Introduction
Amphibians are imperiled globally, largely plagued by the so-called Linnean (knowledge gaps in species taxonomy) and Wallacean (knowledge gaps in species distribution) shortfalls (Hortal et al., 2015). Extensive inventory surveys coupled with an integrative taxonomic approach have catered much to bridge the knowledge gap of the former shortfall evident from the unprecedented rise of species descriptions in the last two decades (see Frost, 2021); however, the latter shortfall is still shrouded in uncertainty and skewed, being heavily biased to surveys across easily accessible areas and that too for only a few charismatic or flagship species. This Wallacean shortfall is even more acute for tropical countries in South and Southeast Asia with significant forest cover and topographic relief having largely remote and inaccessible regions (Bini et al., 2006), despite being hotspots of amphibian diversity and endemism (Stuart et al., 2008). To bridge this growing disparity among the two above-mentioned pitfalls, conservationists’ are increasingly relying upon predictive distribution models to ascertain the geographic distribution of amphibian species (see Barbosa et al., 2012), a knowledge that is a prerequisite parameter for effective management and conservation plan.
Species distribution models (hereafter SDM) are prediction based modeling approaches used for refining distribution data and works on the underlying principle of estimating the fundamental niche of a species (the environmental suite of conditions within which a species can persist) from known samples of occurrences (absence data being seldom used because of its credibility), by establishing a relationship between the associated environmental variables found therein and then projecting it back into the geographical space to identify areas that could be a potentially suitable site (ecologically conducive areas) for the species concerned (Peterson et al., 2011; Anderson, 2012). Being robust in nature, SDMs’ have found wide ecological applications ranging from investigating climate change-induced impact assessment to identifying and delineating conservation priority areas as well as studying underlying evolutionary processes in speciation (Peterson & Soberon, 2012). However, their predictive potential has found limited usage in guiding explorative surveys of rare, endemic, and at-risk amphibians largely owing to the availability of few occurrence records (but see Bourke, Busse & Böhme, 2012; Groff, Marks & Hayes, 2014), hitherto failing to produce accurate niche models (Pawar et al., 2007). Moreover, the tests evaluating the predictive performance of very limited occurrence records to yield reliable predictions of distribution have mostly centered around using discrimination-based test statistics (Fielding & Bell, 1997) and the “jackknife” validation approach (Pearson et al., 2007). But, rarely are these predictive models ground-truthed to evaluate their predictive performance or remodeled incorporating newly discovered localities guided by the model’s prediction with change detection approach (Rebelo & Jones, 2010). Thus, our objective was to assess the potential of SDM based on few occurrence records in guiding surveys aimed at identifying unrecorded populations of Zhangixalus suffry (Bordoloi et al., 2007), endemic to one of the most biodiversity hotspot region of the world– the Indo-Burma Region (Myers et al., 2000). We also intend to identify the suite of environmental factors that limits the distribution of Z. suffry in the region and evaluate the coverage performance of the current protection level existing in the form of Protected Area (PAs) in its predicted potential distribution range. We hypothesize that the environmental cues influencing the habitat suitability of amphibians will strongly reflect their biphasic mode of life.
Study area
Northeast (NE) India, with a spatial coverage of 256,083 km
2 (extending from 88°E to 97°E and 22°N to 29°30’N), covers a significant portion of both the Himalaya and the Indo-Burma biodiversity hotspots (Myers
et al., 2000,
Figure 1). It presents characteristic features of the Eastern Himalayan Ranges in the north with significant topographic relief (above 7000 m), the northeast hill ranges aligned in the north-south direction offering a much lesser imposing relief feature (with Manipur and Naga hills varying between 900-2100 m, while the Mizo hills rarely rising above 900 m) merging with the Meghalaya-Karbi plateau through the intervening Garo, Khasi, Jaintia and Barail Hill Range (1300-1800 m). These orographic features encircle the low-lying river valleys– the Brahmaputra and the Barak Valley (around 100 m.s.l. on an average), endowing the region with substantial environmental gradients (Pawar
et al., 2007). Lying close to the Tropics, NE India enjoys a subtropical climate characterized by hot humid summers and heavy precipitation clustered around four summer months from June to September, largely contributed by the moisture-laden southwest monsoon winds coming from the Bay of Bengal (Jain, Kumar & Saharia, 2013). With a mean annual rainfall of 2068 mm, this region (marked by a steep 1,200 m high escarpment of the Shillong plateau) receives some of the highest rainfall in the world with only a few pocket areas receiving rainfall below 1500 mm, being located in the rain shadow regions of the mountain range (Jain
et al., 2013). Recorded mean annual temperatures vary considerably across the region with a stark contrast between the valleys (15º-28º C), the adjoining mountainous region (9º-21º C) and the upper reaches in the Eastern Himalayan Region (6º-20º C), while the mean relative humidity remains high (70-85%) for most of the year (Dikshit & Dikshit, 2014). With all these factors contributing to diverse habitats (Champion & Seth, 1968) fostering rich diversity, its strategic position as a “gateway” bridging the Indian subcontinent with the rest of Asia have enabled dispersal and subsequent diversification of various anuran lineages creating high species-level regional endemism (Pan
et al., 2017).
Study species and Occurrence Data
Zhangixalus suffry was described based on collections from a tea garden (Suffry Tea Estate, Assam) in the Brahmaputra Valley of NE India (Bordoloi
et al., 2007). Originally described as
Rhacophorus suffry, this frog was recently placed under the genus
Zhangixalus (Jiang
et al., 2019). The species is a medium-sized, green dorsum, red webbed tree frog, without any dark black spots on the flanks, which are its prominent distinguishable characters that make field identification relatively easy. Among the green dorsum tree frogs of the genus
Zhangixalus found to be having overlapping geographical range,
Z. suffy is very similar to
Z. smaragdinus; however, the latter is differentiated from the former by the absence of red-colored webbings and much larger body size. Besides, from the other species of
Rhacophorus (sensu lato) having red-webbings like
R. bipunctatus, R. rhodopus, and
R. subansiriensis,
Z. suffry can be differentiated by the absence of dark black spots on the flanks (
Figure 1 inset).
Post its description, it has been reported from the Brahmaputra Valley (2 localities), the Eastern Himalayas and its adjoining hill range (10 localities), and northeast hill ranges (1 locality) extending its range of occurrence to a few kilometers away from its type locality (summarised in Saikia, Nanda & Sinha, 2017). Subsequently, it was also reported from Mizoram and Meghalaya (Lalremsanga, 2017, Mukhim et al., 2017). All these verified occurrence records within the study area (16 localities) were gleaned from published literature (surveys conducted during 2007-2017) and were used to generate SDM. To reduce the effect of spatial autocorrelation between two adjacent locations (Hampe, 2004; Luoto et al., 2005), the presence localities were spatially thinned (using the package spThin, Aiello-Lammens et al., 2015) into a 1-km2 grid cell to retain only one presence point per grid cell (Brown, Bennett & French, 2017).
Data acquisition
Environmental variables
There has been a global unison of amphibian distribution in an area to be influenced by temperature and precipitation (reviewed in Buckley & Jetz, 2007, Cunningham
et al., 2016) with members representing the Rhacophorid group depending equally on topographic features and habitat matrix quality living close to water sources (Khongwir, Hooroo & Dutta, 2016; Zheng & Natuhara, 2020). With this rationale, we assembled a dataset of 27 environmental variables in our SDMs (
Table 1) to model the potential distributional range of
Z. suffry in NE India. The model covered 19 bioclimatic variables obtained from WorldClim (
http://www.worldclim.org), relative humidity (%) from CHELSA (
http://chelsa-climate.org/), while the rest of environmental predictors covered general aspects of topography (slope, aspect, and elevation), land-use land-cover (LULC) pattern, normalized difference vegetation index (NDVI for the first and second quarter) and night light as a surrogate measure of anthropogenic impact (habitat fragmentation and urbanization), all readily downloaded from within R (see details of acquisition and processing in
Table 1).
All variables were projected to the World Geodetic System 1984 (WGS84) datum and resampled to a 1 × 1 km spatial resolution for further analyses using cubic convolution (cc) approach in R program (v.3.6.2) (RCore Team, 2020).
Data analysis
Model approach
The highly non-systematic and opportunistic nature of faunistic surveys in the past has resulted in datasets with geographic sampling bias for distribution modeling approaches (Hortal et al., 2008). Thus, we employed the Maximum entropy method (MaxEnt; Phillips, Anderson & Schapire, 2006) which have proved to be more efficient for predictive modeling approaches with presence-only data, that too when constrained with sparse occurrence records (Hernandez et al., 2006; Pearson et al., 2007; Tsoar et al., 2007; Wisz et al., 2008).
MaxEnt evaluates the species’ probability of occurrence in a site based on the environmental constraints (Elith
et al., 2011), and thus the suite of environmental predictors considered during the modeling approach can have a profound effect on the probability distribution. In this study, variable retention was based on “data-driven” variable selection to identify the least correlated and most parsimonious model (Vignali
et al., 2020). This was achieved through a two-step method which firstly involves using the
“varSel” function (in SDMtune package) to identify and remove all highly correlated variables (r > 0.75) from the initial set of 26 predictors (LULC being the only categorical variable). Subsequently, for model parsimony, the “
reduceVar” function retained a subset of the most important variables (S-
Table 1) using a threshold set to 5 % permutation importance (see Groff
et al., 2014).
Models were trained by “maxnet” implementation (Phillips et al., 2017) through SDMtune package (Vignali et al.,2019) and run with default settings enabling the application of any feature class combination (i.e., linear, quadratic, product and hinge) and suitable regularization multiplier. With a random sample of 1000 “background” locations to represent the varying environmental condition in the study area, models were run for 500 iterations including the selected environmental variables and available locality records described earlier (Phillips & Dudík, 2008; Elith et al., 2011; Warren & Seifert, 2011).
Post prediction survey
To ground validate the SDM (MaxI) generated using published historical records (n=16), regional clusters of suitable predictions sites were first selected “a posteriori” (within the two river valleys and the Meghalaya plateau), narrowing down to specific survey sites within these clusters that apparently had favorable tree-frog habitats with the help of Google Earth imagery. We directed our survey efforts mainly to private lands (tea gardens) and community-owned forests, as they were less likely to have been previously surveyed.
Field surveys were carried out in 43 such sites between March and June 2018 (after the first monsoon rain) using extensive visual encounter surveys (VES) in shallow, vegetated areas near water sources (63 days of field sampling, about 500 m in each site). The primary focus was to record the presence of Z. suffry individuals and egg mass detection, with each encountered individual identified following Bordoloi et al. (2007), photographed and geo-located using Garmin eTrex map 62 GPS. Findings of these surveys were incorporated to create a final composite distribution model (MaxF) and evaluated for any change in predictions by calculating a two-tailed Wilcoxon-signed rank test using each model’s corresponding species presence cell values (between MaxI and MaxF). Additionally, we calculated pairwise linear regressions of the model’s logistic output values for only localities where Z. suffry is known to occur. The species data frame with occurrence records, geographic layers consisting of the selected environmental variables, and a background spatial mask of NE India for clipping the geographic layers was created, and based on this mask the environmental raster layers were re-projected.
Efficiency of Protected area coverage
To evaluate the efficiency of the existing network of Protected Area (PAs) in terms of spatial coverage over the predicted potential distribution of the species in the study area, we superimposed the predicted distributional range over the boundaries of the PA network of NE India made available online through the Protected Planet website featuring a comprehensive database on Protected Areas of the World (WDPA) (
http://www.protectedplanet.net).
Results
Predicted distributional range
The potential distribution of
Z. suffry modeled based on historical presence-only data and a set of environmental variables (Max
I) presented a diffused distribution of suitable habitat within the study area (
Figure 2a). All the historical occurrence records used for modeling have been superimposed over it and are summarised in S-
Table 2.
Prior to this work, the population of
Z. suffry reported from Meghalaya and Mizoram (Mukhim
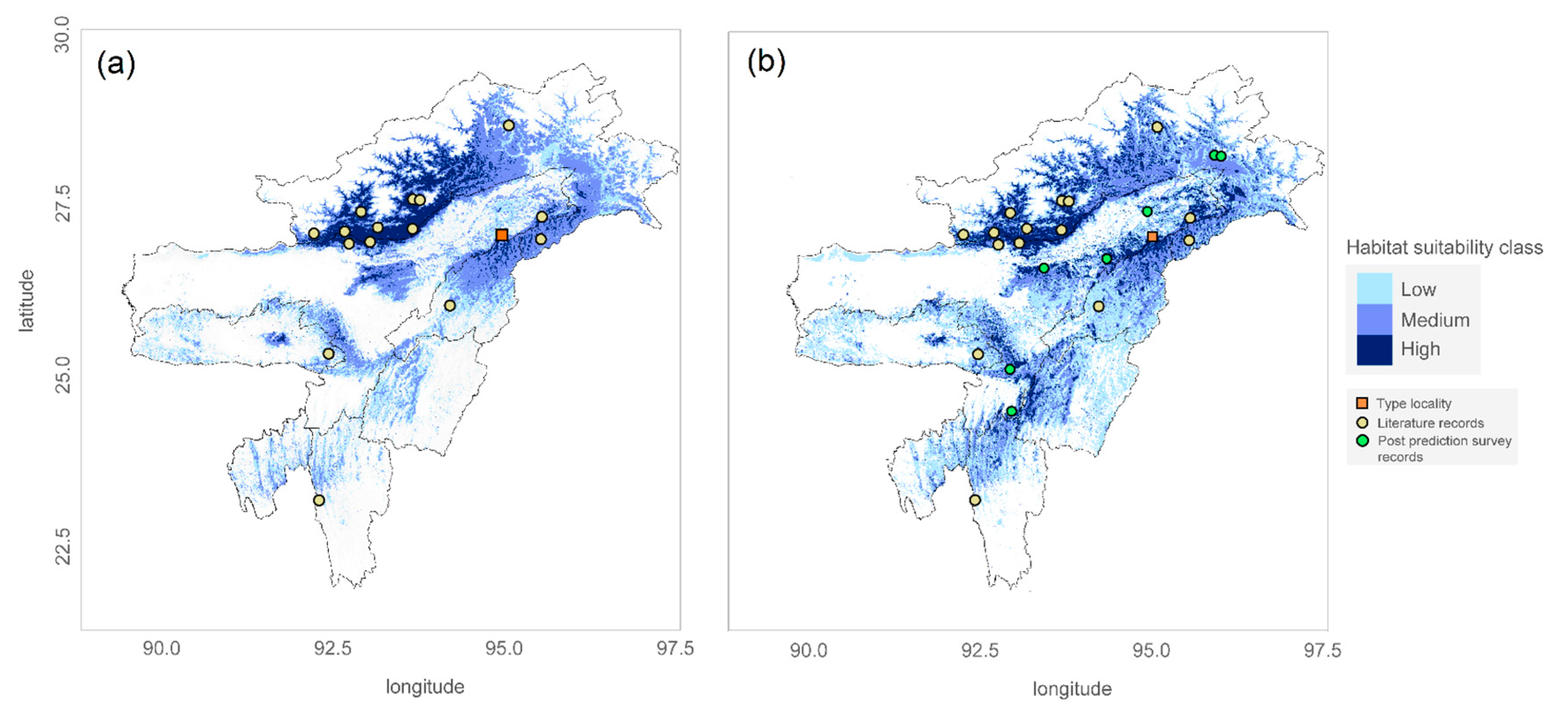
et al., 2017; Lalremsanga, 2017) was considered as two disjunct groups that appeared to be isolated from the main range. However, our MaxEnt modeling predictions suggest that these isolated populations could be linked to the main range via suitable habitats all along the Barail Hill Range and the low elevation hill ranges of Manipur and Mizoram (
Figure 2a). Later, subsequent exploration guided by the model predictions revealed new distributional records from seven previously unrecorded localities, suggesting connectivity between the ranges (forming a continuous distribution) corroborating our model predictions.
We observed one sub-adult male on a private land (Khasi punji) near Barak Reserve forest on 17th March 2018, along a forest trail running parallel to a small stream. The observed individual was identified by its distinct red webbings without any dark black spots on the flanks which set it apart from its congeners. Subsequently, the species presence was also recorded from six other unrecorded sites spanning across both river valleys and adjoining hill ranges (S-
Table 3). However, no reproductive behavior could be recorded in any of these sightings. While survey efforts in and around the Meghalaya plateau did not yield any sighting of
Z. suffry, these new occurrence records (7 localities) confirms the presence of the species at altitudes as low as 100 m to an upper altitude limit of 2000 m (S-
Table 3).
The final composite SDM (Max
F) built using the new distributional and the historical records (23 localities in total) also predicted similar core areas of suitable habitat (spatial coverage,
Figure 2b). The percentage of study areas predicted to be in the low suitability class was fairly consistent among the two models. However, areas predicted as moderate to high suitability class increased in spatial coverage (extent of area) post inclusion of these new records (
Table 2). Interestingly, one opportunistic sighting of
Z. suffry from Jokai Botanical Garden, Dibrugarh comes from an area predicted to be under low habitat suitability class.
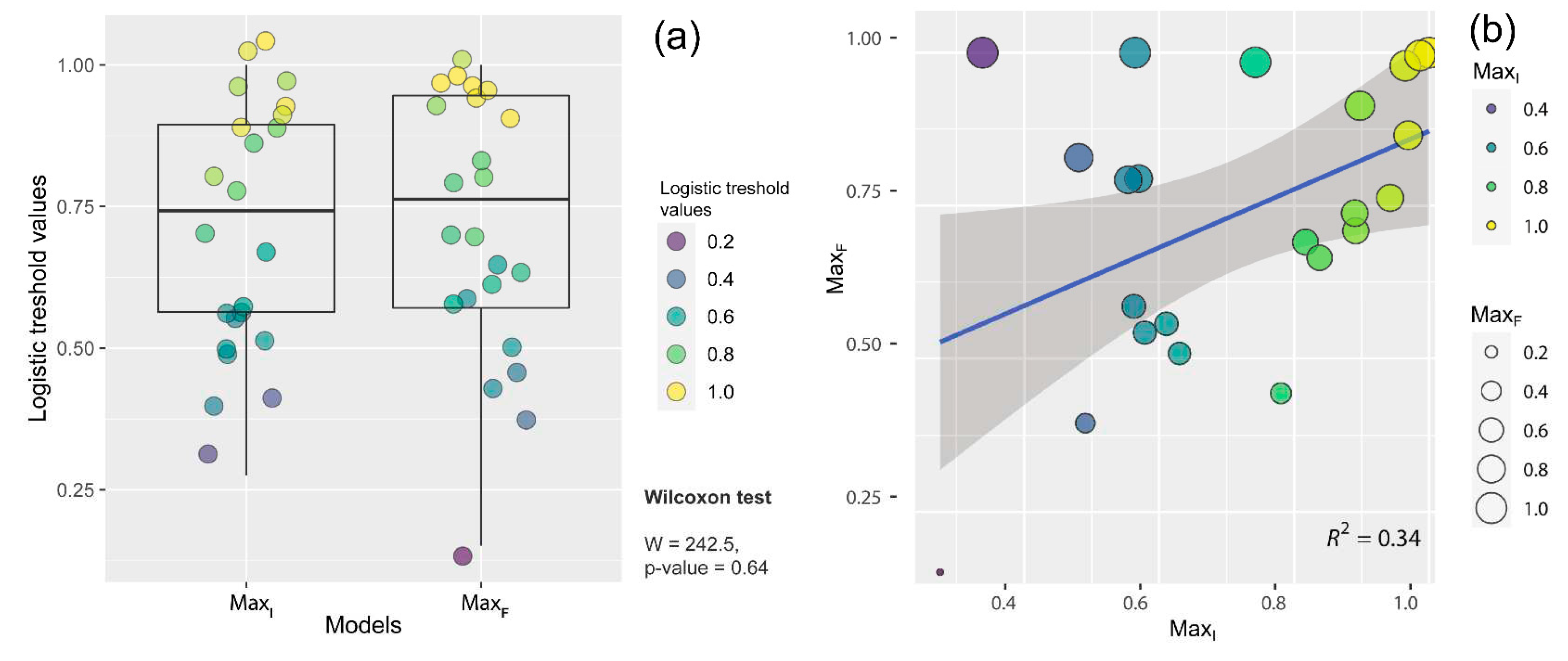
Statistical comparison of model prediction (species presence cell values) showed no significant difference even after the addition of new location records (Median values: Max
I=0.74, Max
F=0.76; Wilcoxon test, W=242; p=0.64) which suggest similarity in prediction pattern (median box-whisker plot given in
Figure 3a). Further, linear regression analysis showed a positive relationship between the models' species presence cell values revealing consistency with the known geographic distribution of the species (R
2= 0.34, scatter-line plot given in
Figure 3b).
Model evaluation and validation
Model performances of Max
I (run using hinge feature combinations and a regularization multiplier of 1.0) resulted in statistically robust predictions (Max
I: AUC = 0.89 ± 0.082 SD, S-
Figure 1a). Here, the model classified a site as a suitable habitat with one having probability values greater than the lowest 10% of all probability values (~0.19).
Jackknife (leave-one-out) validation test (corrected for small sample size) for MaxI produced a moderately high success rate at 62% (p= 0.0042) and was statistically significant when compared to a random assignment of the excluded localities. This corresponds with a 38% omission rate where 10 model runs were able to predict the excluded locality successfully.
The performance however increased substantially when new distribution records from unrecorded localities were incorporated (7 new localities). The consolidated model outperformed (Max
F: AUC = 0.93 ± 0.05 SD, S-
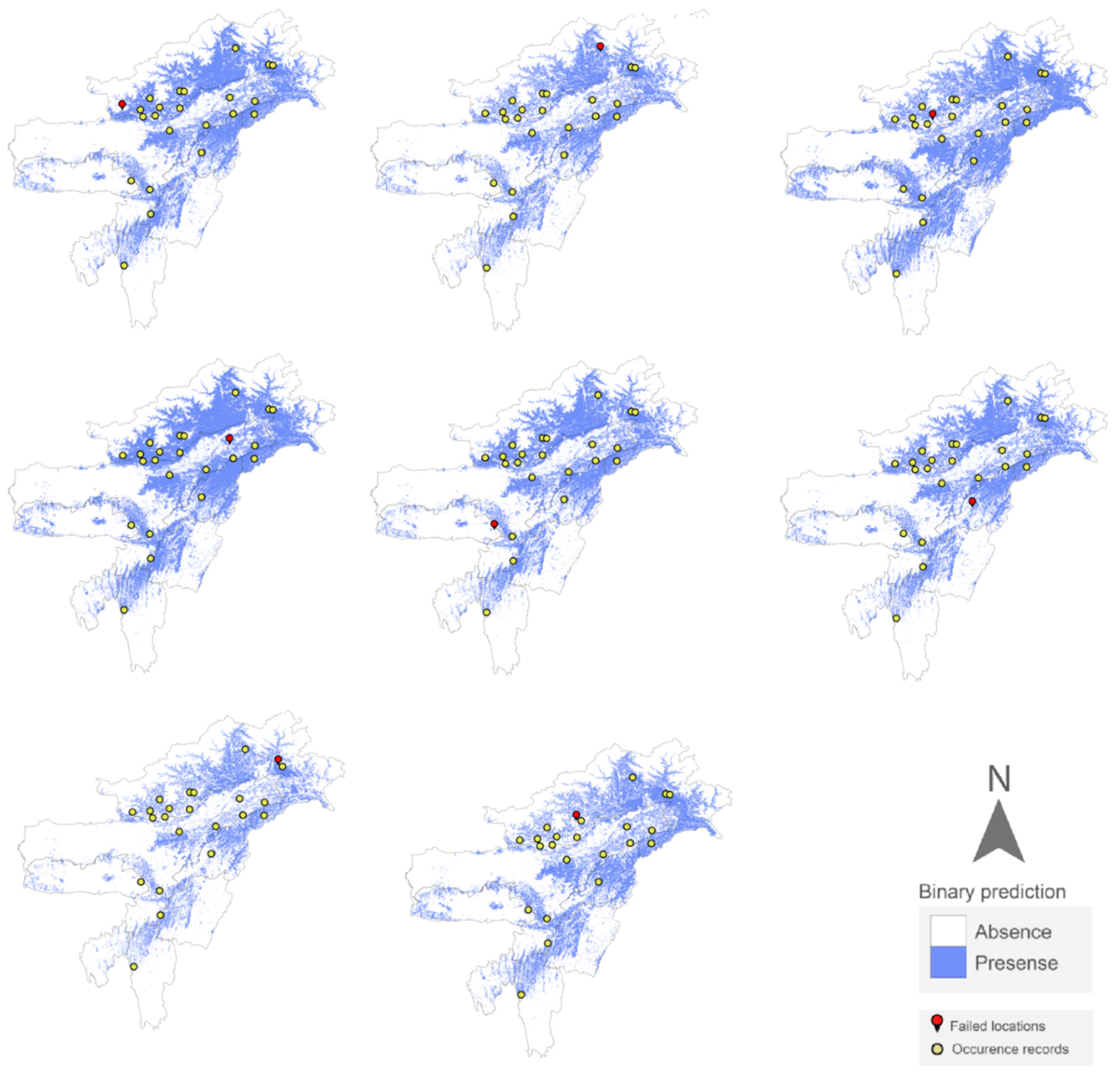
Figure 1b) the previous model. Jack-knife validation test resulted in a rate of 77% success (p=0.0016). However, six localities were consistently excluded in both cases when tested with the fixed (10%) threshold method with one of these localities reporting the frog from a cave entrance (
Figure 4, S-
Figure 2). We additionally assessed the models using True Skill Statistic (TSS) which confirmed the latter one to be a more robust model (Max
I = 0.69, Max
F = 0.73).
Environmental variable contribution
Maxent jackknife test for variable contribution (Max
F) shows precipitation related predictors to be the most important bioclimatic variable explaining the probability of occurrence of
Z. suffry with precipitation of the warmest quarter and precipitation seasonality (40.6 % and 12.7 % respectively) along with aspect (19.6 %) accounting altogether for more than 72 % contribution (S-
Figure 3).
Apart from these strong predictors, elevation and vegetation quality (NDVI) contributes significantly to the model. These results are somewhat consistent under model permutation with the same set of predictors contributing the most to the model. However, precipitation seasonality gained relatively disproportionate importance in this analytic approach (
Table 3).
Precipitation of the warmest quarter displayed a strong peak response curve suggesting high occurrence probability in areas receiving between 1300 and 2000 mm rainfall during the warmest part of the year. Elevation and aspect both impacted the frog's distribution pattern, with their respective response curves pointing towards decreasing suitability as elevation increases, being mostly confined to the shade side (North and North-East) of mountains and steep valleys of the region (S-
Figure 4).
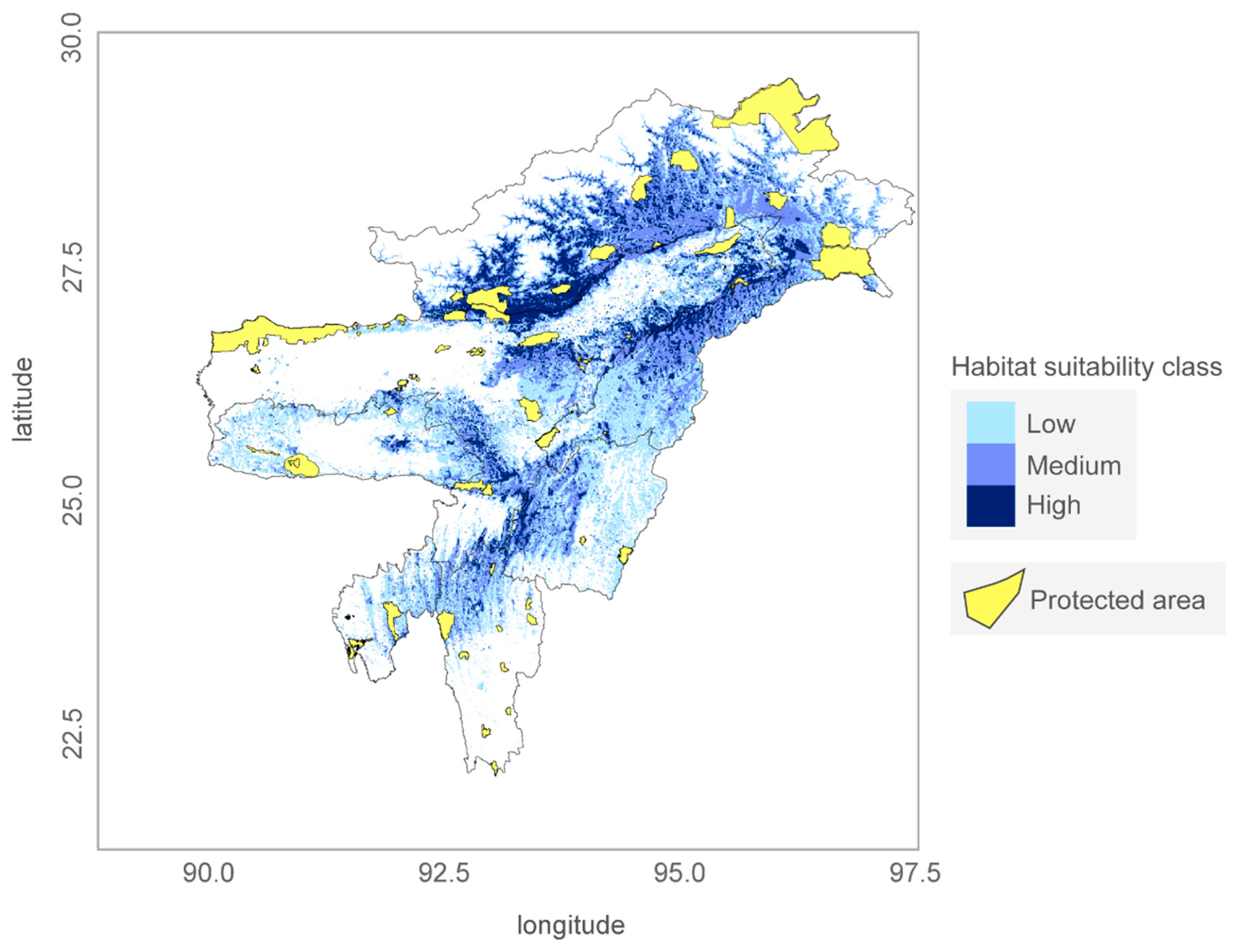
Efficiency of Protected area coverage
Overlaying the current protected area network over the predicted distribution of the frog revealed just 7 % (about 1774 km
2) coverage over its high occurrence probability zone, with many large patches of high suitability zones outside the protected areas network (
Figure 5). Among the seven states of Northeast India, Arunachal Pradesh followed by Assam presents a fair coverage of high suitable zones under legal protection (
Table 4).
Discussion
Species distribution models (SDMs) rely on the concept of niche theory that underpins predictive model(s) of species occurrences along the suitable environmental gradients. This enables one to make generalizations from point locations to larger scales, forming the cornerstone of effective species conservation plan (Austin, 2002; Chase & Leibold, 2003).
Our model prediction with 16 occurrence records projected a diffused distribution of suitable habitat, invariably predicting the empirically known localities of Z. suffry as suitable. Despite predicting a tight distribution of moderate and high suitability zones clustered around known localities, it successfully facilitated exploratory surveys leading to the discovery of seven new populations from previously unrecorded locations, which was our primary objective in the first place. The usage of the MaxEnt’s default regularization parameter(s) which is known to produce models that overfit the input data (Anderson & Raza, 2010; Warren & Seifert, 2011), might have resulted in the concentration of suitable habitats around known localities of the species resulting in a 23% omission rate in our model evaluation process.
The new locality records extend the known distributional range significantly over both the river valleys and also revealed a continuous distribution across the study area giving an impression that the species could be much more widely distributed than previously assumed. Importantly, the new populations recorded near Barak Reserve Forest and Barail Wildlife Sanctuary re-establishes the southernmost distributional range of the species, which extends up to Mizoram (Lalremsanga, 2017). It also asserts the bio-geographic importance of the Barail Hill Range acting as a dispersal corridor for various mid-elevation faunal elements (Pawar et al., 2006). This hill range, a south-western extension of the Patkai Range, connects to the Jaintia Hills of Meghalaya where another report of Z. suffry exists. However, the study's failure to record any sightings in the Meghalaya Plateau (commission error) where suitable habitat was predicted should not be ruled as "true absence," as such assertions require extensive ground probing, which was a constraint in this study.
Furthermore, non-detection of the target species when surveyed beyond its ‘explosive reproductive’ time period could be due to our limited understanding of its microhabitat (see Khongwir et al., 2016) coupled with restricted access to private lands and sacred grooves in Meghalaya. This could be also attributed to the inherent characteristics of niche models, which do not account for biotic interactions, the presence of geographic barriers, and anthropogenic influences (Peterson, 2001; Anderson, Lew & Peterson, 2003). We refrain from postulating local extinction to be a plausible factor as such speculative assertions need proper and long-term monitoring.
Surprisingly five existing literature records of the species constantly failed to be predicted as suitable through our model evaluation, even with a final composite model that incorporates the new locality records. These localities centered around parts of the Eastern Himalayas, and thus formed the highest elevation records for
Z. suffry. In contrast, nearly every new distributional record of this species found in the present study comes from low to mid-elevation areas (see S-
Table 2).
These finding creates an interesting avenue and the possibility that Z. suffry might not be a high mountain taxon, warrants a further investigation dealt with a molecular approach to authenticate the identification of the species. While taxonomic identification of R. bipunctatus seems easy (see Species Description section), it’s the presence of similar red webbings in sub-adults of Z. maximus (now Z. smaragdinus) (Wildenhues et al., 2010) which might have led to erroneous identification obscuring their representation in geographic space. Also, the ecological requirement of both the species is quite similar with largely overlapping distribution in the entire study area.
Its occurrence in a habitat predicted to be less suitable (locality record no. 18, S-
Table 2) shows that some areas predicted to be under low suitability class in our model prediction may hold remnant populations of the species, which might have been overlooked in the present study. Their occurrence in less suitable habitats could suggest the high tolerance of
Z. suffry or could simply imply a relict population holding up in these habitats where conditions might have been conducive in former times. We, therefore, recommend surveys in search of other populations at lower altitudes which can have considerable implications in their conservation status (Bordoloi
et al., 2008).
According to our model, changes and variability in precipitation values (Precipitation Seasonality and Precipitation of the Warmest Quarter) and the factors that can modulate it (e.g. elevation and aspect) were key climatic and topographic determinant for habitat suitability of the species, restricting their distribution to moist areas largely coinciding along the mountains and hill ranges with local changes in aspect (shade side), capturing additional variation typical of tree frog habitats.
This is also reflected in most SDM studies of cold-blooded amphibians where precipitation related variables have received more weight than temperature (Dolgener et al., 2013; but also see Gül, Kumlutaş & Ilgaz, 2018) with extreme values (e.g., precipitation of driest month, precipitation of the warmest quarter) likely to be more influential than averages or range values (reviewed in Carey & Alexander, 2003; Corn, 2005; Soares & Brito, 2007; Henle et al. 2008; Qian, 2010; Baselga et al., 2012). However, this apparent generalization might vary with the geographical location of the study site as well as with the eco-physiology of the target species being modeled. For a study site having a subtropical climate like ours, high seasonality of rainfall pattern can potentially restrict the breeding phenology of Rhacophorid frogs to a narrow window of time (March-April) by affecting the hydro-period of water sources in their environments (Walls, Barichivich & Brown, 2013; Khongwir et al., 2016).
This was evident in the response curve obtained for precipitation seasonality showing high seasonality drastically reducing the suitability of the site for Z. suffry. This is not surprising considering that Rhacophorid anurans (tree frog) life history are characterized by the presence of a unique behavioral adaptation to built foam nest which appears to have evolved mainly for egg or tadpole protection against desiccation and predator avoidance (Duellman & Trueb, 1986). Our study establishes the critical interplay of not only the pattern and timing of rainfall events (precipitation seasonality) but also of ambient temperature (both air and water) during these rainy months (precipitation of warmest quarter) in influencing the distribution pattern of amphibians in tropical countries, especially in relation to their seasonal explosive reproductive strategy. The sharp orographic features and the time of arrival of moisture-laden monsoon winds in this region from the Bay of Bengal, which acts as a critical checkpoint controlling the attainment of maximum temperature (warmest quarter) across the study area plays a pivotal role in the habitat suitability of Rhacophorid anurans like Z. suffry.
On comparing the ecological preferences of Z. suffry with its closest sibling species (Z. burmanus) studied with MaxEnt in the same area, a similar pattern of biotope utilization mostly confined to moist and humid terrestrial habitats such as banks of ponds and ephemeral forest streams with shaded overhanging vegetation was evident (see Sengupta, Das & Ahmed, 2016). We are of the opinion that topography (only when accessed on a finer scale) and preferred microhabitat features if and when classified and incorporated, can have the potential to define a species’ presence within that range (Mazerolle & Villard, 1999; Guerry & Hunter, 2002; Buskirk, 2005).
Species with restricted ecological niches have smaller geographic ranges (such as endemics) providing more robust and precise niche distribution models (Stockwell & Peterson, 2002, Tsoar et al., 2007). The occurrence records reviewed, collected, and used in our modeling approach however present an uneven geographic sampling regime with much more extensive sampling covering the Eastern Himalayan Region and their adjacent hills. Not to mention the level of accuracy associated with some of the historical locality data of species being in the form of textual descriptions rather than pinpoint geographical coordinates. In addition, our study area lacks a dense weather station network, and it provides only a generalized picture of the climate with only 30 weather stations (Dikshit & Dikshit, 2014), which could not well represent actual climatic conditions on ground (Hijmans et al., 2005). On top of it, the environmental dataset assembled for the modeling purpose had a temporal discrepancy (1950-2000 data) not congruent with the occurrence record (post-2007 data). Albeit all these drawbacks, our MaxEnt distribution model for Z. suffry had robust evaluation metrics with AUC value of 0.93, where values > 0.9 are suggestive of substantial predictive performance (Elith, 2002; Merow, Smith & Silander, 2013). Thus, various studies (including ours involving the “ground-truthing” approach) vindicates MaxEnt successful implementation to identify suitable habitat and potential distribution of rare and endemic amphibians across various bio-geographic realms when having very few occurrence localities (for < 20 records, see Bourke et al., 2012; Groff et al., 2014; Fernandez et al., 2009).
In conclusion, we argue that the predicted suitable habitat and potential distribution range delineated in our study can help in developing conservation strategies, land use management, and habitat restoration around these existing populations. Though PAs are widely advocated as successful management tools for ‘in-situ’ conservation of biodiversity in natural ecosystems, they have a poor spatial coverage (less than 15%) globally (Protected Planet Report 2018), coupled with inadequate biodiversity representation (Brooks et al., 2004, Rodrigues et al., 2004). Our results corroborate these lacunae as > 90% of the predicted distribution area of Z. suffry falls outside of the limits of the PA network in the Region, where it is reported to be endemic.
Rhacophorid amphibians with limited dispersal abilities, being largely restricted to breeding migrations before and after spawning to nearest water sources can be severely impeded by fragmentation, intensification of agriculture, and linear infrastructure intrusion into pristine habitats (Collins & Storfer, 2003; Cushman, 2006; Holderegger & Di Giulio, 2010). Evidence of these potent threats is rampant in the study area reeling under tremendous agronomic pressure, resulting in fragmentation of contiguous distribution of wildlife species creating isolated populations prone to local extinctions (Sharma, Madhusudan & Sinha, 2014). Future thrust areas in research and modeling approaches should investigate privately owned lands, especially Tea Gardens as our survey efforts were hindered by our inability to access such private lands. Also, with a continuous decline of monsoon precipitation over the Region since the 1950s (Preethi et al., 2017), the impact of different climate change scenarios in the distributional pattern of suitable habitat for endemic amphibians should be addressed to develop climate change-adapted conservation strategies.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Samrat Sengupta: Conceptualization, Methodology, Formal analysis, Investigation, Data Curation, Writing - original draft, Writing - Review & Editing, Visualization, Project administration. Subhasish Arandhara: Methodology, Formal analysis, Writing - original draft, Writing - Review & Editing. Bhaskar Saikia: Investigation. Methodology, Writing – Review and Editing.
Acknowledgments
We thank Abhijit Das (WII) for suggesting valuable reference material and for providing incredible support. We also like to thank Priyanuz Goswami for helping us with the study area map and Monsoonjyoti Gogoi for accompanying us in field surveys. Our heartist thanks to Anindita Deka for painfully going through each references and pointing out the mistakes.
References
- Aiello-Lammens, M.E. , Boria, R.A., Radosavljevic, A., Vilela, B. & Anderson, R.P. (2015). spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography. 38(5), 541–545. [CrossRef]
- Allouche, O. , Tsoar, A. & Kadmon, R. (2006). Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 43(6), 1223–1232. [CrossRef]
- Anderson, R. P. (2012). Harnessing the world’s biodiversity data: Promise and peril in ecological niche modeling of species distributions. Ann. N. Y. Acad. Sci. 1260(1), 66–80. [CrossRef]
- Anderson, R. P., Lew, D. & Peterson, A.T. (2003). Evaluating predictive models of species’ distributions: criteria for selecting optimal models. Ecol. Modell. 162: 211–232.
- Anderson, R.P.; Raza, A. The effect of the extent of the study region on GIS models of species geographic distributions and estimates of niche evolution: Preliminary tests with montane rodents (genus Nephelomys) in Venezuela. J. Biogeogr. 37(7), 1378–1393. [CrossRef]
- Austin, M.P. (2002). Spatial prediction of species distribution: an interface between ecological theory and statistical modelling. Ecol. Modell. 157(2-3), 101-118.
- Barbosa, A. M. , Sillero, N., Martínez-Freiría, F. & Real, R. (2012). Ecological niche models in Mediterranean herpetology: Past, present and future. Ecol. Modell. 8, 173–204. [CrossRef]
- Baselga, A., Lobo, J. M., Svenning, J. C. & Araújo, M. B. (2012). Global patterns in the shape of species geographical ranges reveal range determinants. J. Biogeogr. 39(4), 760–771. 4. [CrossRef]
- Bean, W. T., Stafford, R. & Brashares, J. S. (2012). The effects of small sample size and sample bias on threshold selection and accuracy assessment of species distribution models. Ecography. 35(3), 250–258. 250–258. [CrossRef]
- Bini, L. M., Diniz-Filho, J. A. F., Rangel, T. F. L. V. B., Bastos, R. P. & Pinto, M. P. (2006). Challenging Wallacean and Linnean shortfalls: Knowledge gradients and conservation planning in a biodiversity hotspot. Divers. Distrib. 12(5), 475–482. [CrossRef]
- Bordoloi, S., Bortamuli, T., & Ohler, A. (2007). Systematics of the genus Rhacophorus (Amphibia, Anura): identity of red-webbed forms and description of a new species from Assam. Zootaxa. 1653: 1–20.
- Bordoloi, S., Sengupta, S., Ohler, A. & Agarwal, I. (2008). Rhacophorus suffry. The IUCN Red List of Threatened Species 2008: E.T136092A4231921, 8235. [CrossRef]
- Bourke, J., Busse, K. & Böhme, W. (2012). Searching for a lost frog (Rhinoderma rufum): Identification of the most promising areas for future surveys and possible reasons of its enigmatic decline. North-West. J. Zool. 8(1), 99–106.
- Brooks, T. M., Bakarr, M. I., Boucher, T., Da Fonseca, G. A. B., Hilton-Taylor, C., Hoekstra, J.M.,Morritz, T., Olivieri, S., Parrish, J., Pressey, R.L., Rodrigues, A.S.L., Sechrest, W., Stattersfield, A., Strahm, W., & Stuart S.N. (2004). Coverage provided by the global protected-area system: Is it enough? BioScience. 54(12), 1081–1091. [CrossRef]
- Brown, J. L., Bennett, J. R., & French, C. M. (2017). SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ, (12). [CrossRef]
- Buckley, L. B. & Jetz, W. (2007). Environmental and historical constraints on global patterns of amphibian richness. Proc. R. Soc. B. Biol. Sci. 274(1614), 1167–1173. [CrossRef]
- Busetto, L., & Ranghetti, L. (2016). MODIStsp: An R package for automatic preprocessing of MODIS Land Products time series. Comp. geoscis. 97, 40-48.
- Buskirk, J. V. (2005). Local and Landscape Influence on Amphibian Occurrence. Ecology, 86(7), 1936–1947.
- Carey, C. & Alexander, M. A. (2003). Climate change and amphibian declines: Is there a link? Divers. Distrib. 9(2), 111–121. [CrossRef]
- Champion, H. G. & Seth, S. K. (1968). A Revised survey of Forest types of India. Government of India Press, Nasik.
- Chase, J. M. & Leibold, M. A. (2003) Ecological niches: linking classical and contemporary approaches. University of Chicago Press, Chicago, Illinois.
- Collins, J. P. & Storfer, A. (2003). Global amphibian declines: Sorting the hypotheses. Divers. Distrib. 9(2), 89–98. [CrossRef]
- Corn, P. S. (2005). Climate change and amphibians. Anim. Biodivers. Conserv. 28(1), 59–67.
- Cunningham, H. R., Rissler, L. J., Buckley, L. B. & Urban, M. C. (2016). Abiotic and biotic constraints across reptile and amphibian ranges. Ecography. 39(1), 1–8. 1. [CrossRef]
- Cushman, S. A. (2006). Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol. Conserv. 128(2), 231–240.
- Dikshit, K. R. & Dikshit, J. K. (2014). Weather and climate of North-East India. North-East India: Land, People and Economy, Advances in Asian Human-Environmental Research, Springer Science . [CrossRef]
- Dolgener, N., Freudenberger, L., Schneeweiss, N., Ibisch, P. L. & Tiedemann, R. (2013). Projecting current and potential future distribution of the Fire-bellied toad Bombina bombina under climate change in north-eastern Germany. Reg. Environ. Chang. 14(3), 1063–1072. [CrossRef]
- Duellman, W. E. & Trueb, L. (1986). Biology of Amphibians, McGrawHill, New York.
- Elith, J. (2002). Quantitative Methods for Modeling Species Habitat: Comparative Performance and an Application to Australian Plants. In: Quantitative Methods for Conservation Biology New York: Springer. P: 39–58. [CrossRef]
- Elith, J., Phillips, S. J., Hastie, T., Dudík, M., Chee, Y. E. & Yates, C. J. (2011). A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 17(1), 43–57. 1. [CrossRef]
- Fernández, M., Cole, D., Heyer, W. R., Reichle, S. & De Sá, R. O. (2009). Predicting Leptodactylus (Amphibia, Anura, Leptodactylidae) Distributions: Broad-Ranging Versus Patchily Distributed Species Using a Presence-Only Environmental Niche Modeling Technique. South Am. J. Herpetol. 4(2), 103–116. [CrossRef]
- Fielding, A. H., & Bell, J. F. (1997). A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 24(1), 38–49. [CrossRef]
- Frost, D. R. 2021. Amphibian Species of the World: an Online Reference. Version 6.0 (accessed on 20 May, 2021). Electronic database accessible at http://reserch.amnh.org/herpetology/amphibia/index.html, Am. Mus. Nat. Hist., New York, USA.
- Groff, L. A., Marks, S. B., & Hayes, M. P. (2014). Using ecological niche models to direct rare amphibian surveys: A case study using the oregon spotted frog (Rana pretiosa). Herpetol. Conserv. Biol. 9(2), 354–368.
- Guerry, A. D., & Hunter. M. L. Jr. (2002). Amphibian distributions in a landscape of forests and agriculture: an examination of landscape composition and configuration. Conserv. Biol. 16(3), 745–754. /: http.
- Gül, S., Kumlutaş, Y., & Ilgaz, Ç. (2018). Potential distribution under different climatic scenarios of climate change of the vulnerable Caucasian salamander (Mertensiella caucasica): A case study of the Caucasus Hotspot. Biol. (Poland), 73(2), 175–184. [CrossRef]
- Hampe, A. (2004). Bioclimate envelope models: what they detect and what they hide. Glob. Ecol. Biogeogr. 13(5), 469-471. [CrossRef]
- Henle, K., Dick, D., Harpke, A., Kuhn, I., Schweiger, O., & Settele, J. (2008). Climate Change Impacts on European Amphibians and Reptiles. Convention on the conservation of European Wildlife and natural habitats, Strasbourg.
- Hernandez, P. A. Hernandez, P. A., Graham, C. H., Master, L. L., & Albert, D. L. (2006). The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography, 29(5), 773–785. [CrossRef]
- Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. Intl. J. Climatol. 25(15), 1965–1978. 15. [CrossRef]
- Hijmans, R. J. , Van Etten, J., Cheng, J., Mattiuzzi, M., Sumner, M., Greenberg, J. A.,... & Hijmans, M. R. J. (2015). Package ‘raster’. R package, 734.
- Hijmans, R. J. , Bivand, R., Forner, K., Ooms, J., & Pebesma, E. (2021). Package ‘terra’.
- Holderegger, R., & Di Giulio, M. (2010). The genetic effects of roads: A review of empirical evidence. Basic Appl. Ecol. 11(6), 522–531. [CrossRef]
- Hortal, J., De Bello, F., Diniz-Filho, J. A. F., Lewinsohn, T. M., Lobo, J. M., & Ladle, R. J. (2015). Seven Shortfalls that Beset Large-Scale Knowledge of Biodiversity. Annu. Rev. Ecol. Evol. Syst. 46, 523–549. [CrossRef]
- Hortal, J., Jiménez-Valverde, A., Gómez, J. F., Lobo, J. M., & Baselga, A. (2008). Historical bias in biodiversity inventories affects the observed environmental niche of the species. Oikos, 117(6), 847–858. [CrossRef]
- Jain, S. K., Kumar, V., & Saharia, M. (2013). Analysis of rainfall and temperature trends in northeast India. Intl. J. Climatol. 33(4), 968–978. 4. [CrossRef]
- Jiang, D., Jiang, K., Ren, J., Wu, J., & Li, J. (2019). Resurrection of the genus Leptomantis, with description of a new genus to the family Rhacophoridae (Amphibia: Anura). Asian Herpetol. Res. 10(1), 1–12. [CrossRef]
- Khongwir, S., Hooroo, R. N. K., & Dutta, S. K. (2016). Breeding and nesting behaviour of Rhacophorus maximus (Anura: Rhacophoridae) in Meghalaya, North East India. Curr. Sci. 110(6), 1102–1105. [CrossRef]
- Kwok, R., 2018. Ecology’s remote-sensing revolution. Nature 556, 137. [CrossRef]
- Lalremsanga, H.T. (2017). Geographical Distribution record on Rhacophorus suffry. Herpetol. Rev. 48(1), 120-121.
- Luoto, M., Pöyry, J., Heikkinen, R. K., & Saarinen, K. (2005). Uncertainty of bioclimate envelope models based on the geographical distribution of species. Glob. Ecol. Biogeogr. 14(6), 575–584. [CrossRef]
- Mazerolle, M. J., & Villard, M. A. (1999). Patch characteristics and landscape context as predictors of species presence and abundance: A review. Ecoscience, 6(1), 117–124. [CrossRef]
- Merow, C., Smith, M. J., & Silander, J. A. (2013). A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography, 36(10), 1058–1069. 1069. [CrossRef]
- Mukhim, D. K. B., Saikia, B., Imam, I., Kharkongor, I. J., Sarma, D., & Arbenz, T. (2017). Rhacophorus suffry bordoloi, bortamuli and ohler, 2007 - A new record from Meghalaya, North-East India. J. Bom. Nat. Hist. Soc. 114, 8–9. [CrossRef]
- Myers, N., Mittermeier, R.A., Mittermeier, C.G., da Fonseca, G.A.B., & Kent, J.(2000). Biodiversity hotspots for conservation priorities. Nature, 403: 853-858.
- Pan, T., Zhang, Y., Wang, H., Wu, J., Kang, X., Qian, L., Chen, J., Rao, D., Jiang, J., & Zhang, B. (2017). The reanalysis of biogeography of the Asian tree frog, Rhacophorus (Anura: Rhacophoridae): Geographic shifts and climatic change influenced the dispersal process and diversification. PeerJ, 11, 1-21. [CrossRef]
- Pawar, S., Koo, M. S., Kelley, C., Ahmed, M. F., Chaudhuri, S., & Sarkar, S. (2007). Conservation assessment and prioritization of areas in Northeast India: Priorities for amphibians and reptiles. Biol. Conserv. 136(3), 346–361. [CrossRef]
- Pawar, S. S., Birand, A. C., Ahmed, M. F., Sengupta, S., & Raman, T. R. S. (2006). Conservation biogeography in North-east India: Hierarchical analysis of cross-taxon distributional congruence. Divers. Distrib. 13(1), 53–65. [CrossRef]
- Pearson, R. G., Raxworthy, C. J., Nakamura, M., & Peterson, A. T. (2007). Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 34(1), 102–117. [CrossRef]
- Pebesma, E. , Bivand, R., Pebesma, M. E., RColorBrewer, S., & Collate, A. A. A. (2012). Package ‘sp’. The Comprehensive R Archive Network.
- Peterson, A. T. (2001). Predicting species’ geographic distributions based on ecological niche modeling. Condor, 103(3), 599–605. [CrossRef]
- Peterson, A. T, & Soberón, J. (2012). Species distribution modeling and ecological niche modeling: Getting the Concepts Right. Nat. a Conserv. 10(2), 102–107. [CrossRef]
- Peterson, A. T, Soberón, J., Pearson, R. G., Anderson, R. P., Martínez-Meyer, E., Nakamura, M., & Araújo, M. B. (2011). Ecological Niches and Geographic Distributions. Princeton University Press, Princeton, New Jersey. [CrossRef]
- Phillips, S. J., Andeson, R. P., & Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecol. Modell. 190, 231–259.
- Phillips, S. J., Anderson, R. P., Dudík, M., Schapire, R. E., & Blair, M. E. (2017). Opening the black box: an open-source release of Maxent. Ecography, 40(7), 887–893. [CrossRef]
- Phillips, S. J., & Dudík, M. (2008). Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography, 31(2), 161–175. [CrossRef]
- Preethi, B., Mujumdar, M., Kripalani, R. H., Prabhu, A., & Krishnan, R. (2017). Recent trends and tele-connections among South and East Asian summer monsoons in a warming environment. Clim. Dyn. 48(7–8), 2489–2505. [CrossRef]
- Protected Planet Report (2018). UNEP-WCMC, IUCN, & NGS. Cambridge UK; Gland, Switzerland; and Washington, D.C., USA.
- Qian, H. (2010). Environment-richness relationships for mammals, birds, reptiles, and amphibians at global and regional scales. Ecol. Res. 25(3), 629–637. [CrossRef]
- R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Rebelo, H., & Jones, G. (2010). Ground validation of presence-only modelling with rare species: A case study on barbastelles Barbastella barbastellus (Chiroptera: Vespertilionidae). J. Appl. Ecol. 47(2), 410–420. [CrossRef]
- Rodrigues, A. S. L., Andelman, S.J., Bakarr, M.I., Boitani, L., Brooks, T.M., Cowling, R.M., Fishpool, L.D.C., da Fonseca, G.A.B., Gaston, K. J., Hoffmann, M., Long, J.S., Marquet, P.A.,Pilgrim, J.D., Pressey, R.L., Schipper, J., Sechrest, W., Stuart, S. N., Underhill, L. G., Waller, R. W.,Watts, M. E. J., & Yan, X. (2004). Effectiveness of the global protected area network in representing species diversity. Nature, 428, 640–643. [CrossRef]
- Saikia, B., Nanda, P., & Sinha, B. (2017). Atlas of endemic Rhacophorus (Amphibia : Anura) of Northeast India, Bull. Ar. Forest Res. 32(1&2), 91-95.
- Sengupta, S., Das, A., Ahmed, M. F. (2016). Distribution of Rhacophorus burmanus (Andersson , 1939), with notes on its natural history. Herpetozoa, 29(3/4), 194–198.
- Sharma, N., Madhusudan, M. D., & Sinha, A. (2014). Local and landscape correlates of primate distribution and persistence in the Remnant Lowland Rainforests of the Upper Brahmaputra Valley, Northeastern India. Conserv. Biol. 28(1), 95–106. [CrossRef]
- Soares, C., & Brito, J. C. (2007). Environmental correlates for species richness among amphibians and reptiles in a climate transition area. Biodivers. Conserv. 16(4), 1087–1102. 16, 4, 1087–1102. [CrossRef]
- Stockwell, D. R. B., & Peterson, A. T. (2002). Effects of sample size on accuracy of species distribution models. Ecol. Modell. 148(1), 1–13. [CrossRef]
- Stuart, S.N., Hoffmann, M., Chanson, J.S., Cox, N.A., Berridge, R.J., Ramani, P., & Young, B.E. (eds.) (2008). Threatened Amphibians of the World. Lynx Edicions, Barcelona, Spain; IUCN, Gland, Switzerland; and Conservation International, Arlington, Virginia, USA.
- Tsoar, A., Allouche, O., Steinitz, O., Rotem, D., & Kadmon, R. (2007). A comparative evaluation of presence-only methods for modelling species distribution. Divers. Distrib. 13(4), 397–405. [CrossRef]
- Vignali, S. , Barras, A., & Braunisch, V. (2019). SDMtune: Species distribution model selection. R Package Version, 101.
- Vignali, S., Barras, A. G., Arlettaz, R., & Braunisch, V. (2020). SDMtune: An R package to tune and evaluate species distribution models. Ecol. Evol. 10(20), 11488–11506. [CrossRef]
- Walls, S. C., Barichivich, W. J., & Brown, M. E. (2013). Drought, deluge and declines: The impact of precipitation extremes on amphibians in a changing climate. Biol. 2(1), 399–418. [CrossRef]
- Warren, D. L., & Seifert, S. N. (2011). Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 21(2), 335–342. [CrossRef]
- Wildenhues, M. J., Gawor, A., Nguyen, T. Q., Nguyen, T. T., Schmitz, A., & Ziegler, T. (2010). First description of larval and juvenile stages of Rhacophorus maximus Günther, 1859 “1858” (Anura: Rhacophoridae) from Vietnam. Rev. Suisse Zool. 117(4), 679–696. [CrossRef]
- Wisz, M. S., Hijmans, R. J., Li, J., Peterson, A. T., Graham, C. H., Guisan, A., and NCEAS Predicting Species Distributions Working Group (2008). Effects of sample size on the performance of species distribution models. Divers. Distrib. 14(5), 763–773. [CrossRef]
- Zheng, X., & Natuhara, Y. (2020). Landscape and local correlates with two green tree-frogs, Rhacophorus (Amphibia: Rhacophoridae) in different habitats, central Japan. Landsc. Ecol. Eng. 16(2), 199–206. [CrossRef]
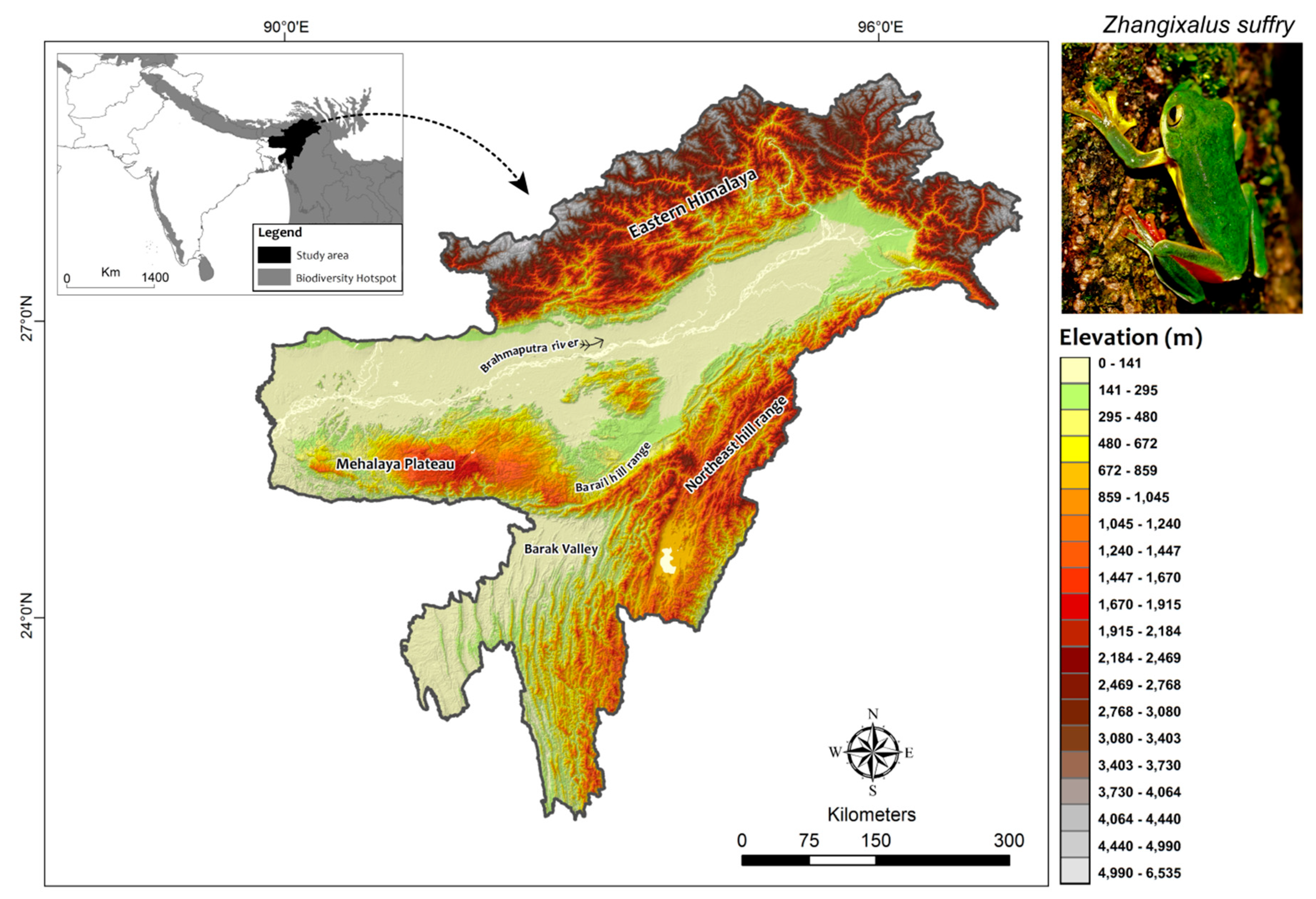
Figure 1.
Map of the Northeast India delineating the elevation range of the study area. Insetleft: location of the study area within Biodiversity Hotspot regions of the world. Inset top right: shows live individual of Zhangixalus suffry near Barak Reserve Forest, Assam, India (Photographed by Samrat Sengupta).
Figure 1.
Map of the Northeast India delineating the elevation range of the study area. Insetleft: location of the study area within Biodiversity Hotspot regions of the world. Inset top right: shows live individual of Zhangixalus suffry near Barak Reserve Forest, Assam, India (Photographed by Samrat Sengupta).
Figure 2.
The species distribution model of Z. suffry showing the predicted probability of occurrence within Northeast India based on maximum entropy modelling approach corrected for small sample size. a) MaxI represent SDM based on only historical records available in existing literatures, b) MaxF represent SDM based on the historical records as well as incorporating the new distributional records guided by model predictions during ground-truthing process. Habitat suitability classes in discrete scale: lowest: cyan blue, medium: cobalt blue highest: navy blue. Point markers, yellow round: historical database records; green round: new locality records (present study); red square: type locality (Suffry Tea Estate, Assam).
Figure 2.
The species distribution model of Z. suffry showing the predicted probability of occurrence within Northeast India based on maximum entropy modelling approach corrected for small sample size. a) MaxI represent SDM based on only historical records available in existing literatures, b) MaxF represent SDM based on the historical records as well as incorporating the new distributional records guided by model predictions during ground-truthing process. Habitat suitability classes in discrete scale: lowest: cyan blue, medium: cobalt blue highest: navy blue. Point markers, yellow round: historical database records; green round: new locality records (present study); red square: type locality (Suffry Tea Estate, Assam).
Figure 3.
Comparison between each model’s corresponding species presence cell values where Z. suffry is known to occur. a) median box-whisker plot representing overlapping confidence intervals and Wilcoxcon test shows insignificant difference (p=0.64), b) linear regression model showing positive relationship between two models (R2=0.34). Model abbreviations given in materials and methods.
Figure 3.
Comparison between each model’s corresponding species presence cell values where Z. suffry is known to occur. a) median box-whisker plot representing overlapping confidence intervals and Wilcoxcon test shows insignificant difference (p=0.64), b) linear regression model showing positive relationship between two models (R2=0.34). Model abbreviations given in materials and methods.
Figure 4.
Projected distribution of n-1 different models of Z. suffry using MaxEnt following the Jackknife approach (eight failed prediction models).
Figure 4.
Projected distribution of n-1 different models of Z. suffry using MaxEnt following the Jackknife approach (eight failed prediction models).
Figure 5.
Predicted distribution map of Z. suffry, showing the Protected Area (PA) network in the study area.
Figure 5.
Predicted distribution map of Z. suffry, showing the Protected Area (PA) network in the study area.
Table 1.
Description of environmental variables used to build the MaxEnt model with details of source, default resolution, method of extraction and processing. All the Bioclimatic variables were resampled to a working resolution of 30 arc-sec (~1 km) for further analysis (see Methods section).
Table 1.
Description of environmental variables used to build the MaxEnt model with details of source, default resolution, method of extraction and processing. All the Bioclimatic variables were resampled to a working resolution of 30 arc-sec (~1 km) for further analysis (see Methods section).
| Data |
Source |
Default resolution |
Extraction and Processing |
| Elevation |
Aster DEM-Earth Data |
30 m |
Using “Raster” and “sp” package in R (Hijmans et al., 2015; Pebesma et al., 2012) |
| Aspect |
Same as above |
30 m |
Analysis of aspect using terrain function in raster package in R |
| Slope |
Same as above |
30 m |
Analysis of slope similar to aspect |
| Land use and land cover (LULC) |
USGS-Landsat 8 |
30 m |
Extraction using “getSpatialData” package
(Kwok, 2018) and classification using “terra” package (Hijmans et al., 2021)
|
Normalized difference vegetation index
(NDVI) |
MODIS |
250 m |
Analysis using Modistsp and Raster package in R (Busetto & Ranghetti, 2016) |
| Bio-climatic variables |
BIOCLIM (consisting of 19 variables) |
1 km |
Using getData function trough raster package in R |
| Relative humidity |
CHELSA |
1 km |
same as above |
| Night light |
DMSP/OLS |
1 km |
same as above |
Table 2.
Percent of study area predicted by each maximum entropy model corrected for small sample size (MaxI and MaxF) according to four habitat suitability classes: unsuitable, low suitability, moderate suitability and high suitability based on the chosen 10th percentile training presence logistic threshold.
Table 2.
Percent of study area predicted by each maximum entropy model corrected for small sample size (MaxI and MaxF) according to four habitat suitability classes: unsuitable, low suitability, moderate suitability and high suitability based on the chosen 10th percentile training presence logistic threshold.
| |
Percent of study area |
| Models (n) |
Unsuitable |
Low |
Moderate |
High |
| MaxI (n=16 localities) |
59.85 |
25.6 |
8.28 |
6.28 |
| MaxF (n=23 localities) |
45.63 |
25.5 |
19.11 |
9.77 |
Table 3.
Contribution of the environmental variables to the MaxEnt model (MaxI and MaxF). The percentage contribution assesses the model's gain through each environmental variable, while the model is built. The permutation importance value is the drop of the AUC value based on the final MaxEnt model, when randomly permuting the values of the variable among the training points.
Table 3.
Contribution of the environmental variables to the MaxEnt model (MaxI and MaxF). The percentage contribution assesses the model's gain through each environmental variable, while the model is built. The permutation importance value is the drop of the AUC value based on the final MaxEnt model, when randomly permuting the values of the variable among the training points.
| MaxI |
Variables |
Percent contribution |
Permutation importance |
| Precipitation of Warmest Quarter |
24.7 |
18.4 |
| NDVIQ2 |
21.8 |
17.3 |
| Aspect |
15.9 |
7.9 |
| Precipitation Seasonality |
13.1 |
23.9 |
| Slope |
12.8 |
20.1 |
| Isothermality |
11.8 |
12.4 |
| MaxF |
Precipitation of Warmest Quarter |
40.6 |
35.7 |
| Aspect |
19.6 |
16.2 |
| NDVIQ2 |
17.4 |
21.3 |
| Elevation |
9.7 |
1.7 |
| Precipitation Seasonality |
12.7 |
25.1 |
Table 4.
Extent of coverage of the predicted potential distribution of Zhangixalus suffry under the existing Protected Area (PA) network in the study area.
Table 4.
Extent of coverage of the predicted potential distribution of Zhangixalus suffry under the existing Protected Area (PA) network in the study area.
| Levels |
AR |
AS |
ML |
MN |
MZ |
NL |
TR |
PA coverage (km2) |
NE coverage (km2) |
| <10% threshold |
5052 |
2035 |
520 |
55 |
324 |
193 |
244 |
8423 |
116853 |
| Low |
1929 |
1768 |
319 |
137 |
280 |
68 |
310 |
4811 |
65258 |
| Moderate |
1897 |
688 |
53 |
25 |
247 |
8 |
55 |
2973 |
48940 |
| High |
1038 |
662 |
7 |
8 |
29 |
9 |
21 |
1774 |
25032 |
| Total area |
9916 |
5153 |
899 |
226 |
880 |
278 |
629 |
17981 |
256083 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).