Submitted:
09 May 2023
Posted:
11 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
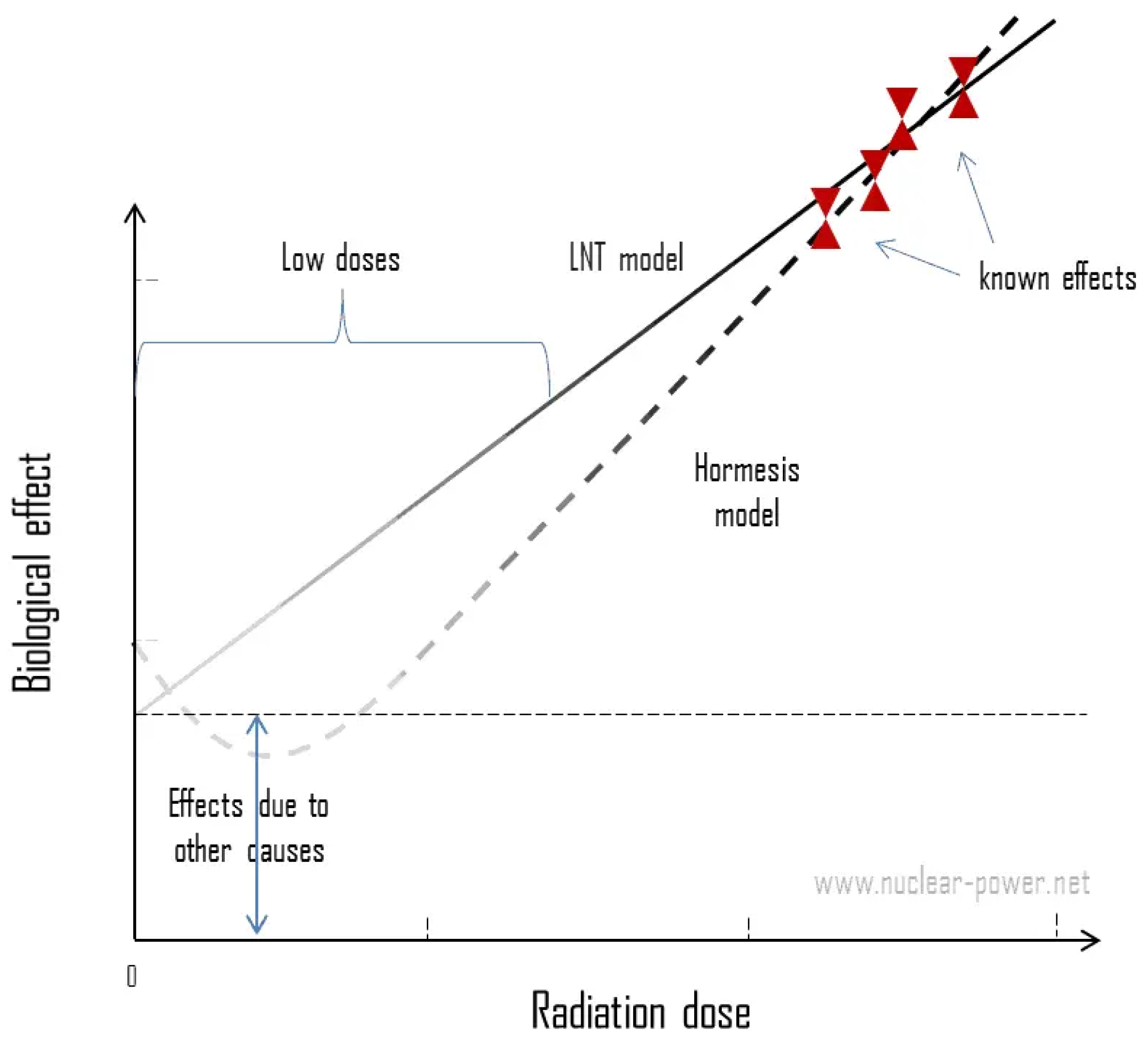
2. Ionizing radiation dose response models
- -
- DNA damage in mammalian cells is proportional to the dose with additional possible Bystander effects.
- -
- DNA damage comes overwhelmingly from non-radiation sources at background radiation exposure levels.
- -
- The probability of radiation induced adaptive protection measurably outweighs that of damage from doses well below 200 mSv low-LET (linear energy transfer) radiation.
- -
- The delayed and temporarily adaptive protection at low doses involves damage prevention, damage repair and immune response.
- -
- Cell and DNA damage appear increasingly to overrule, negate, or annihilate the more subtle signalling effects seen after low doses, at higher doses in tissue.
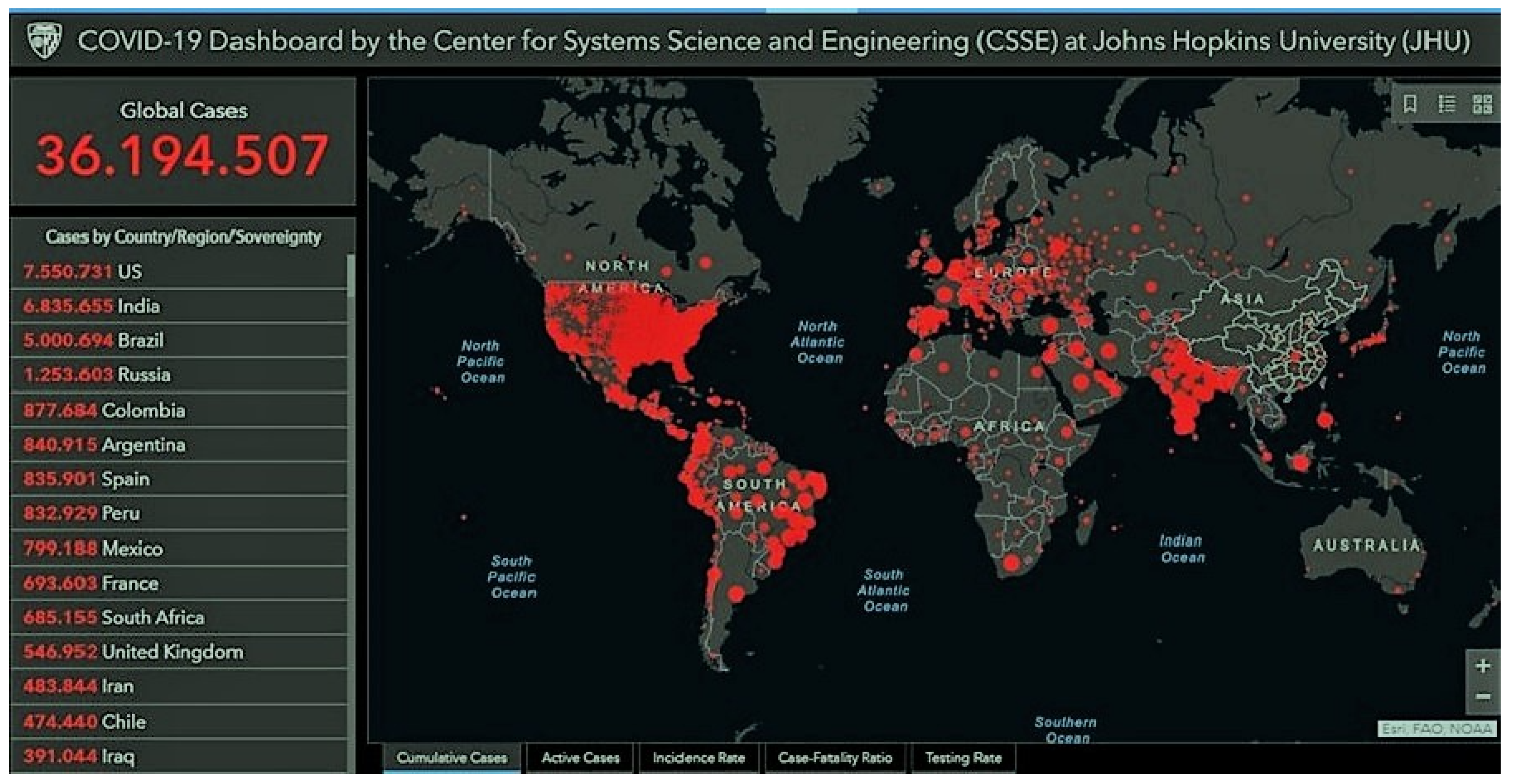
3. Inflammation biomarkers due to abnormal levels of radiation
4. Inflammation biomarkers on COVID-19 cases
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- UNSCEAR 2006. United Nations Scientific Committee on the Effects of Atomic Radiation Sources, effects, and risks of ionizing radiation. UNSCEAR 2006 Report.
- Lumniczky, K.; Impens, N.; Armengol, G.; Candéias, S.; Georgakilas, A.G.; Hornhardt, S.; Martin, O.A.; Rödel, F.; Schaue, D. Low dose ionizing radiation effects on the immune system. Environ Int. 2021, 149, 106212. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Hall, E.J. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Zoran, M.A.; Savastru, R.S.; Savastru, D.M.; Tautan, M.N. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan, Italy. Sci Total Environ. 2020, 738, 139825. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet 2022, 399, 1513–1536; Erratum in Lancet 2022, 399, 1468. [Google Scholar] [CrossRef]
- Cui, J.; Yang, G.; Pan, Z.; Zhao, Y.; Liang, X.; Li, W.; Cai, L. Hormetic Response to Low-Dose Radiation: Focus on the Immune System and Its Clinical Implications. Int J Mol Sci. 2017, 18, 280. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- UNSCEAR 2012. United Nations Scientific Committee on the Effects of Atomic Radiation Sources, effects, and risks of ionizing radiation. UNSCEAR 2012 Report.
- Kaminski, C.Y.; Dattoli, M.; Kaminski, J.M. Replacing LNT: The Integrated LNT-Hormesis Model. Dose Response. 2020, 18, 1559325820913788. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Giordano, J. LNTgate: How LNT benefited from editorial actions. Chem Biol Interact. 2022, 362, 109979. [Google Scholar] [CrossRef]
- Calabrese, E.J. Linear non-threshold (LNT) fails numerous toxicological stress tests: Implications for continued policy use. Chem Biol Interact. 2022, 365, 110064. [Google Scholar] [CrossRef]
- Feinendegen, L.E. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005, 78, 3–7. [Google Scholar] [CrossRef]
- Feinendegen, L.E.; Pollycove, M.; Sondhaus, C.A. Responses to low doses of ion- izing radiation in biological systems. Nonlinearity in Biology, Toxicology, and Medicine 2004, 2, 143–171. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Giordano, J.J.; Kozumbo, W.J.; Leak, R.K.; Bhatia, T.N. Hormesis mediates dose-sensitive shifts in macrophage activation patterns. Pharmacol Res. 2018, 137, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E. The linear Non-Threshold (LNT) dose response model: A comprehensive assessment of its historical and scientific foundations. Chemico-Biological Interactions 2019, 301, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Kozumbo, W.J.; Kapoor, R.; Dhawan, G.; Lara, P.C.; Giordano, J. Nrf2 activation putatively mediates clinical benefits of low-dose radiotherapy in COVID-19 pneumonia and acute respiratory distress syndrome (ARDS): Novel mechanistic considerations. Radiother Oncol. 2021, 160, 125–131. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.T.; Kim, K.; Norris, A.J.; Vlashi, E.; Phillips, T.M.; Lagadec, C.; Della Donna, L.; Ratikan, J.; Szelag, H.; Hlatky, L.; McBride, W.H. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010, 70, 8886–8895. [Google Scholar] [CrossRef] [PubMed]
- Kuikka, J.T. Low-dose radiation risk and the linear no-threshold model. Int J Low Radiat 2009, 6, 157–163. [Google Scholar] [CrossRef]
- Doss, M. Are We Approaching the End of the Linear Non-Threshold Era? J Nucl Med 2018, 59, 1786–1793. [Google Scholar] [CrossRef]
- Li, K.; Chen, Y.; Li, X.; et al. Alteration of Cytokine Profiles in Uranium Miners Exposed to Long-Term Low-dose Ionizing Radiation. Scientific World Journal 2014. [CrossRef]
- Hendry, J.H.; Simon, S.L.; Wojcik, A.; et al. Human exposure to high natural background radiation: what can it teach us about radiation risks? J Radiol Prot. 2009, 29, 29–42. [Google Scholar] [CrossRef]
- Yahyapour, R.; Amini, P.; Rezapour, S.; Cheki, M.; Rezaeyan, A.; Farhood, B.; Shabeeb, D.; Musa, A.E.; Fallah, H.; Najafi, M. Radiation-induced inflammation and autoimmune diseases. Mil Med Res. 2018, 5, 9. [Google Scholar] [CrossRef]
- Analytical Methodology for the Determination of Radium Isotopes in Environmental Samples. IAEA Analytical Quality in Nuclear Applications Series No. 19. IAEA /AQ /19.
- Nyhan, M.M.; Ricec, M.; Blomberg, A.; et al. Associations between ambient particle radioactivity and lung function. Environment International 2019, 130. [Google Scholar] [CrossRef] [PubMed]
- De Flora, S.; Grassi, C.; Carati, L. Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment. Eur Respir J. 1997, 10, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Metryka, E.; Chibowska, K.; Gutowska, I.; et al. Lead (Pb) Exposure Enhances Expression of Factors Associated with Inflammation. Int J Mol Sci. 2018, 19, 1813. [Google Scholar] [CrossRef] [PubMed]
- USEPA (United States Environmental Protection Agency). RadNet Monitoring Network. http://www.epa.gov/radnet.
- Zagà, V.; Lygidakis, C.; Chaouachi, K.; et al. Polonium and Lung Cancer. J Oncol. 2011. [Google Scholar] [CrossRef]
- Papastefanou, C. Radioactivity of Tobacco Leaves and Radiation Dose Induced from Smoking. Int. J. Environ. Res. Public Health 2009, 6, 558–567. [Google Scholar] [CrossRef]
- Radford, E.P., Jr.; Hunt, V.R. Polonium- 210: A volatile radioelement in cigarette. Science 1964, 143, 247–249. [Google Scholar] [CrossRef]
- Winters, T.H.; Di Franza, J.R. Radioactivity in cigarette smoking. N. Engl. J. Med. 1982, 306, 364–365. [Google Scholar]
- Ghiassi-nejad, M.; Mortazavi, S.M.; Cameron, J.R.; Niroomand-rad, A.; Karam, P.A. Very high background radiation areas of Ramsar, Iran: preliminary biological studies. Health Phys. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Attar, M.; Molaie Kondolousy, Y.; Khansari, N. Effect of high dose natural ionizing radiation on the immune system of the exposed residents of Ramsar Town, Iran. Iran J Allergy Asthma Immunol. 2007, 6, 73–78. [Google Scholar]
- Zhao, N.; Di, B.; Xu, L.L. The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev. 2021, 61, 2–15. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; Niu, P.; Zhan, F.; Ma, X.; Wang, D.; Xu, W.; Wu, G.; Gao, G.F.; Tan, W. China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Torres, J.L.; Verdiá-Báguena, C.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A.; Castaño-Rodriguez, C.; Fern, ez-Delgado, R. ; Torres, J.; Aguilella, V.M.; Enjuanes, L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology 2015, 485, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S.Y. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J Thorac Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev. 2020, 19, 102537. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; et al. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Aggarwal, N.R.; King, L.S.; D’Alessio, F.R. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014, 306, L709–L725. [Google Scholar] [CrossRef]
- Lu, H.L.; Huang, X.Y.; Luo, Y.F.; Tan, W.P.; Chen, P.F.; Guo, Y.B. Activation of M1 macrophages plays a critical role in the initiation of acute lung injury. Biosci Rep. 2018, 38, BSR20171555. [Google Scholar] [CrossRef]
- Mettler, F.A.; et al. Effective Doses in Radiology and Diagnostic Nuclear Medicine: A Catalog. Radiology 2008, 248, 254–263. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Wu, H.; Hu, J.; Zhang, J. Repeat chest ct scans in moderate- to-severe patient’s management during the COVID-19 pandemic observations from a single centre in Wuhan, China. Radiat Prot Dosimetry 2020, 190, 269–275. [Google Scholar] [CrossRef]
- Homayounieh, F.; Holmberg, O.; Umairi, R.A.; et al. Variations in CT Utilization, Protocols, and Radiation Doses in COVID-19 Pneumonia: Results from 28 countries in the IAEA study. Radiology 2021, 298, E141–E151. [Google Scholar] [CrossRef] [PubMed]
- ICRP 103. Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 37 (2-4). 2007.
- Liu, S.Z. Nonlinear Dose-Response Relationship in the Immune System Following Exposure to Ionizing Radiation: Mechanisms and Implications. Nonlinearity Biol Toxicol Med. 2003, 1, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Ruhm, W.; Harrison, R.M. High CT doses return to the agenda. Radiat Environ Biophys 2020, 59, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Charles, L. Sanders. Radiobiology and Radiation Hormesis. New Evidence and its Implications for Medicine and Society. Springer: 2017. ISBN 978-3-319-56371-8. ISBN 978-3-319-56372-5 (eBook).
- Travis, W.D.; Costabel, U.; Hansell, D.M.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Teresa Pinto, A.; Laranjeiro Pinto, M.; Patrícia Cardoso, A.; Monteiro, C.; Teixeira Pinto, M.; Filipe Maia, A.; Castro, P.; Figueira, R.; Monteiro, A.; Marques, M.; Mareel, M.; Dos Santos, S.G.; Seruca, R.; Adolfo Barbosa, M.; Rocha, S.; José Oliveira, M. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci Rep 2016, 6, 18765. [Google Scholar] [CrossRef]
- Frey, B.; Hehlgans, S.; Rödel, F.; Gaipl, U.S. Modulation of inflammation by low and high doses of ionizing radiation: Implications for benign and malign diseases. Cancer Lett 2015, 368, 230–237. [Google Scholar] [CrossRef]
- Lindee, S. Survivors and scientists: Hiroshima, Fukushima, and the Radiation Effects Research Foundation, 1975-2014. Soc Stud Sci. 2016, 46, 184–209. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Kozumbo, W.J. The hormetic dose-response mechanism: Nrf2 activation. Pharmacol Res. 2021, 167, 105526. [Google Scholar] [CrossRef]
- Oakley, P.A.; Harrison, D.E. Death of the ALARA Radiation Protection Principle as Used in the Medical Sector. Dose Response 2020, 18, 1559325820921641. [Google Scholar] [CrossRef]
- EURATOM 2013. Council Directive 2013/59/EURATOM, of , laying down basic safety standards for protection against the dangers arising from exposure to ionizing radiation. Official Journal of the European Union. 5 December.
- Backert, S. (Editor). Inflammasome signaling and bacterial infections. Current topics in microbiology and immunology. Springer: 2016; Volume 397, ISBN 978-3-319-41170-5.
- Calabrese, E.J.; Selby, P.B. Cover up and cancer risk assessment: Prominent US scientists suppressed evidence to promote adoption of LNT. Environ Res 2022, 210, 112973. [Google Scholar] [CrossRef]
- Cuadrado, A.; Pajares, M.; Benito, C.; Jiménez-Villegas, J.; Escoll, M.; Fernández-Ginés, R.; Garcia Yagü, A.J.; Lastra, D.; M. ; a G.; Rojo, A.I.; Dinkova-Kostova, A.T. Can Activation of NRF2 Be a Strategy against COVID-19? Trends Pharmacol Sci. 2020, 41, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Dalan, R.; Bornstein, S.R.; El-Armouche, A.; Rodionov, R.N.; Markov, A.; Wielockx, B.; Beuschlein, F.; Boehm, B.O. The ACE-2 in COVID-19: Foe or Friend? Horm Metab Res 2020, 52, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Hamarsheh, S.; Zeiser, R. NLRP3 Inflammasome Activation in Cancer: A Double-Edged Sword. Front Immunol 2020, 11, 1444. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A. Natural levels of Polonium-210 in urine. J Radiol Prot 2018, 38, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.M.; Wright, F.A.; Broide, D.H.; W. ; erer, A.A.; Kolodner, R.D. Identification of a locus on chromosome 1q44 for familial cold urticaria. American Journal of Human Genetics 2000, 66, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Su, I.J.; Theron, M.; et al. An interferon-γ-related cytokine storm in SARS patients. J Med Virol 2005, 75, 185–194. [Google Scholar] [CrossRef]
- Ichikado, K.; Muranaka, H.; Gushima, Y.; Kotani, T.; Nader, H.M.; Fujimoto, K.; Johkoh, T.; Iwamoto, N.; Kawamura, K.; Nagano, J.; Fukuda, K.; Hirata, N.; Yoshinaga, T.; Ichiyasu, H.; Tsumura, S.; Kohrogi, H.; Kawaguchi, A.; Yoshioka, M.; Sakuma, T.; Suga, M. Fibroproliferative changes on high-resolution CT in the acute respiratory distress syndrome predict mortality and ventilator dependency: a prospective observational cohort study. BMJ Open 2012, 2, e000545. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- ; Lee, C. Managing Radiation Dose from Chest CT in Patients with COVID-19. Radiology 2021, 298, E158–E159. [Google Scholar] [CrossRef]
- Lee, E.K.; Kim, J.A.; Park, S.J.; Kim, J.K.; Heo, K.; Yang, K.M.; Son, T.G. Low-dose radiation activates Nrf1/2 through reactive species and the Ca(2+)/ERK1/2 signaling pathway in human skin fibroblast cells. BMB Rep 2013, 46, 258–263. [Google Scholar] [CrossRef]
- Lippi, G.; South, A.M.; Henry, B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann Clin Biochem 2020, 57, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Metabolism and function of glutathione. Dolphin, D.; Poulson, R., Avramovich, O., Eds.; eds. Glutathione: Chemical, biochemical, and medical aspects. Wiley-Interscience: New York. 1989; pp. 367–374. [Google Scholar]
- Mense, M.; Stark, G.; Apell, H.J. Effects of Free Radicals on Partial Reactions of the Na,K-ATPase. J. Membrane Biol. 2020, 156, 63–71. [Google Scholar] [CrossRef]
- Petruzzi, S.; Musim, B.; Bignami, G. Acute and chronic sulfur dioxide (SO2) exposure: and overview of its effects on humans and laboratory animals. Review. Ann. Ist. Super Sanita. 1994, 30, 151–156. [Google Scholar] [PubMed]
- Sheppard, S.C.; Sheppard, M.I. Modeling estimates of the effect of acid rain on background radiation dose. Environ Health Perspect. 1998, 78, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Puyo, C.A. N-Acetylcysteine to Combat COVID-19: An Evidence Review. Ther Clin Risk Manag. 2020, 16, 1047–1055. [Google Scholar] [CrossRef]
- Shprentz, D.S.; Bryner, G.C.; Shprentz, J.S.; Hawkings, D.G. Breath taking: premature mortality due to air pollution in 239 American cities. National Resources Defense Council, Washington, DC. 1996, 99.
- Suter, P.M.; Domenighetti, G.; Schaller, M.D.; et al. N-Acetylcysteine enhances recovery from acute lung injury in man: a randomized, double-blind, placebo-controlled clinical study. Chest 1994, 105, 190–194. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 2019, 19, 477–489. [Google Scholar] [CrossRef]
- WHO Regional Office for. Europe, Copenhagen, Denmark; 2000.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




