Introduction
Different parameters such as incubation time, competent cell concentration, and plasmid-to-cell ratio affects transformation efficiency [
1,
2]. And transformation is a physio-biological process that remains incompletely understood, particularly with respect to how different parameters cross-interact to affect the final outcome: transformation efficiency. This work sought to understand through conventional microtube-based experiments how different parameters affect transformation efficiency.
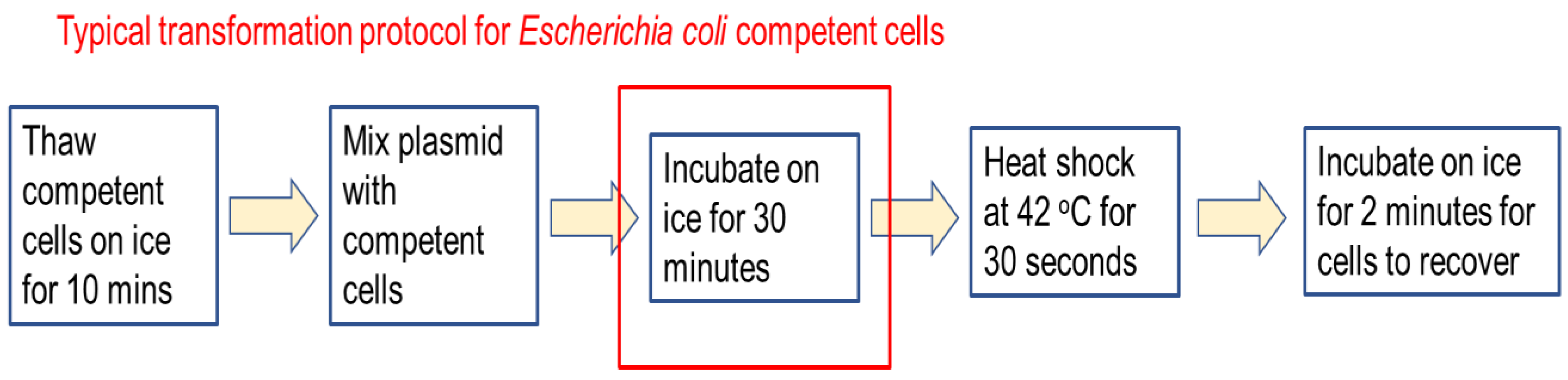
The parameters selected for study are incubation time on ice, competent cell concentration, and plasmid-to-cell ratio. Each parameter represents some aspect of the transformation system that can significantly affect transformation efficiency, and critically, where some trade-off in system design can help improve bacterial transformation. Firstly, conventional transformation in
Escherichia coli would require a 30 min incubation on ice [
3]. This is to a significant extent too time consuming for implementation in a microfluidic chip-based system. Hence, this work sought to examine the possibility of reducing incubation time on ice. Secondly, competent cells, especially chemically competent cells, are hard and time-consuming to prepare, but there is a popular notion that high concentration competent cells would result in higher transformation efficiency. Using microtube-based experiments, a series of experiments were performed to first understand the effect of competent cell concentration on transformation efficiency, and possibly to reduce the concentration of competent cells used. Thirdly, plasmid-to-cells ratio was examined to understand if there is a less understood biological effect governing how plasmid interact with cells, especially from the perspective of single cells taking up more than one plasmid that give rise to heightened metabolic burden, reduced cell growth, and reduced colony formation. The key question here is not solely the cost of plasmid or competent cells, but rather, how do we conduct transformation to ensure high transformation efficiency as perceived through colony counting on agar plates after transformation.
Materials and Methods
Competent Cell Preparation
Escherichia coli DH5α chemically competent cells were prepared as per instructions in the Zymo Research “Mix and go” competent cell preparation kit. Prepared cells were in aliquots of 200 µL per 1.5 mL microcentrifuge tube and stored at -80 oC prior to use.
Plasmid used in this study
pNAR plasmid of approximate size 10 kilobases and with ampicillin antibiotic resistance marker was used in this study. Purified plasmid was stored in elution buffer of Qiagen MiniPrep plasmid extraction kit and stored at -20 oC prior to use.
Discussion
From the theoretical perspective, bacterial transformation is the process whereby we manipulate experimental conditions to facilitate the movement of a plasmid close to a competent cell, and helping the cell to uptake the plasmid to endow it with new functions and properties. Along the way, many factors need to be considered to help optimise the journey and improve the efficiency of bacterial transformation. In terms of expediency, incubation time on ice is perhaps one area in need for improvement.
Current protocols for
Escherichia coli transformation require 30 minutes incubation on ice to ensure higher transformation efficiency [
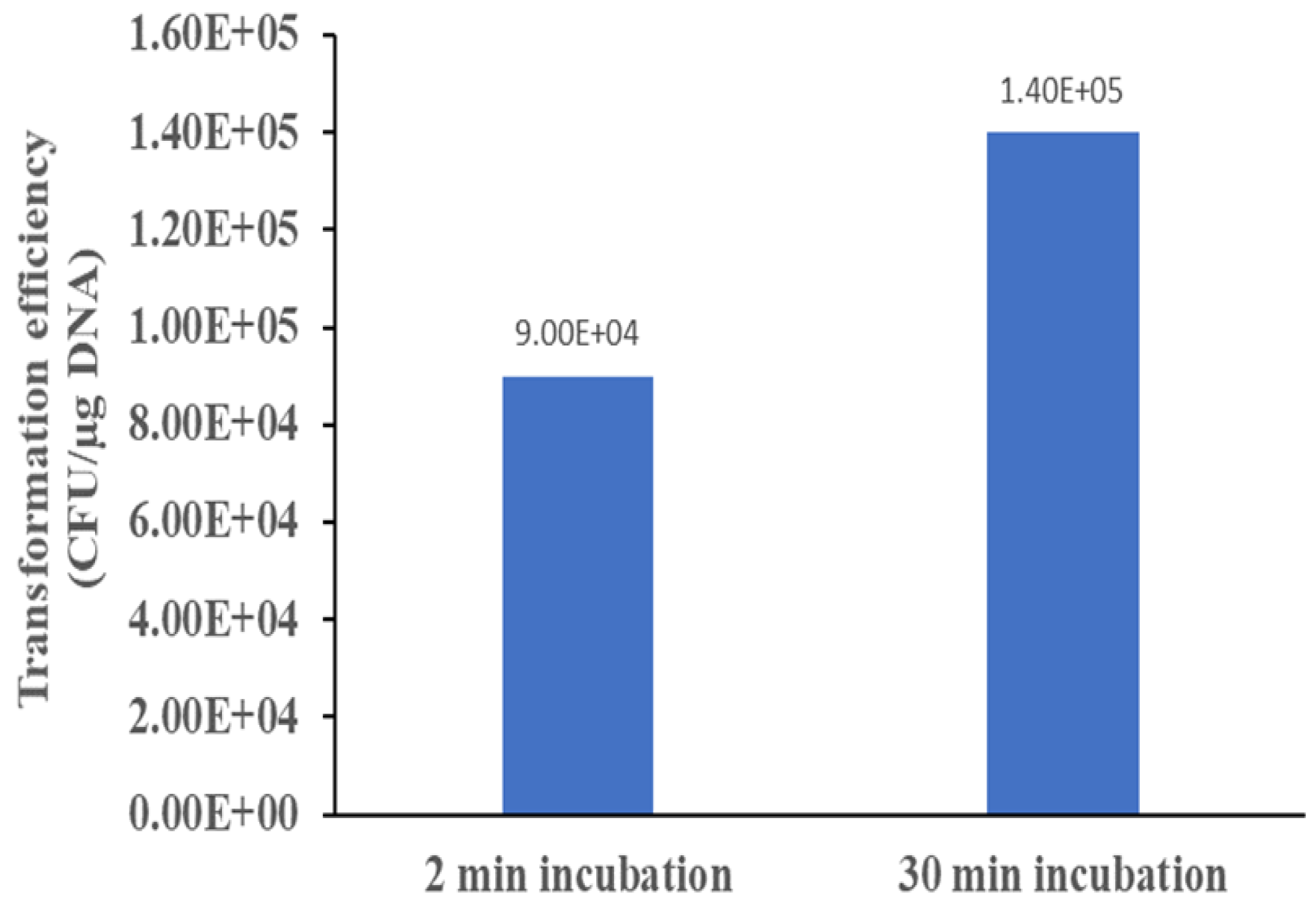
4]. But, could we achieve similar transformation efficiency with a shorter time? This work demonstrates that incubation time positively correlates with transformation efficiency since more time is available for the plasmid to get close to the cells and be taken up by it. However, a short 2 minute incubation still yield good transformation efficiency suitable for most applications in biotechnology where the desire is to transformation the cell with one plasmid.
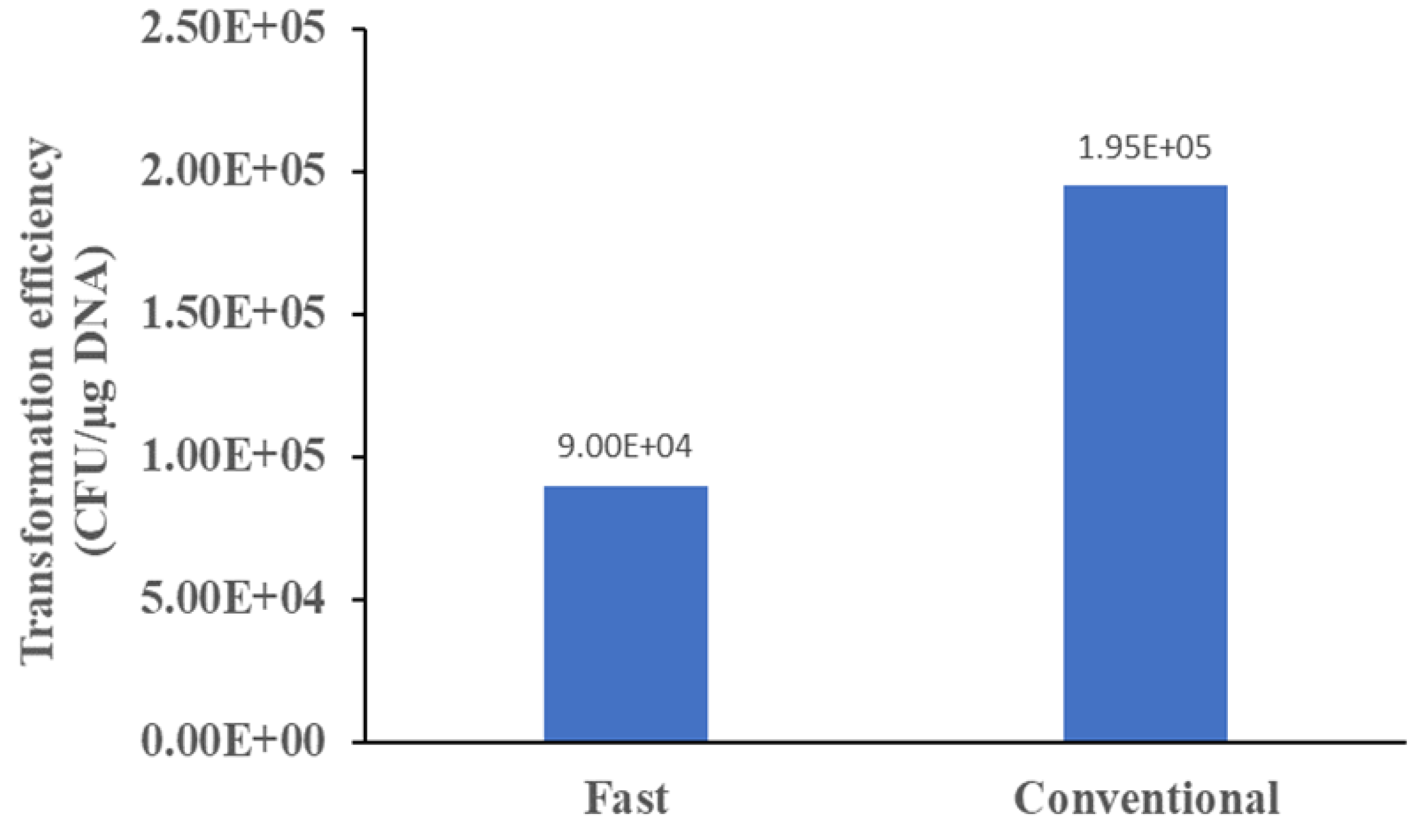
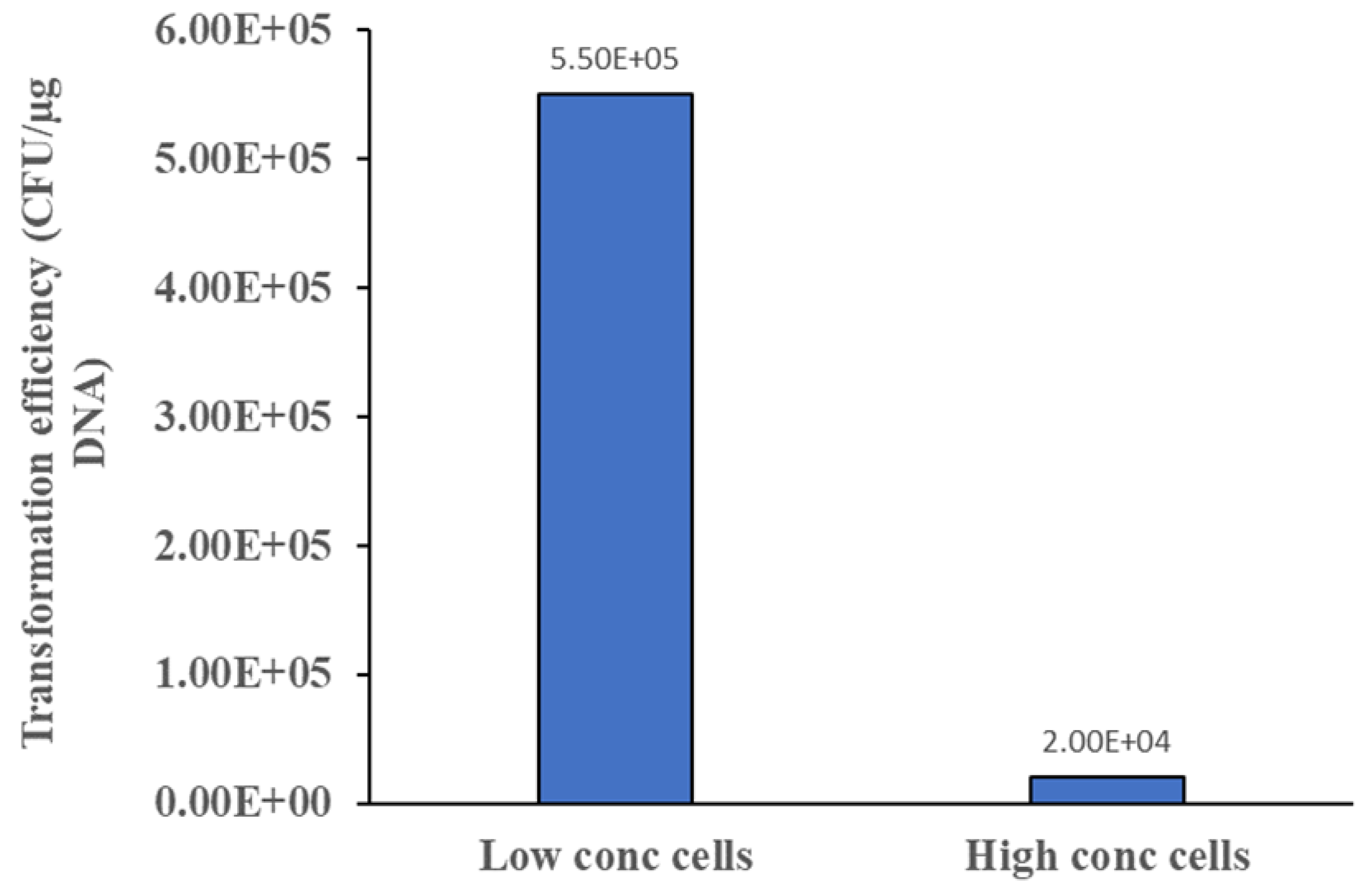
Perhaps the most intriguing finding from this work is the realisation that high concentration competent cells (108 CFU/mL) delivered poorer transformation efficiency compared to low concentration competent cells (106 CFU/mL). This is indeed counter-intuitive since conventional notion is that higher concentration of competent cells preparation will have more cells to uptake a given amount of plasmid. However, experiment results indicated that this is not true. One reason could be high concentration competent cells presenting a highly negatively charged microenvironment where the plasmid is surrounded by many negatively charged competent cells. Charge-charge repulsion would then present a significant hindrance for the facile diffusion of the plasmid to the cell surface, and subsequent uptake by the cell. This aspect of transformation is perhaps the least suspecting facet of the process, and its elucidation may help optimise many transformation protocols used in academic and industrial research labs.
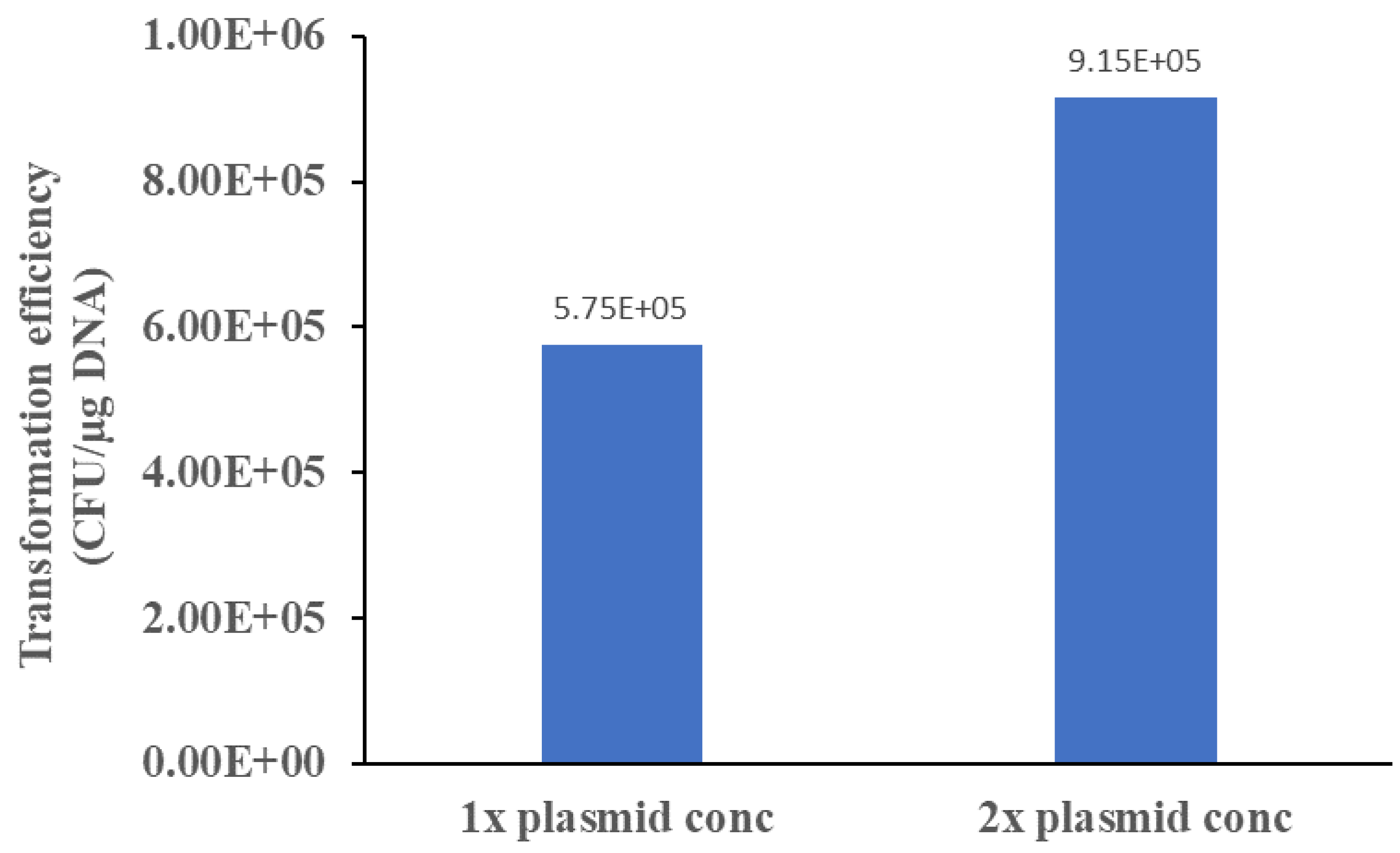
Finally, plasmid-to-cell ratio concerns the usage and consumption of precious plasmid, which is still produced by the cell-based approach coupled with plasmid extraction [
5]. Higher plasmid-to-cell ratio is perhaps the natural choice for many researchers interested in ensuring success of the experiment. However, possibility exists of high plasmid-to-cell ratio resulting in too many plasmids taken up by the cells, which may exhibit retarded growth due to high metabolic burden of expressing genes on the plasmid [
6]. Coupled with strong replication origins on the plasmid, a high plasmid-to-cell ratio may lead to smaller and fewer colonies on the transformation plate. This work observes that a one-fold increase in plasmid-to-cell ratio improves transformation efficiency. The results suggests that the pNAR plasmid may be too large (10 kB in size) for efficient uptake by the cells. Plasmid smaller in size, and thus, more efficiently uptake by cells may result in a reverse correlation. Hence, plasmid size likely determines the effect of plasmid-to-cell ratio on transformation efficiency.
Conclusions
This work has elucidated new understanding in three areas of bacterial transformation. Firstly, possibility exists in reducing the incubation time on ice while still retaining appreciable level of transformation efficiency for most applications. Secondly, low concentration competent cells may deliver better transformation efficiency compared to high concentration competent cells. Finally, experiments should be conducted to examine species-specific effect of plasmid-to-cell ratio. In the case of E. coli, only one plasmid is taken up by each cell in the case of large plasmid like pNAR, and hence, higher plasmid-to-cell ratio leads to higher transformation efficiency in these cases.
Funding
The author thank the National University of Singapore for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- H. Tan, L. Fu, and M. Seno, “Optimization of Bacterial Plasmid Transformation Using Nanomaterials Based on the Yoshida Effect. Int. J. Mol. Sci. 2010, 11, 12. [CrossRef]
- W.-T. Chan, C. S. W.-T. Chan, C. S. Verma, D. P. Lane, and S. K.-E. Gan, A comparison and optimization of methods and factors affecting the transformation of Escherichia coli. Biosci. Rep. 2013, 33, e00086. [Google Scholar] [CrossRef] [PubMed]
- A. Froger and J. E. Hall, “Transformation of Plasmid DNA into E. coli Using the Heat Shock Method. JoVE J. Vis. Exp. 2007, e253. [CrossRef]
- C. T. Chung, S. L. C. T. Chung, S. L. Niemela, and R. H. Miller, One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc. Natl. Acad. Sci. USA 1989, 86, 2172–2175. [Google Scholar] [CrossRef] [PubMed]
- J. Ohlson, Plasmid manufacture is the bottleneck of the genetic medicine revolution. Drug Discov. Today 2020, 25, 1891–1893. [CrossRef] [PubMed]
- F. Silva, J. A. Queiroz, and F. C. Domingues, Evaluating metabolic stress and plasmid stability in plasmid DNA production by Escherichia coli. Biotechnol. Adv. 2012, 30, 691–708. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).