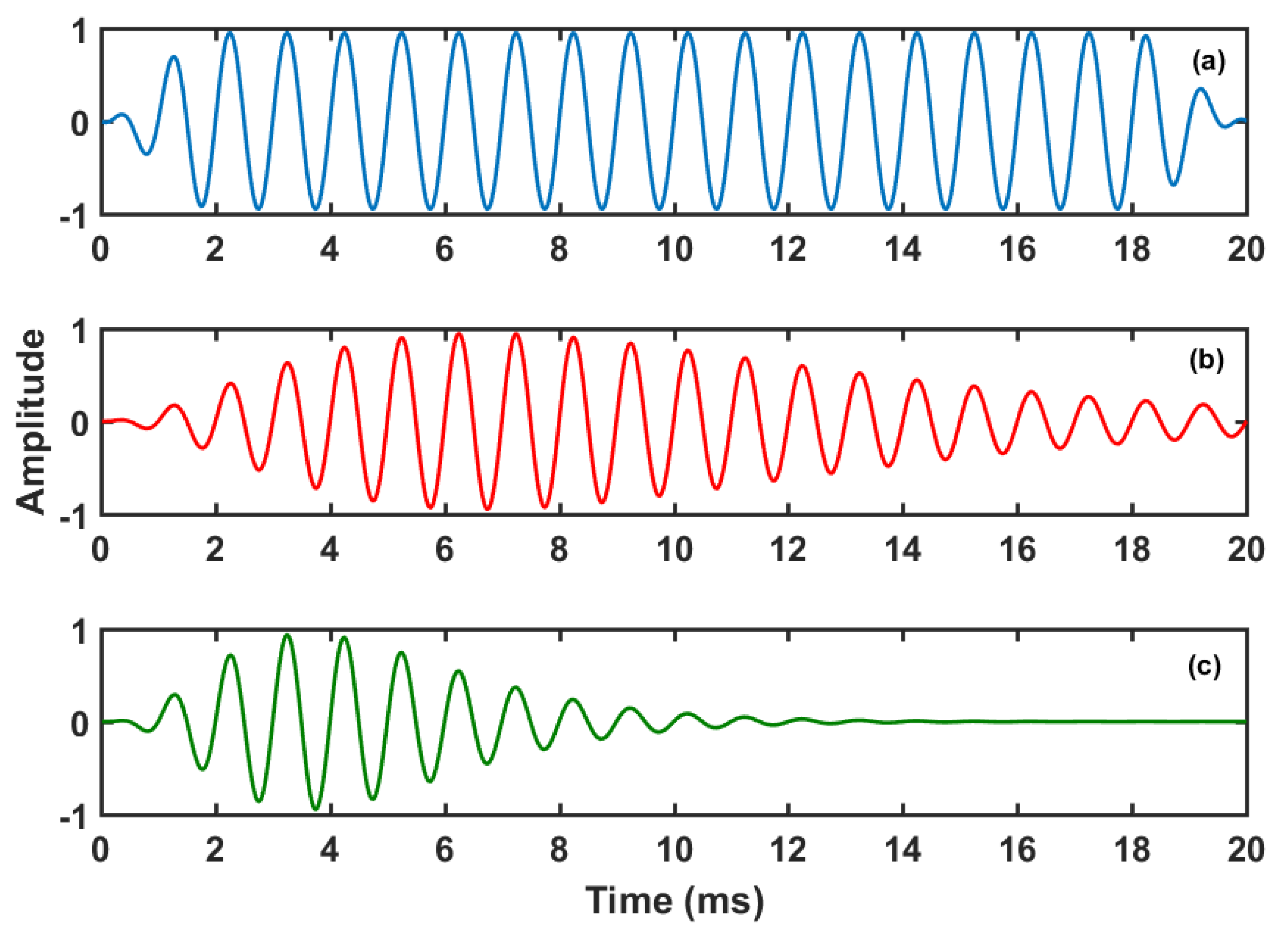
Figure 3 shows a tone-burst with Blackman-Harris window [
33], a tone-pip with (
α,γ)=(3,300) and a gammatone at 1000 Hz.
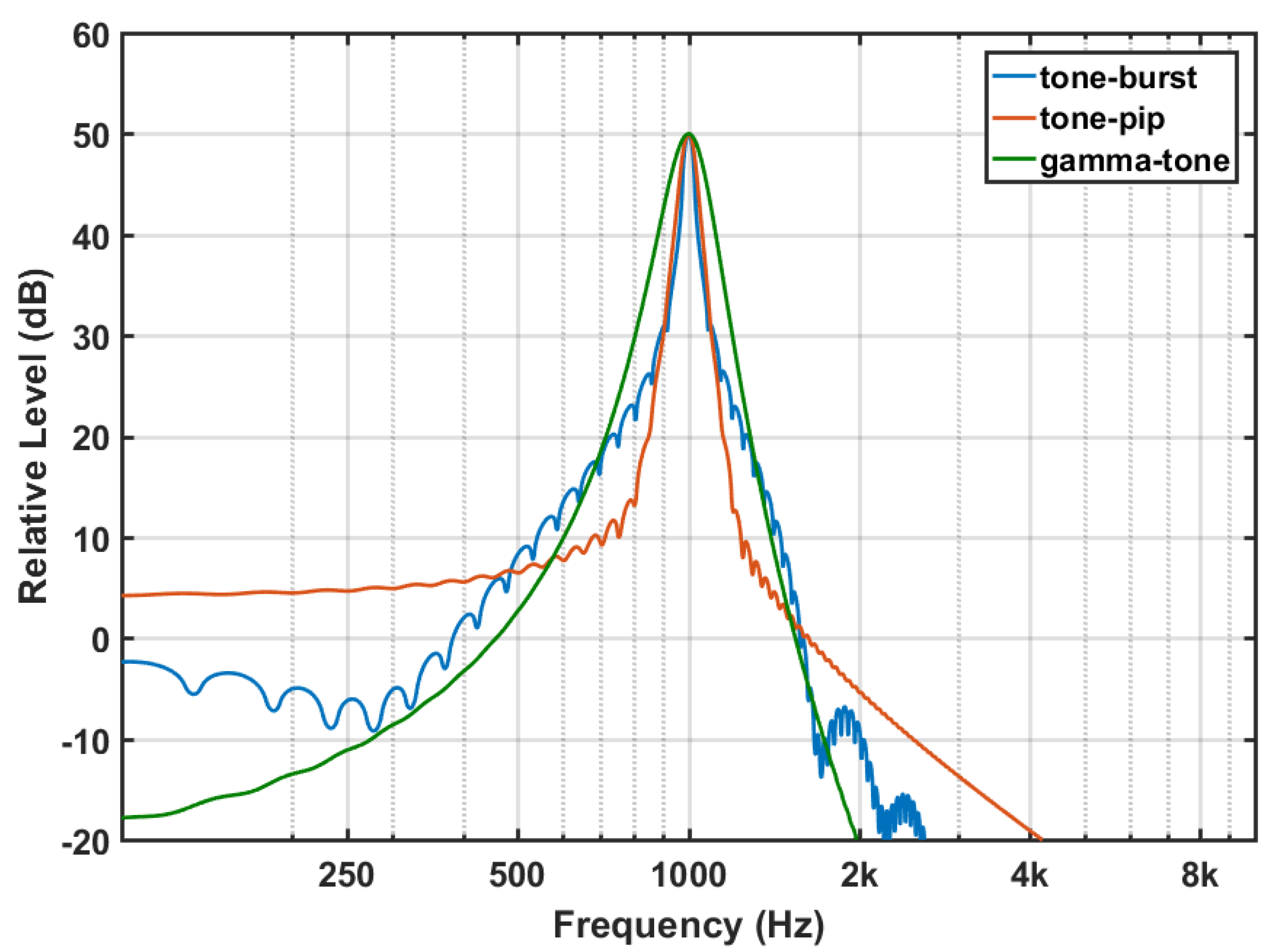
Figure 4 shows the corresponding log-spectra. The tone-burst has a symmetric envelope. Since this pulse is larger in the time domain, its main lobe in the frequency domain is shorter and has the typical side-lobes. The EAE with tone-burst is the same that the EAE proposed by Noreña and Chery-Croze [
29]. The tone-pip and gammatone are asymmetric and have also asymmetric log-spectra. The gammatone is shorter in the time domain and has a wider main-lobe. The EAE with tone-pips was already used by Noreña and Eggermont in animal models [
25,
26].
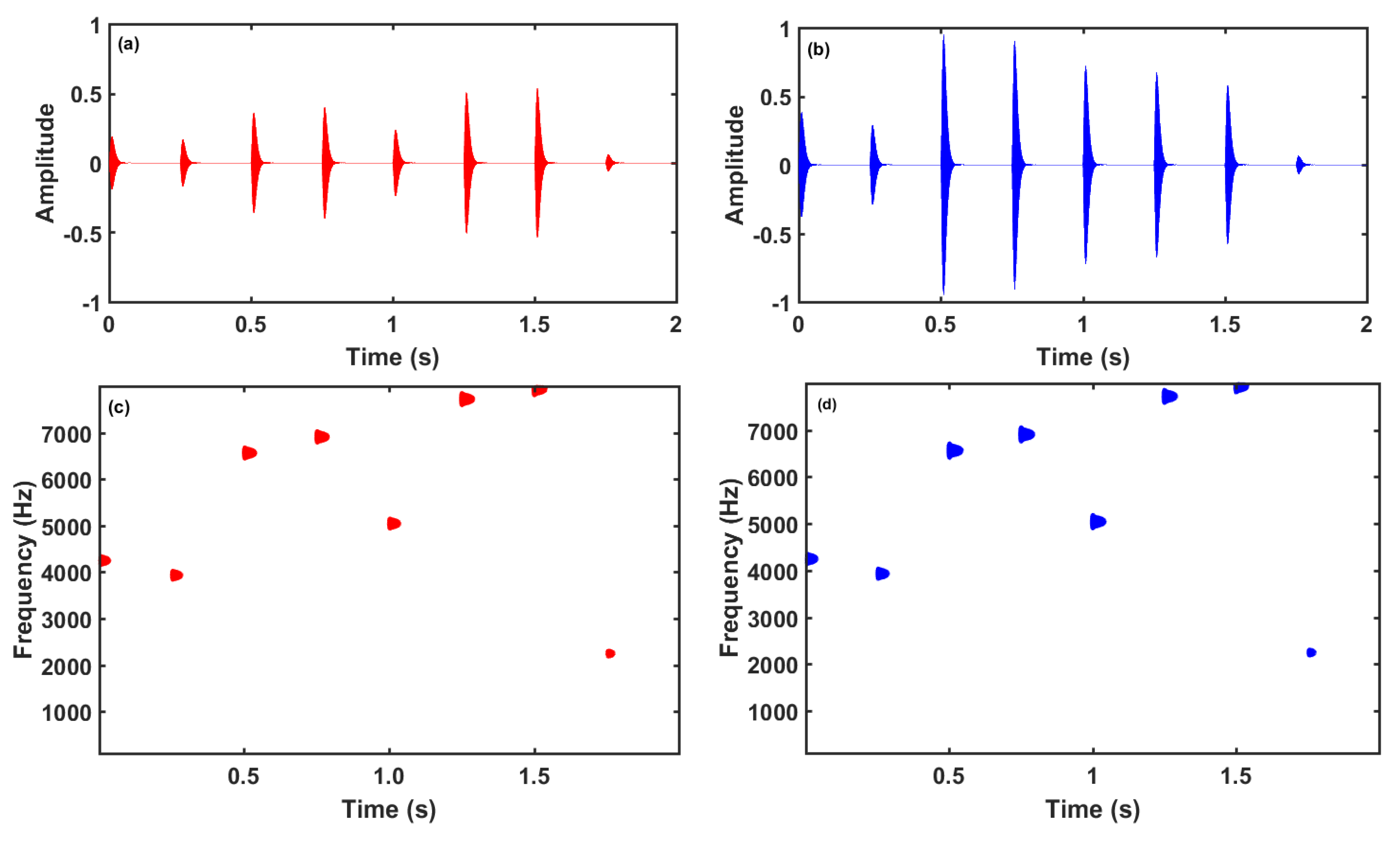
Let us illustrate the sequential EAE stimulus with an example.
Figure 5 shows the HL curves of a tinnitus subject, consisting of high frequency hearing losses in both ears. Furthermore, a slight scotoma at 6 kHz is observed in the left ear.
Figure 6 shows the first 2 seconds of a sequence of tone-pips with parameters (
α,γ)=(3,300) and
τ=0.25 s.
Since the HL is different in the right and left ears, the corresponding tone-pips have also distinct amplitudes. The random nature of the tone frequencies is better seen in the spectrograms of the sequences,
Figure 6c,d. Note also that the sequence of tone-pip is a diotic stimulus (the left and right tone-pips at each instant have the same pitch).
2.5.2. Continuous EAE
The equation for a continuous EAE is
where
f is the frequency (in the audio frequency range 125 Hz-8 kHz),
A(f) is an amplitude factor given by the interpolation of Equation (2) in the full frequency range,
RAND(f) is a random function, and
stands for inverse Fourier transform. The continuous EAE is the implementation of the broadband stimulus matched to the hearing loss curve proposed by Schaette and Kempter [
23,
24]. The log-spectra of a continuous EAE stimulus adjusted to the HL curves of
Figure 5 are shown in
Figure 7. The HL curves are superimposed for comparison. As expected, the log-spectra of the continuous EAE match exactly the HL curves.
2.6. Tinnitus outcome
The tinnitus severity of participants was evaluated firstly using a validated Spanish version of the THI [
32]. According to the severity scale of THI [
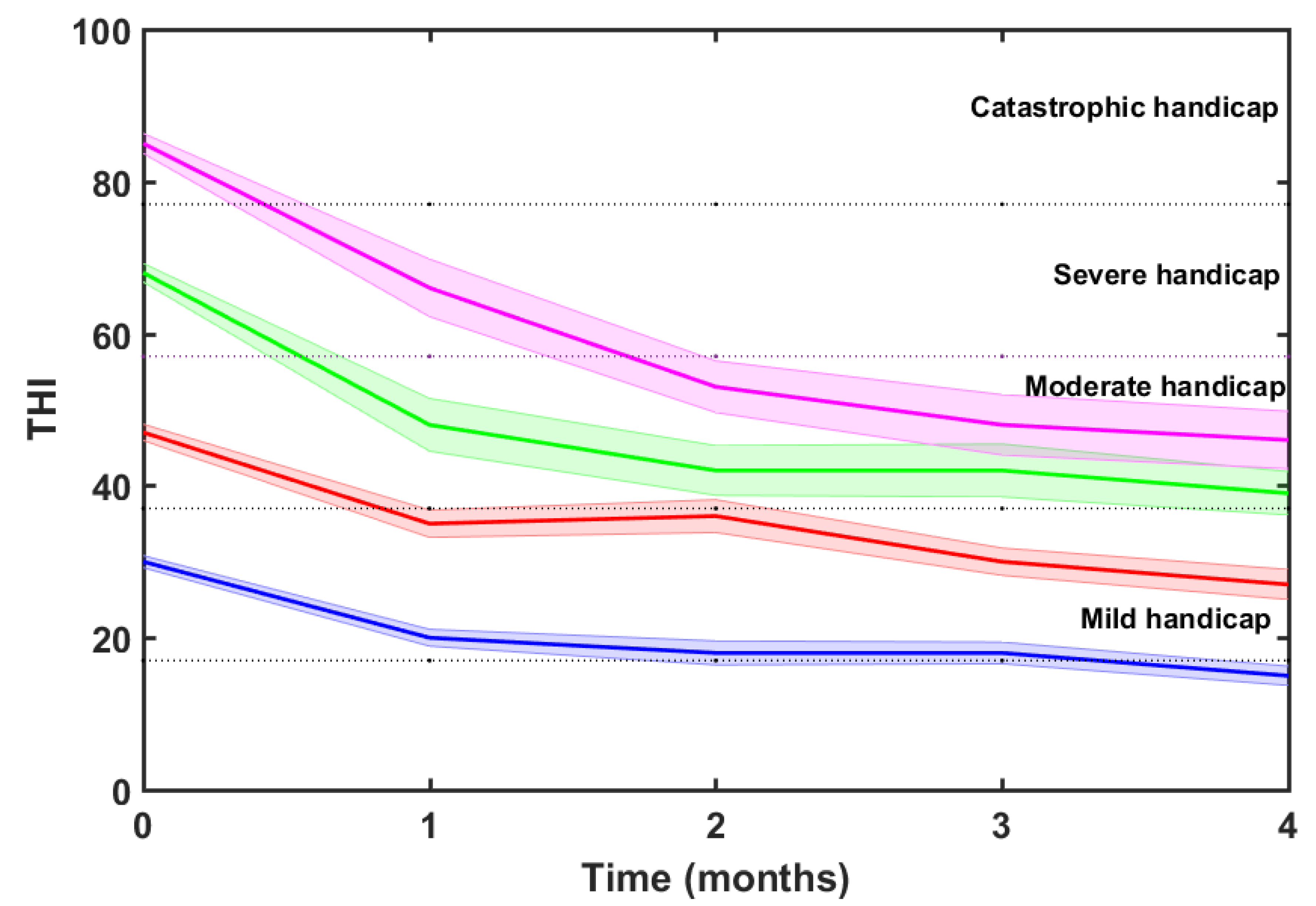
36], 40/123 (33%) of participants suffered of mild handicap (18≤THI≤36), 31/123 (25%) were concerned by moderate handicap (38≤THI≤56), 24/123 (20%) had severe handicap (58≤THI≤76), and 28/123 (23%) suffered of catastrophic handicap (THI≥78).
Since April 2021, participant also completed a new questionnaire, the Tinnitus Functional Index (TFI) [
37,
38] which was developed specifically to detect treatment-related changes of tinnitus. Fernández
et al. [
22] demonstrated that both questionnaires were correlated (r = 0.74), behaved almost identically as evaluators of the tinnitus severity at baseline, and detected equally the changes related to the application of the EAE therapy.
3. Results
As explained above, tinnitus relief was assessed by the THI and the TFI questionnaires. Since these questionnaires evaluate the tinnitus-related distress, the success of the therapy was calculated through the scores drop along the four months. As anticipated in
Section 2.1, 137 of 230 subjects (59.6%) completed the 4 months of sound therapy. From these, 123 (90%) achieved some tinnitus relief, 5 (3.5%) did not, and 9 (6.5%) were inconclusive (see
Figure 1). For the successful subjects both scores decreased, for the unsuccessful participants both scores increased, and these for which one questionnaire score increased but the other decreased were considered as inconclusive. Fernandez
et al. [
22] demonstrated that both questionnaires (THI and TFI) are highly correlated and give almost equal results to evaluate the tinnitus severity at baseline as well as to identify changes related to a treatment. Therefore, only results related to the THI score will be provided in the following.
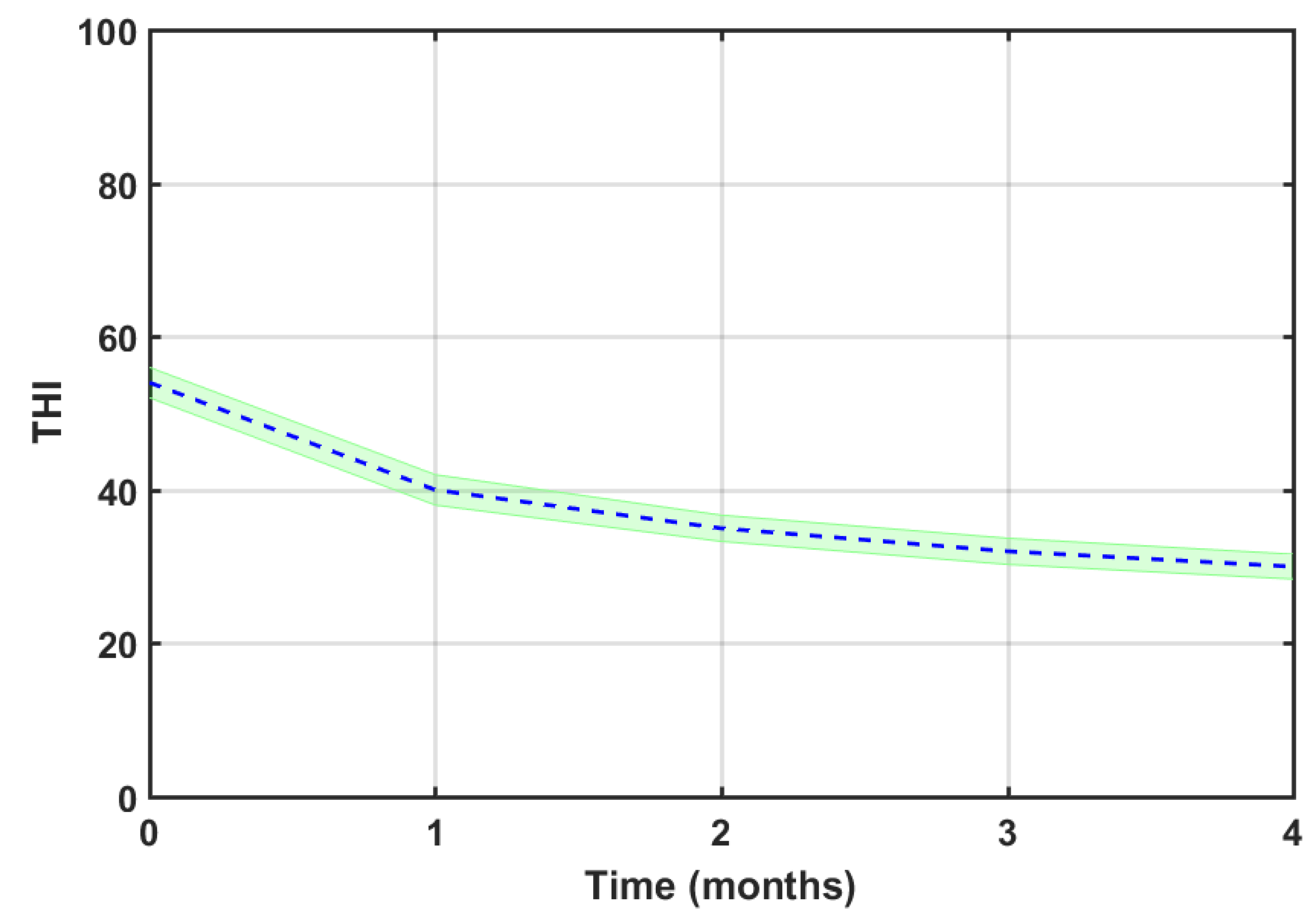
Figure 8 displays the average THI score drop of the 123 successful participants after the four months of EAE therapy. The overall mean THI drop, ΔTHI= THI
baseline -THI
final, was 24.3. According to Jastreboff [
9], this THI reduction is clinically relevant. Furthermore, the potential of THI reduction is proportional to the THI score at baseline, i.e. the higher the THI score is at baseline, the more THI score reduction can be achieved [
21]. This is observed in
Figure 9, which shows the mean THI score decreases for each tinnitus severity grade. The tinnitus-related severity of participants descended two (catastrophic) or one (severe, moderate and mild) degree.
Table 3 summarizes the number of subjects within each severity scale, their baseline and final (after the four months of EAE therapy) scores, and the THI reduction (ΔTHI). A column is added with the significance levels (p) of theses score reductions estimated applying an ANOVA test. Results show that the THI score decrease for each severity grade is statistically significant at p<0.001.
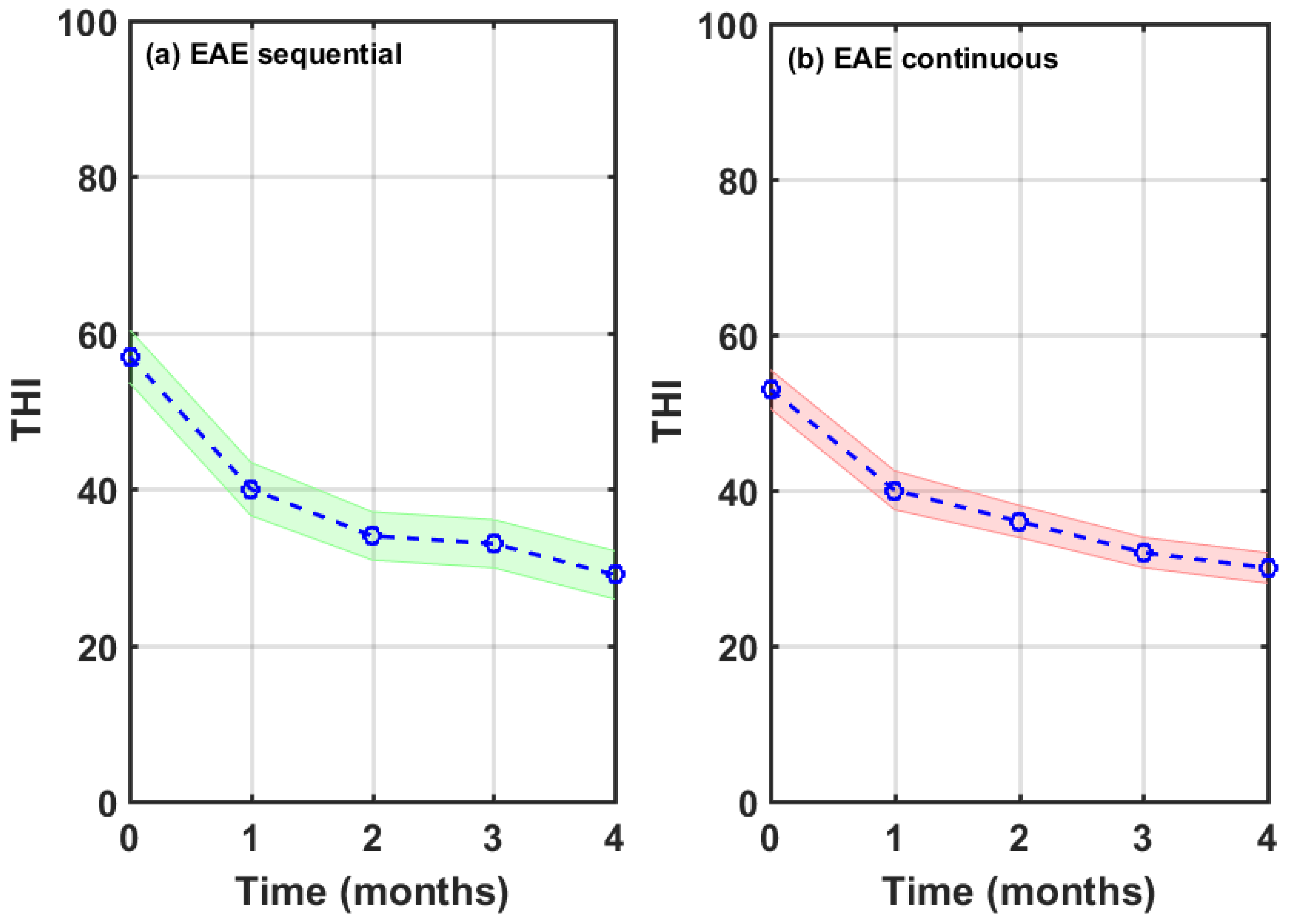
Participants were allowed to choose which EAE stimulus, either sequential or continuous, use for their four-month therapy, based uniquely on their hearing comfort feeling. 85 of 123 (69%) chose the continuous while 38 of 123 (31%) selected the sequential EAE stimuli. From these last 38 subjects, 31 chose the sequence of gammatones, 6 selected the sequence of tone-pips, and 1 picked the sequence of tone-bursts.
Figure 10 shows the average THI score drop of each subgroup.
Baseline and final mean values of THI score, as well as the ΔTHI after the four-moth therapy, are summarized in
Table 4. The sequential EAE subgroup has a greater mean THI score at baseline. It is seen that the THI score drop of sequential EAE is, on average, 5.1 points greater than that of the continuous EAE. Again, a column is added with the significance levels provided by ANOVA tests. Thus, THI score drops for each subgroups are statistically significant at p<0.001.
4. Discussion
Our tinnitus treatment could be considered as an improved version of the TRT [
9]. It combines also counselling with sound therapy. The sound therapy of the TRT applies usually a broadband noise, although other sound types have been proposed, such as colored (white, red and pink) noises [
39], custom sounds [
40], and band-filtered noise [
41]. Our proposal combines also counselling with sound therapy but differs in the use of EAE as a personalized sound stimulus. Since hearing loss is considered as one of the major risks of deafferentation to the auditory system, and hence, one of the main tinnitus trigger, we propose to compensate the EAE sound stimulus with an amplitude function (see Equation (2)) proportional to this hearing loss. Note that some subjects could suffer of tinnitus with an apparent normal audiometry [
5,
21,
42]. In this case, the continuous EAE proposed here becomes just a TRT.
The TRT applied in most clinics requires hearing the sound broadband stimulus for 3-4 hours/day during 12-18 months, affording reduced tinnitus-related distress in about 80% of subjects [
9]. One of our aims was to demonstrate that it is possible to achieve similar (o even better) results with lower exposition time. Specifically, 90% of our participants reached an average THI score drop of 24.3 points in just four months. When compared with other TRT results, only those of Jastreboff [
9] and Barozzi et
al. [
43] achieved a comparable THI score drop of 23 and 20 points, respectively, but with higher exposition time. Henry
et al. [
44], Bauer
et al. [
45], and Oishi
et al. [
46] reported THI score drops of 7, 13 and 10 points in four months, respectively.
Primarily, sequential EAE seemed to provide higher tinnitus-related distress relief than continuous EAE. However, we should take into account that the proportional composition of severity grades in each subgroup is different, as the EAE stimuli were chosen by participants paying attention just to their hearing comfort feeling.
Table 5 summarizes the number of participants within each severity scale in both subgroups.
The continuous EAE subgroup contains more proportion of participants with mild, but less ratio of them with catastrophic, tinnitus. As discussed above, participants in the overall mild scale achieved much lesser mean THI score drop (14.1 points) than the subjects in the overall catastrophic scale (41.2 points). Could this explain that the 85 participants exposed with continuous EAE reached lesser tinnitus-related distress relief? We can estimate the relative contribution of each severity grade in the whole THI score drop using the data in
Table 3. Specifically
where, for all participants in
Table 3,
fmild=(40/123),
fmode=(31/123),
fseve=(24/123), and
fcatas=(28/123), and
N is a factor. This results in
N=1.0919. Assuming the same relative weight of each severity scale in the continuous and sequential EAE subgroups, we obtain that the predicted ΔTHI for both subgroups should be ΔTHI
cont= 23.7, ΔTHI
seq=25.5, being the difference between both THI score drops ΔTHI
seq- ΔTHI
cont =1.8. Since the difference of both THI score drops in our study is 5.1, we conclude that the sequential EAE stimulus provides more tinnitus-related distress relief than the continuous EAE.
The 10% of participants who did not achieve tinnitus relief (3.5% unsuccessful and 6.5% inconclusive) deserve some mention. In general, they are refractory subjects unable to change their attitude regarding their tinnitus along the therapy time. Their monthly THI can slightly fluctuate around their baseline score but, at the end of treatment, they fail to reach any progress. Although the results of the study could improve by detecting and excluding these patients at the recruitment phase, we prefer to keep them in the study. Furthermore, finding a treatment able to alleviate the suffering of these subjects is the target of our future research.
5. Conclusions
This study aimed to optimize a tinnitus sound therapy regarding its duration as well as the sound stimulus type. The sound stimulus consists of a customized Enriched Acoustic Environment, continuous or sequential, matched to the hearing level curves of the subject. 137 tinnitus subjects were treated with this type of stimuli.
A mean decrease of 24.3 points on the Tinnitus Handicap Inventory (THI) score was obtained in 90% of these subjects. This tinnitus-related distress relief was clinically relevant and statistically significant. Subjects exposed with sequential stimuli (31%) achieved greater distress relief (5.1 points more in their THI score) than those stimulated with continuous sound (69%). Therefore, although both type of stimuli provided tinnitus-related distress decrease, sequential Enriched Acoustic Environment should be recommended in this type of sound therapy.
Author Contributions
Both authors contributed equally to the conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of the CSIC (protocol code 004/2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are unavailable due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eggermont, J.J.; Tass, P.A. Maladaptive Neural Synchrony in Tinnitus: Origin and Restoration. Front. Neurol. 2015, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Gallus, S.; Hall, D.A.; Kleinjung, T.; Langguth, B.; Maruotti, A.; Meyer, M.; Norena, A.; Probst, T.; Pryss, R.; et al. Editorial: Towards an Understanding of Tinnitus Heterogeneity. Front. Aging Neurosci. 2019, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, M.; Cobo, P. A Heterogeneous Sample of a Spanish Tinnitus Cohort. Brain Sci. 2023, 13, 652. [Google Scholar] [CrossRef] [PubMed]
- Brueggemann, P.; Szczepek, A.J.; Rose, M.; McKenna, L.; Olze, H.; Mazurek, B. P Impact of Multiple Factors on the Degree of Tinnitus Distress. Front. Human Neurosc. 2016, 10, 3093. [Google Scholar] [CrossRef] [PubMed]
- McFerran, D.J., Stockdale, D., Holme, R., Large, C.H., Baguley, D.M. Why Is There No Cure for Tinnitus? Front. Neurosc. 2019, 13, 802.
- Cima, R.F.F.; Mazurek, B.; Haider, H.; Kikidis, D.; Lapira, A.; Noreña, A.; Hoare, D.J. A multidisciplinary European guideline for tinnitus: diagnostics, assessment, and treatment. HNO 2019, 67, 10–42. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J. The neuroscience of tinnitus. Oxford University Press: Oxford, UK, 2012.
- Liberman, M.C.; Epstein, M.J.; Cleveland, S.S.; Wang, H.; Maison, S.F. Toward a Differential Diagnosis of Hidden Hearing Loss in Humans. PLOS ONE 2016, 11, e0162726–e0162726. [Google Scholar] [CrossRef]
- Jastreboff, P. 25 Years of tinnitus retraining therapy. HNO 2015, 63, 307–311. [Google Scholar] [CrossRef]
- Kraus, K.S.; Canlon, B. Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear. Res. 2012, 288, 34–46. [Google Scholar] [CrossRef]
- Searchfield, G.D.; Durai, M.; Linford, T. A State-of-the-Art Review: Personalization of Tinnitus Sound Therapy. Front. Psychol. 2017, 8, 1599–1599. [Google Scholar] [CrossRef]
- Sereda, M.; Xia, J.; El Refai,e A.; Hall, D.A.; Hoare, D.J. Sound therapy (using amplification devices and/or sound generators) for tinnitus in adults (Protocol). Cochrane Database of Systematic Reviews 2018, 8, CD013094.
- Pienkowski, M. Rationale and Efficacy of Sound Therapies for Tinnitus and Hyperacusis. Neuroscience 2019, 407, 120–134. [Google Scholar] [CrossRef]
- Jastreboff, P.J.; Jastreboff, M.M. Decreased Sound Tolerance: hyperacusis, misophonia, diplacousis, and polyacousis. Handb. Clin. Neurol. 2015, 129, 375–387. [Google Scholar] [CrossRef]
- Vermeire, K.; Heyndrickx, K.; De Ridder, D.; Van De Heyning, P. Phase-shift tinnitus treatment: an open prospective clinical trial. B-ENT 2007, 3, 65–69. [Google Scholar]
- Herraiz, C.; Diges, I.; Cobo, P.; Aparicio, J.M.; Toledano, A. Auditory discrimination training for tinnitus treatment: the effect of different paradigms. Eur. Arch. Oto-Rhino-Laryngology 2010, 267, 1067–1074. [Google Scholar] [CrossRef]
- Adamchic, I.; Hauptmann, C.; Tass, P.A. Changes of oscillatory activity in pitch processing network and related tinnitus relief induced by acoustic CR neuromodulation. Front. Syst. Neurosci. 2012, 6, 18. [Google Scholar] [CrossRef]
- Pantev, C.; Okamoto, H.; Teismann, H. Music-induced cortical plasticity and lateral inhibition in the human auditory cortex as foundations for tonal tinnitus treatment. Front. Syst. Neurosci. 2012, 6, 50. [Google Scholar] [CrossRef]
- Cobo, P. Preliminary results of the enriched acoustic environment as personalized sound-therapy for tinnitus. Auditio 2021, 5(3), 1–9. [Google Scholar] [CrossRef]
- Cobo, P.; Cuesta, M.; de la Colina, C. Customised enriched acoustic environment for sound therapy of tinnitus. Acta Acust. 2021, 5, 34. [Google Scholar] [CrossRef]
- Cuesta, M.; Garzón, C.; Cobo, P. Efficacy of Sound Therapy for Tinnitus Using an Enriched Acoustic Environment with Hearing-Loss Matched Broadband Noise. Brain Sci. 2022, 12, 82. [Google Scholar] [CrossRef]
- Fernández, M.; Cuesta, M.; Sanz, R.; Cobo, P. Comparison of Tinnitus Handicap Inventory and Tinnitus Functional Index as Treatment Outcomes. Audiol. Res. 2022, 13, 23–31. [Google Scholar] [CrossRef]
- Schaette, R.; Kempter, R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: a computational model. Eur. J. Neurosci. 2006, 23, 3124–3138. [Google Scholar] [CrossRef]
- Schaette, R.; Kempter, R. Development of hyperactivity after hearing loss in a computational model of the dorsal cochlear nucleus depends on neuron response type. Hear. Res. 2008, 240, 57–72. [Google Scholar] [CrossRef]
- Noreña, A.; Eggermont, J. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear. Res. 2003, 183, 137–153. [Google Scholar] [CrossRef]
- Noreña, A.J.; Eggermont, J.J. Enriched acoustic environment alter noise trauma reduces hearing loss and prevents cortical map reorganization. J. Neurosci. 2005, 25, 699–705. [Google Scholar] [CrossRef]
- Hermes, D.J.; Eggermont, J.J.; Aertsen, A.M.H.J.; Johannesma, P.I.M. Spectro-temporal characteristics of single units in the auditory midbrain of slightly anaesthetised grass fogs investigated with tone stimuli. Hear. Res. 1983, 6, 103–126. [Google Scholar] [CrossRef]
- Eggermont, J.J. Cortical tonotopic map reorganization and its implications for treatment of tinnitus. Acta Oto-Laryngologica 2006, 126, 9–12. [Google Scholar] [CrossRef]
- Noreña, A.J.; Chery-Croze, S. Enriched acoustic environment rescales auditory sensitivity. NeuroReport 2007, 18, 1251–1255. [Google Scholar] [CrossRef]
- Bauer, C.A.; Berry, J.L.; Brozoski, T.J. The effect of tinnitus retraining therapy on chronic tinnitus: A controlled trial. Laryngoscope Investig. Otolaryngol. 2017, 2, 166–177. [Google Scholar] [CrossRef]
- Cuesta, M.; Cobo, P. Broadband sound equalized by the hearing loss curves as an improved stimulus for tinnitus retraining therapy—A pilot, non-controlled observational study. J. Int. Adv. Otol. 2020, 16, 207–212. [Google Scholar] [CrossRef]
- Herráiz, C., Hernández Calvín, F.J., Plaza, G., Tapia, M.C., De los Santos, G. Evaluación de la incapacidad en los pacientes con acúfenos (Evaluation of handicap in tinnitus patients). Acta Otorrinolaringol Esp. 2001, 52, 142–145. [CrossRef]
- Nuttall, A.H. Some windows with very good side-lobe behavior. IEEE Transactions on Acoustics, Speech and Signal Processing, 1981, ASSP-29, 84–91. [CrossRef]
- Glasberg, B.R.; Moore, B.C. Derivation of auditory filter shapes from notched-noise data. Hear. Res. 1990, 47, 103–138. [Google Scholar] [CrossRef]
- Katsiamis, A.G.; Drakakis, E.M.; Lyon, R.F. Practical Gammatone-Like Filters for Auditory Processing. EURASIP J. Audio, Speech, Music. Process. 2007, 2007, 1–15. [Google Scholar] [CrossRef]
- McCombe, A.; Baguley, D.; Coles, R.; McKenna, L.; McKinney, C.; Windle-Taylor, P. Guidelines for the grading of tinnitus severity: the results of a working group commissioned by the British Association of Otolaryngologists, Head and Neck Surgeons, 1999. Clin. Otolaryngol. 2001, 26, 388–393. [Google Scholar] [CrossRef]
- Meikle, M. B.; Henry, J. A.; Griest, S. E.; Stewart, B. J.; Abrams, H. B.; McArdle, R.; Myers, P. J.; Newman, C. W.; Sandridge, S.; Turk, D. C.; Folmer, R. L.; Frederick, E. J.; House, J. W.; Jacobson, G. P.; Kinney, S. E.; Martin, W. H.; Nagler, S. M.; Reich, G. E.; Searchfield, G.; Sweetow, R.; Vernon, J. A. The Tinnitus Functional Index: Development of a New Clinical Measure for Chronic, Intrusive Tinnitus. Ear Hear. 2012, 33, 153–176. [Google Scholar] [CrossRef]
- Henry, J.A.; Griest, S.; Thielman, E.; McMillan, G.; Kaelin, C.; Carlson, K.F. Tinnitus Functional Index: Development, validation, outcomes research, and clinical application. Hear. Res. 2016, 334, 58–64. [Google Scholar] [CrossRef]
- Barozzi, S.; Ambrosetti, U.; Callaway, S.L.; Behrens, T.; Passoni, S.; Del Bo, L. Effects of Tinnitus Retraining Therapy with Different Colours of Sound. Int. Tinnitus J. 2017, 21, 139–143. [Google Scholar] [CrossRef]
- Henry, J.A.; Rheinsburg, B.; Zaugg, T. Comparison of Custom Sounds for Achieving Tinnitus Relief. J. Am. Acad. Audiol. 2004, 15, 585–598. [Google Scholar] [CrossRef]
- Kim, B.J.; Chung, S.-W.; Jung, J.Y.; Suh, M.-W. Effect of Different Sounds on the Treatment Outcome of Tinnitus Retraining Therapy. Clin. Exp. Otorhinolaryngol. 2014, 7, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, M.; Cobo, P. A Heterogeneous Sample of a Spanish Tinnitus Cohort. Brain Sci. 2023, 13, 652. [Google Scholar] [CrossRef] [PubMed]
- Barozzi, S.; Del Bo, L.; Crocetti, A.; Dyrlund, O.; Passoni, S.; Zolin, A.; Panicucci, E.; Mancuso, A.; Kaur, M.; Searchfield, G.D. A Comparison of Nature and Technical Sounds for Tinnitus Therapy. Acta Acust. United Acust. 2016, 102, 540–546. [Google Scholar] [CrossRef]
- Henry, J.A.; Schechter, M.A.; Zaugg, T.L.; Griest, S.; Jastreboff, P.J.; Vernon, J.A.; Kaelin, C.; Meikle, M.B.; Lyons, K.S.; Stewart, B.J. Outcomes of Clinical Trial: Tinnitus Masking versus Tinnitus Retraining Therapy. J. Am. Acad. Audiol. 2006, 17, 104–132. [Google Scholar] [CrossRef]
- Bauer, C.A.; Berry, J.L.; Brozoski, T.J. The effect of tinnitus retraining therapy on chronic tinnitus: A controlled trial. Laryngoscope Investig. Otolaryngol. 2017, 2, 166–177. [Google Scholar] [CrossRef]
- Oishi, N.; Shinden, S.; Kanzaki, S.; Saito, H.; Inoue, Y.; Ogawa, K. Effects of tinnitus retraining therapy involving monaural noise generators. Eur. Arch. Oto-Rhino-Laryngology 2012, 270, 443–448. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).