Submitted:
12 May 2023
Posted:
15 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Search and Retrieval Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
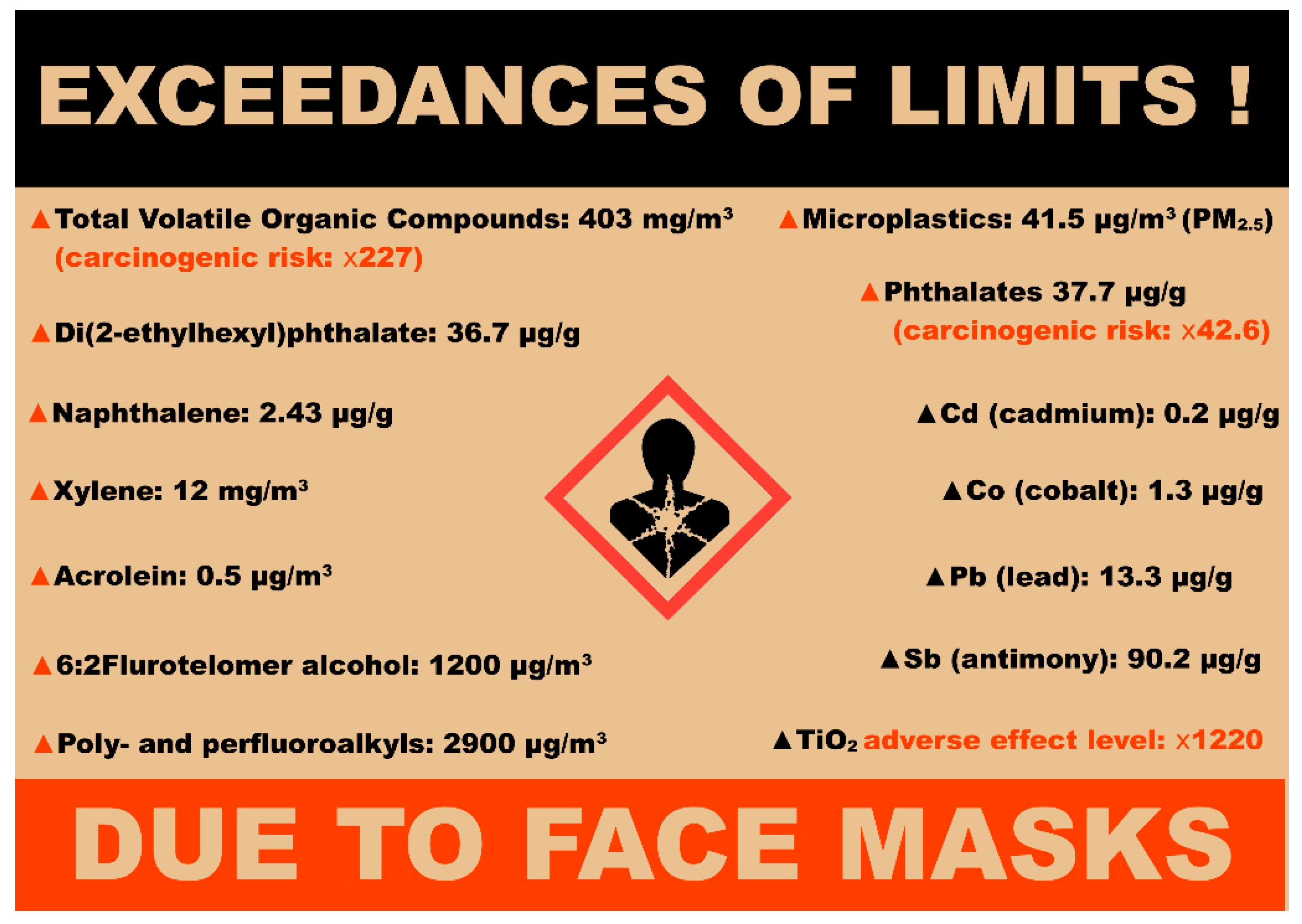
2.4. Calculations and Exceedance Analysis
3. Results
3.1. General Findings
3.2. Analysed Substance Classes
3.3. Special Findings
4. Discussion
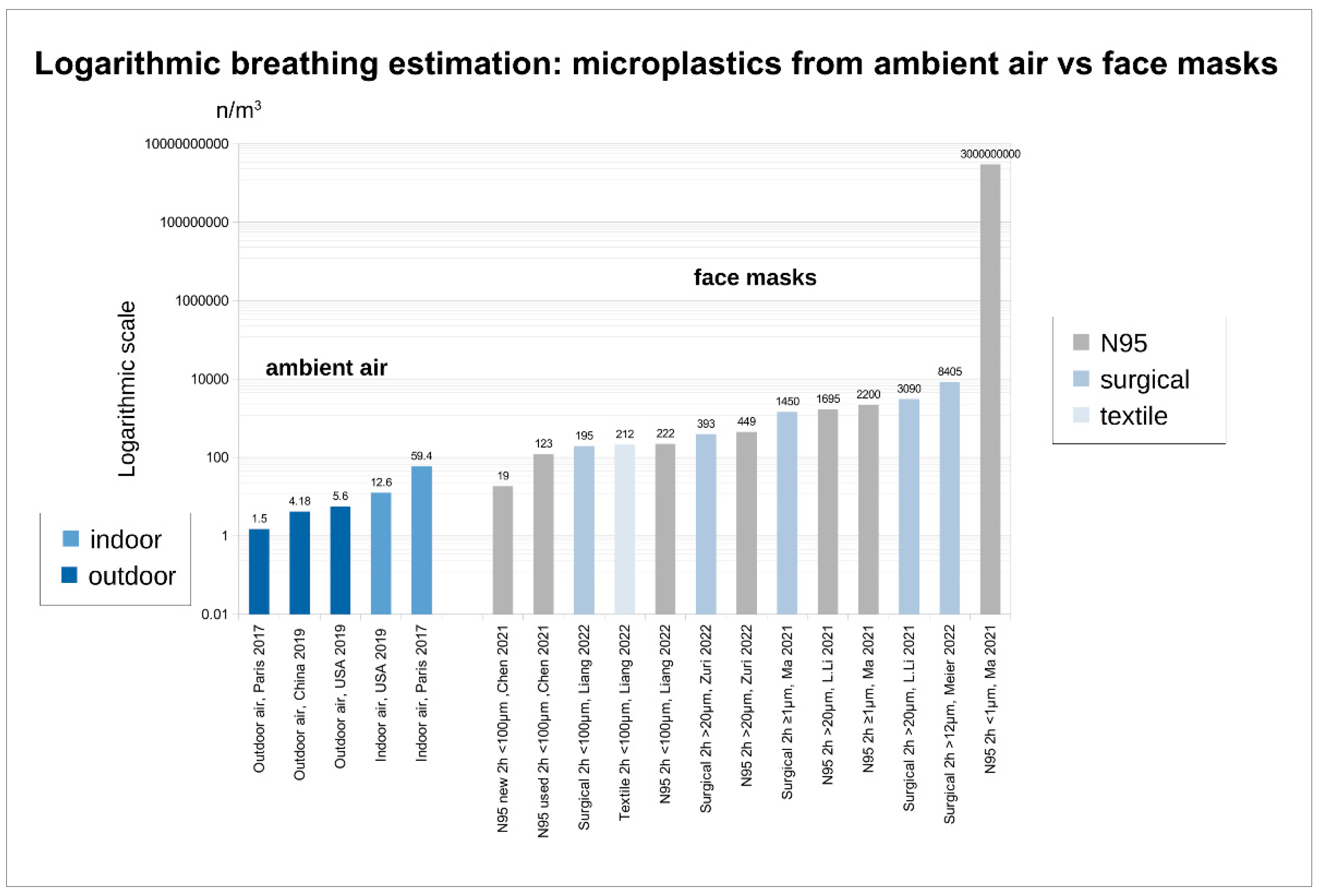
4.1. Microfibers, Micro- and Nanoplastics (MPs and NPs)
4.1.1. MP and NP from Masks - Origin
4.1.2. MP and NP from Masks - Release and Intake
4.1.3. Limits for MPs (Nps)
| Publication | Mask type | Outcome | Result * | AQG WHO [106] threshold value ** | Factor of exceedance |
|---|---|---|---|---|---|
|
Liang 2022 [67] (Ma 2022 [32]) |
N95 | MP (PM2.5) release |
41.55 µg/m3 (72 min use) |
5 µg/m3 (PM2.5) annual average |
8.31 |
|
Liang 2022 [67] (Ma 2022 [32]) |
surgical | MP (PM2.5) release |
33.9 µg/m3 (72 min use) |
5 µg/m3 (PM2.5) annual average |
6.78 |
|
Liang 2022 [67] (Ma 2022 [32]) |
N95 | MP (PM2.5) release |
41.55 µg/m3 (72 min use) |
15 µg/m3 (PM2.5) 3 to 4 days (24 h) per year |
2.77 |
|
Liang 2022 [67] (Ma 2022 [32]) |
surgical | MP (PM2.5) release |
33.9 µg/m3 (72 min use) |
15 µg/m3 (PM2.5) 3 to 4 days (24 h) per year |
2.26 |
4.1.4. MP and NP Risks
4.2. Organic Compounds and Organic Contaminants: Volatile Organic Compounds (VOCs) in General, Including total VOCs (TVOCs)
4.2.1. VOCs from Masks - Origin
4.2.2. VOCs - Release/Intake
4.2.3. Limits for VOCs
4.2.4. VOCs - Risks
4.3. Specific Organic Compounds: Organophosphate Esters (OPEs) and Organophosphate Flame Retardants (OPFRs)
4.3.1. OPEs and OPFRs from Masks - Origin
4.3.2. OPEs and OPFRs from Masks - Release/Intake
4.3.3. Limits for OPEs and OPFRs
4.3.4. OPEs and OPFRs - Risks
4.4. Specific Organic Compounds: UV-filters
4.4.1. UV-Filters from Masks - Origin
4.4.2. UV-Filters from Masks - Release/Intake
4.4.3. Limits for UV-Filters
4.4.4. UV-Filters - Risks
4.5. Specific Organic Compounds: Phthalates and Phthalate esters (PAEs)
4.5.1. Phthalates and PAEs from Masks - Origin
4.5.2. Phthalates and PAEs from Masks - Release/Intake
4.5.3. Limits for Phthalates and PAEs
4.5.4. Phthalates and PAEs - Risks
4.6. Specific Organic Compounds: Polycyclic Aromatic Hydrocarbons (PAHs)
4.6.1. PAHs from Masks - Origin
4.6.2. PAHs from Masks - Release/Intake
4.6.3. Limits for PAHs
4.6.4. PAHs - Risks
4.7. Specific Organic Compounds: Per- and Polyfluoroalkyl Substances (PFAS)
4.7.1. PFAS from Masks - Origin
4.7.2. PFASs from Masks - Release/Intake
4.7.3. Limits for PFAS
4.7.4. PFAS - Risks
4.8. Trace Elements and (Heavy) Metals Including TiO2
4.8.1. Trace Elements and Heavy Metals from Masks - Origin
4.8.2. Trace Elements and Heavy Metals from Masks - Release/Intake
4.8.3. Limits for Trace Elements and Heavy Metals
4.8.4. Trace Elements and Heavy Metals - Risks
4.9. Consequences for Science and Supervisory Authorities
| Publication | Mask type | Outcome | Result * | Threshold value Institution/Organisation ** |
Factor of exceedance |
|---|---|---|---|---|---|
| Kerkeling 2021 [72] | N95 |
TVOC release |
403 mg/m3 (17 min) |
0.3 mg/m3 target guideline European Community [157,161,162,164] German Federal Environment Agency [158,159,160,164,165] |
1343 |
|
Kerkeling 2021 [72] |
N95 |
TVOC release |
403 mg/m3 (17 min) |
0.5 mg/m3 Oeko-Tex [154] |
806 |
|
Xie 2022 [55] |
textile |
DEHP content |
36.7 µg/g | 0.01% of weight Oeko-Tex [154] |
367 |
|
Xie 2021 [56] |
textile |
SVOC carcinogenic risk (CR) |
2.27 × 10−4 |
≤1 × 10−6 US EPA [186,187] |
227 |
|
Xie 2022 [55] |
textile |
Phthalates content |
37.7 µg/g | 0.025% of weight Oeko-Tex [154] |
150.8 |
|
Muensterman 2022 [9] |
textile (coated) |
PFAS content |
2900 µg/m2 | 250 µg/kg Oeko-Tex [154] |
107 |
|
Kerkeling 2021 [72] |
N95 |
Xylene release |
12 mg/m3 (17 min) |
10 mg/kg Oeko-Tex [154] |
70.8 |
|
Xie 2022 [55] |
N95 |
DEHP content |
6.3 µg/g | 0.01% of weight Oeko-Tex [154] |
63 |
|
Muensterman 2022 [9] |
textile (coated) |
FTOH content |
1200 µg/m2 | 250 µg/kg Oeko-Tex [154] |
44.2 |
|
Xie 2022 [55] |
textile (for children) |
Phthalate carcinogenic risk (CR) |
4.26 × 10−5 |
≤1 × 10−6 US EPA [186,187] |
42.6 |
|
Kerkeling 2021 [72] |
N95 |
TVOC release |
403 mg/m3 (17 min) |
10 mg/m3 AgBB, German Federal Environment Agency [164,165] |
40 |
|
Muensterman 2022 [9] |
textile |
PFAS content |
910 µg/m2 | 250 µg/kg Oeko-Tex [154] |
33.5 |
|
Zuri 2022 [59] |
N95 |
phthalates content/release |
8.16 µg/g | 0.025% of weight Oeko-Tex [154] |
32 |
|
Zuri 2022 [59] |
surgical |
phthalates content/release |
7.56 µg/g | 0.025% of weight Oeko-Tex [154] |
30 |
|
Jin 2021 [24] |
surgical |
Acrolein release |
0.5 μg/m3 (30 min) |
0.02 μg/m3 US EPA [166,167] |
25 |
|
Xie 2021 [56] |
N95 (for children) |
SVOC carcinogenic risk (CR) |
2.5 × 10−5 |
≤1 × 10−6 US EPA [186,187] |
25 |
|
Kerkeling 2021 [72] |
N95 |
Xylene release |
12 mg/m3 (17 min) |
500 µg/m3 AgBB, German Federal Environment Agency [158,159,160,164,165] |
24 |
|
Xie 2021 [56] |
N95 |
SVOC carcinogenic risk (CR) |
1.59 × 10−5 |
≤1 × 10−6 US EPA [186,187] |
15.9 |
|
Xie 2022 [55] |
textile |
Phthalate carcinogenic risk (CR) |
1.45 × 10−5 |
≤1 × 10−6 US EPA [186,187] |
14.5 |
| Chang 2022 [25] | surgical |
TVOC release |
>1 mg/m3 (1 h) |
0.3 mg/m3 target guideline European Community, [157,161,162,164] German Federal Environment Agency [158,159,160,164,165] |
>3 |
| Chang 2022 [25] | surgical |
TVOC release |
>1 mg/m3 (1 h) |
0.5 mg/m3 Oeko-Tex [154] |
>2 |
|
Muensterman 2022 [9] |
surgical |
PFAS content |
46 µg/m2 | 250 µg/kg Oeko-Tex [154] |
1.4 |
|
Muensterman 2022 [9] |
textile |
FTOH intake estimation 10 h mask use |
7.04 µg/kg-bw/day | 5 µg/kg-bw/day Danish Ministry of Environment [184] |
1.4 |
|
Xie 2021 [56] |
N95 |
Naphthalene content |
2.43 µg/g | 2 mg/kg Oeko-Tex [154] |
1.2 |
| Publication | Mask type | Outcome | Result * | Threshold value Institution/Organisation ** |
Factor of exceedance |
|---|---|---|---|---|---|
|
Verleysen 2022 [77] |
textile, reusable |
TiO2 exposure Adverse effect level (AELmask) two mask per day, 8h |
4394 μg | 3.6 µg ANSES, France [188,189,190] |
1220 |
| Bussan 2022 [76] | surgical |
Pb content |
13.3 µg/g | 0.2 mg/kg Oeko-Tex [154] |
66.5 |
| Bussan 2022 [76] | surgical |
Cu content |
410 µg/g | 50 mg/kg Oeko-Tex [154] |
8.2 |
| Sullivan 2021 [70] | textile |
Pb content |
0.68 µg/g | 0.2 mg/kg Oeko-Tex [154] |
3.4 |
| Bussan 2022 [76] | N95 |
Sb content |
90.18 µg/g | 30 mg/kg Oeko-Tex [154] |
3 |
|
Z. Liu 2022 [68] |
surgical |
Cd content |
0.22 µg/g | 0.1 mg/kg Oeko-Tex [154] |
2.2 |
| Sullivan 2021 [70] | textile |
Cd content |
0.19 µg/g | 0.1 mg/kg Oeko-Tex [154] |
1.9 |
|
Z. Liu 2022 [68] |
surgical |
Co content |
1.33 µg/g | 1 mg/kg Oeko-Tex [154] |
1.33 |
| Sullivan 2021 [70] | textile |
Sb content |
39.3 µg/g | 30 mg/kg Oeko-Tex [154] |
1.3 |
|
Z. Liu 2022 [68] |
surgical |
Pb content |
0.22 µg/g | 0.2 mg/kg Oeko-Tex [154] |
1.1 |
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Face Covering Policies during the COVID-19 Pandemic. Available online: https://ourworldindata.org/grapher/face-covering-policies-covid (accessed on 29 December 2022).
- WHO Recommendations on Mask Use by Health Workers, in Light of the Omicron Variant of Concern: WHO Interim Guidelines, 22 December 2021. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-IPC_Masks-Health_Workers-Omicron_variant-2021.1 (accessed on 16 March 2023).
- Verordnung Zum Schutz Vor Neuinfizierungen Mit Dem Coronavirus SARS-CoV-2 (Coronaschutzverordnung–CoronaSchVO) Volume 24. January 2023.
- Knobloch, J.K.; Franke, G.; Knobloch, M.J.; Knobling, B.; Kampf, G. Overview of Tight Fit and Infection Prevention Benefits of Respirators (Filtering Face Pieces, FFP). Journal of Hospital Infection 2023, 134, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Helios führt allgemeine Maskenpflicht ein. Available online: https://www.helios-gesundheit.de/unternehmen/aktuelles/pressemitteilungen/detail/news/helios-fuehrt-allgemeine-maskenpflicht-ein/ (accessed on 16 March 2023).
- Helios führt Maskenscanner in allen Kliniken ein. Available online: https://www.helios-gesundheit.de/unternehmen/aktuelles/pressemitteilungen/detail/news/helios-fuehrt-maskenscanner-in-allen-kliniken-ein/ (accessed on 16 March 2023).
- Ladhani, S.N. Face Masking for Children—Time to Reconsider. Journal of Infection 2022, 85, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S. Mask Mandates for Children during the COVID-19 Pandemic: An International Human Rights Perspective. Scand J Public Health 2022, 50, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Muensterman, D.J.; Cahuas, L.; Titaley, I.A.; Schmokel, C.; De la Cruz, F.B.; Barlaz, M.A.; Carignan, C.C.; Peaslee, G.F.; Field, J.A. Per- and Polyfluoroalkyl Substances (PFAS) in Facemasks: Potential Source of Human Exposure to PFAS with Implications for Disposal to Landfills. Environ. Sci. Technol. Lett. 2022, 9, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Shruti, V.C. A Critical Synthesis of Current Peer-Reviewed Literature on the Environmental and Human Health Impacts of COVID-19 PPE Litter: New Findings and next Steps. Journal of Hazardous Materials 2022, 422, 126945. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.; Wang, W.; Xing, J.; Zhang, Q.; Ma, Q.; Lv, Q. Non-Targeted Analysis of Unknown Volatile Chemicals in Medical Masks. Environment International 2022, 161, 107122. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, X.; Li, Z.; Song, K. COVID-19: Performance Study of Microplastic Inhalation Risk Posed by Wearing Masks. J Hazard Mater 2021, 411, 124955. [Google Scholar] [CrossRef]
- Khan, A.; Jia, Z. Recent Insights into Uptake, Toxicity, and Molecular Targets of Microplastics and Nanoplastics Relevant to Human Health Impacts. iScience 2023, 26. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Q.; Zhang, Q.; Zuo, C.; Shi, H. An Overview of Chemical Additives on (Micro)Plastic Fibers: Occurrence, Release, and Health Risks. Reviews Env.Contamination (formerly:Residue Reviews) 2022, 260, 22. [Google Scholar] [CrossRef]
- Ganesapillai, M.; Mondal, B.; Sarkar, I.; Sinha, A.; Ray, S.S.; Kwon, Y.-N.; Nakamura, K.; Govardhan, K. The Face behind the Covid-19 Mask—A Comprehensive Review. Environ Technol Innov 2022, 28, 102837. [Google Scholar] [CrossRef]
- Aerts, O.; Dendooven, E.; Foubert, K.; Stappers, S.; Ulicki, M.; Lambert, J. Surgical Mask Dermatitis Caused by Formaldehyde (Releasers) during the COVID-19 Pandemic. Contact Dermatitis 2020, 83, 172–173. [Google Scholar] [CrossRef] [PubMed]
- Raval, H.; Sangani, H. Certain Face Masks Contain Toxic Chemicals, Inhalation of Which Has the Potential to Affect the Upper Respiratory System. J. Trop. Dis 2021, 9. [Google Scholar]
- Li, M.; Hou, Z.; Meng, R.; Hao, S.; Wang, B. Unraveling the Potential Human Health Risks from Used Disposable Face Mask-Derived Micro/Nanoplastics during the COVID-19 Pandemic Scenario: A Critical Review. Environment International 2022, 170, 107644. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zeng, Z.; Song, B.; Yi, H.; Hu, T.; Zhang, Y.; Zeng, G.; Xiao, R. Neglected Microplastics Pollution in Global COVID-19: Disposable Surgical Masks. Sci Total Environ 2021, 790, 148130. [Google Scholar] [CrossRef] [PubMed]
- Morgana, S.; Casentini, B.; Amalfitano, S. Uncovering the Release of Micro/Nanoplastics from Disposable Face Masks at Times of COVID-19. Journal of Hazardous Materials 2021, 419, 126507. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.; Patrício Silva, A.L.; Soares, A.M.V.M.; Barceló, D.; Duarte, A.C.; Rocha-Santos, T. Current Knowledge on the Presence, Biodegradation, and Toxicity of Discarded Face Masks in the Environment. Journal of Environmental Chemical Engineering 2023, 11, 109308. [Google Scholar] [CrossRef] [PubMed]
- Masud, R.I.; Suman, K.H.; Tasnim, S.; Begum, M.S.; Sikder, M.H.; Uddin, M.J.; Haque, M.N. A Review on Enhanced Microplastics Derived from Biomedical Waste during the COVID-19 Pandemic with Its Toxicity, Health Risks, and Biomarkers. Environmental Research 2023, 216, 114434. [Google Scholar] [CrossRef] [PubMed]
- Potluri, P.; Needham, P. 6—Technical Textiles for Protection. In Textiles for Protection; Woodhead Publishing Series in Textiles; Scott, R.A., Ed.; Woodhead Publishing, 2005; pp. 151–175. ISBN 978-1-85573-921-5. [Google Scholar]
- Jin, L.; Griffith, S.M.; Sun, Z.; Yu, J.Z.; Chan, W. On the Flip Side of Mask Wearing: Increased Exposure to Volatile Organic Compounds and a Risk-Reducing Solution. Environ. Sci. Technol. 2021, 55, 14095–14104. [Google Scholar] [CrossRef]
- Chang, Y.; Huang, R.-J.; Cheng, K.; Lin, C.; Ling, Q.; Haque, M.M.; Ovadnevaite, J.; O’Dowd, C. Highly Time-Resolved and Nontargeted Characterization of Volatile Organic Compound Emissions from Face Masks. Environ. Sci. Technol. Lett. 2022, 9, 1007–1013. [Google Scholar] [CrossRef]
- Kisielinski, K.; Giboni, P.; Prescher, A.; Klosterhalfen, B.; Graessel, D.; Funken, S.; Kempski, O.; Hirsch, O. Is a Mask That Covers the Mouth and Nose Free from Undesirable Side Effects in Everyday Use and Free of Potential Hazards? International Journal of Environmental Research and Public Health 2021, 18, 4344. [Google Scholar] [CrossRef]
- Wyszyńska, M.; Czelakowska, A.; Rosak, P.; Białożyt-Bujak, E.; Gruca, O.; Rosak-Szyrocka, J.; Kasperski, J.; Skucha-Nowak, M. Changes in the Oral Cavity Mucosal Surface under the Influence of Wearing Protective Face Masks—Nitric Oxide Concentration Analysis—Preliminary Report. Coatings 2022, 12, 1164. [Google Scholar] [CrossRef]
- De-la-Torre, G.E.; Pizarro-Ortega, C.I.; Dioses-Salinas, D.C.; Ammendolia, J.; Okoffo, E.D. Investigating the Current Status of COVID-19 Related Plastics and Their Potential Impact on Human Health. Current Opinion in Toxicology 2021, 27, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Faustman, E.M.; Silbernagel, S.M.; Fenske, R.A.; Burbacher, T.M.; Ponce, R.A. Mechanisms Underlying Children’s Susceptibility to Environmental Toxicants. Environ Health Perspect 2000, 108 (Suppl 1), 13–21. [Google Scholar] [CrossRef] [PubMed]
- Estevan, C.; Vilanova, E.; Sogorb, M.A. Case Study: Risk Associated to Wearing Silver or Graphene Nanoparticle-Coated Facemasks for Protection against COVID-19. Arch Toxicol 2022, 96, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Huppertz, T.; Alali, A.; Spielhaupter, M.; Hörmann, K.; Matthias, C.; Hagemann, J. A New Form of Irritant Rhinitis to Filtering Facepiece Particle (FFP) Masks (FFP2/N95/KN95 Respirators) during COVID-19 Pandemic. World Allergy Organ J 2020, 13, 100474. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, F.; Xu, H.; Jiang, H.; Liu, J.; Li, P.; Chen, C.C.; Pan, K. Face Masks as a Source of Nanoplastics and Microplastics in the Environment: Quantification, Characterization, and Potential for Bioaccumulation. Environmental Pollution 2021, 288, 117748. [Google Scholar] [CrossRef] [PubMed]
- Habich, I. Nanosilber in Alltagsmasken: BfR Warnt Vor Ungeklärten Risiken. Available online: https://www.deutsche-apotheker-zeitung.de/news/artikel/2020/08/04/nanosilber-in-alltagsmasken-bfr-warnt-vor-ungeklaerten-risiken (accessed on 17 March 2023).
- Masken-Rückruf bei Müller: potenziell krebserregendes Anilin nachgewiesen. Available online: https://www.oekotest.de/gesundheit-medikamente/Masken-Rueckruf-bei-Mueller-potenziell-krebserregendes-Anilin-nachgewiesen-_11623_1.html (accessed on 17 March 2023).
- BfArM: Dringende Sicherheitsinformation—Rückrufbetreffend ViralProtect 7—Monatsmaske Der Farbe Schwarz Grösse M Und L Charge T100406. Available online: https://www.bfarm.de/SharedDocs/Kundeninfos/DE/02/2020/21532-20_kundeninfo_de.pdf?__blob=publicationFile&v=1.
- Corona-Maske im Rückruf: Hochgiftiger Stoff entdeckt—es besteht Lebensgefahr! Available online: https://www.hna.de/verbraucher/corona-coronavirus-maske-rueckruf-covid-19-mueller-mundschutz-gefahr-gesundheit-kassel-zr-90129285.html (accessed on 17 March 2023).
- Azoulay, D.; Thomas, J.; Napierska, D.; Ruffinengo, E. Sale and Distribution of Toxic Nanographene Masks on the European Market.
- Maynard, A. How Safe Are Graphene-Based Face Masks? Edge of Innovation 2021.
- Information de sécurité—Action de sécurité de Santé publique—ANSM. Available online: https://ansm.sante.fr/informations-de-securite/action-de-securite-de-sante-publique-france-masques-ffp2-particle-filtering-half-mask-labellises-biomass-graphene-shandong-shengquan-new-materials (accessed on 17 March 2023).
- Government of Canada. Graphene Face Masks—Recalls, Advisories and Safety Alerts—Canada. Available online: https://recalls-rappels.canada.ca/en/alert-recall/graphene-face-masks (accessed on 17 March 2023).
- La AEMPS informa de los resultados de la investigación efectuada sobre las mascarillas quirúrgicas tipo IIR con grafeno, fabricadas por Shandong Shenquan New Materials Co., Ltd., China. Available online: https://www.aemps.gob.es/informa/la-aemps-informa-de-los-resultados-de-la-investigacion-efectuada-sobre-las-mascarillas-quirurgicas-tipo-iir-con-grafeno-fabricadas-por-shandong-shenquan-new-materials-co-ltd-china/ (accessed on 17 March 2023).
- Mast, J.; Blaude, M.-N.; Siciliani, L.; Cheyns, K.; Waegeneers, N.; Loco, J.V.; Vleminckx, C.; Verleysen, E. Identification, physicochemical characterisation and preliminary risk analysis of titanum dioxide particles in face masks. Intermediate report TiO2-Mask COVID-19 project September 2021. Available online: https://www.sciensano.be/en/biblio/identification-physicochemical-characterisation-and-preliminary-risk-analysis-titanium-dioxide-0 (accessed on 17 March 2023).
- Palmieri, V.; De Maio, F.; De Spirito, M.; Papi, M. Face Masks and Nanotechnology: Keep the Blue Side Up. Nano Today 2021, 37, 101077. [Google Scholar] [CrossRef]
- Mast, J.; Van Miert, E.; Siciliani, L.; Cheyns, K.; Blaude, M.-N.; Wouters, C.; Waegeneers, N.; Bernsen, R.; Vleminckx, C.; Van Loco, J.; et al. Application of Silver-Based Biocides in Face Masks Intended for General Use Requires Regulatory Control. Sci Total Environ 2023, 870, 161889. [Google Scholar] [CrossRef]
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 17 March 2023).
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a Knowledge Representation for Clinical Questions. AMIA Annu Symp Proc 2006, 359–363. [Google Scholar]
- Calc|LibreOffice—Free Office Suite—Based on OpenOffice—Compatible with Microsoft. Available online: https://www.libreoffice.org/discover/calc (accessed on 17 March 2023).
- U.S. Environmental Protection Agency. Air Topics. Available online: https://www.epa.gov/environmental-topics/air-topics (accessed on 17 March 2023).
- What Are the WHO Air Quality Guidelines? Available online: https://www.who.int/news-room/feature-stories/detail/what-are-the-who-air-quality-guidelines (accessed on 17 March 2023).
- Luft. Available online: https://www.umweltbundesamt.de/themen/luft (accessed on 17 March 2023).
- Air Quality. Available online: https://environment.ec.europa.eu/topics/air/air-quality_en (accessed on 17 March 2023).
- Oeko-Tex®. Oeko-Tex Service GmbH, Genferstrasse 23, 8002 Zürich, Switzerland. Available online: https://www.oeko-tex.com/en/our-standards/oeko-tex-standard-100 (accessed on 17 March 2023).
- LibreOffice—Free Office Suite—Based on OpenOffice—Compatible with Microsoft. Available online: https://www.libreoffice.org/ (accessed on 17 March 2023).
- Fernández-Arribas, J.; Moreno, T.; Bartrolí, R.; Eljarrat, E. COVID-19 Face Masks: A New Source of Human and Environmental Exposure to Organophosphate Esters. Environment International 2021, 154, 106654. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Han, W.; Xie, Q.; Xu, T.; Zhu, M.; Chen, J. Face Mask—A Potential Source of Phthalate Exposure for Human. Journal of Hazardous Materials 2022, 422, 126848. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Du, J.; Han, W.; Tang, J.; Li, X.; Chen, J. Occurrence and Health Risks of Semi-Volatile Organic Compounds in Face Masks. Science Bulletin 2021, 66, 1601–1603. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, S.; Miller, A.; Eimer, B.C.; Shaffer, R.E. Filtration Performance of FDA-Cleared Surgical Masks. J Int Soc Respir Prot 2009, 26, 54–70. [Google Scholar] [PubMed]
- Roberge, R.J.; Bayer, E.; Powell, J.B.; Coca, A.; Roberge, M.R.; Benson, S.M. Effect of Exhaled Moisture on Breathing Resistance of N95 Filtering Facepiece Respirators. Ann Occup Hyg 2010, 54, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Zuri, G.; Oró-Nolla, B.; Torres-Agulló, A.; Karanasiau, A.; Lacorte, S. Migration of Microplastics and Phthalates from Face Masks to Water. Molecules 2022, 27, 6859. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Risk Assessment Guidance for Superfund (RAGS) Vol 1: Human Health Evaluation Manual, Part A, Interim Final, Office of Emergency and Remedial Response. EPA/540/1-89-002; U.S. Environmental Protection Agency: Washington D.C., 1989. [Google Scholar]
- Benchetrit, G. Breathing Pattern in Humans: Diversity and Individuality. Respir Physiol 2000, 122, 123–129. [Google Scholar] [CrossRef]
- Kisielinski, K.; Wagner, S.; Hirsch, O.; Klosterhalfen, B.; Prescher, A. Possible Toxicity of Chronic Carbon Dioxide Exposure Associated with Face Mask Use, Particularly in Pregnant Women, Children and Adolescents—A Scoping Review. Heliyon 2023. [Google Scholar] [CrossRef]
- Kisielinski, K.; Hirsch, O.; Wagner, S.; Wojtasik, B.; Funken, S.; Klosterhalfen, B.; Kanti Manna, S.; Prescher, A.; Sukul, P.; Sönnichsen, A. Physio-Metabolic and Clinical Consequences of Wearing Face Masks—Systematic Review with Meta-Analysis and Comprehensive Evaluation. Frontiers in Public Health 2023, 11. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Liu, Q.; Zhao, Q.; Xiong, X.; Wu, C. Used Disposable Face Masks Are Significant Sources of Microplastics to Environment. Environmental Pollution 2021, 285, 117485. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Gallardo, J.; Sullivan, G.L.; Tokaryk, M.; Russell, J.E.; Davies, G.R.; Johns, K.V.; Hunter, A.P.; Watson, T.M.; Sarp, S. Disposable FFP2 and Type IIR Medical-Grade Face Masks: An Exhaustive Analysis into the Leaching of Micro- and Nanoparticles and Chemical Pollutants Linked to the COVID-19 Pandemic. ACS EST Water 2022, 2, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, J.; Torres-Quiroz, C.; Mahato, J.; Park, J. Facemasks: A Looming Microplastic Crisis. International Journal of Environmental Research and Public Health 2021, 18, 7068. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ji, Y.; Ge, W.; Wu, J.; Song, N.; Yin, Z.; Chai, C. Release Kinetics of Microplastics from Disposable Face Masks into the Aqueous Environment. Science of The Total Environment 2022, 816, 151650. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, J.; Yang, X.; Huang, Q.; Zhu, K.; Sun, Y.; Van Hulle, S.; Jia, H. Generation of Environmental Persistent Free Radicals (EPFRs) Enhances Ecotoxicological Effects of the Disposable Face Mask Waste with the COVID-19 Pandemic. Environmental Pollution 2022, 301, 119019. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.; Zabara, M.; Hirsch, C.; Gogos, A.; Tscherrig, D.; Richner, G.; Nowack, B.; Wick, P. Evaluation of Fiber and Debris Release from Protective COVID-19 Mask Textiles and in Vitro Acute Cytotoxicity Effects. Environment International 2022, 167, 107364. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.L.; Delgado-Gallardo, J.; Watson, T.M.; Sarp, S. An Investigation into the Leaching of Micro and Nano Particles and Chemical Pollutants from Disposable Face Masks—Linked to the COVID-19 Pandemic. Water Research 2021, 196, 117033. [Google Scholar] [CrossRef]
- Hui Li, A.S.; Sathishkumar, P.; Selahuddeen, M.L.; Asyraf Wan Mahmood, W.M.; Zainal Abidin, M.H.; Wahab, R.A.; Mohamed Huri, M.A.; Abdullah, F. Adverse Environmental Effects of Disposable Face Masks Due to the Excess Usage. Environmental Pollution 2022, 308, 119674. [Google Scholar] [CrossRef]
- Kerkeling, S.; Sandten, C.; Schupp, T.; Kreyenschmidt, M. VOC Emissions from Particle Filtering Half Masks—Methods, Risks and Need for Further Action. EXCLI Journal 2021, 20, 995–1008. [Google Scholar] [CrossRef]
- Min, K.; Weng, X.; Long, P.; Ma, M.; Chen, B.; Yao, S. Rapid In-Situ Analysis of Phthalates in Face Masks by Desorption Corona Beam Ionization Tandem Mass Spectrometry. Talanta 2021, 231, 122359. [Google Scholar] [CrossRef]
- Wang, X.; Okoffo, E.D.; Banks, A.P.; Li, Y.; Thomas, K.V.; Rauert, C.; Aylward, L.L.; Mueller, J.F. Phthalate Esters in Face Masks and Associated Inhalation Exposure Risk. Journal of Hazardous Materials 2022, 423, 127001. [Google Scholar] [CrossRef] [PubMed]
- Vimalkumar, K.; Zhu, H.; Kannan, K. Widespread Occurrence of Phthalate and Non-Phthalate Plasticizers in Single-Use Facemasks Collected in the United States. Environment International 2022, 158, 106967. [Google Scholar] [CrossRef] [PubMed]
- Bussan, D.D.; Snaychuk, L.; Bartzas, G.; Douvris, C. Quantification of Trace Elements in Surgical and KN95 Face Masks Widely Used during the SARS-COVID-19 Pandemic. Science of The Total Environment 2022, 814, 151924. [Google Scholar] [CrossRef] [PubMed]
- Verleysen, E.; Ledecq, M.; Siciliani, L.; Cheyns, K.; Vleminckx, C.; Blaude, M.-N.; De Vos, S.; Brassinne, F.; Van Steen, F.; Nkenda, R.; et al. Titanium Dioxide Particles Frequently Present in Face Masks Intended for General Use Require Regulatory Control. Sci Rep 2022, 12, 2529. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Tao, J.; Cheng, M.; Deng, R.; Chen, S.; Yin, L.; Li, R. Microplastics and Nanoplastics in the Environment: Macroscopic Transport and Effects on Creatures. Journal of Hazardous Materials 2021, 407, 124399. [Google Scholar] [CrossRef] [PubMed]
- Aragaw, T.A. Surgical Face Masks as a Potential Source for Microplastic Pollution in the COVID-19 Scenario. Marine Pollution Bulletin 2020, 159, 111517. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.O.; Okoffo, E.D. Covid-19 Face Masks: A Potential Source of Microplastic Fibers in the Environment. Sci Total Environ 2020, 737, 140279. [Google Scholar] [CrossRef]
- Hasan, N.A.; Heal, R.D.; Bashar, A.; Haque, M.M. Face Masks: Protecting the Wearer but Neglecting the Aquatic Environment?—A Perspective from Bangladesh. Environmental Challenges 2021, 4, 100126. [Google Scholar] [CrossRef]
- Parashar, N.; Hait, S. Plastics in the Time of COVID-19 Pandemic: Protector or Polluter? Science of The Total Environment 2021, 759, 144274. [Google Scholar] [CrossRef]
- Xu, E.G.; Ren, Z.J. Preventing Masks from Becoming the next Plastic Problem. Front Environ Sci Eng 2021, 15, 125. [Google Scholar] [CrossRef]
- Hutten, I.M. CHAPTER 5—Processes for Nonwoven Filter Media. In Handbook of Nonwoven Filter Media; Hutten, I.M., Ed.; Butterworth-Heinemann: Oxford, 2007; pp. 195–244. ISBN 978-1-85617-441-1. [Google Scholar]
- Prata, J.C.; Silva, A.L.P.; da Costa, J.P.; Mouneyrac, C.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. Solutions and Integrated Strategies for the Control and Mitigation of Plastic and Microplastic Pollution. International Journal of Environmental Research and Public Health 2019, 16, 2411. [Google Scholar] [CrossRef] [PubMed]
- Tesfaldet, Y.T.; Ndeh, N.T. Public Face Masks Wearing during the COVID-19 Pandemic: A Comprehensive Analysis Is Needed for Potential Implications. Journal of Hazardous Materials Advances 2022, 7, 100125. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; D’Alessandro, M.M.; Ireland, K.J.; Burel, W.G.; Wencil, E.B.; Rasmussen, S.A. Personal Protective Equipment Supply Chain: Lessons Learned from Recent Public Health Emergency Responses. Health Security 2017, 15, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Huang, S.; Wang, J. Environmental Risks of Polymer Materials from Disposable Face Masks Linked to the COVID-19 Pandemic. Science of The Total Environment 2022, 815, 152980. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; He, S. Need for Assessing the Inhalation of Micro(Nano)Plastic Debris Shed from Masks, Respirators, and Home-Made Face Coverings during the COVID-19 Pandemic. Environmental Pollution 2021, 268, 115728. [Google Scholar] [CrossRef] [PubMed]
- Buzzin, A.; Domènech-Gil, G.; Fraschetti, E.; Giovine, E.; Puglisi, D.; Caputo, D. Assessing the Consequences of Prolonged Usage of Disposable Face Masks. Sci Rep 2022, 12, 16796. [Google Scholar] [CrossRef] [PubMed]
- Gaston, E.; Woo, M.; Steele, C.; Sukumaran, S.; Anderson, S. Microplastics Differ Between Indoor and Outdoor Air Masses: Insights from Multiple Microscopy Methodologies. Appl Spectrosc 2020, 74, 1079–1098. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and Potential Risk Assessment of Suspended Atmospheric Microplastics in Shanghai. Science of The Total Environment 2019, 675, 462–471. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A First Overview of Textile Fibers, Including Microplastics, in Indoor and Outdoor Environments. Environmental Pollution 2017, 221, 453–458. [Google Scholar] [CrossRef]
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Moore, F.; Akhbarizadeh, R. Microplastic Pollution in Deposited Urban Dust, Tehran Metropolis, Iran. Environ Sci Pollut Res 2017, 24, 20360–20371. [Google Scholar] [CrossRef]
- Abbasi, S.; Jaafarzadeh, N.; Zahedi, A.; Ravanbakhsh, M.; Abbaszadeh, S.; Turner, A. Microplastics in the Atmosphere of Ahvaz City, Iran. Journal of Environmental Sciences 2023, 126, 95–102. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Setting and Reviewing Standards to Control Particulate Matter (PM) Pollution. Available online: https://www.epa.gov/pm-pollution/setting-and-reviewing-standards-control-particulate-matter-pm-pollution (accessed on 18 March 2023).
- Kelly, F.J.; Fussell, J.C. Size, Source and Chemical Composition as Determinants of Toxicity Attributable to Ambient Particulate Matter. Atmospheric Environment 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Wieland, S.; Balmes, A.; Bender, J.; Kitzinger, J.; Meyer, F.; Ramsperger, A.F.; Roeder, F.; Tengelmann, C.; Wimmer, B.H.; Laforsch, C.; et al. From Properties to Toxicity: Comparing Microplastics to Other Airborne Microparticles. Journal of Hazardous Materials 2022, 428, 128151. [Google Scholar] [CrossRef] [PubMed]
- Allegri, M.; Bianchi, M.G.; Chiu, M.; Varet, J.; Costa, A.L.; Ortelli, S.; Blosi, M.; Bussolati, O.; Poland, C.A.; Bergamaschi, E. Shape-Related Toxicity of Titanium Dioxide Nanofibres. PLOS ONE 2016, 11, e0151365. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L.; Hirvonen, J. Physicochemical Characterization of Nano- and Microparticles. Current Nanoscience 2008, 4, 101–107. [Google Scholar] [CrossRef]
- Silva, T.; Pokhrel, L.R.; Dubey, B.; Tolaymat, T.M.; Maier, K.J.; Liu, X. Particle Size, Surface Charge and Concentration Dependent Ecotoxicity of Three Organo-Coated Silver Nanoparticles: Comparison between General Linear Model-Predicted and Observed Toxicity. Science of The Total Environment 2014, 468–469, 968–976. [Google Scholar] [CrossRef]
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A Review of Human and Animals Exposure to Polycyclic Aromatic Hydrocarbons: Health Risk and Adverse Effects, Photo-Induced Toxicity and Regulating Effect of Microplastics. Science of The Total Environment 2021, 773, 145403. [Google Scholar] [CrossRef]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential Human Health Risks Due to Environmental Exposure to Nano- and Microplastics and Knowledge Gaps: A Scoping Review. Science of The Total Environment 2021, 757, 143872. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Global Air Quality Guidelines. Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide 2021.
- Donaldson, K.; Seaton, A. A Short History of the Toxicology of Inhaled Particles. Part Fibre Toxicol 2012, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Riediker, M.; Zink, D.; Kreyling, W.; Oberdörster, G.; Elder, A.; Graham, U.; Lynch, I.; Duschl, A.; Ichihara, G.; Ichihara, S.; et al. Particle Toxicology and Health—Where Are We? Part Fibre Toxicol 2019, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Kisielinski, K.; Mumme, T.; Reinartz, P.; Buell, U. FDG-PET Detects Foreign-Body Reactions to Polyethylene Wear Debris in Non-Infected Knee Endoprostheses. Nuklearmedizin 2004, 43, N3–N6. [Google Scholar]
- Kisielinski, K.; Cremerius, U.; Büll, U.; Hermanns, B.; Wirtz, D.C.; Niethard, F.U. [First experiences with fluorodeoxyglucose-positron-emission tomography (FDG-PET) in the evaluation of painful total knee and hip joint replacements]. Z Orthop Ihre Grenzgeb 2003, 141, 153–159. [Google Scholar] [CrossRef]
- Kisielinski, K.; Cremerius, U.; Reinartz, P.; Niethard, F.U. Fluordeoxyglucose Positron Emission Tomography Detection of Inflammatory Reactions Due to Polyethylene Wear in Total Hip Arthroplasty. The Journal of Arthroplasty 2003, 18, 528–532. [Google Scholar] [CrossRef]
- Klosterhalfen, B.; Junge, K.; Klinge, U. The Lightweight and Large Porous Mesh Concept for Hernia Repair. Expert Rev Med Devices 2005, 2, 103–117. [Google Scholar] [CrossRef]
- Klinge, U.; Klosterhalfen, B. Mesh Implants for Hernia Repair: An Update. Expert Review of Medical Devices 2018, 15, 735–746. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in Air: Are We Breathing It In? Current Opinion in Environmental Science & Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Howie, R.M.; Addison, J.; Cherrie, J.; Robertson, A.; Dodgson, J. Fibre release from filtering facepiece respiators. The Annals of Occupational Hygiene 1986, 30, 131–133. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne Microplastics: Consequences to Human Health? Environmental Pollution 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Baeza-Martínez, C.; Olmos, S.; González-Pleiter, M.; López-Castellanos, J.; García-Pachón, E.; Masiá-Canuto, M.; Hernández-Blasco, L.; Bayo, J. First Evidence of Microplastics Isolated in European Citizens’ Lower Airway. Journal of Hazardous Materials 2022, 438, 129439. [Google Scholar] [CrossRef] [PubMed]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using ΜFTIR Spectroscopy. Science of The Total Environment 2022, 831, 154907. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.S.R.; de Souza, C.B. The Impact of Microplastic on Human Health. Current Biotechnology 2021, 10, 158–167. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D. Microplastic Diagnostics in Humans: “The 3Ps” Progress, Problems, and Prospects. Science of The Total Environment 2023, 856, 159164. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Science of The Total Environment 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Wang, D.Y. Objective Assessment of Increase in Breathing Resistance of N95 Respirators on Human Subjects. Ann Occup Hyg 2011, 55, 917–921. [Google Scholar] [CrossRef]
- Thomas, R.J. Particle Size and Pathogenicity in the Respiratory Tract. Virulence 2013, 4, 847–858. [Google Scholar] [CrossRef]
- Heyder, J.; Gebhart, J.; Rudolf, G.; Schiller, C.F.; Stahlhofen, W. Deposition of Particles in the Human Respiratory Tract in the Size Range 0.005–15 Μm. Journal of Aerosol Science 1986, 17, 811–825. [Google Scholar] [CrossRef]
- Human Respiratory Tract Model for Radiological Protection. A Report of a Task Group of the International Commission on Radiological Protection. Ann ICRP 1994, 24, 1–482. [Google Scholar]
- Everard, M.L.; Hardy, J.G.; Milner, A.D. Comparison of Nebulised Aerosol Deposition in the Lungs of Healthy Adults Following Oral and Nasal Inhalation. Thorax 1993, 48, 1045–1046. [Google Scholar] [CrossRef]
- Barnoya, J.; Glantz, S.A. Cardiovascular Effects of Secondhand Smoke. Circulation 2005, 111, 2684–2698. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environment International 2022, 163, 107199. [Google Scholar] [CrossRef]
- Kannan, K.; Vimalkumar, K. A Review of Human Exposure to Microplastics and Insights Into Microplastics as Obesogens. Frontiers in Endocrinology 2021, 12. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for Europe. Noncommunicable Diseases and Air Pollution: WHO European High-Level Conference on Noncommunicable Diseases: Time to Deliver—Meeting NCD Targets to Achieve Sustainable Development Goals in Europe: 9–10 April 2019, Ashgabat, Turkmenistan; World Health Organization. Regional Office for Europe, 2019. [Google Scholar]
- WHO Air Quality Guidelines Global Update 2005 Report on a Working Group Meeting, Bonn, Germany, 2005.
- Chen, J.; Hoek, G. Long-Term Exposure to PM and All-Cause and Cause-Specific Mortality: A Systematic Review and Meta-Analysis. Environ Int 2020, 143, 105974. [Google Scholar] [CrossRef] [PubMed]
- Orellano, P.; Reynoso, J.; Quaranta, N.; Bardach, A.; Ciapponi, A. Short-Term Exposure to Particulate Matter (PM10 and PM2.5), Nitrogen Dioxide (NO2), and Ozone (O3) and All-Cause and Cause-Specific Mortality: Systematic Review and Meta-Analysis. Environment International 2020, 142, 105876. [Google Scholar] [CrossRef] [PubMed]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A Review on Microplastics and Nanoplastics in the Environment: Their Occurrence, Exposure Routes, Toxic Studies, and Potential Effects on Human Health. Marine Pollution Bulletin 2022, 181, 113832. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef]
- Kwak, J.I.; An, Y.-J. Post COVID-19 Pandemic: Biofragmentation and Soil Ecotoxicological Effects of Microplastics Derived from Face Masks. Journal of Hazardous Materials 2021, 416, 126169. [Google Scholar] [CrossRef]
- Li, S.; Wang, Q.; Yu, H.; Yang, L.; Sun, Y.; Xu, N.; Wang, N.; Lei, Z.; Hou, J.; Jin, Y.; et al. Polystyrene Microplastics Induce Blood–Testis Barrier Disruption Regulated by the MAPK-Nrf2 Signaling Pathway in Rats. Environ Sci Pollut Res 2021, 28, 47921–47931. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. International Journal of Environmental Research and Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Kisielinski, K.; Wojtasik, B.; Kisielinski, K.; Wojtasik, B. Suitability of Rose Bengal Sodium Salt Staining for Visualisation of Face Mask Contamination by Living Organisms. AIMSES 2022, 9, 218–231. [Google Scholar] [CrossRef]
- Fögen, Z. The Foegen Effect: A Mechanism by Which Facemasks Contribute to the COVID-19 Case Fatality Rate. Medicine (Baltimore) 2022, 101, e28924. [Google Scholar] [CrossRef] [PubMed]
- Spira, B. Correlation Between Mask Compliance and COVID-19 Outcomes in Europe. Cureus 2022, 14, e24268. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, C.; Can, İ.H. The Effect of N95 and Surgical Masks on Mucociliary Clearance Function and Sinonasal Complaints. Eur Arch Otorhinolaryngol 2022, 279, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, T.; Doenhardt, M.; Diffloth, N.; Berner, R.; Armann, J.P. High Burden of RSV Hospitalizations in Germany 2021–2022. Infection 2022, 50, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.C. Increase in Acute Respiratory Illnesses Among Children and Adolescents Associated with Rhinoviruses and Enteroviruses, Including Enterovirus D68—United States, July–September 2022. MMWR Morb Mortal Wkly Rep 2022, 71. [Google Scholar] [CrossRef] [PubMed]
- New COVID-19 Cases Worldwide. Available online: https://coronavirus.jhu.edu/data/new-cases (accessed on 1 February 2023).
- Fearnley, L.; Wu, X. Beyond Asian ‘Mask Culture’: Understanding the Ethics of Face Masks during the Covid-19 Pandemic in Singapore. Critical Public Health 2022, 0, 1–12. [Google Scholar] [CrossRef]
- Brohi, R.D.; Wang, L.; Talpur, H.S.; Wu, D.; Khan, F.A.; Bhattarai, D.; Rehman, Z.-U.; Farmanullah, F.; Huo, L.-J. Toxicity of Nanoparticles on the Reproductive System in Animal Models: A Review. Frontiers in Pharmacology 2017, 8. [Google Scholar] [CrossRef]
- Bonner, J.C. Nanoparticles as a Potential Cause of Pleural and Interstitial Lung Disease. Proc Am Thorac Soc 2010, 7, 138–141. [Google Scholar] [CrossRef]
- Hansen, T.; Clermont, G.; Alves, A.; Eloy, R.; Brochhausen, C.; Boutrand, J.P.; Gatti, A.M.; James Kirkpatrick, C. Biological Tolerance of Different Materials in Bulk and Nanoparticulate Form in a Rat Model: Sarcoma Development by Nanoparticles. Journal of The Royal Society Interface 2006, 3, 767–775. [Google Scholar] [CrossRef]
- Rowan, D.D. Volatile Metabolites. Metabolites 2011, 1, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Shrubsole, C.; Dimitroulopoulou, S.; Foxall, K.; Gadeberg, B.; Doutsi, A. IAQ Guidelines for Selected Volatile Organic Compounds (VOCs) in the UK. Building and Environment 2019, 165, 106382. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Consumer Products: National Volatile Organic Compound Emission Standards. Available online: https://www.epa.gov/stationary-sources-air-pollution/consumer-products-national-volatile-organic-compound-emission (accessed on 18 March 2023).
- Salthammer, T. TVOC—Revisited. Environment International 2022, 167, 107440. [Google Scholar] [CrossRef] [PubMed]
- Oeko-Tex® Standard 100 2023.
- World Health Organization. Occupational and Environmental Health Team. In Guidelines for air quality; World Health Organization, 2000. [Google Scholar]
- World Health Organization. Regional Office for Europe. WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization. Regional Office for Europe, 2010; ISBN 978-92-890-0213-4. [Google Scholar]
- Tsai, W.-T. An Overview of Health Hazards of Volatile Organic Compounds Regulated as Indoor Air Pollutants. Reviews on Environmental Health 2019, 34, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Debiak, M.; Sagunski, H.; Röhl, C.; Kraft, M.; Kolossa-Gehring, M. The German Approach to Regulate Indoor Air Contaminants. International Journal of Hygiene and Environmental Health 2019, 222, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Seifert, B. Richtwerte für die Innenraumluft. In Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz; Springer: Berlin, Heidelberg, 1999; pp. 270–278. ISBN 978-3-662-38283-7. [Google Scholar]
- Mølhave, L.; Clausen, G.; Berglund, B.; De Ceaurriz, J.; Kettrup, A.; Lindvall, T.; Maroni, M.; Pickering, A.C.; Risse, U.; Rothweiler, H.; et al. Total Volatile Organic Compounds (TVOC) in Indoor Air Quality Investigations*. Indoor Air 1997, 7, 225–240. [Google Scholar] [CrossRef]
- Tedd Nathanson, Federal-Provincial Advisory Committee on Environmental and Occupational Health (Canada). Indoor Air Quality in Office Buildings: A Technical Guide: A Report of the Federal-Provincial Advisory Committee on Environmental and Occupational Health. H46-2/93-166Erev-PDF—Government of Canada Publications—Canada. 2002. [Google Scholar]
- Tuomi, T.; Vainiotalo, S. The Guideline and Target Values for Total Volatile Organic Compound Concentrations in Industrial Indoor Environments in Finland. Indoor and Built Environment 2016, 25, 424–434. [Google Scholar] [CrossRef]
- Jantunen, M.; Jaakkola, J.J.K.; Krzyzanowski, M. Assessment of Exposure to Indoor Air Pollutants; World Health Organization. Regional Office for Europe, 1997; ISBN 978-92-890-1342-0. [Google Scholar]
- Umweltbundesamt Beurteilung von Innenraumluftkontaminationen mittels Referenz- und Richtwerten. Bundesgesundheitsbl 2007, 50, 990–1005. [CrossRef]
- Umweltbundesamt Ausschuss zur gesundheitlichen Bewertung von Bauprodukten. Available online: https://www.umweltbundesamt.de/themen/gesundheit/kommissionen-arbeitsgruppen/ausschuss-zur-gesundheitlichen-bewertung-von (accessed on 19 March 2023).
- US EPA National Center for Environmental Assessment. R.T.P.N. IRIS Toxicological Review of Acrolein (2003 Final). Available online: https://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=51977 (accessed on 19 March 2023).
- US EPA National Center for Environmental Assessment. Acrolein; CASRN 107-02-8 2003.
- Bayati, M.; Vu, D.C.; Vo, P.H.; Rogers, E.; Park, J.; Ho, T.L.; Davis, A.N.; Gulseven, Z.; Carlo, G.; Palermo, F.; et al. Health Risk Assessment of Volatile Organic Compounds at Daycare Facilities. Indoor Air 2021, 31, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, P.; Ma, S.; Lu, S.; Yu, Y.; An, T. A Critical Review of Human Internal Exposure and the Health Risks of Organophosphate Ester Flame Retardants and Their Metabolites. Critical Reviews in Environmental Science and Technology 2022, 52, 1528–1560. [Google Scholar] [CrossRef]
- US EPA. Regional Screening Levels (RSLs)—Generic Tables. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 19 March 2023).
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006 (Text with EEA Relevance); 2008; Volume 353.
- He, C.; Lin, C.-Y.; Mueller, J.F. Chapter Ten—Organophosphate Flame Retardants in the Environment: Source, Occurrence, and Human Exposure. In Comprehensive Analytical Chemistry; Emerging Halogenated Flame Retardants in the Environment; Oh, J.-E., Ed.; Elsevier, 2020; Volume 88, pp. 341–365. [Google Scholar]
- Huang, Y.; Law, J.C.-F.; Lam, T.-K.; Leung, K.S.-Y. Risks of Organic UV Filters: A Review of Environmental and Human Health Concern Studies. Science of The Total Environment 2021, 755, 142486. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No. 126/2013 Amending Annex XVII to Regulation (EC) No. 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH).|FAOLEX. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC120590/ (accessed on 19 March 2023).
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Polycyclic Aromatic Hydrocarbons (PAHs): What Are the Standards and Regulations for PAHs Exposure?|Environmental Medicine|ATSDR. Available online: https://www.atsdr.cdc.gov/csem/polycyclic-aromatic-hydrocarbons/standards_and_regulations_for_exposure.html (accessed on 19 March 2023).
- Directive 2004/37/EC of the European Parliament and of the Council of 29 April 2004 on the Protection of Workers from the Risks Related to Exposure to Carcinogens or Mutagens at Work (Sixth Individual Directive within the Meaning of Article 16(1) of Council Directive 89/391/EEC) (Codified Version) (Text with EEA Relevance); 2014.
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo[a]Pyrene—Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. International Journal of Molecular Sciences 2022, 23, 6348. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J Expo Sci Environ Epidemiol 2019, 29, 131–147. [Google Scholar] [CrossRef]
- US EPA. Questions and Answers: Drinking Water Health Advisories for PFOA, PFOS, GenX Chemicals and PFBS. Available online: https://www.epa.gov/sdwa/questions-and-answers-drinking-water-health-advisories-pfoa-pfos-genx-chemicals-and-pfbs (accessed on 19 March 2023).
- Per- and Polyfluoroalkyl Substances (PFASs)—ECHA. Available online: https://echa.europa.eu/hot-topics/perfluoroalkyl-chemicals-pfas (accessed on 19 March 2023).
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast) (Text with EEA Relevance); 2020; Volume 435.
- Jensen, A.A.; Warming, M. Short-Chain Polyfluoroalkyl Substances (PFAS). A Literature Review of Information on Human Health Effects and Environmental Fate and Effect Aspects of Short-Chain PFAS; Danish Environmental Protection Agency, 2015. [Google Scholar]
- Forouzandeh, P.; O’Dowd, K.; Pillai, S.C. Face Masks and Respirators in the Fight against the COVID-19 Pandemic: An Overview of the Standards and Testing Methods. Saf Sci 2021, 133, 104995. [Google Scholar] [CrossRef]
- Calculating Hazard Quotients and Cancer Risk Estimates. Available online: https://www.atsdr.cdc.gov/pha-guidance/conducting_scientific_evaluations/epcs_and_exposure_calculations/hazardquotients_cancerrisk.html (accessed on 19 March 2023).
- US EPA. Guidelines for Carcinogen Risk Assessment 2005.
- ANSES Dioxyde de titane sous forme nanoparticulaire: recommandation de valeurs limites d’exposition professionnelle. Available online: https://www.anses.fr/fr/content/dioxyde-de-titane-sous-forme-nanoparticulaire-recommandation-de-valeurs-limites-d%E2%80%99exposition (accessed on 19 March 2023).
- ANSES Valeurs Limites d’exposition En Milieu Professionnel Le Dioxyde de Titane Sous Forme Nanométrique (TiO2-NP, P25). Rapport d’expertise Collective, Décembre 2020—Expertise Scientifique. Saisine N° 2019-SA-0109—LEP TiO2-NP 2019.
- Bermudez, E.; Mangum, J.B.; Wong, B.A.; Asgharian, B.; Hext, P.M.; Warheit, D.B.; Everitt, J.I. Pulmonary Responses of Mice, Rats, and Hamsters to Subchronic Inhalation of Ultrafine Titanium Dioxide Particles. Toxicol Sci 2004, 77, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Zannoni, N.; Lakey, P.S.J.; Won, Y.; Shiraiwa, M.; Rim, D.; Weschler, C.J.; Wang, N.; Ernle, L.; Li, M.; Bekö, G.; et al. The Human Oxidation Field. Science 2022, 377, 1071–1077. [Google Scholar] [CrossRef]
- World Health Organization. Mask Use in the Context of COVID-19: Interim Guidance, 1 December 2020. 2020. [Google Scholar]
- Jefferson, T.; Dooley, L.; Ferroni, E.; Al-Ansary, L.A.; Driel, M.L.; van Bawazeer, G.A.; Jones, M.A.; Hoffmann, T.C.; Clark, J.; Beller, E.M.; et al. Physical Interventions to Interrupt or Reduce the Spread of Respiratory Viruses. Cochrane Database of Systematic Reviews 2023. [Google Scholar] [CrossRef]
- Huckelba, A.L.; Van Lange, P.A.M. The Silent Killer: Consequences of Climate Change and How to Survive Past the Year 2050. Sustainability 2020, 12, 3757. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Sharif, Y.; Sughra, K.; Sajid, M.; Khan, K.A.; Sneharani, A.H.; Li, S. Health and Environmental Effects of Silent Killers Organochlorine Pesticides and Polychlorinated Biphenyl. Journal of King Saud University - Science 2021, 33, 101511. [Google Scholar] [CrossRef]
- Nawrot, T.S.; Staessen, J.A. Low-Level Environmental Exposure to Lead Unmasked as Silent Killer. Circulation 2006, 114, 1347–1349. [Google Scholar] [CrossRef] [PubMed]
- Alasfar, R.H.; Isaifan, R.J. Aluminum Environmental Pollution: The Silent Killer. Environ Sci Pollut Res 2021, 28, 44587–44597. [Google Scholar] [CrossRef]
- Shaldon, S.; Vienken, J. Beyond The Current Paradigm: Recent Advances in The Understanding of Sodium Handling - Guest Editors: Stanley Shaldon and Joerg Vienken: Salt, the Neglected Silent Killer. Seminars in Dialysis 2009, 22, 264–266. [Google Scholar] [CrossRef]
- Houston, T.P. The Silent Killer: Environmental Tobacco Smoke. J Fam Pract 1991, 32, 457–458. [Google Scholar]
- Redlich, C.A.; Sparer, J.; Cullen, M.R. Sick-Building Syndrome. Lancet 1997, 349, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Directorate-General for Health and Consumers (European Commission) Now known as Making Risk Assessment More Relevant for Risk Management. Publications Office of the European Union: LU, 2013; ISBN 978-92-79-31205-2.
- World Health Organization (WHO). WHO—Advice on the Use of Masks in the Context of COVID-19: Interim Guidance, 5 June 2020. 2020. [Google Scholar]
- Mitteilungen der Ad-hoc-Arbeitsgruppe Innenraumrichtwerte der Innenraumlufthygiene-Kommission des Umweltbundesamtes und der Obersten Landesgesundheitsbehörden [Health evaluation of fine particulate matter in indoor air]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2008, 51, 1370–1378. [CrossRef]
- Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe; 2008; Volume 152.
- Delanghe, L.; Cauwenberghs, E.; Spacova, I.; De Boeck, I.; Van Beeck, W.; Pepermans, K.; Claes, I.; Vandenheuvel, D.; Verhoeven, V.; Lebeer, S. Cotton and Surgical Face Masks in Community Settings: Bacterial Contamination and Face Mask Hygiene. Front Med (Lausanne) 2021, 8, 732047. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, R.; Garg, K.; Singh, G.; Mehrotra, V. Is Safeguard Compromised? Surgical Mouth Mask Harboring Hazardous Microorganisms in Dental Practice. J Family Med Prim Care 2020, 9, 759–763. [Google Scholar] [CrossRef]
- Luksamijarulkul, P.; Aiempradit, N.; Vatanasomboon, P. Microbial Contamination on Used Surgical Masks among Hospital Personnel and Microbial Air Quality in Their Working Wards: A Hospital in Bangkok. Oman Med J 2014, 29, 346–350. [Google Scholar] [CrossRef]
- Park, A.-M.; Khadka, S.; Sato, F.; Omura, S.; Fujita, M.; Hashiwaki, K.; Tsunoda, I. Bacterial and Fungal Isolation from Face Masks under the COVID-19 Pandemic. Sci Rep 2022, 12, 11361. [Google Scholar] [CrossRef]
- Zhiqing, L.; Yongyun, C.; Wenxiang, C.; Mengning, Y.; Yuanqing, M.; Zhenan, Z.; Haishan, W.; Jie, Z.; Kerong, D.; Huiwu, L.; et al. Surgical Masks as Source of Bacterial Contamination during Operative Procedures. J Orthop Translat 2018, 14, 57–62. [Google Scholar] [CrossRef]
- Jefferson, T.; Mar, C.B.D.; Dooley, L.; Ferroni, E.; Al-Ansary, L.A.; Bawazeer, G.A.; Driel, M.L.; van Jones, M.A.; Thorning, S.; Beller, E.M.; et al. Physical Interventions to Interrupt or Reduce the Spread of Respiratory Viruses. Cochrane Databaseof Systematic Reviews 2020. [Google Scholar] [CrossRef]
| Author and year | Type of study, method | Aim | Mask Types | Outcomes | Findings | Special risks mentioned | Maximal face mask content * |
|---|---|---|---|---|---|---|---|
| Bussan 2022 [76] | Experimental and analytical study, ICP-MS, saliva leaching (6 h) and breathing experiments (15 min). | Determining Concentration of trace elements measured by Inductively Coupled Plasma Mass Spectrometry ICP-MS) in leachates and breathing release. | 24 masks: 21 surgical and 3 KN95 | 12 trace elements: Cr, Mn, Ni, Cu, Zn, As, Se, Mo, Cd, Sb, Tl, and Pb (206Pb, 207Pb, and 208Pb) | Detectable concentration levels for Cu, Sb, Pb and Zn. Cu detected in most of the surgical masks (2.24 to 410 μg/g). Sb was detected in both surgical and KN95 masks, (0.97 to 90.18 μg/g) with KN95>surgical. Pb was detected in surgical and KN95 masks (0.15 to 13.33 μg/g). Noticeably, Pb was detected in 76% of black colored masks. Zn in surgical masks: 15.93 to 56.80 μg. |
Sb is a possible carcinogen. Sb in amounts greater than 8.87 mg/m3 can cause pneumoconiosis, also chronic bronchitis, chronic emphysema, inactive tuberculosis, pleural adhesions, and respiratory irritation. Inhaled and ingested Pb can cause severe brain damage, reproductive system damage and death. Excess of Zn can cause lethargy and respiratory tract problems such as metal fume fever (MFF). |
Cu: 1230 µg (surgical) Sb: 360.7 µg (KN95) Pb: 39.9 µg (surgical) Zn: 170.4 µg (surgical) |
| Fernández-Arribas 2021 [54] | Experimental-analytical in vitro study (6 h), electrospray 4 h simulation of mask wearing, ionisation mass spectrometry, chemical organic trace analysis. |
Estimatig the Organo-phosphate ester (OPE) content (ng/mask) for 16 substances, additional inhalation estimation while testing with two paper-mache dummy heads representing an adult human’s head (indoors and outdoors). | 20 masks, surg. (8), KN95 (3), FFP2 (3), FFP3 (2), and reusable face masks (4) |
12 OPEs: TCEP, TCIPP, THP, TEHP, IDPP, TEP, TPP, DCP, TNBP, TPHP, TPPO, TDClPP, TCP, T2IPPP. |
Highest OPE mean concentrations obtained for KN95 masks (11.6 µg/mask) and the lowest for surgical masks (0.24 µg/mask). TEP, TPHP, TNBP, TEHP and TClPP being the most common OPEs at the highest concentrations. The highest inhalation percentages were for TNBP (between 1 and 13%) and TDClPP (between 6 and 9%). Comparing indoor to outdoor use, no differences found. Face mask is not considered to be dangerous for citizens regarding exposure to OPEs. Human exposure to OPEs via indoor air inhalation is doubled by the use of a KN95 mask per day. |
OPEs are associated with asthma and allergies. TNBP is observed to disrupt endocrine and reproductive functions, nervous system development and is suspected carcinogen. TDClPP is associated with decline of semen quality. |
Σ OPE: 20.4 µg (KN95) Σ OPE: 0.717 µg (surgical) Σ OPE: 27.7 µg (FFP3) TNBP 44.9 ng (N95) TNBP 657 ng (surgical) TDCIPP 23.5 ng (N95) TDCIPP 10.4 ng (surgical) |
| Jin 2021 [24] | Analytical and experimental study (1 h), behind mask breathing-zone VOC-analysis, GC–MS, HPLC–FLD | Estimating the increased human exposure to volatile organic compounds (VOCs) through wearing surgical |
60 surgical | 11 Organic compounds: Formaldehyde, Acetaldehyde, Acrolein, Glyoxal, Methylglyoxal, Furfural, Hexanal, Octanal, Decanal, Benzaldehyde, p-Tolualdehyde 16 polycyclic aromatic hydrocarbons (PAH): Naphthalene, Acenaphthene, Acenaphthylene, Fluorene. Phenanthrene, Anthracene, Fluoranthene, Pyrene, Benz[a]anthracene, Chrysene, Benzo[b]fluoranthene, Benzo[k]fluoranthene, Benzo[a]pyrene (equivalent calculations), Dibenz[a,h]anthracene, Benzo[ghi]perylene, Indeno [1,2,3-cd]pyrene 6 Phthalate esters: DMP, DEP, DPP, BBP, DBP, DEHP |
VOC concentrations in the breathing zone of the mask were positively correlated with the levels of VOC residues in the masks. Surgical masks from around the world are loaded with semivolatile and volatile organic compounds (VOCs), including alkanes, polycyclic aromatic hydrocarbons (PAHs), phthalate esters, and reactive carbonyls at ng to μg/mask levels. Naphthalene was the most abundant mask-borne PAH, accounting for over 80% of total PAH levels. Acrolein, a mutagenic carbonyl, was detected in most of the mask samples, and DEHP, an androgen antagonist, was detected in one-third of the samples, exceeding the inhalation reference concentration (RfC; a daily inhalation exposure concentration below which yields no appreciable risk) for acrolein (0.02 µg/m3) set by EPA. Furthermore, wearing the mask containing the highest level of acrolein residues (0.64 µg/mask) increased acrolein concentrations in the /m3 behind-mask breathing zone to over 0.5 µg and remained above the RfC for 1 h. DEP and DBP, both of which are highly volatile, accounted for over 85% of the total detected phthalate content |
Alarmingly, wearing surgical mask increased the VOC amount in the breathing zone by a factor of ~5, whereas wearing highly polluted masks further increased the total VOC. VOCa are respiratory irritants and suspected or known carcinogens. Acrolein and glyoxal are both highly mutagenic and strong irritants to the skin, eyes, and nasal passages. Acrolein is a well-known lung cancer causing agent. PAHs are 1B carcinogens. Epidemiological studies have shown the elevated risk of bladder, lung, skin, and gastrointestinal cancer and other chronic health effects, including cataracts, jaundice, and kidney and liver damage. Dermal contact with naphthalene can cause skin redness and inflammation, and inhalation of excess naphthalene is associated with hemolysis. Phthalate exposure is associated with asthma, obesity, impaired reproductive development, endocrine disruption, and infertility. DEHP is known as an androgen antagonist and has been demonstrated to have a lasting effect on male reproductive function and carcinogenicity. Masks containing more residue VOCs lead to significantly higher exposure levels and associated disease risks to the wearer, which should warrant the attention of the general public and regulatory agencies. |
Σ VOC 36.8 µg/mask Acrolein 637 ng/mask (0.5 μg/m3 in the mask breathing zone) Glyoxal 862 ng/mask Σ PAH 5563 ng/mask (Naphthalene 80%) Naphthalene 5296 ng/mask Σ Phthalates 2305 ng/mask (DEP + DEB > 85% phthalates) DEHP 1450 ng/mask |
| ASH. Li 2022 [71] | Analytical and experimental study. Leachates (24 h), GFAAS, ICP-OES, FESEM-EDX, GC-MS |
Identifying and quantifying the major chemicals released from face masks including the facemasks’ fibers | 100 surgical masks | Microfiber degradation, 3 heavy metals: Pb, Cd, Cr, 7 VOCs (4-methylheptane, 2,4 dimethylhept-1-ene, Heptacosane, Heneicosane, Octadecane, Octacosane, Pyridine-3-carboxamide) |
pH-dependent degradation of microfibers. Pb (3.238% ppb), Cd (0.672 ppb) and Cr (0.786 ppb) were found. Additionally, 2,4-dimethylhept-1-ene and 4-methylheptane were identified as the VOCs. | The experiments indicate a pH-related degraded material. VOC emissions can vary over the lifespan of the polymer because polymers deteriorate due to several factors such as thermal stress and UV exposure, even under normal circumstances. Pb, Cr, and Cd hold high potential to harm human health and the environment. |
Pb 69.36 ± 0.535 ng (surgical) Cd 3.343 ± 0.009 ng (surgical) Cr 84.01 ± 6.538 ng (surgical) |
| Y. Liu 2022 [11] | Analytical study. Non-targeted analysis method with GC-Orbitrap HRMS, Full scan MS, GC–MS |
Explore the unknown volatile chemicals in medical masks. | 60 medical masks, thereof: 5 N95, 25 surgical, 30 medical, thereof 20 children masks, |
Volatile substances | 69 volatile substances were identified in 60 masks, alkanes, esters, benzenes, and alcohols were the top four groups of substances identified in masks and accounted for 34.8%, 15.9%, 10.1%, and 7.2% of the total substances, respectively. In addition, ketones, ethers, phenolics, amides, and other substances were identified. 12 high-risk volatile chemicals in medical masks were: 1,4-Dichlorobenzene, Toluene, Xylenes (p, m, o), Ethylene oxide, Ethylbenzene, Caprolactam, N,N-Dimethylacetamide, N,N-Dimethylformamide. N-Methylpyrrolidone, Dimethyl glutarate. | Some of volatile chemicals were considered carcinogenic. For example, ethylene oxide was classified as group 1 carcinogens (carcinogenic to humans) by the International Agency for Research on Cancer (IARC, 2020). 1,4-Dichlorobenzene and ethylbenzene were classified as group 2B carcinogen (possibly carcinogenic to humans). Toluene, and xylene were categorized as group 3 carcinogens (not classifiable as to their carcinogenicity to humans). Some substances were restricted in textile related regulations. For example, 1,4-dichlorobenzene, N,N-dimethylacetamide, and N,N-dimethylformamide were restricted by the International Environmental Textile Association Oeko-Tex Standard 100. The latter two were also listed in the RSL list of the American Apparel and Footwear Association. N-Methylpyrrolidone was restricted by REACH regulations. Other substances, such as dimethyl glutarate, can irritate the human eye, respiratory system, and skin. |
Caprolactam 205.2 µg N95 Caprolactam 153.9 µg surgical Ethylene 20.8 µg N95 Ethylene 15.6 µg surgical N-methylpyrrolidon 25.6 µg N95 N-methylpyrrolidon 19.2 µg N95 |
| Min 2021 [73] | Analytical study. Analysis with DCBI-MS LC-MS. |
To establish a rapid screening of the phthalate esters (PAEs) in face masks. | Surgical (3), N95 (2), activated charcoal (2) |
13 PAEs: DMEP, DEP, DAP), DPhP, BBP), DBP, DBEP, DPP, DHXP, DEHP, DNOP, DINP, DDP. |
DAP, BBP, DBP, DPP, DHXP and DEHP were detected in all masks with an overall detection rate of 100%. The highest values were found for DHXP. The maximal content values for surgical masks were: DAP 54.1, BBP 32.4, DBP 34.7, DPP 65.8, DHXP 168.7 and DEHP 34.8 µg/m2 mask surface. For N95 masks the maximal content values were: DAP 18.2, BBP 38.8, DBP 6.8, DPP 12.5, DHXP 201.3, DEHP 19.3 µg/m2 mask surface. |
Some PAEs such as DHXP were detected in a concentration of more than 0.9 μg/g or 200 μg/m2, which is a safety issue for susceptible population, such as the elderly, children, pregnant women. Phthalates (PAEs) from masks will enter the human body directly from the respiratory system thus potentially threatening human health. PAEs are known as endocrine disruptors that can have adverse effects on human hormonal balance and development, some PAEs and their metabolites are suspected to be human carcinogenic. |
DAP 1.2443 ± 0.0368 µg (surgical) DAP 0.3185 ± 0.01225 µg (N95) BBP 0.7452 ± 0.0345 µg (surgical) BBP 0.679 ± 0.028 µg (N95) DBP 1.5134 ± 0.046 µg (surgical) DBP 0.119 ± 0.007 µg (N95) DPP 1.5134 ± 0.0414 µg (surgical) DPP 0.21875 ± 0.01225 µg (N95) DHXP 3.8801 ± 0.0897 µg (surgical) DHXP 3.5 ± 0.05425 µg (N95) DEHP 1.0396 ± 0.0437 µg (surgical) DEHP 0.33775 ± 0.0175 µg (N95) |
| Muenster-man 2022 [9] | Analytical study, LC-qTOF, GC-MS, PIGE. Additional human exposure and risk estimates, landfill contamination estimation with leachates | To characterize per- and polyfluoroalkyl substances (PFAS) associated with different types of facemasks. | 9 masks: 1 N95, 6 cloth, 1 other, 1 surgical |
50 target and 4886 suspect nonvolatile PFAS by LC-qTOF |
Total fluorine was quantifiable in 5 of 9 facemasks and ranged up to 40,000 nmol F/cm2. Summed PFAS concentrations ranged from 15 to 2900 µg/m2. The surgical and N95 masks gave the lowest measured total PFAS. Of the nonvolatile PFAS, perfluoroalkyl carboxylates (PFCAs) gave the highest detection frequency, followed by fluorotelomer-based PFAS, and perfluoroalkyl sulfonates (PFSAs). Nonvolatile PFAS suspect screening revealed tentative identification of only three PFAS. Fluorotelomer alcohol (FTOH), was estimated to be the dominant exposure route, accounting for over 40% (children) and 50% (adults) of total median exposure to PFAS in facemasks. High physical activity increased inhalation exposure estimates to over 70% (children), 700% (women), and 400% (men) more than the summed ingestion and dermal exposure routes. |
In the estimates of human exposure wearing masks treated with high levels of PFAS for extended periods of time can be a notable source of exposure and have the potential to pose a health risk. |
Σ Flourine 1.747862 ± 0.786531 ng/ cloth mask Σ PFAS: 1.058 ± 0.368 µg/surgical Σ PFAS: 0.2625 µg/ N95 Σ PFAS: 20.93 ± 4.37 µg/cloth mask Σ PFAS: 66.7 µg/special cloth mask volatile PFAS 5.75 ± 0.391 µg/cloth mask volatile PFAS 27.6 µg/special cloth mask |
| Verleysen 2022 [77] | Analytical study and estimation of the fraction of TiO2 particles at the fiber surface. STEM-EDX analysis, ICP-OES, TEM imaging and analysis, |
To evaluate whether the TiO2 particles in face masks possibly present a health risk, their amounts, their properties and their localization were analysed. |
Textile masks (12) | Size, morphology and agglomeration state of TiO2 particles | STEM-EDX analysis on sections of a variety of single use and reusable face masks visualized agglomerated near-spherical TiO2 particles in non-woven fabrics, polyester, polyamide and bi-component fibers. Median sizes of constituent particles ranged from 89 to 184 nm, implying an important fraction of nano-sized particles (< 100 nm). The total TiO2 mass determined by ICP-OES ranged from 791 to 152,345 µg per mask. | The estimated TiO2 mass at the fiber surface ranged from 17 to 4394 µg, and systematically exceeded the estimated acceptable exposure level to TiO2 by inhalation (3.6 µg). In animal experiments, toxic effects were reported when TiO2 particles were inhaled, as well as when they were ingested orally. In 2017, the Risk Assessment Committee (RAC) of the European Chemical Agency (ECHA) reviewed the carcinogenic potential of TiO2 and proposed to classify Titanium dioxide as Carc. 2, H351 (suspected human carcinogen) by inhalation. |
Particle size 89-184 nm TiO2 2386 ± 286 µg (single use textile mask) TiO2 152,345 ± 18,281 µg (reusable community mask) |
| Vimalkumar 2021 [75] | Analytical and experimental study. Analysis with GC-MS, additionally inhalation exposure assessment for 24-h (loss of analytes measured). Correlation analysis of plasticisers composition |
To determine the occurrence of plasticizers in facemasks. | 66 textile masks | nine phthalate diesters: DMP, DEP, DBP, DiBP, BbzP, DCHP, DnHP, DEHP, DNOP. four adipates; DEA, DBA, DiBA, DEHA. and TBP, and DBS. |
DEHP, DBP, BBzP, and DEHA were found at mean concentrations> 500 ng/g, whereas DBS was the most predominant plasticizer, with an overall median concentration of > 3200 ng/g. Among nine phthalate diesters measured (mean (±SD in ng/g), DiBP 405 ± 399, DBP 620 ± 497, and DEHP 732 ± 1060 were found in all facemask samples. BBzP was found in 67% of the samples analysed, at a mean concentration of 598 ± 1050 ng/g. At detection frequencies of between 21% and 61% at concentrations in ng/g, DMP 34, DEP 276, DnHP 14, and DnOP 210 were found. Among non-phthalate plasticizers, dibutyl sebacate (median: 3390 ng/g) and di(2-ethylhexyl)adipate (352 ng/g) were found at notable concentrations. Inhalation exposure to select phthalate and non-phthalate plasticizers from the use of facemasks was estimated to range from 0.1 to 3.1 and 3.5 to 151 ng/kg-bw/d, respectively. DBP, DiBP, and BBzP were significantly correlated (Spearman’s r = 0.253–0.599, p< 0.05). Also DiBA, DEHA, and DBS were significantly correlated with each other (Spearman’s r = 0.674–0.748, p < 0.01). |
Several plasticizers are used in combination in face masks. Little is known about the toxicity of non-phthalate plasticizers. Non-phthalates plasticizer exposure for children was higher than for adults. Face masks are not a significant source of human exposure to phthalates, but exposure to non-phthalate plasticizers from face masks is “notable”. |
Disposable textile masks: DEP 5.85 µg DiBP 6.325 µg DBP 5.025 µg DEHP 19.175 µg BBzP 13.75 µg DBA 4.725 µg DEHA 14.15 µg |
| Wang 2022 [74] | Experimental and analytical study, Pyrolysis-GC/ MS analysis of mask material. PAEs sampling (24 h), with volume of 4 m3. One volunteer used mask for 4.7 h and urine samples collected before and after and analysed with LC-MS. |
To assess and quantify phthalate esters (PAEs) in face mask materials and evaluate associated inhalation exposure risk. | Surgical (12), N95 (4) |
2 Polymers: PP and PET, 8 PAEs: DMP, DEP, DnBP, DiBP, BBzP, DEHP, DCHP, DNOP. |
Mask samples were identified to be made of polypropylene (PP), with polyethylene terephthalate (PET). PAE detection frequency (DF) was the highest for DMP (88%), followed by DnBP (75%), DEP (69%), DiBP (50%) and DEHP (44%). DEHP and DiBP were higher and detected in all of the N95/P1/P2 masks but in only ~30% of the 3-layer surgical masks. Mass loss (%) of PAEs on the masks during the course was calculated as from 12% to 82%. The highest loss was observed from DEP (60 – 82%). No obvious increase was observed for the urinary concentration of any phthalate metabolite. |
Although the exposure may not be a concern during a single mask wearing event for an individual, such unprecedented use of face masks worldwide means long-term exposure at the population level. This require a particular attention for frontline workers who may need to wear face masks more frequently and for longer periods of time. |
Σ PAE 1700 ± 140 ng/surgical masks Σ PAE 5200 ± 800 ng/N95 DEP 98 ± 60 ng (N95) DEP 41 ± 32 ng (surgical) DnBP 57 ± 32 ng (surgical) DnBP 510 ± 630 ng (N95) DiBP 140 ± 54 ng (N95) DEHP 750 ± 270 ng (N95) |
| Xie 2021 [56] | Analytical study, GC-MS, estimation of SVOCs exposure | To explore the occurrence and health risks of the semi-volatile organic compounds (SVOCs) exposure from face masks. | 53 masks (16 N95, 1KN90, 36 textile masks), including 25 children masks |
Three categories of 31 SVOCs 14 polycyclic aromatic hydrocarbons (PAHs): naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(a)pyrene, indeno(1,2,3-cd)pyrene, dibenz(a,h)anthracene, benzo(g,h,i)perylene 4 organophosphate flame retardants (OPFRs): TBP, 2-ethylhexyl diphenyl phosphate, tris (2-chloroethyl) phosphate, triphenyl phosphate 13 UV-filters: benzothiazole, oxybenzone, octocrylene, 2-methylbenzothiazole, benzophenone, octyl salicylate, 2-(2-hydroxy-5-methyl-phenyl)benzotriazole, octyl methoxycinnamate, 2-(3-t-butyl-2-hydroxy-5-methylphenyl)5-chlorobenzotriazole, 2-(2-Hydroxy-5-tert-octylphenyl)benzotriazole, 2,4-di-t-butyl-6-(5-chloro-2Hbenzotriazole-2-yl)phenol, 2-(2H-benzotriazole-2yl)4,6-di-t-pentylphenol, octocrylene, 2[3,5-bis(1-methyl-1-phenylethyl)-2-hydroxyphenyl]benzotriazole, hexamethylbenzene |
26 compounds were detected (10 PAHs, 12 UV-filters and 4 OPFRs). The total concentrations of the SVOCs ranged from 8.83 to 9200 ng/g, with a median value of 263 ng/g. The PAHs, UV-filters and OPFRs were detected in 90.6%, 96.2% and 92.5% of the mask samples, respectively. N95 masks have significantly higher concentrations of PAHs and OPFRs than the surgical mask. The detection frequencies of individual compound for the OPFRs were found to be generally higher than those for the PAHs and UV-filters. For the UV-filters content, no significant difference was observed between the two types of masks. The median values of the exposures for the OPFRs, PAHs and UV-filters from the 53 face masks were 0.63, 0.98 and 0.99 ng/kg bw/d. The median values of total concentrations of the OPFRs and PAHs in the KN95 masks were 224 and 57.1 ng/g, significantly higher than those in the disposable masks with values of 63.4 and 26.7 ng/g. While for the UV-filters content, no significant difference was observed between the two types of masks. |
Face mask can be a potential source of SVOCs exposure to humans. The cumulative carcinogenic risks (CCRs) for 39 masks exceeded the safe level for the carcinogenic risks, which accounted for 73.6% of the whole mask samples. |
Σ SVOC 29 µg/mask Σ UV-filters 3.43 µg/mask Naphthalene 10.206 µg (N95) Phenanthrene 0.101 µg (N95) anthracene 0.126 µg (N95) fluoranthene 0.287 µg (N95) 2-(3-t-butyl-2-hydroxy-5-methylphenyl)5-chlorobenzotriazole 0.305 µg (N95) tributyl phosphate (TBP) 4.104 µg (N95) benzothiazole 22.444 µg (N95) benzophenone 49.978 µg (N95) 2-ethylhexyl diphenyl phosphate 0.161 µg (KN90) disposable textile masks: triphenyl phosphate 14.4039 µg 2-(2-Hydroxy-5-tert-octylphenyl)benzotriazole 0.013 µg 2-(2H-benzotriazole-2yl)4,6-di-t-pentylphenol 0.063 µg pyrene 0.056 µg benzo(a)anthracene 0.042 µg chrysene 0.054 µg benzo(a)pyrene 3.046 µg benzo(g,h,i)perylene 0.023 µg tris (2-chloroethyl) phosphate 0.092 µg fluorene 0.114 µg |
| Xie 2022 [55] | Analytical study, GC-MS, estimation of phthalate exposure | To analyse levels of phthalates in face masks and to estimate daily intake (EDI). | 56 masks (16 N95, 1KN90, 1KF94, 38 textile masks), including 16 children masks | 12 phthalates: DMP, DEP, DiBP, DBP, DMEP, DPP, DHXP, DCHP, DEHP, DphP, DNOP, DNP. Three deuterated compounds were used as surrogates, DiBP-d4, DMP-d4, DEP-d4. |
11 phthalates were determined ranging from 115 ng/g to 37,700 ng/g with a median level of 1950 ng/g. Estimated daily intakes (EDIs) ranged from 3.71 to 639 ng/kg-bw/day, and the EDIs of the phthalates from masks for toddlers were approximately 4–5 times higher than those for adults. Regarding phthalates, masks seem to have only additional influence on daily intake rate. |
89.3% of the mask samples exhibited potential carcinogenic effects to humans. Phthalate exposure is reported to affect testosterone and semen parameters as well as fetal growth and have reproductive toxicity. Bis(2-ethylhexyl)phthalate (DEHP) was also found to be associated with penile birth defects and other effects related to androgen disruption. |
Σ Phthalates 191.64 µg (textile mask) DBP 9.66 µg (textile mask) DBP 1.60 µg (N95) DEHP 186.59 µg (textile mask) DEHP 26.91µg (N95) DiBP 3.00 µg (N95) DiBP 2.84 µg (textile mask) |
| Author and year | Type of study, method | Aim | Mask Types | Outcomes | Findings | Special risks mentioned | Maximal face mask release * |
|---|---|---|---|---|---|---|---|
| Chang 2022 [25] | Analytical study, flow-cell-experiment (surgical 6 h, N95 12 h), PTR-QiTOF |
Highly time-resolved and non-targeted measurements of volatile organic compounds (VOCs) emitted from face masks | 11 masks: 7 surg., 4 N95 |
9 VOCs: Methanol-d, propyne, propene, 1-butene and 2-butene, 1-pentene and 2-pentene and 3-methyl-1-pentene/4-methyl-1-pentene |
Typical thermoplastic materials used for filtration fibers were found (e.g., 1-butene and 2-butene, 1-pentene and 2-pentene, 3-methyl-1-pentene and 4-methyl-1-pentene). High concentrations of VOCs emitted from surgical masks (predominant mask type) were all concentrated in the initial 1h with >1000 µg/m3 and then dropped rapidly to an acceptable level after a process of naturally airing out. Surgical masks generally had higher TVOC concentrations than N95 respirators, especially in the first 2 h. Higher emissions from a surgical mask for children are likely due to their colourful cartoon patterns. Despite the lowest emissions, the N95 respirator with an active carbon layer required 6 h to remove the toxic methanol (52% of N95 total VOC emissions). | Diverse VOC species emitted, some of which are toxic (e.g., methanol). As an acutely toxic VOC, short-term exposure of healthcare workers to methanol by inhalation may result in dizziness, blurred vision, and headache. Great health concern since the emitted total VOC concentration exceeds the WHO guideline of Level 4 for TVOCs (only temporary exposure is acceptable). Humans can inhale VOC emissions from the mask at zero distance. In this regard, mask wearing may exert a higher risk of VOC exposure than many environmental sources. |
average TVOC (6 h) 445 µg/m3 (surgical, adult) average TVOC (6 h) 839 µg/m3 (surgical, children) average TVOC (12 h) 406 µg/m3 (N95) average TVOC (12 h) 91 µg/m3 (N95 with active carbon layer) specific VOC release: Propene >40 µg/m3 (surg., 40 min) Propene <10 µg/m3 approx. 8 (N95, 40 min) Methanol-d 48.23 µg/m3 (N95) |
| Chen 2021 [64] | Experimental and analytical: 24 h water release experiment, microplastics retained on the filter (0.8 μm pore size) were examined under stereo-microscope, Raman spectra analysis |
To evaluate the ability of new and used masks of different types to release microplastics |
18 masks: 7 surg., 2 N95, 5 medical, 4 dispo-sable textile |
MP release capacity, characteristics of released MP (shape, color, and size), four size categories (<100 μm, 100–500 μm, 500–1000 μm, 1000–2000 μm and >2000 μm). |
Released MPs were either fibrous or fragmentary. Medium size (100–500 μm) microplastics were predominant both in fibers and fragments. Fibers were predominant, accounting for more than 70% of the total released microplastic. Average amount of microplastics released was 183.00 ± 78.42 particles/piece while microplastics release from used DFMs was 1246.62 ± 403.50 particles/piece in 24 h. Microplastics released from used ones increased significantly than the new ones from 6.0 to 8.1 times. N95 released more MPs than surgical. |
Microplastics released from used ones increased significantly than the new ones. Large amount of fibers carried by the fabric material of the masks themselves, but also because of the process of use that would further promote the production and release of microplastics from the masks. |
MP 222.17 ± 98.79/new N95 mask (24 h) MP 1478.00 ± 265.80/used N95 mask (24 h) |
| Delgado-Gallardo 2022 [65] | Analytical and experimental; Leaching (4 h) and separation of particles, 0.02 μm pore size inorganic membranes were used to retain and subsequently analyze nanoparticles (>20 nm). Optical Microscopy, FEG-SEM with Energy-Dispersive Spectroscopy, Elemental characterisation of particles, LC–MS analysis, ICP-MS Elemental Analysis for heavy metals. |
To study the release of micro- and nanopollutants into the environment from medical masks |
Surgical (3) and N95 (3) masks | Micro- and naoparticles, 11 heavy metals (As, Cd, Cr, Co, Cu, Mo, Ni Pb, Sb, Ti, and Hg), organic contaminants |
FFP2 and surgical masks release MP, NP and fiber, most likely made from polypropylene, in the micro- and nanoscale. FFP2 emit more fibers than surgical masks (significant amounts of additional microplastic particles). Chemical elements found in particles were 3.65% of As, 3.47% of Cd, 3.73% of Cu, 4.71% of Hg, 3.96% of Ni, 5.65% of Pb, and 4.92% of Sn, Masks emit heavy metals (antimony up to 2.41 μg/L and copper up to 4.68 μg/L). Polar leachable organic species related to plastic additives and contaminants, polyamide-66 monomer and oligomers (nylon-66 synthesis), surfactant molecules, and PEG. |
The presence of particles containing heavy metals in the masks is of particular concern. These results claim for stricter regulations to be put in place. Also, a complete investigation must be done to clarify the extent of the risks and the potential impacts of the fibers and particles released. The presence of particles containing heavy metals in the masks is of particular concern as it is unknown how strongly they are bonded to the mask fibers. |
Cd 0.001 µg (surgical) Co 0.003 µg (N95) Cr 0.029 µg (N95) Cu 4.676 µg (surgical) Mo 0.019 µg (N95) Ni 0.025µg (surgical) Pb 0.052 µg (surgical) Sb 2.413 µg (N95) Ti 0.083 µg (surgical) V 0.002 µg surgical |
| Dissanayake 2021 [66] | Experimental in-vitro analytical study, FTIR, leaching (48 h), 0.45 µm nitrocellulose filter, digital. microscopy (400x). |
Preliminary quantification of number of bigger (light microscopic) microplastic fibers released by different face masks to aqueous medium | 13 masks: 3 surgical 3 KF94 3 KF-AD 4 FFP1 |
Fiber count and composition | >84% polypropylene (outer layer), and polystyrene. (inner layer). Microplastic <3mm with fibers less 1mm: Surgical masks released higher number (>100). | Microplastics are carriers of biofilm and pathogenic microorganisms. |
81 ± 7 MP fibers (KF-AD) 147 ± 18 MP fibers (KF94) 169 ± 31 MP fibers (surgical) 143 ± 16 MP fibers (FFP1) |
| Kerkeling 2021 [72] | Analytical study, emission measurements: 17-170 min, TD, GC, MS, FID |
Investigations into volatile organic compound (VOC) emissions from polymer fleeces used in particle filtering half masks, evaluation against the German hygienic guide values and provide an initial, tentative toxicological evaluation. |
47 masks: 31 FFP2, and 16 KN95 |
Aromatics, Siloxanes, Terpenes, Caprolactam, Aldehydes, Alkanes, Alcohols, Esters, Amin, Phthalates |
All masks showed emission of xylene. in most cases, aromatic compounds such as toluene and other alkylated benzenes and a variety of different alkanes. In 94 % of samples, up to 24 additional aromatic compounds were found. 17 % of samples showed terpenes, 53 % emitted aldehydes, 77 % exhibited caprolactam and 98 % released siloxanes. Exponential decline of VOC levels. emission rate declines rapidly over the first few hours and emissions seem to stabilize at 16 mg/m3. Half of the measured emissions are inhaled while the other half is exhaled. | All masks exceeded the TVOC hygienic guidance value level 5 of 10 mg/m3. Emissions reach a constant level after an initial decrease. The user might already be exposed to individual VOCs in indoor air, which would increase the total VOC intake. |
Total VOCs 403 mg/m3 (N95) Xylene 12 mg/m3 (N95) |
| ASH. Li 2022 [71] | Analytical and experimental study. Leachates (24 h), GFAAS, ICP-OES, FESEM-EDX, GC-MS |
Identifying and quantifying the major chemicals released from face masks including the facemasks’ fibers | 100 surgical masks | Microfiber degradation, 3 heavy metals: Pb, Cd, Cr, 7 VOCs (4-methylheptane, 2,4 dimethylhept-1-ene, Heptacosane, Heneicosane, Octadecane, Octacosane, Pyridine-3-carboxamide |
pH-dependent degradation of microfibers. Pb (3.238% ppb), Cd (0.672 ppb) and Cr (0.786 ppb) were found. Additionally, 2,4-dimethylhept-1-ene and 4-methylheptane were identified as the VOCs. | The experiments indicate a pH-related degraded material. VOC emissions can vary over the lifespan of the polymer because polymers deteriorate due to several factors such as thermal stress and UV exposure, even under normal circumstances. Pb, Cr, and Cd hold high potential to harm human health and the environment. |
Pb 2.322 ± 0.138 ng (surgical) Cd 0.672 ± 0.009 ng (surgical) Cr 0.747 ± 0.071 ng (surgical) |
| L. Li 2021 [12] | Experimental, with 2 h (up to 720 h) breathing simulation (collection of filtrated microplastic), microscopic analysis with Raman spectroscopy, FTIR, LDIR. |
Investigating microplastic inhalation risk. Microplastic inhalation caused by reusing masks that underwent various treatment processes was also tested. | 7 masks: 1 N95, 2 surgical, 4 other types |
Microplastic and particles 20-500µm |
Inhaled microplastics were mostly fiber-like and spherical types, 20 µm to 500 µm, over 90% of the identified particles are 20–100 µm. When suction time was 2 h, the spherical-type particles observed with the N95, surgical-A, cotton, fashion, nonwoven, surgical-B, and activated carbon masks, and without a mask were 1695, 1808, 2241, 3110, 2152, 3090, 2212, and 3918, respectively). The amount of fiber-like microplastics was determined to be 25, 38, 92, 69, 47, 112, 153, and 172 particles after the continuous use of N95, surgical-A, cotton, fashion, nonwoven, surgical-B, and activated carbon masks, and in the blank case, respectively, based on 2 h of simulated respiration. Mask disinfection processes led to varying extents of microplastic inner structure damage, increasing the risk of microplastic inhalation. |
Wearing masks poses microplastic inhalation risk, reusing masks increases the risk. This study was not conducted in super-clean laboratory, no contamination control measures were applied, thus it is not clear whether the control air in the blank measurements (no mask) does not correspond to the air already contaminated by mask handling. |
>90% of face mask particles 20-100 µm Spherical-type particles: 1695 MP (N95, 2 h) 3090 MP (surgical, 2 h) Fiber-like particles: 25 (N95, 2 h) 112 (surgical, 2 h) |
| Liang 2022 [67] | Analytical and experimental study. Water based 24 h to 168 h release experiment (0.45 µm cellulose ester membrane filter), optical microscope, Raman microscope | To identify the microplastics released and measure their quantities, also analysing microplastic release kinetics | 12 medical masks, thereof 4 N95, 4 medical 4 surgical |
Microplastics: length, shape, and colour. release kinetics: mass loss of mask, microplastic release change over time. |
Microplastics of 100–500 μm and of <100 μm were released in large quantities and at rapid rates. Fiber and transparent microplastics accounted for a large proportion and their daily release proportion increased with time. Polypropylene microplastics fibers and debris were released. N95 masks released 801 ± 71 to 2667 ± 97 microplastic particles (piece/24 h), surgical masks released 1136 ± 87 to 2343 ± 168 microplastic particle (piece/24 h), and normal medical masks released 1034 ± 119 to 2547 ± 185 microplastic particles (piece/ 24 h). The mass loss ranged from 0.293 ± 0.03 to 0.831 ± 0.035 mg/piece/ 24h. The percentage mass loss of masks in this study ranged from 0.006% to 0.019%. The cumulative release quantities increased from1034 ± 119–2457 ± 135 particles/piece on the first day to1737 ± 82 to 4270 ±185 particles/piece on the seventh day. Microplastics release was rapid with the increase in release quantity on the first day. The Elovich equation described the release kinetics of microplastics well. |
Wearing masks poses risks of microplastic inhalation and ingestion. Plastic pollution from face masks has become a major environmental and health concern (indirectly and directly). |
MP (24 h) 0.831 ± 0.035 mg/N95 MP (24 h) 2667 ± 97 particles/N95 MP (24 h) 2343 ± 168 particles/surgical MP 2547 ± 185 particles/medical |
| Z. Liu 2022 [68] | Experimental in-vitro analytical study with leachates (15 d), stereo-microscope analysis, SEM, FTIR, GC-SM and ICP-OES and cell culture toxicological measurements (24 h) |
Verifying the release of chemical compounds and generation of environmental persistent free radicals (EPFRs) after exposing face masks to water, and assess the toxicity of the leachate | 8 masks: 6 surg., 2 N95 |
MP release, non-organic and organic chemical substances, EPFRs, Viability of mc3t3e1cell |
MP’s being fibrous (80.3-97.4%), rarer particle (<10%), consisting of polypropylene >89.2%, range of 76-276 items/L (blue and transparent). Abundance of MP’s 40-75µm (37.1-47.6%). Metals as Co (8.0µg/L), Cu (8.3 µg/L), Ni (2.8µg/L), Sr (14.4µg/L), Ti (9.2µg/L) and Zn (17.7µg/L) detected in all samples Cd (1.3µg/L), Cr (0.8µg/L), Mn (2.9µg/L) and Pb (1.3µg/L), presented in the surgical masks. Organics, such as acetophenone (6.8 µg/L), 2,4-Di-tert-butylphenol -DTBP (3.8µg/L), benzothiazole (9.2µg/L), bisphenol-A (3.2µg/L), phthalide (4.1µg/L), but also tributyl acetylcitrate and benzaldehyde detected. Environmentally persistent free radicals (EPFRs) generated in the leachates with characteristic g-factors in a range of 2.003–2.004 G, identified as mixture of carbon- and oxygen-centered radicals (superoxide radical and methyl radical). Viability of mc3t3e1cell was significantly decreased after exposing to leachate (excessive oxidative stress to the test cells). |
Contact allergy to Cr, Ni and Co is the most common metal allergy (1–3%). Cd, Co, Cr and Pb was reported to have potential carcinogenic risk. Multiple metal–metal interactions of, e.g., Cd, Cu, Ni, and Zn, may contribute to a higher toxicity in a mixture. EPFR’s cause cytotoxicity and oxidative stress. By inducing reactive oxygen species (ROS) and overloaded ROS may induce oxidative stress, further causing cardiopulmonary dysfunction and chronic respiratory diseases. |
Co 4.0 µg (surgical) Cu 4.15 µg (surgical) Ni 1.4 µg (surgical) Sr 7.2 µg (surgical) Ti 4.6 µg (surgical) Zn 8.85 µg (surgical) Cd 0.65 µg (surgical) Cr 0.4 µg (surgical) Mn 1.45 µg (surgical) Pb 0.65 µg (surgical) Acetophenone 3.4 µg/L 2,4-Di-tert-butylphenol -DTBP 1.9 µg Benzothiazole 4.6 µg Bisphenol-A 1.6 µg Phthalide 2.05 µg g-factors 1.002 G |
| Ma 2021 [32] | Experimental in-vitro and in-vivo qualitative and quantitative analytical study, leachates (4 h) analysed on silicon wafer with SEM, FTIR but also retention of MPs in human nasal mucus after wearing a mask for 1-2 h with fluorescence microscope of nasal rinsings. |
Quantify and characterise face mask released particles and evaluate their potential for accumulation in humans | 8 surg. and 2 N95 masks (10) |
Microparticles- (MPs) and Nanoparticles (NPs) |
>1,000,000,000 of NPs and MPs were released from each surgical or N95 face mask, mostly irregularly-shaped particles sized from 5 nm to 600 μm. Most of them <1 μm. N95 masks release more and smaller NPs than surgical masks (p < 0.05). MPs were detected in the nasal mucus of mask wearers. Higher breathing frequency resulted in a larger number of particles detected in the nasal mucus (p<0-05). | MPs >1 μm occupied only a minor fraction of the particles, ranging from 1.3 to 4.4 × 103 per mask. Most particles in the masks were nano scale sized<1 μm. PM2.5 (Particulate matter < 2.5 μm) is well-known for generating adverse effects in humans. PM0.1 (<0.1 μm) have even more harmful effects such as alveolar inflammation and exacerbation of pre-existing cardiopulmonary diseases. |
6 × 109 NPs (N95 > surgical, 4 h) 4.4 × 103 MPs (N95, 4 h) 2.9 × 103 MPs (surgical, 4 h) |
| Meier 2022 [69] | Experimental in-vitro qualitative and quantitative analytical study. Air based (12.0 µm Nuclepore filter membrane) debris extraction (1 h and 8 h), liquid fiber and particle (0.4 µm Nuclepore filter membrane) extraction (45 min), optical analysis (NanoSight LM20), ICP-MS. Cell culture (48 h) |
To quantify the debris release (fibers and particles) and metals from a textile-based facemask in comparison to a surgical mask and a reference cotton textile using both liquid and air extraction, possible adverse effects on cell culture. | Surgical masks (2), textile based face masks (5) |
fiber and particle release, metal content (Cr, Co, Cu, Fe, Pb, Mn, Ni, Ag, Zn). |
Release of 740 particles per surgical mask (SM) in breathing simulation (air based extraction 8h), of which 404 with 0.3 µm. Under liquid extractions, SM released up to 1030 ± 115 fibers g−1 textile, corresponding to 3152 ± 352 fibers per mask. The sum metal content of calibrated elements (Cr, Co, Cu, Fe, Pb, Mn, Ni, Ag, Zn) was 43 ± 2 µg g−1 for SM. Several metals including copper (up to 40.8 ± 0.9 µg g−1) and iron (up to 7.0 ± 0.3 µg g−1). Mask debris show no acute in vitro cytotoxicity to human lung cells | The in vitro acute cytotoxicity assessment does not allow prediction of possible long-term exposure effects (long-term toxicity assessment on in vitro and in vivo lung exposure models). |
Σ Fibers 3152 ± 352 (surgical, average) Σ metal release: 131.6 ± 6.1 µg (surgical) Σ metal release: 211.7 ± 39,7 µg (coated cotton) Cu 125.5 ± 3.06 µg (surgical) Fe 92.61 ± 10.6µg (coated cotton) |
| Sullivan 2021 [70] | Experimental in-vitro qualitative and quantitative analytical study, leachates (4 h) analysed with FTIR, SEM-EDX, light microscopy, ICP-MS and LC-MS. |
To identify and characterize various released pollutants (heavy metals), emitted/leached from face masks including micro (<1 mm) and nano-particles (0.1–1 µm). | Textile masks (7) | Micro and nano-fibers and particles (MP’s and NP’s), heavy metals: Cd, Co, Cu, Pb, Sb, and Ti |
Significant amount of grain-sized particles measured between 360 nm-500 µm, micro- and nano-scale corresponding to MP and NP. Polymeric fibers (25 µm to 2.5 mm) found. Fibrous particles had high percentage of carbon, the grains contained high percentages of Si and oxygen. Polar organic species pollutants: Polyamide-66, polyamide-6 and various oligomers of polyamide (PA) found, also polyethylene glycol (PEG) derivatives and aromatic amines. Heavy metals: Cd (1.92 µg/L), Co (0.59 µg/L), Cu (4.17 µg/L), Pb (6.79 µg/L), Sb (393 µg/L) and Ti (0.64 µg/L) found in masks. | Even low exposures to Pb can lead to neurological damage and be detrimental to foetal development. MPs and NPs exhibit cytotoxic and genotoxic effects including neurotoxicity and oxidative stress. |
Cd 0.48 µg (textile mask) Cu 1.04 µg (textile mask) Co 0.14 µg (textile mask) Pb 1.69 µg (textile mask) Sb 98.3 µg (textile mask) Ti 0.16 µg (textile mask) |
|
Zuri 2022 [59] |
Analytical and experimental study, migration water experiment, (24h), collection with 20 µm nylon filters, Stereo-microscope, µ-FTIR, UPLC-MS |
To evaluate the migration of microplastics (MP) and phthalates. Migration was evaluated according to the conditions stated in EU Regulation No 10/2011 on plastic materials and articles intended to come into contact with food. |
3 FFP2, 1 surgical |
MP-morphological analysis (shape, dimension, particle count), 11 phthalates: DMP, DEP, BBP, DBP, DPP, BMPP, DnHP, HEHP, DEHP, DNOP and DNP |
All masks released particles in form of fibers and fragments. Polypropylene (PP) and polyamide (PA) were released as fragments, while both PP and polyester (PES) were released as fibers. Each mask could potentially release from 2040 to 4716 MP/mask. Additionally, phthalates including DBP, BBP, DNOP, and DEHP were also released. | MP affect biota and also represent a health hazard for humans, specifically a risk of MP inhalation through breathing. Additionally, MP could carry other potentially harmful compounds and heavy metals that can be introduced in the human body. Concerning phthalates DEHP has been identified as an endocrine disruptor, BBP is classified as a reproductive toxicant. |
5390 MP (FFP2, 24 h) 4716 MP (surgical, 24 h) Σ Phtalates 35 µg (FFP2) Σ Phtalates 25.3 µg (surgical) DBP 21.1 µg/FFP2 BBP 13.6 µg/surgical DNOP 4.96 µg/FFP2 DEHP 4.59 µg/FFP2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





