Submitted:
11 May 2023
Posted:
12 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
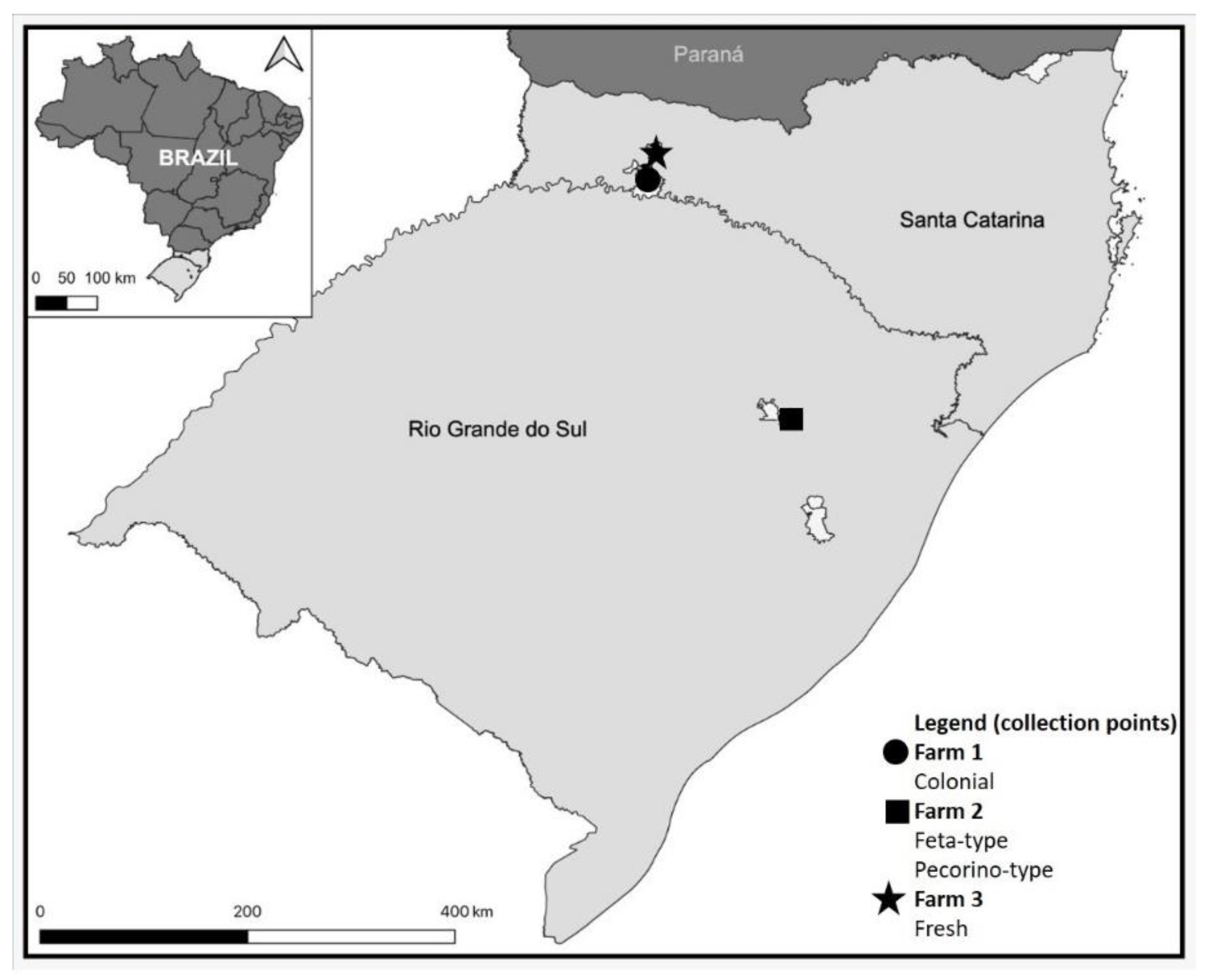
2.1. Sampling and processing
2.2. Sampling and processing
2.3. Analysis of staphylococcal enterotoxins
2.4. Staphylococcus spp. Isolation
2.5. Antimicrobial susceptibility analysis
2.6. Detection of resistance genes
2.7. Statistical analysis
3. Results and discussion
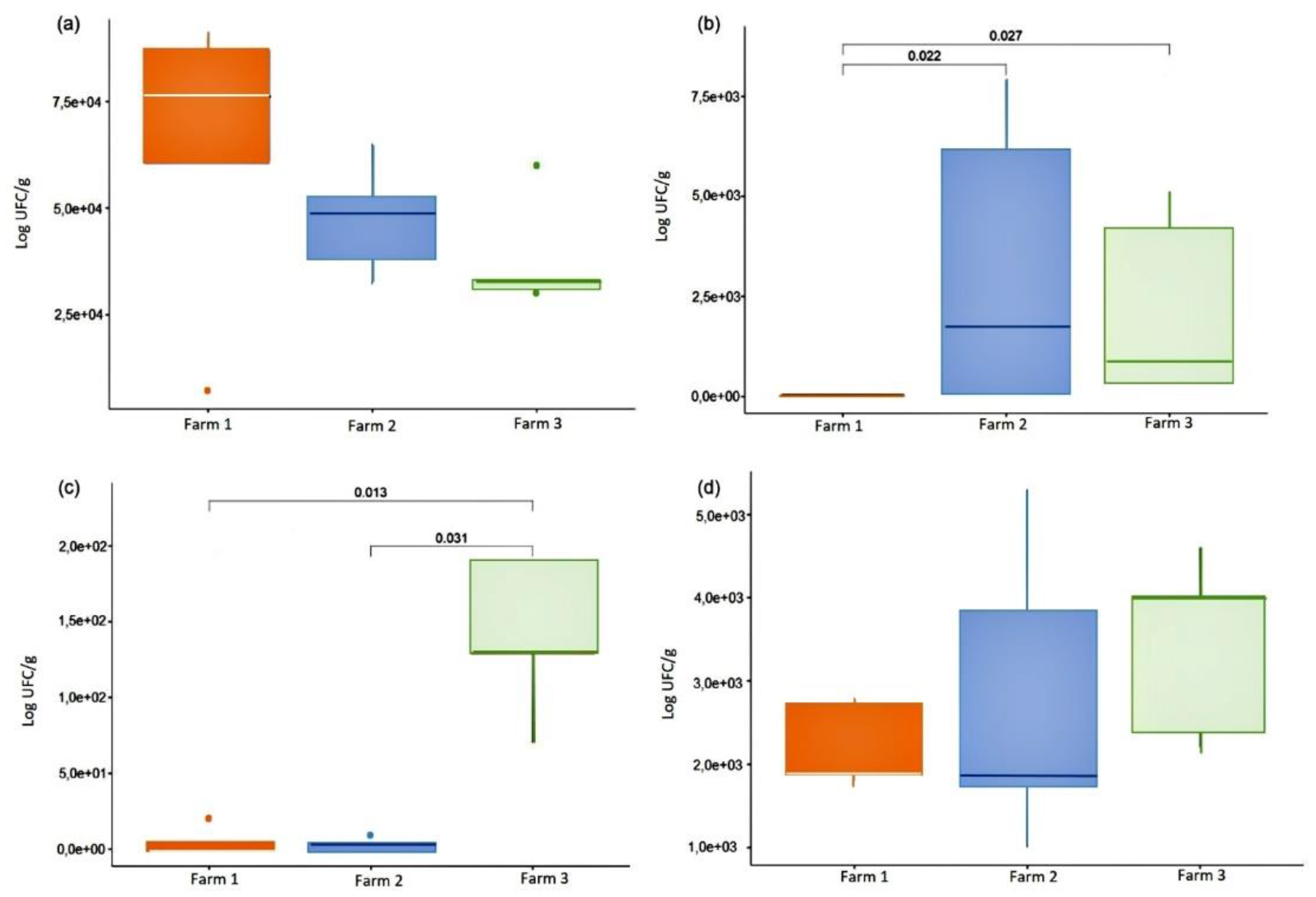
3.1. Microbiological quality of milk samples
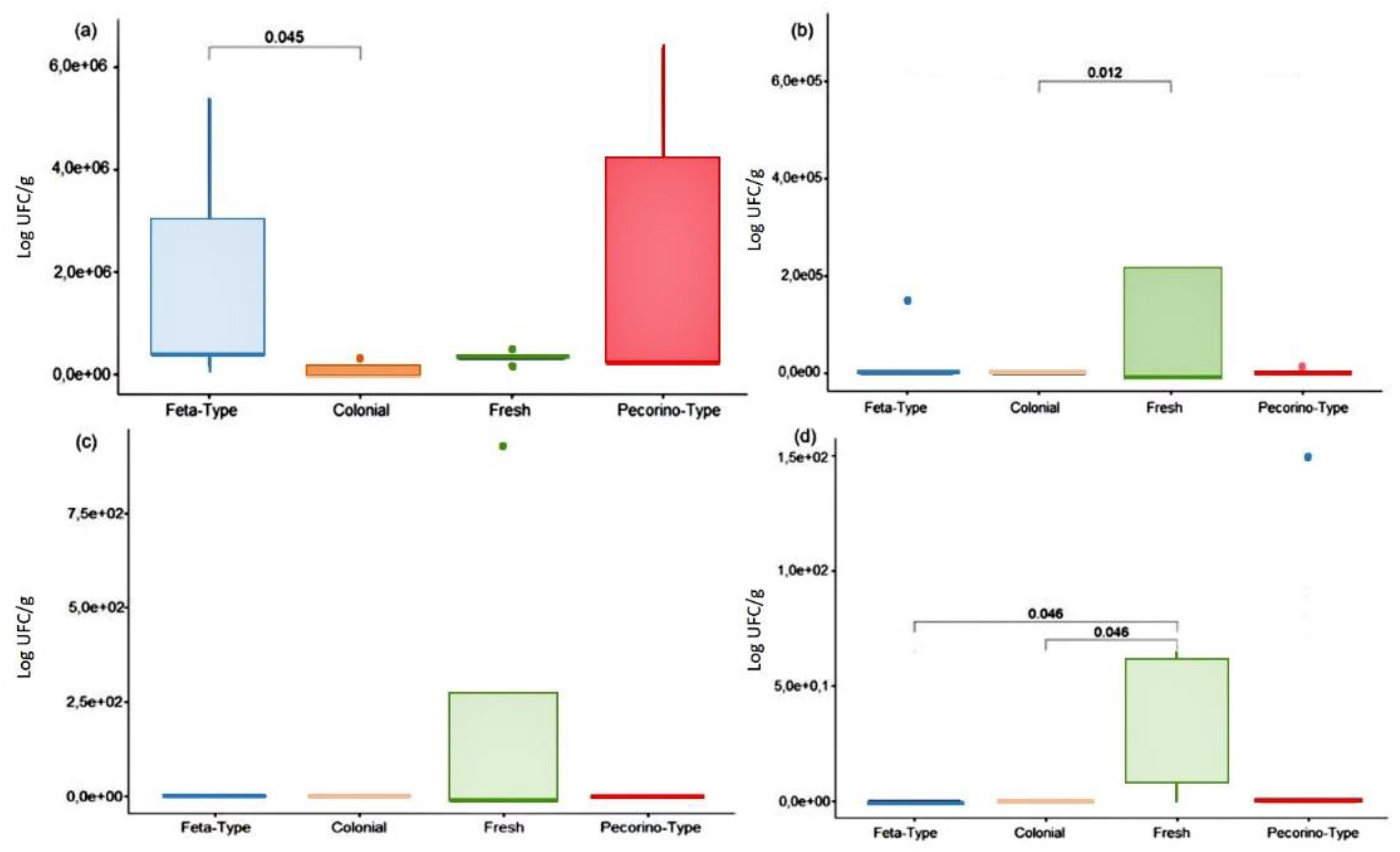
3.2. Microbiological quality of cheese samples
3.3. Enterotoxin investigation
3.4. Staphylococcus spp. isolation
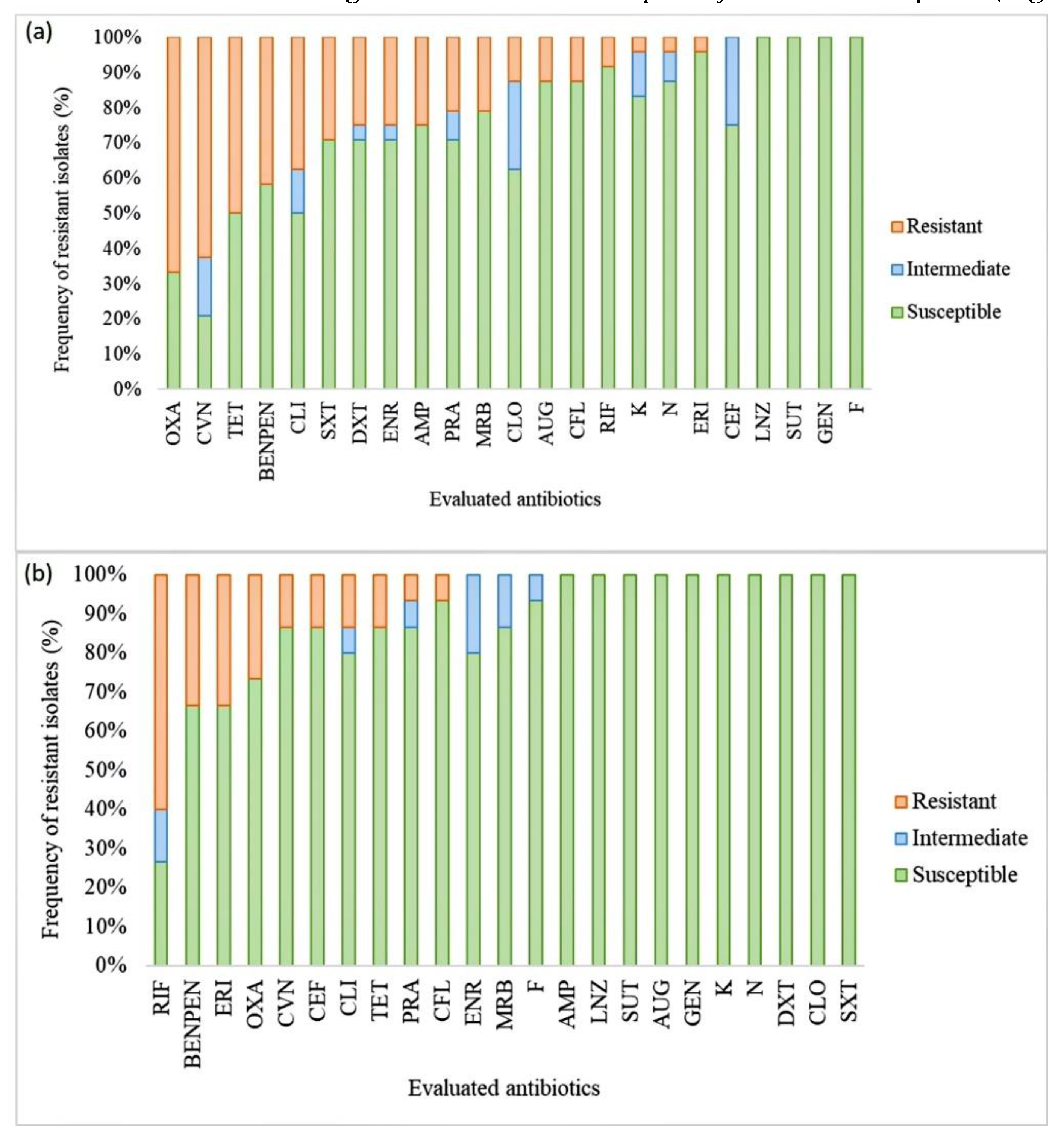
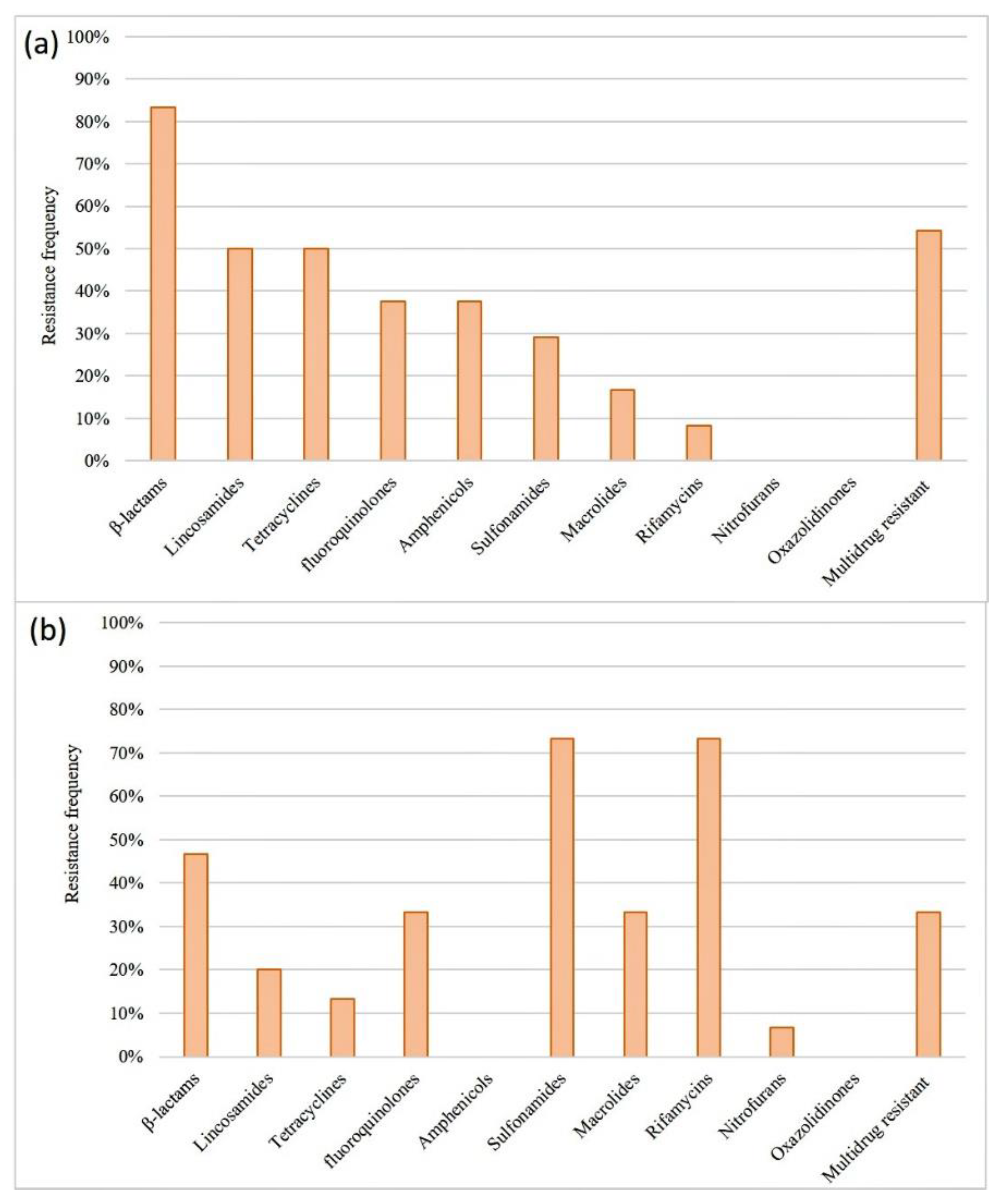
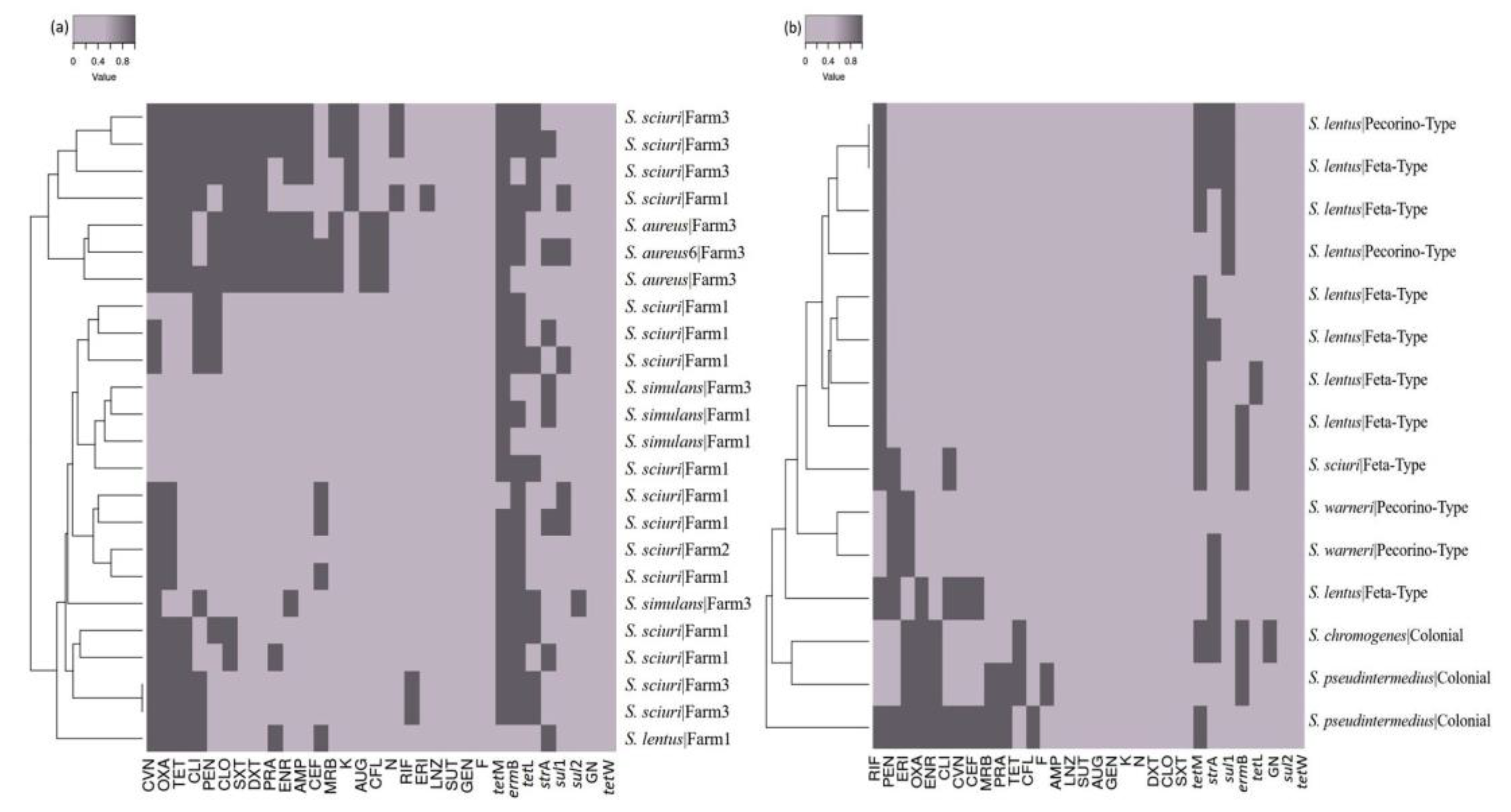
3.5. Antimicrobial susceptibility tests and the detection of resistance genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Embrapa, C. e O. Panorama Da Ovinocultura e Da Caprinocultura a Partir Do Censo Agropecuário 2017 Panorama Da Ovinocultura e Da Caprinocultura a Partir Do Censo Agropecuário 2017. Bol. Embrapa Caprino e Ovinos - n. 07 2018, 5–26.
- Bianchi, A. E. Avaliação de sistemas produtivos de ovinos leiteiros em diferentes regiões do brasil, Universidade Federal Do Paraná, 2018.
- Boor, K. J.; Wiedmann, M.; Murphy, S.; Alcaine, S. A 100-Year Review: Microbiology and Safety of Milk Handling. J. Dairy Sci. 2017, 100, 9933–9951. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G. do N.; Rosa, R. da S.; Dias, A. A.; Gonçalves, D. J. S.; Seribelli, A. A.; Pinheiro-Hubinger, L.; Eller, L. K. W.; de Carvalho, T. B.; Pereira, V. C. Characterization of the Virulence, Agr Typing and Antimicrobial Resistance Profile of Aureus Strains Isolated from Food Handlers in Brazil. Brazilian J. Infect. Dis. 2022, 26. [Google Scholar] [CrossRef]
- Kadiroǧlu, P.; Korel, F.; Ceylan, C. Quantification of Staphylococcus aureus in White Cheese by the Improved DNA Extraction Strategy Combined with TaqMan and LNA Probe-Based QPCR. J. Microbiol. Methods 2014, 105, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Silva, L. F. B. da; Bortoluci, F.; Vivan, A. C. P. Análise Microbiológica de Queijos Tipo Minas Frescal Oriundos de Diferentes Formas de Produção. Rev. Salusvita 2019, 329–343. [Google Scholar]
- Jørgensen, H. J.; Mørk, T.; Rørvik, L. M. The Occurrence of Staphylococcus aureus on a Farm with Small-Scale Production of Raw Milk Cheese. J. Dairy Sci. 2005, 88, 3810–3817. [Google Scholar] [CrossRef] [PubMed]
- Soares, D. B.; Monteiro, G. P.; Fonseca, B. B.; Freitas, E. A.; Mendonça, E. P.; De Melo, R. T.; Iasbeck, J. R.; Rossi, D. A. Análise sanitária e físico-química e adequação bacteriológica do queijo minas artesanal produzido em duas propriedades. Ciência Anim. Bras. 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Paiva, W. de S.; Neto, F. E. de S.; Oliveira, L. L. B. de; Bandeira, M. G. L.; Paiva, E. de S.; Batista, A. C. de L. Staphylococcus aureus : A Threat to Food Safety Staphylococcus aureus : Uma Ameaça à Segurança Alimentar Staphylococcus aureus : Una Amenaza Para La Seguridad Alimentaria. Res. Soc. Dev. 2021, 2021, 1–9. [Google Scholar]
- Mutuku, C.; Gazdag, Z.; Melegh, S. Occurrence of Antibiotics and Bacterial Resistance Genes in Wastewater: Resistance Mechanisms and Antimicrobial Resistance Control Approaches. World J. Microbiol. Biotechnol. 2022, 38, 1–27. [Google Scholar] [CrossRef]
- Murray, C. J.; Ikuta, K. S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; Johnson, S. C.; Browne, A. J.; Chipeta, M. G.; Fell, F.; Hackett, S.; Haines-Woodhouse, G.; Kashef Hamadani, B. H.; Kumaran, E. A. P.; McManigal, B.; Agarwal, R.; Akech, S.; Albertson, S.; Amuasi, J.; Andrews, J.; Aravkin, A.; Ashley, E.; Bailey, F.; Baker, S.; Basnyat, B.; Bekker, A.; Bender, R.; Bethou, A.; Bielicki, J.; Boonkasidecha, S.; Bukosia, J.; Carvalheiro, C.; Castañeda-Orjuela, C.; Chansamouth, V.; Chaurasia, S.; Chiurchiù, S.; Chowdhury, F.; Cook, A. J.; Cooper, B.; Cressey, T. R.; Criollo-Mora, E.; Cunningham, M.; Darboe, S.; Day, N. P. J.; De Luca, M.; Dokova, K.; Dramowski, A.; Dunachie, S. J.; Eckmanns, T.; Eibach, D.; Emami, A.; Feasey, N.; Fisher-Pearson, N.; Forrest, K.; Garrett, D.; Gastmeier, P.; Giref, A. Z.; Greer, R. C.; Gupta, V.; Haller, S.; Haselbeck, A.; Hay, S. I.; Holm, M.; Hopkins, S.; Iregbu, K. C.; Jacobs, J.; Jarovsky, D.; Javanmardi, F.; Khorana, M.; Kissoon, N.; Kobeissi, E.; Kostyanev, T.; Krapp, F.; Krumkamp, R.; Kumar, A.; Kyu, H. H.; Lim, C.; Limmathurotsakul, D.; Loftus, M. J.; Lunn, M.; Ma, J.; Mturi, N.; Munera-Huertas, T.; Musicha, P.; Mussi-Pinhata, M. M.; Nakamura, T.; Nanavati, R.; Nangia, S.; Newton, P.; Ngoun, C.; Novotney, A.; Nwakanma, D.; Obiero, C. W.; Olivas-Martinez, A.; Olliaro, P.; Ooko, E.; Ortiz-Brizuela, E.; Peleg, A. Y.; Perrone, C.; Plakkal, N.; Ponce-de-Leon, A.; Raad, M.; Ramdin, T.; Riddell, A.; Roberts, T.; Robotham, J. V.; Roca, A.; Rudd, K. E.; Russell, N.; Schnall, J.; Scott, J. A. G.; Shivamallappa, M.; Sifuentes-Osornio, J.; Steenkeste, N.; Stewardson, A. J.; Stoeva, T.; Tasak, N.; Thaiprakong, A.; Thwaites, G.; Turner, C.; Turner, P.; van Doorn, H. R.; Velaphi, S.; Vongpradith, A.; Vu, H.; Walsh, T.; Waner, S.; Wangrangsimakul, T.; Wozniak, T.; Zheng, P.; Sartorius, B.; Lopez, A. D.; Stergachis, A.; Moore, C.; Dolecek, C.; Naghavi, M. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis -Meneral Determination. Off. Methods Anal. 2019, 1 (2017.02 (2.6.38)), 6.
- Bobenchik, A. M.; Hindler, J. A.; Giltner, C. L.; Saeki, S.; Humphries, R. M. Performance of Vitek 2 for Antimicrobial Susceptibility Testing of Staphylococcus Spp. and Enterococcus Spp. J. Clin. Microbiol. 2017, 52, 392–397. [Google Scholar] [CrossRef]
- Caporaso, J. G.; Lauber, C. L.; Walters, W. A.; Berg-Lyons, D.; Lozupone, C. A.; Turnbaugh, P. J.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc Natl Acad Sci U S A 2011. [CrossRef] [PubMed]
- Sutcliffe, J.; Tait-Kamradt, A.; Wondrack, L. Streptococcus Pneumoniae and Streptococcus Pyogenes Resistant to Macrolides but Sensitive to Clindamycin: A Common Resistance Pattern Mediated by an Efflux System. Antimicrob. Agents Chemother. 1996, 40, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Van de Klundert JAM, V. J. PCR Detection of Genes Coding for Aminoglycoside-Modifying Enzymes. Diagnostic Mol. Microbiol. Princ. Appl. 1993, 547–552. [Google Scholar]
- Ana Paula Guedes Frazzon, Bianca Almeida Gama, Vanessa Hermes, Christine Garcia Bierhals, Rebeca Inhoque Pereira, Arthur Gomes Guedes, P.A. d’Azevedo; Frazzon, J. Prevalence of Antimicrobial Resistance and Molecular Characterization of Tetracycline Resistance Mediated by Tet(M) and Tet(L) Genes in Enterococcus Spp. Isolated from Food in Southern Brazil. World J. Microbiol. Biotechnol. 2010, 26, 365–370.
- Martins, J. M. Características Físico-Químicas e Microbiológicas Durante a Maturação Do Queijo Minas Artesanal Da Região Do Serro, Universidade Federal de Viçosa, Viçosa, 2006. Available online: https://locus.ufv.br//handle/123456789/499 (accessed on 3 December 2021).
- de Medeiros, M. I. M.; Filho, A. N.; de Souza, V.; Melo, P.de C.; Ferreira, L. M.; Medina Canalejo, L. M. Epidemiologia Molecular Aplicada Ao Monitoramento de Estirpes de Staphylococcus aureus Na Produção de Queijo Minas Frescal. Ciência Anim. Bras. 2013, 14, 98–105. [Google Scholar] [CrossRef]
- Lopes Júnior, W. D.; Monte, D. F. M. do; de Leon, C. M. G. C.; Moura, J. F. P. de; Silva, N. M. V. da; Queiroga, R. de C. R. do E.; Gonzaga Neto, S.; Givisiez, P. E. N.; Pereira, W. E.; Oliveira, C. J. B. de. Logistic Regression Model Reveals Major Factors Associated with Total Bacteria and Somatic Cell Counts in Goat Bulk Milk. Small Rumin. Res. 2021, 198, 0–7. [Google Scholar] [CrossRef]
- Akineden, Ö.; Hassan, A. A.; Schneider, E.; Usleber, E. Enterotoxigenic Properties of Staphylococcus aureus Isolated from Goats’ Milk Cheese. Int. J. Food Microbiol. 2008, 124, 211–216. [Google Scholar] [CrossRef]
- Endres, C. M.; Castro, Í. M. S.; Trevisol, L. D.; Severo, J. M.; Mann, M. B.; Varela, A. P. M.; Frazzon, A. P. G.; Mayer, F. Q.; Frazzon, J. Molecular Characterization of the Bacterial Communities Present in Sheep’s Milk and Cheese Produced in South Brazilian Region via 16S RRNA Gene Metabarcoding Sequencing. LWT 2021, 147, 111579. [Google Scholar] [CrossRef]
- Martins, K. B.; Faccioli-Martins, P. Y.; Riboli, D. F. M.; Pereira, V. C.; Fernandes, S.; Oliveira, A. A.; Dantas, A.; Zafalon, L. F.; de Lourdes Ribeiro de Souza da Cunha, M. Clonal Profile, Virulence and Resistance of Staphylococcus aureus Isolated from Sheep Milk. Brazilian J. Microbiol. 2015, 46, 535–543. [Google Scholar] [CrossRef]
- Spanu, V.; Spanu, C.; Cossu, F.; Virdis, S.; Scarano, C.; Santis, E. P. L. De. Prevalence of Staphylococcus aureus Strains in Raw Sheep Milk Cheese and Enterotoxigenic Profile. Ital. J. Food Saf. 2012, 1, 91–95. [Google Scholar] [CrossRef]
- Lu, M.; Wang, N. S. Spoilage of Milk and Dairy Products; Elsevier Ltd, 2017. [CrossRef]
- Campos, G. Z.; Lacorte, G. A.; Jurkiewicz, C.; Hoffmann, C.; Landgraf, M.; Gombossy de Melo Franco, B. D.; Pinto, U. M. Microbiological Characteristics of Canastra Cheese during Manufacturing and Ripening. Food Control 2021, 121, 107598. [Google Scholar] [CrossRef]
- Dash, K. K.; Fayaz, U.; Dar, A. H.; Shams, R.; Manzoor, S.; Sundarsingh, A.; Deka, P.; Khan, S. A. A Comprehensive Review on Heat Treatments and Related Impact on the Quality and Microbial Safety of Milk and Milk-Based Products. Food Chem. Adv. 2022, 1, 100041. [Google Scholar] [CrossRef]
- Abolghait, S. K.; Fathi, A. G.; Youssef, F. M.; Algammal, A. M. Methicillin-Resistant Staphylococcus aureus (MRSA) Isolated from Chicken Meat and Giblets Often Produces Staphylococcal Enterotoxin B (SEB) in Non-Refrigerated Raw Chicken Livers. Int. J. Food Microbiol. 2020, 328, 108669. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, K.; Islam, M. R.; Siddiky, N. A.; Samad, M. A.; Chowdhury, S.; Hossain, K. M. M.; Rume, F. I.; Hossain, M. K.; Mahbub-E-Elahi, A.; Ali, M. Z.; Rahman, M.; Amin, M. R.; Masuduzzaman, M.; Ahmed, S.; Ara Rumi, N.; Hossain, M. T. Antimicrobial Resistance Profile of Common Foodborne Pathogens Recovered from Livestock and Poultry in Bangladesh. Antibiotics 2022, 11, 1551. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, H.; Rahimi, E.; Moshkelani, S. Molecular Detection of Antimicrobial Resistance Genes in E. coli Isolated from Slaughtered Commercial Chickens in Iran. Vet. Med. (Praha). 2012, 57, 193–197. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Bhandari, B.; Yang, C. Investigação Sobre Gel de Surimi de Peixe Como Material Alimentar Promissor Para Impressão 3D Palavras-Chave. 2020, 1–17.
- Ferasyi, T. R.; Abrar, M.; Subianto, M.; Afrianandra, C.; Hambal, M.; Razali, R.; Ismail, I.; Nurliana, N.; Rastina, R.; Sari, W. E.; Safika, S.; Vierman, V.; Mutia, N.; Barus, R. A.; Yusmadi, Y.; Rosa, T. S.; Ramadhan, R. Isolation, Identification, and Critical Points of Risk of Escherichia coli O157:H7 Contamination at Aceh Cattle Breeding Centre. E3S Web Conf. 2020, 151, 8–12. [Google Scholar] [CrossRef]
- Schmid, A.; Hörmansdorfer, S.; Messelhäusser, U.; Käsbohrer, A.; Sauter-Louis, C.; Mansfeld, R. Prevalence of Extended-Spectrum β-Lactamase-Producing Escherichia coli on Bavarian Dairy and Beef Cattle Farms. Appl. Environ. Microbiol. 2013, 79, 3027–3032. [Google Scholar] [CrossRef]
- Aguiar, R. A. C.; Ferreira, F. A.; Dias, R. S.; Nero, L. A.; Miotto, M.; Verruck, S.; De Marco, I.; De Dea Lindner, J. Graduate Student Literature Review: Enterotoxigenic Potential and Antimicrobial Resistance of Staphylococci from Brazilian Artisanal Raw Milk Cheeses. J. Dairy Sci. 2022, 105, 5685–5699. [Google Scholar] [CrossRef]
- Ertas Onmaz, N.; Abay, S.; Karadal, F.; Hizlisoy, H.; Telli, N.; Al, S. Occurence and Antimicrobial Resistance of Staphylococcus aureus and Salmonella Spp. in Retail Fish Samples in Turkey. Mar. Pollut. Bull. 2015, 90, 242–246. [Google Scholar] [CrossRef]
- Ministério da saude. Ministério Da Saúde; 2020. Available online: http://bvsms.saude.gov.br/bvs/saudelegis/svs1/1998/prt0027_13_01_1998.html (accessed on 7 December 2020).
- Penna, A. L. B.; Gigante, M. L.; Todorov, S. D. Artisanal Brazilian Cheeses—History, Marketing, Technological and Microbiological Aspects. Foods 2021, 10, 1562. [Google Scholar] [CrossRef]
- Borelli, B. M.; Ferreira, E. G.; Lacerda, I. C. A.; Santos, D. A.; Carmo, L. S.; Dias, R. S.; Silva, M. C. C.; Rosa, C. A. Enteroxigenic Staphylococcus Spp. and Other Microbial Contaminants during Production of Canastra Cheese, Brazil. Brazilian J. Microbiol. 2006, 37, 545–550. [Google Scholar] [CrossRef]
- Morandi, S.; Brasca, M.; Lodi, R.; Cremonesi, P.; Castiglioni, B. Detection of Classical Enterotoxins and Identification of Enterotoxin Genes in Staphylococcus aureus from Milk and Dairy Products. Vet. Microbiol. 2007, 124, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Sabike, I. I.; Fujikawa, H.; Sakha, M. Z.; Edris, A. M. Production of Staphylococcus aureus Enterotoxin a in Raw Milk at High Temperatures. J. Food Prot. 2014, 77, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Tribst, A. A. L.; Falcade, L. T. P.; Carvalho, N. S.; Leite Júnior, B. R. de C.; Oliveira, M. M. de. Manufacture of a Fermented Dairy Product Using Whey from Sheep’s Milk Cheese: An Alternative to Using the Main by-Product of Sheep’s Milk Cheese Production in Small Farms. Int. Dairy J. 2020, 111, 104833. [Google Scholar] [CrossRef]
- Argemi, X.; Hansmann, Y.; Prola, K.; Prévost, G. Coagulase-Negative Staphylococci Pathogenomics. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Nanoukon, C.; Argemi, X.; Sogbo, F.; Orekan, J.; Keller, D.; Affolabi, D.; Schramm, F.; Riegel, P.; Baba-Moussa, L.; Prévost, G. Pathogenic Features of Clinically Significant Coagulase-Negative Staphylococci in Hospital and Community Infections in Benin. Int. J. Med. Microbiol. 2017, 307, 75–82. [Google Scholar] [CrossRef]
- Feyissa, N.; Alemu, T.; Jirata Birri, D.; Dessalegn, A. Isolation, Identification, and Determination of Antibiogram Characteristics of Staphylococcus aureus in Cow Milk and Milk Products (Yoghurt and Cheese) in West Showa Zone, Ethiopia. Int. Dairy J. 2023, 137, 105503. [Google Scholar] [CrossRef]
- Goerges, S.; Mounier, J.; Rea, M. C.; Gelsomino, R.; Heise, V.; Beduhn, R.; Cogan, T. M.; Vancanneyt, M.; Scherer, S. Commercial Ripening Starter Microorganisms Inoculated into Cheese Milk Do Not Successfully Establish Themselves in the Resident Microbial Ripening Consortia of a South German Red Smear Cheese. Appl. Environ. Microbiol. 2008, 74, 2210–2217. [Google Scholar] [CrossRef]
- Rahmdel, S.; Hosseinzadeh, S.; Shekarforoush, S. S.; Torriani, S.; Gatto, V.; Pashangeh, S. Safety Hazards in Bacteriocinogenic Staphylococcus Strains Isolated from Goat and Sheep Milk. Microb. Pathog. 2018, 116, 100–108. [Google Scholar] [CrossRef]
- Sasaki, T.; Tsubakishita, S.; Tanaka, Y.; Sakusabe, A.; Ohtsuka, M.; Hirotaki, S.; Kawakami, T.; Fukata, T.; Hiramatsu, K. Multiplex-PCR Method for Species Identification of Coagulase-Positive Staphylococci. J. Clin. Microbiol. 2010, 48, 765–769. [Google Scholar] [CrossRef]
- Lasagno, M.; Ortiz, M.; Vissio, C.; Yaciuk, R.; Bonetto, C.; Pellegrino, M.; Bogni, C.; Odierno, L.; Raspanti, C. Pathogenesis and Inflammatory Response in Experimental Caprine Mastitis Due to Staphylococcus Chromogenes. Microb. Pathog. 2018, 116, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Kiossis, E.; Brozos, C. N.; Petridou, E.; Zdragas, A.; Papadopoulos, T.; Boscos, C. Study on the Possible Survival of Staphylococcus Chromogenes through the Dry Period in Dairy Ewes. Small Rumin. Res. 2013, 115, 124–129. [Google Scholar] [CrossRef]
- Valckenier, D.; Piepers, S.; De Visscher, A.; De Vliegher, S. The Effect of Intramammary Infection in Early Lactation with Non-Aureus Staphylococci in General and Staphylococcus Chromogenes Specifically on Quarter Milk Somatic Cell Count and Quarter Milk Yield. J. Dairy Sci. 2020, 103, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Khazandi, M.; Al-Farha, A. A. B.; Coombs, G. W.; O’Dea, M.; Pang, S.; Trott, D. J.; Aviles, R. R.; Hemmatzadeh, F.; Venter, H.; Ogunniyi, A. D.; Hoare, A.; Abraham, S.; Petrovski, K. R. Genomic Characterization of Coagulase-Negative Staphylococci Including Methicillin-Resistant Staphylococcus Sciuri Causing Bovine Mastitis. Vet. Microbiol. 2018, 219, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ye, Q.; Chen, M.; Li, F.; Xiang, X.; Shang, Y.; Wang, C.; Zhang, J.; Xue, L.; Wang, J.; Wu, S.; Pang, R.; Ding, Y.; Wu, Q. Novel Species-Specific Targets for Real-Time PCR Detection of Four Common Pathogenic Staphylococcus Spp. Food Control 2022, 131, 108478. [Google Scholar] [CrossRef]
- Azimi, T.; Mirzadeh, M.; Sabour, S.; Nasser, A.; Fallah, F.; Pourmand, M. R. Coagulase-Negative Staphylococci (CoNS) Meningitis: A Narrative Review of the Literature from 2000 to 2020. New Microbes New Infect. 2020, 37, 100755. [Google Scholar] [CrossRef]
- Xiao, Z.; Xue, M.; Wu, X.; Zeng, L.; Zhu, Y.; Jiang, N.; Fan, Y.; Zhou, Y. Isolation and Identification of Staphylococcus Warneri from Diseased Coreius Guichenoti. Aquac. Reports 2022, 22, 100988. [Google Scholar] [CrossRef]
- Ruaro, A.; Andrighetto, C.; Torriani, S.; Lombardi, A. Biodiversity and Characterization of Indigenous Coagulase-Negative Staphylococci Isolated from Raw Milk and Cheese of North Italy. Food Microbiol. 2013, 34, 106–111. [Google Scholar] [CrossRef]
- Mahros, M. A.; Abd-Elghany, S. M.; Sallam, K. I. Multidrug-, Methicillin-, and Vancomycin-Resistant Staphylococcus aureus Isolated from Ready-to-Eat Meat Sandwiches: An Ongoing Food and Public Health Concern. Int. J. Food Microbiol. 2021, 346, 109165. [Google Scholar] [CrossRef]
- Xavier, M. R.; Freitas, T. S.; Pereira, R. L. S.; Marinho, E. M.; Bandeira, P. N.; de Sousa, A. P.; Oliveira, L. S.; Bezerra, L. L.; Neto, J. B. A.; Silva, M. M. C.; Cruz, B. G.; Rocha, J. E.; Barbosa, C. R. S.; da Silva, A. W.; de Menezes, J. E. S. A.; Coutinho, H. D. M.; Marinho, M. M.; Marinho, E. S.; dos Santos, H. S.; Teixeira, A. M. R. Anti-Inflammatory Effect, Antibiotic Potentiating Activity against Multidrug-Resistant Strains of Escherichia coli and Staphylococcus aureus, and Evaluation of Antibiotic Resistance Mechanisms by the Ibuprofen Derivative Methyl 2-(-4-Isobutylphenyl)Propan. Microb. Pathog. 2022, 170, 1–8. [Google Scholar] [CrossRef]
- Zhen, X.; Lundborg, C. S.; Zhang, M.; Sun, X.; Li, Y.; Hu, X.; Gu, S.; Gu, Y.; Wei, J.; Dong, H. Clinical and Economic Impact of Methicillin-Resistant Staphylococcus aureus: A Multicentre Study in China. Sci. Reports 2020 101 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Colautti, A.; Arnoldi, M.; Comi, G.; Iacumin, L. Antibiotic Resistance and Virulence Factors in Lactobacilli: Something to Carefully Consider. Food Microbiol. 2022, 103, 103934. [Google Scholar] [CrossRef]
- Zheng, X. R.; Sun, Y. H.; Chang, M. X.; Jiang, H. X. Plasmid and Chromosomal Copies of BlaCMY-2 Mediate Resistance to Third-Generation Cephalosporins in Escherichia coli from Food Animals in China. Vet. Microbiol. 2022, 271, 109493. [Google Scholar] [CrossRef] [PubMed]
- Buelow, E.; Ploy, M. C.; Dagot, C. Role of Pollution on the Selection of Antibiotic Resistance and Bacterial Pathogens in the Environment. Curr. Opin. Microbiol. 2021, 64, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Chung, H. Y.; Kim, Y. T.; Kwon, J. G.; Im, H. H.; Ko, D.; Lee, J. H.; Choi, S. H. Molecular Interaction between Methicillin-Resistant Staphylococcus aureus (MRSA) and Chicken Breast Reveals Enhancement of Pathogenesis and Toxicity for Food-Borne Outbreak. Food Microbiol. 2021, 93, 103602. [Google Scholar] [CrossRef] [PubMed]
- Avila-Novoa, M. G.; González-Gómez, J. P.; Guerrero-Medina, P. J.; Cardona-López, M. A.; Ibarra-Velazquez, L. M.; Velazquez-Suarez, N. Y.; Morales-del Río, J. A.; Gutiérrez-Lomelí, M. Staphylococcus aureus and Methicillin-Resistant S. aureus (MRSA) Strains Isolated from Dairy Products: Relationship of Ica-Dependent/Independent and Components of Biofilms Produced in Vitro. Int. Dairy J. 2021, 119, 105066. [Google Scholar] [CrossRef]
- Lemma, F.; Alemayehu, H.; Stringer, A.; Eguale, T. Prevalence and Antimicrobial Susceptibility Profile of Staphylococcus aureus in Milk and Traditionally Processed Dairy Products in Addis Ababa, Ethiopia. Biomed Res. Int. 2021, 2021. [Google Scholar] [CrossRef]
- Basanisi, M. G.; Nobili, G.; La Bella, G.; Russo, R.; Spano, G.; Normanno, G.; La Salandra, G. Molecular Characterization of Staphylococcus aureus Isolated from Sheep and Goat Cheeses in Southern Italy. Small Rumin. Res. 2016, 135, 17–19. [Google Scholar] [CrossRef]
- Angelidis, A. S.; Komodromos, D.; Giannakou, R.; Arsenos, G.; Gelasakis, A. I.; Kyritsi, M.; Filioussis, G.; Hadjichristodoulou, C.; Torounidou, P.; Papa, A.; Sergelidis, D. Isolation and Characterization of Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus (MRSA) from Milk of Dairy Goats under Low-Input Farm Management in Greece. Vet. Microbiol. 2020, 247, 108749. [Google Scholar] [CrossRef]
- Titouche, Y.; Hakem, A.; Houali, K.; Meheut, T.; Vingadassalon, N.; Ruiz-Ripa, L.; Salmi, D.; Chergui, A.; Chenouf, N.; Hennekinne, J. A.; Torres, C.; Auvray, F. Emergence of Methicillin-Resistant Staphylococcus aureus (MRSA) ST8 in Raw Milk and Traditional Dairy Products in the Tizi Ouzou Area of Algeria. J. Dairy Sci. 2019, 102, 6876–6884. [Google Scholar] [CrossRef]
- Phiri, B. S. J.; Hang’ombe, B. M.; Mulenga, E.; Mubanga, M.; Maurischat, S.; Wichmann-Schauer, H.; Schaarschmidt, S.; Fetsch, A. Prevalence and Diversity of Staphylococcus aureus in the Zambian Dairy Value Chain: A Public Health Concern. Int. J. Food Microbiol. 2022, 375, 109737. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yuan, X.; Hu, M.; Zhang, Y.; Li, L.; Zhang, Q.; Yuan, X.; Wang, W.; Liu, Y. Prevalence and Characterization of Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus Isolated from Bulk Tank Milk in Shandong Dairy Farms. Food Control 2021, 125, 107836. [Google Scholar] [CrossRef]
- Ferreira, J. S.; Costa, W. L. R.; Cerqueira, E. S.; Carvalho, J. S.; Oliveira, L. C.; Almeida, R. C. C. Food Handler-Associated Methicillin-Resistant Staphylococcus aureus in Public Hospitals in Salvador, Brazil. Food Control 2014, 37, 395–400. [Google Scholar] [CrossRef]
- Godziszewska, J.; Pogorzelska-Nowicka, E.; Brodowska, M.; Jagura-Burdzy, G.; Wierzbicka, A. Detection in Raw Cow’s Milk of Coliform Bacteria - Reservoir of Antibiotic Resistance. Lwt 2018, 93, 634–640. [Google Scholar] [CrossRef]
- Abatcha, M. G.; Effarizah, M. E.; Rusul, G. Prevalence, Antimicrobial Resistance, Resistance Genes and Class 1 Integrons of Salmonella Serovars in Leafy Vegetables, Chicken Carcasses and Related Processing Environments in Malaysian Fresh Food Markets. Food Control 2018, 91, 170–180. [Google Scholar] [CrossRef]
- Pahlavanzadeh, S.; Khoshbakht, R.; Kaboosi, H.; Moazamian, E. Antibiotic Resistance and Phylogenetic Comparison of Human, Pet Animals and Raw Milk Staphylococcus aureus Isolates. Comp. Immunol. Microbiol. Infect. Dis. 2021, 79, 101717. [Google Scholar] [CrossRef]
- Águila-Arcos, S.; Álvarez-Rodríguez, I.; Garaiyurrebaso, O.; Garbisu, C.; Grohmann, E.; Alkorta, I. Biofilm-Forming Clinical Staphylococcus Isolates Harbor Horizontal Transfer and Antibiotic Resistance Genes. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
| Target | Annealing temperature (°C) | Amplicon size (bp) | Sequence (5′ to 3′) | Reference |
|---|---|---|---|---|
| 16S rRNA | 55 | 375 | F CACGGTCGKCGGCGCCATT | [14] |
| R GGACTACHVGGGTWTCTAAT | ||||
| ermB | 52 | 639 | F GAAAAGGTACTCAACCAAATA | [15] |
| R AGTAACGGTACTTAAATTGTTTAC | ||||
| AAC(6)’ | 60 | 219 | F CCAAGAGCAATAAGGGCATA | [16] |
| R CACTATCATAACCACTACCG | ||||
| tetL | 58 | 628 | F ACTCGTAATGGTTGTAGTTGC | [17] |
| R TGTAACTCCGATGTTTAACACG | ||||
| tetM | 52 | 657 | F GTTAAATAGTGTTCTTGGAG | [18] |
| R CTAAGATATGGCTCTAACAA | ||||
| tetW | 60 | 168 | F GAGAGCCTGCTATATGCCAGC | [19] |
| R GGGCGTATCCACAATGTTAAC | ||||
| Sul1 | 60 | 99 | F GGATCAGACGTCGTGGATGT | [20] |
| R GTCTAAGAGCGGCGCAATAC | ||||
| Sul2 | 57 | 99 | F CGCAATGTGATCCATGATGT | [20] |
| R GCGAAATCATCTGCCAAACT | ||||
| strA | 59 | 99 | F CCAGTTCTCTTCGGCGTTAG | [20] |
| R ACTCTTCAATGCACGGGTCT |
| Sample | Species | Frequency (%) | Coagulase test | AMR frequency (%)* |
|---|---|---|---|---|
| Milk | S. sciuri | 16 | CoNS | 15 |
| S. Simulans | 4 | CoNS | 3 | |
| S. aureus | 3 | CoPS | 3 | |
|
S. lentus |
1 |
CoNS |
1 |
|
| Total | 24 | 22 | ||
| Cheese |
S. lentus |
9 |
CoNS |
8 |
|
S. warneri |
2 |
CoNS |
2 |
|
|
S. pseudintermedius |
2 |
CoPS |
2 |
|
|
S. chromogenes |
1 |
CoNS |
1 |
|
|
S. sciuri |
1 |
CoNS |
1 |
|
| Total | 15 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





