Submitted:
11 May 2023
Posted:
12 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Culture conditions
2.3. 16S rRNA sequencing
2.4. Gas and organic acid concentrations
3. Results
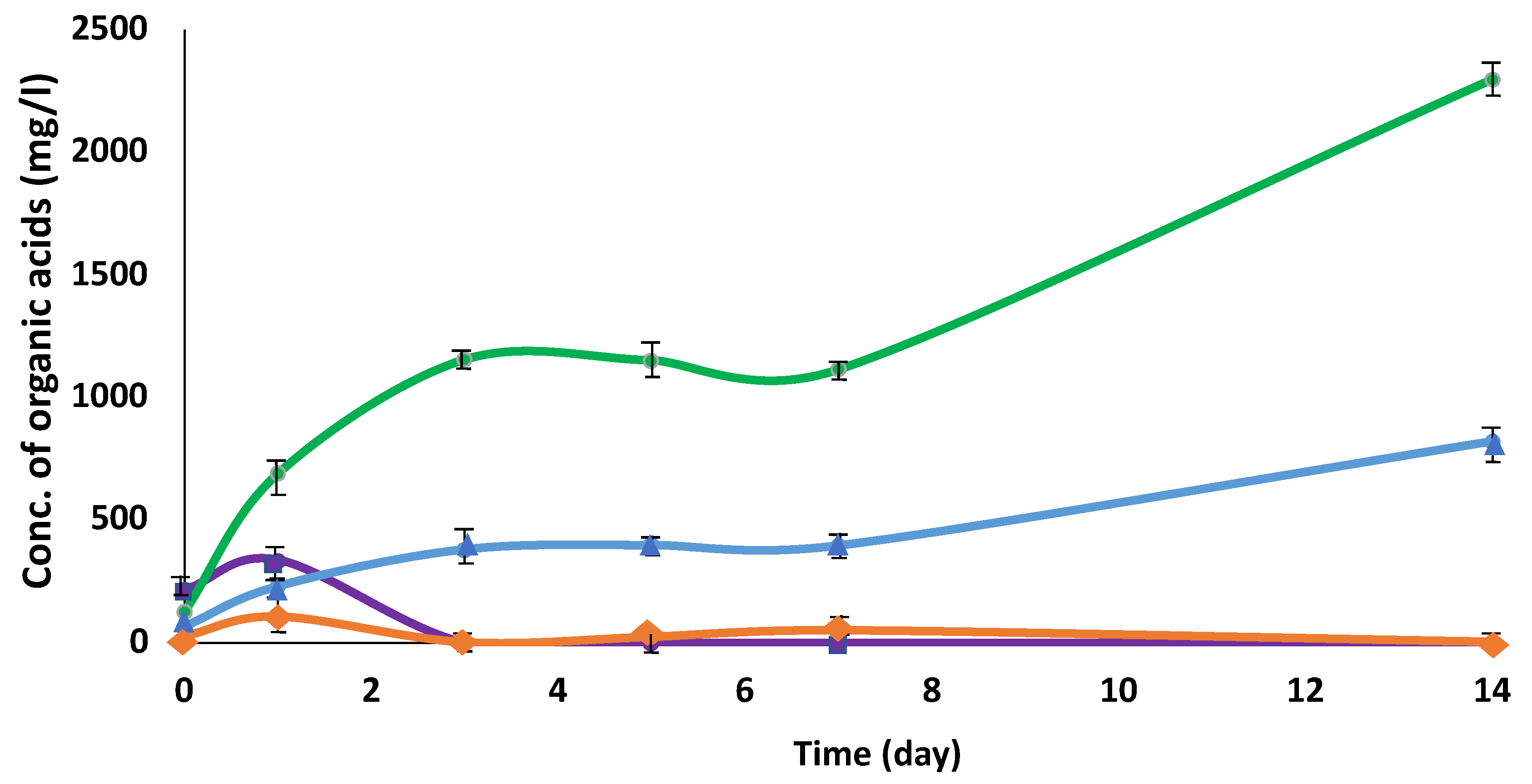
3.1. Cultivation of C. cellulovorans with pig manure
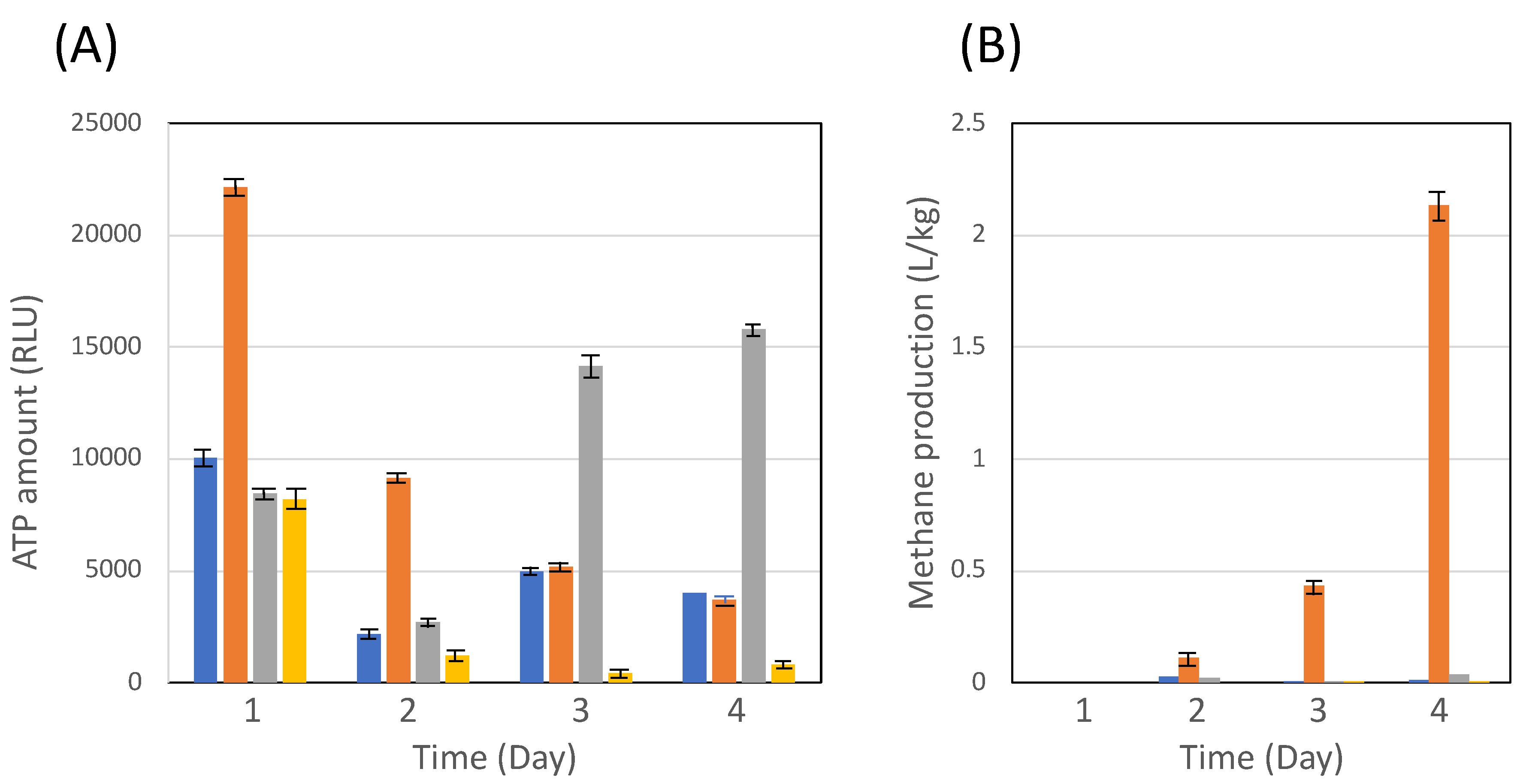
3.2. Co-cultivation of C. cellulovorans with methanogens or M. mazei
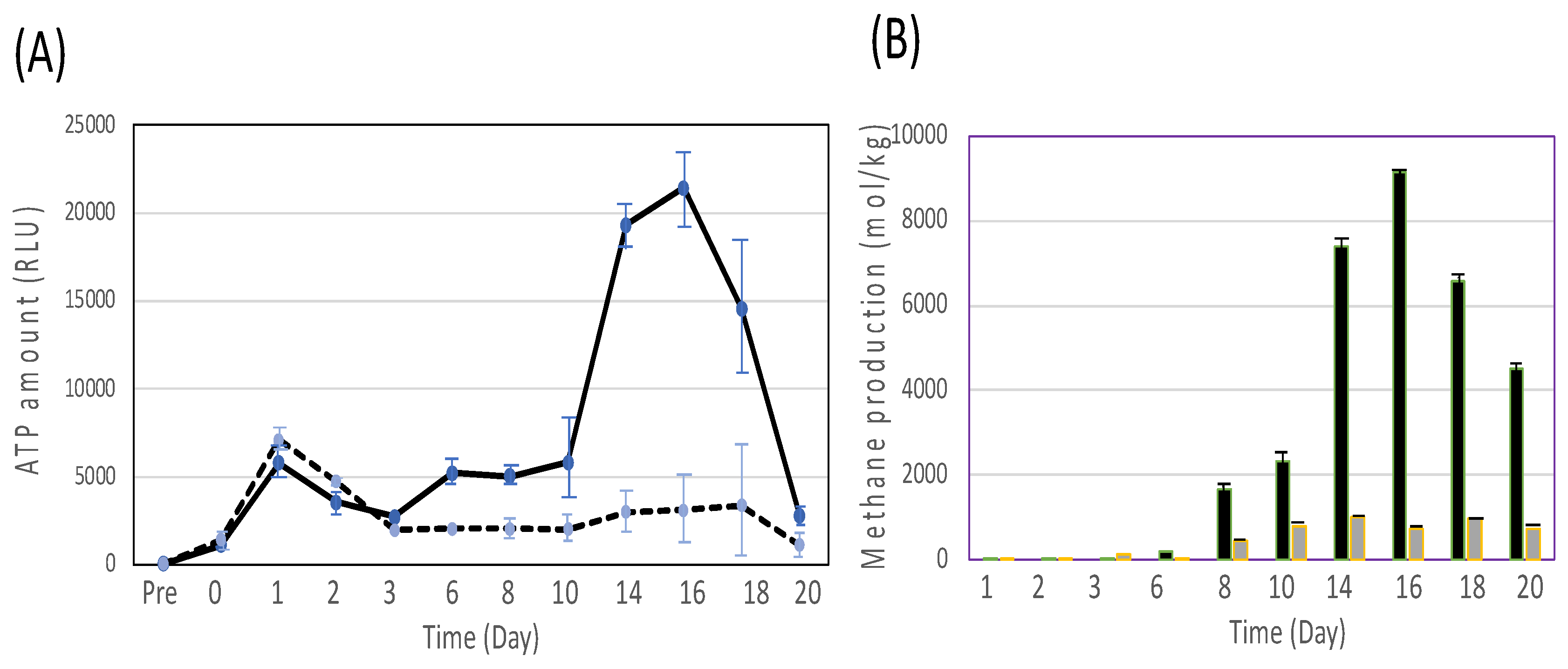
3.3. Effect of carbon sources with methanogens
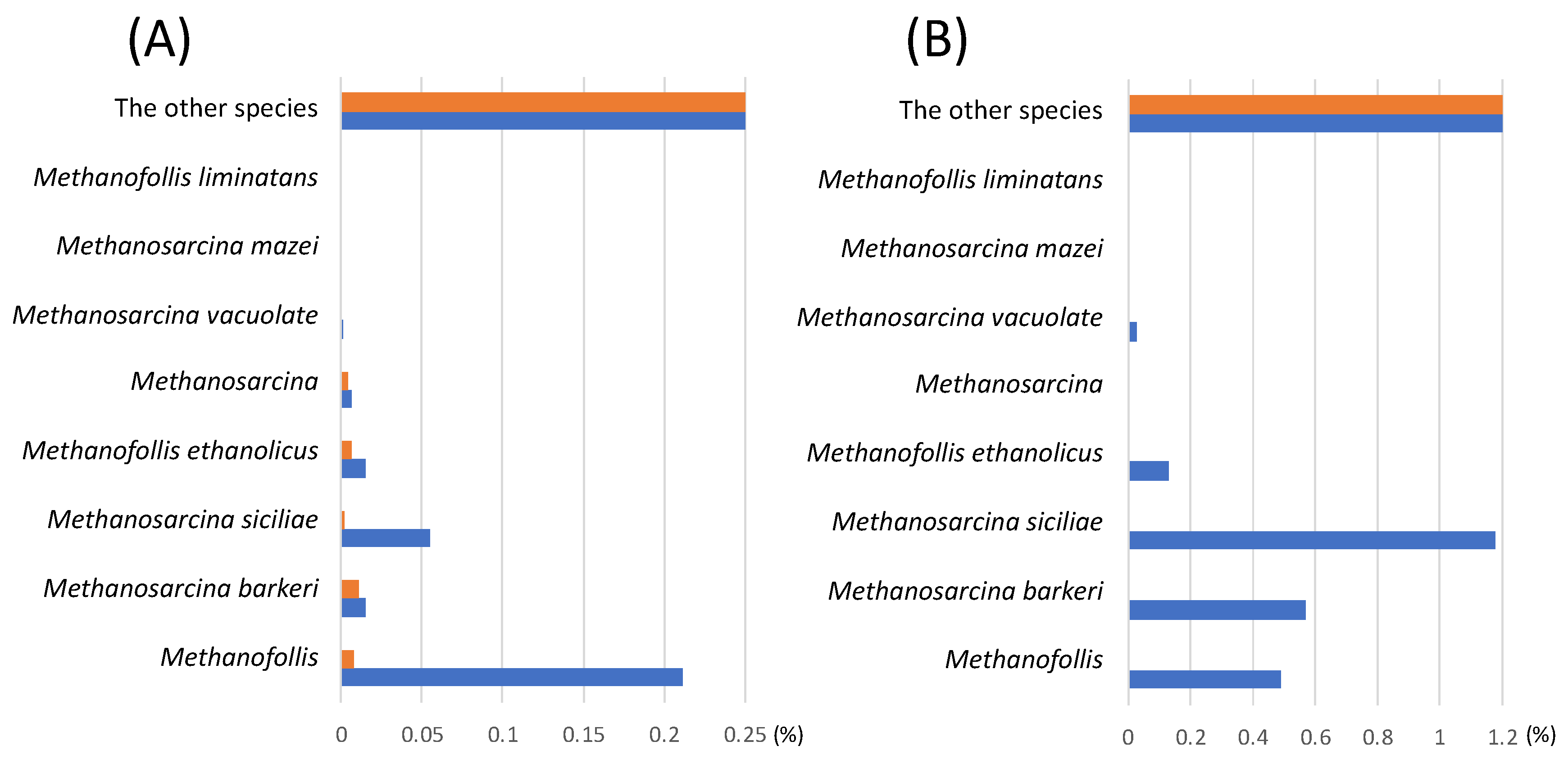
3.4. Identification of methanogens cultivatied with the different carbon sources
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, X.; Angelidaki, I.; Alvarado-Morales, M.; Liu, H.; Liu, Y.; Huang, X.; Zh, G. Methane production from formate, acetate and H2/CO2; focusing on kinetics and microbial characterization. Bioresour. Technol. 2016, 218, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.R.; Johansson, T.; Åkerman, H.J.; Mastepanov, M.; Malmer, N.; Friborg, T.; Crill, P.; Svenssonet, B.H. Thawing sub-arctic permafrost: effect sonvegetation and methane emissions. Geophys. Res. Lett. 2004, 31, L04501. [Google Scholar] [CrossRef]
- Woodcroft, B.J.; Singleton, C.M.; Boyd, J.A.; Evans, P.N.; Emerson, J.B.; Zayed, A.F.Z.; Hoelzle, R.D.; Lamberton, T.O.; Mccalley, C.K.; Hodgkins, S.B.; Wilson, R.M.; Purvine, S.O.; Nicora, C.D.; Li, L.; Frolking, S.; Chanton, J.P.; Patrick, M.; Crill, P.M.; Saleska, S.R.; Rich, V.I.; Tyson, G.W. Genome-centric view of carbon processing in thawing permafrost. Nature 2018, 560, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S. Syntrophic acetate-oxidizing microbes in methanogenic environments. Microbes Environ. 2008, 23, 118–127. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef]
- Garcia, J.L.; Patel, B.K.C; Ollivier, B. Taxonomic, phylogenetic, and ecological diversity of methanogenic Archaea. Anaerobe 2000, 6, 205–226. [Google Scholar] [CrossRef]
- Deppenmeier, U.; Müller, V.; Gottschalk, G. Pathways of energy conservation in methanogenic archaea. Arch. Microbiol. 1996, 165, 149–163. [Google Scholar] [CrossRef]
- Kruse, S.; Goris, T.; Westermann, M.; Adrian, L.; Diekert, G. Hydrogen production by Sulfurospirillum species enables syntrophic interactions of Epsilonproteobacteria. Nat. Commun. 2018, 9, 4872. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Segre, J.A. Signaling in host-associated microbial com- munities. Cell 2016, 164, 1288–1300. [Google Scholar] [CrossRef]
- Kenny, D.J.; Balskus, E.P. Engineering chemical interactions in microbial communities. Chem Soc Rev. 2018, 47, 1705–1729. [Google Scholar] [CrossRef]
- Brenner, K.; You, L.; Arnold, F.H. Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 2008, 26, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.Z.; Song, H.; Wang, E.X.; Liu, Y.; Yuan, Y.J. Design and construction of synthetic microbial consortia in China. Synth Syst Biotechnol. 2016, 1, 230–235. [Google Scholar] [CrossRef] [PubMed]
- De Bok, F.A.; Plugge, C.M.; Stams, A.J. Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res. 2004, 38, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zou, W.; Zhang, K.; Ye, G.; Yang, J. Advances and applications of Clostridium co-culture systems in biotechnology. Front. Microbiol. 2020, 11, 560223. [Google Scholar] [CrossRef] [PubMed]
- He, F; Hu, W. ; Li, Y. Biodegradation mechanisms and kinetics of azo dye 4BS by a microbial consortium. Chemosphere. 2004, 57, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Khouni, I.; Marrot, B.; Amar, R.B. Treatment of reconstituted textile waste-water containing a reactive dye in an aerobic sequencing batch reactor using a novel bacterial consortium. Sep Purif Technol. 2012, 87, 110–119. [Google Scholar] [CrossRef]
- Safonova, E.; Kvitko, K.V.; Iankevitch, M.I.; Surgko, L.F.; Afti, I.A.; Reisser, W. Biotreatment of industrial wastewater by selected algal-bacterial consortia. Eng Life Sci. 2004, 4, 347–353. [Google Scholar] [CrossRef]
- Xu, X.H.; Liu, X.M.; Zhang, L.; Mu, Y.; Zhu, X.Y.; Fang, J.Y.; Li, S.P.; Jiang, J.D. Bioaugmentation of chlorothalonil-contaminated soil with hydrolytically or reductively dehalogenating strain and its effect on soil microbial community. J Hazard Mater. 2018, 351, 240–249. [Google Scholar] [CrossRef]
- Sabra, W.; Dietz, D.; Tjahjasari, D.; Zeng, A.P. Biosystems analysis and engineering of microbial consortia for industrial biotechnology. Eng Life Sci. 2010, 10, 407–421. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite induction via microorganism co-culture: a potential way to enhance chemical diversity for drug discovery. Biotechnol Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Eiteman, M.A.; Lee, S.A.; Altman, R.; Altman, E. A substrate-selective co-fermentation strategy with Escherichia coli produces lactate by simultaneously consuming xylose and glucose. Biotechnol Bioeng. 2009, 102, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.X.; Ding, M.Z.; Ma, Q.; Dong, X.T.; Yuan, Y.J. Reorganization of a synthetic microbial consortium for one-step vitamin C fermentation. Microb Cell Fact. 2016, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Schroeckh, V.; Scherlach, K.; Nützmann, H.W.; Shelest, E.; Schmidtheck, W.; Schuemann, J.; Martin, K.; Hertweck, C.; Brakhage, A.A. Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc Natl Acad Sci USA. 2009, 106, 14558–14563. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.X.; He, J.Z. Characterization of a thermostable xylanase from a newly isolated Kluyvera species and its application for biobutanol production. Bioresour Technol. 2013, 135, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.L.; Goyal, G.; Chen, W. Surface display of a functional minicelluloome by intracellular complementation using a synthetic yeast consortium and its application to cellulose hydrolysis and ethanol production. Appl Environ Microbiol. 2010, 76, 7514–7520. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Happy together: microbial communities that hook up to swap electrons. ISME J. 2016, 11, 327–336. [Google Scholar] [CrossRef]
- Charubin, K.; Bennett, R.K.; Fast, A.G.; Papoutsakis, E.T. Engineering Clostridium organisms as microbial cell-factories: challenges & opportunities. Metab. Eng. 2018, 50, 173–191. [Google Scholar]
- Tamaru, Y.; Miyake, H.; Kuroda, K.; Nakanishi, A.; Kawade, Y.; Yamamoto, K.; Uemura, M.; Fujita, Y.; Doi, R.H.; Ueda, M. Genome sequence of the cellulosome-producing mesophilic organism Clostridium cellulovorans 743B. J. Bacteriol. 2010, 192, 901–902. [Google Scholar] [CrossRef]
- Tamaru,Y.; Miyake, H.; Kuroda, K.; Nakanishi, A.; Matsushima, C.; Doi, R.H.; Ueda, M. Comparison of the mesophilic cellulosome- producing Clostridium cellulovorans genome with other cellulosome-related clostridial genomes. Micro. Biotechnol. 2011, 4, 64–73. [CrossRef]
- Tomita, H.; Okazaki, F.; Tamaru, Y. Direct IBE fermentation from mandarin orange wastes by combination of Clostridium cellulovorans and Clostridium beijerinckii. AMB Express 2019, 9, 1. [Google Scholar] [CrossRef]
- Tomita, H.; Okazaki, F.; Tamaru, Y. Biomethane production from sugar beet pulp under cocultivation with Clostridium cellulovorans and methanogens. AMB Express 2019, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Tamaru, Y. The second-generation biomethane from mandarin orange peel under cocultivation with methanogens and the armed Clostridium cellulovorans. Fermentation 2019, 5, 95. [Google Scholar] [CrossRef]
- Lu, H.; Ng, S.-K.; Jia, Y.; Cai, M.; Lee, P.K.H. Physiological and molecular characterizations of the interactions in two cellulose-to-methane cocultures. Biotechnol. Biofuels 2017, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.; Kim, B.S. Green hydrogen production through consolidated bioprocessing of lignocellulosic biomass using nanobiotechnology approach. Biores. Technol. 2022, 365, 128108. [Google Scholar] [CrossRef] [PubMed]
- Goevert, D.; Conrad, R. Effect of substrate concentration on carbon isotope fractionation during acetoclastic methanogenesis by Methanosarcina barkeri and M. acetivorans and in rice field soil. Appl. Environ. Microbiol. 2009, 75, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Ministry of the Environment. MOE Discloses the Estimated Amount of Japan’s Food Loss and Waste Generated in FY 2018. 2021. Available online: https://www.env.go.jp/en/headline/2515.html (accessed on 10 November 2022).
- Watanabe, E.; Seike, N.; Motoki, Y.; Inao, K.; Otani, T. Potential application of immunoassays for simple, rapid and quantitative detections of phytoavailable neonicotinoid insecticides in cropland soils. Ecotoxicol. Environ. Saf. 2016, 132, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.N.B.; Huang, G.H.; Yu, H. Microbial-growth inhibition during composting of food waste: Effect of organic acids. Bioresour. Technol. 2010, 101, 5925–5934. [Google Scholar] [CrossRef] [PubMed]
- Nakasaki, K.; Hirai, H. Temperature control strategy to enhance the activity of yeast inoculated into compost raw material for accelerated composting. Waste Manag. 2017, 65, 29–36. [Google Scholar] [CrossRef]
- Wang, H.; Lim, T.T.; Duong, C.; Zhang, W.; Xu, C.; Yan, L.; Mei, Z.; Wang, W. Long-Term Mesophilic Anaerobic Co-Digestion of Swine Manure with Corn Stover and Microbial Community Analysis. Microorganisms 2020, 8, 188. [Google Scholar] [CrossRef]
- Córdoba, V.; Fernández, M.; Santalla, E. The effect of different inoculums on anaerobic digestion of swine wastewater. J. Environ. Chem. Eng. 2016, 4, 115–122. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Fernández, C.; Gómez, X.; Moran, A. Anaerobic co-digestion of swine manure with energy crop residues. Biotechnol. Bioprocess Eng. 2011, 16, 1044–1052. [Google Scholar]
- Dennehy, C.; Lawlor, P.G.; McCabe, M.S.; Cormican, P.; Sheahan, J.; Jiang, Y.; Zhan, X.; Gardiner, G.E. Anaerobic co-digestion of pig manure and food waste: Effects on digestate dewaterability, and microbial community dynamics. Waste Manag. 2018, 71, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo-Salces, B.; González-Fernández, C.; Gómez, X.; García-González, M.C.; Morán, A. Vegetable processing wastes addition to improve swine manure anaerobic digestion: Evaluation in terms of methane yield and SEM characterization. Appl. Energy 2012, 91, 36–42. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Macé, S.; Astals, S. Codigestion of solid wastes: A review of its uses and perspectives including modeling. Crit. Rev. Biotechnol. 2011, 31, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Anaerobic co-digestion of pig manure and crude glycerol at mesophilic conditions: Biogas and digestate. Bioresour. Technol. 2012, 110, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Moestedt, J.; Müller, B.; Westerholm, M.; Schnürer, A. Ammonia threshold for inhibition of anaerobic digestion of thin stillage and the importance of organic loading rate. Microb. Biotechnol. 2016, 9, 180–194. [Google Scholar] [CrossRef]
- Hartmann, H.; Ahring, B.K. Strategies for the anaerobic digestion of the organic fraction of municipal solid waste: An overview. Water Sci. Technol. 2006, 53, 7–22. [Google Scholar] [CrossRef]
- Zamanzadeh, M.; Hagen, L.H.; Svensson, K.; Linjordet, R.; Horn, S.J. Biogas production from food waste via co-digestion and digestion- effects on performance and microbial ecology. Sci. Rep. 2017, 7, 17664. [Google Scholar] [CrossRef]
- Font-Palma, C. Methods for the treatment of cattle manure. A review. C-J. Carbon Res. 2019, 5, 27. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different live-stock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Rabee, A.E.; Sayed Alahl, A.A.; Lamara, M.; Ishaq, S.L. Fibrolytic rumen bacteria of camel and sheep and their applications in the bioconversion of barley straw to soluble sugars for biofuel production. PLoS ONE 2022, 17, e0262304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Campanaro, S.; Treu, L.; Seshadri, R.; Ivanova, N.; Kougias, P.G; Kyrpides, N.; Angelidaki, I. Metabolic dependencies govern microbial syntrophies during methanogenesis in an anaerobic digestion ecosystem. Microbiome 2020, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621. [Google Scholar] [CrossRef]
- De Bok, F.A.; Plugge, C.M.; Stams, A.J. Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res. 2004, 38, 1368–1375. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, R.; Zhou, J.; He, A.; Xu, J.; Xin, F.; Zhang, W.; Ma, J.; Jiang, M.; Dong, W. Recent advances of biofuels and biochemicals production from sustainable resources using co-cultivation systems. Biotechnol. Biofuels 2019, 12, 155. [Google Scholar] [CrossRef]
- Sasaki, D.; Morita, M.; Sasaki, K.; Watanabe, A.; Ohmura, N. Acceleration of cellulose degradation and shift of product via methanogenic co-culture of a cellulolytic bacterium with a hydrogenotrophic methanogen. J. Biosci. Bioeng. 2012, 114, 435–439. [Google Scholar] [CrossRef]
| Reactions | ΔG0′ (kJ/mol CH4) | Microorganisms |
|---|---|---|
| I. Hydrogen 4H2 + CO2 → CH4 + 2H2O |
-135 | Most methanogens |
| II. Formate 4HCOOH → CH4 + 3CO2 + 2H2O |
-130 | Many hydrogenotrophic methanogens |
| III. Acetate CH3COOH → CH4 + CO2 |
-33 | Methanosarcina and Methanosaeta |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





