1. Introduction
The vagina provides a humid, nutritious and warm habitat for microbiota, which is composed of a large number of protective microorganisms and others that turn out to be opportunistic pathogens [
1,
2,
3,
4]. The vaginal microbiota is responsible for the production of antimicrobial and anti-inflammatory factors, that provide a protection mechanism against non-native microorganisms, as well as a balance between the microorganisms that make up the microbiota [
2,
4,
5,
6,
7,
8]. This balance can be altered by both internal factors (hormonal status, age, and the immune system) and external factors (use of antibiotics, infections, and microbial exposure to the environment), which favors the development of dysbiosis [
2,
4,
5,
6,
7,
8]. So far, five different microbial communities have been identified that maintain a symbiotic relationship with the host and, in particular,
Candida spp., are considered commensals of the vaginal mucosa and coexist as part of the complex vaginal ecosystem [
1,
2,
9,
10,
11,
12,
13,
14].
During pregnancy, physiological changes in the immune system, increased estrogen and progesterone levels, glycogen storage, low vaginal pH, and decreased cell-mediated immunity favor the development of
Candida infection [
9,
10,
11,
12,
13,
14]. Vulvovaginal candidiasis during pregnancy has a high prevalence, which presents a wide geographical variation (
Table 1), e.g, in Selangor in Malaysia they report 17.2%, Burkina Faso 22.71%, and Argentina 24.78%, are the countries with the lowest prevalence and, on the other hand those with the highest prevalence are the state of Enugu in Nigeria with 62.2%, Taif in Saudi Arabia 70.2% and Kenya 90.38%. In Latin America, a prevalence between 24.8 and 44.8% has been reported (Argentina and Brazil); as for Mexico there are no representative and recent studies on the prevalence of
Candida in the general population, the latest report on its study in pregnancy dates from 2014 by Solís-Árias et al. where we found a prevalence of 12.6%. Vulvovaginal candidiasis, or
Candida vaginitis, is caused primarily by
Candida albicans in 85-95% of cases and the remainder by non-
albicans Candida species (
C. glabrata,
C. parapsilosis, and
C. tropicalis) [
7,
9,
10,
11,
12,
19,
20].
Vulvovaginal candidiasis can be classified as uncomplicated or complicated depending on factors including the severity of the infection, the yeast species, and the integrity of the immune system. Complicated vulvovaginal candidiasis is generally difficult to treat, making it necessary to administer more aggressive treatment regimens to cure it. For uncomplicated vaginal candidiasis, azole antifungals are the mainstay of treatment and are adequate to resolve
Candida infections in most cases. These medications come in a variety of formulations and can be administered orally or topically as vaginal creams, ointments, or suppositories. [
7]
Azole antifungals (
Table 2) are a group of medicines that contain an azole ring and inhibit the growth of a wide range of fungi. They are classified into two groups: those with two nitrogens in the azole ring (the imidazoles; examples include clotrimazole, econazole, ketoconazole, miconazole, and tioconazole) and those with three nitrogens in the azole ring (the triazoles; examples include fluconazole, itraconazole, posaconazole, and voriconazole). Azole antifungals work by inhibiting the cytochrome P450 dependent enzyme lanosterol 14-alpha-demethylase, which converts lanosterol to ergosterol, the main sterol in the fungal cell membrane. Depletion of ergosterol damages the cell membrane resulting in cell death.[
21]
Rotem et al. evaluate the risk for major malformations following first-trimester exposure to vaginal azoles was not associated with either major or specific malformations according to organ systems.[
22]
For recurrent vulvovaginal candidiasis (three or more symptomatic episodes in a 12-month period), treatment options include fluconazole, itraconazole, miconazole, clotrimazole, terconazole, and nystatin, with the recent addition of oteseconazole and a vaccine directed at a hyphal virulence factor of
Candida albicans. When vulvovaginal candidiasis is caused by non-albicans
Candida, the recommended treatment options are fluconazole or boric acid. The greatest experience in the treatment of vulvovaginal candidiasis is with fluconazole, and a systematic review on congenital malformations and first trimester use of fluconazole found a potential association between general malformations (odds ratio [OR] 1.10, confidence interval of 95% [CI] 0.98–1.25), cardiac defects (OR 1.29, 95% CI 1.05-1.58), craniofacial defects (OR 1.25, 95% CI 0.88-1 .77) and abortion, although the rate of general malformations and craniofacial defects were not significant. Despite these findings, current guidelines state that only topical azole therapy should be used to treat vulvovaginal candidiasis during pregnancy.[
7]
Several studies have shown the increase in the prevalence of Candida during pregnancy, which can alter the vaginal microbiota, causing an increase in proinflammatory cytokines with induction of the inflammatory cascade and generate adverse pregnancy outcomes such as preterm labor, premature rupture of membranes, preeclampsia, spontaneous abortion, restriction in the fetal growth, low birth weight, fetal death and neonatal sepsis. On the other hand, a positive culture result not associated with symptoms is considered a sign of colonization and not of infection, and therefore does not generate any consequences. The aim of this study is to contribute to identifying whether there is an association between colonization by Candida and adverse perinatal outcomes. [
3,
8,
9,
10,
11,
16,
23,
24,
25,
26,
27].
2. Materials and Methods
2.1. Study design
Descriptive, observational, prospective study that included pregnant patients without signs or symptoms of vaginal infection, and without antimicrobial treatment, who attended the Hospital Regional de Alta Especialidad de Ixtapaluca (HRAEI) obstetric triage service for a period of six months (May to October 2019), agreed to participate in the study and were followed up until the end of pregnancy.
2.2. Cervicovaginal sampling
Samples of cervicovaginal exudate (posterior cul-de-sac and cervix) were taken with a sterile swab, after speculum placement. Vaginal samples were immediately inoculated into Petri dishes with Sabouraud agar and incubated at 37°C for 24-72h.
From the primary isolations, a colony was taken, reseeded on Sabouraud agar plates and incubated at 37°C for 24h, for subsequent identification.
2.3. Phenotypic identification of Candida spp.
Initially, the yeasts were identified by germ tube production, through the inoculation of the yeasts in 500 µL of human serum and incubation at 37 °C for 2 h. Subsequently, 20 µL of the serum inoculated with Candida were placed between slides and coverslips and microscopic observation (40X) was performed to search for the germ tube. The yeasts that presented a germ tube were considered C. albicans, and those that did not develop a germ tube were considered C. non-albicans.
2.4. Genotypic identification of Candida spp.
DNA was extracted from each of the vaginal isolates using the commercial Yeast DNA Preparation kit (Jena Bioscience, GE), following the instructions by the company. The DNA was amplified with the oligonucleotides CandF (5´-AGCTTGCGTTGATTACGTCCCTGCCC-3´) and CandR (5´-TTCACTCGCCGCTACTAAGGCAATCCC-3´), which identify, based on the size of the amplicon,
C. albicans (850 bp),
C. glabrata (1000 bp),
C. tropicalis (790 bp),
C. parapsilosis (731 bp),
C. krusei (800 bp),
C. guilliermondii (1100 bp),
C. lusitaniae (590 bp) and
C. dubliniensis (810 bp)[
28]. As positive controls, DNAs from the reference strains of
C. albicans ATCC 10231 and
C. glabrata ATCC 90030 were used. [
27]
2.5. Perinatal results
The digital record of the patients with positive results for Candida was reviewed to see the maternal, fetal, and neonatal results at the end of the pregnancy (preterm delivery, premature rupture of membranes, small for gestational age, and perinatal mortality).
2.6. Statistic analysis
The possible associations between vaginal colonization by Candida and perinatal complications were determined using Fisher's test (95% CI, p<0.05) using the GraphPad Prism 8.0 statistics software.
3. Results
During the study period, 981 pregnant patients were attended to in the HRAEI obstetric triage; 385 patients were admitted to the hospital, 112 for control of some maternal or fetal complication and 273 for termination of pregnancy, 15 patients were sent to another less complex unit for termination of pregnancy, of the remaining 581 patients, 221 attended for symptoms of vaginal infection, 58 for urinary tract infection, and 302 for non-urgent care, of which only 83 patients out of them met the inclusion criteria and agreed to participate in the study. The age of the patients was between 15 and 45 years old, with a gestational age of 13.2 to 42 weeks. The number of gestation was from 1 to 5. The BMI of the patients ranged from 18.5 to 44.8 Kg/m
2 (
Table 3).
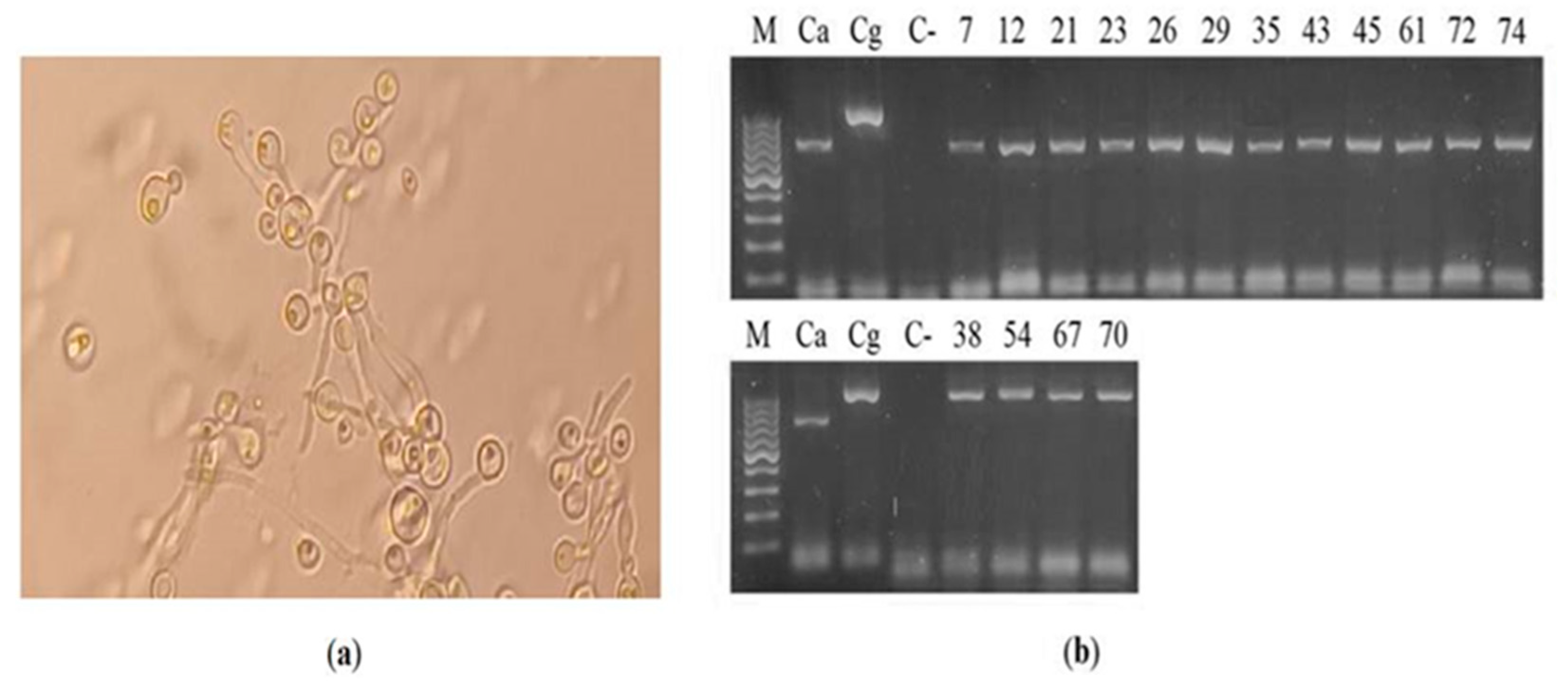
Out of the cultures of the 83 cervicovaginal samples, bacterial colonies were observed in 45 (54.2%), yeast-like colonies developed in 16 (19.3%) (7, 12, 21, 23, 26, 29, 35, 38, 43, 45 , 54, 61, 67, 70, 72, 74), and in 22 (26.5%) no development of microorganisms was observed (
Table 3). Regarding the 16 yeast isolates, 12 (75.0%) developed germ tubes (7, 12, 21, 23, 26, 29, 35, 43, 45, 61, 72, 74), suggestive of the species
C. albicans, while 4 (25%) corresponded to
Candida non-albicans (
Figure 1a). Similarly, amplification with Cand oligonucleotides generated an 850 bp amplicon in 12 samples, while an amplicon of 1000 bp was obtained in the four remaining samples, which corresponds to the identification of the species
C. albicans and
C. glabrata, respectively (
Figure 1b).
Regarding antimicrobial susceptibility (
Table 4), eleven of the samples positive for
C. albicans are sensitive to fluconazole and only one was resistant; all the samples positive for
C. glabrata were resistant to fluconazole.
According to the antecedents in the clinical history, all the patients presented uncomplicated vulvovaginal candidiasis, there were no recurrent or complicated ones.
The perinatal results of the positive group for
Candida and of the patients who had no development in the cultures for preterm delivery, premature rupture of membranes, small for gestational age, and perinatal mortality are described in
Table 5 and
Table 6.
In the group of patients with Candida, the maternal age range was from 15 to 36 years old, of which 7 were adolescents and 2 with advanced maternal age, with an average of 2 gestations, the range of body mass index was from 19.3 to 42.4, of which 4 were overweight and 7 obese. Regarding perinatal complications, one presented chorioamnionitis and 2 neonates were small for gestational age. The patients who had small neonates had a history with epilepsy and the other with anemia.
In the patients that did not develop in the cultures, the age range was from 18 to 45 years old (2 adolescents and 2 with advanced maternal age), with 2 pregnancies on average, the range of body mass index was from 19 to 36.8 (7 overweight and 10 obese), of the perinatal complications, one presented premature rupture of membranes and two small neonates for gestational age. No data were found in the medical record of the patients with a small-for-gestational-age fetus that could be associated with this outcome.
4. Discussion
Epidemiological reviews have shown that at least 75% of all women will present a picture of vulvovaginal candidiasis (VVC) during their lives and that it will be associated with pregnancy between 9 and 55%; in this study the frequency of colonization was 19.3 % [6.24].
Regarding the etiology, the species
C. albicans is the most frequent; however, other emerging species such as
C.glabrata,
C.parapsilosis and
C. tropicalis may be present, in this work 75% were
C. albicans and 25%
C. non-albicans. [
6,
24]
Traditionally, it was considered that the fetoplacental unit was free of germs and that the newborn's first exposure to microorganisms occurred during delivery, and that any microbial growth in the amniotic fluid or placenta originated in the lower genital tract, leading to poor perinatal outcomes (preterm labor, congenital cutaneous candidiasis, neonatal candidiasis, chorioamnioitis, and premature delivery), although it has recently been shown that the bacteria break throug the intact maternal-fetal membranes without causing any consequences. When analyzing adverse perinatal outcomes in this study, a higher frequency was not identified in patients with VVC, compared to healthy pregnant women, which is consistent with the systematic review and meta-analysis by Schuster et al. and may be explained by the fact that complex changes in the maternal immune system protect the pairing (mother-fetus) from infections by favoring the development of fetal immunity, avoiding rejection of the fetus and its attachments by the mother. In addition, the microbiome of the maternal genital tract regulates this immunitary behavior, generating a greater tolerance to microorganisms, through an increase in anti-inflammatory cytokines, the initiation of tolerance to endotoxins, and the suppression of autophagy, which leads to a downward modulation of the immune response or as referred by Farr et al. and Sust et al. that a positive result in the culture is a sign of colonization but not of infection and therefore it is asymptomatic (He et al.), so that it is important to differentiate between colonization and infection. Colonization has been described as the presence of
Candida in the intact vaginal tract of immunocompetent individuals that are generally unrelated to symptoms and when colonization progresses to infection frequently manifests by vaginal itching, burning, pain, dysuria, flushing, dyspareunia, and vaginal discharge (Chatzivasileiou and Vyzantiadis). On the other hand, it is known that any alteration in the vaginal microbiota can lead to an increase in proinflammatory cytokines with induction of the inflammatory cascade and adverse pregnancy outcomes such as preterm labor, premature rupture of membranes, preeclampsia, spontaneous abortion, restriction in the fetal growth, low birth weight, fetal death and neonatal sepsis [
6,
10,
12,
13,
18,
19,
23,
24,
28,
29].
As a result of the reduced estrogenization of the vagina at the extremes of life (before menarche and postmenopause, in the absence of hormone replacement therapy), the probability of colonization by
Candida is lower. In this study, it is striking that the frequency of VVC in adolescents (43.7%) versus healthy patients (9%), probably due to high estrogen levels that generate an increase in glycogen content in vaginal fluid and contrary to what was reported by Chatzivasileiou - Vyzantiadis, Sasani et al. and Bender et al., where adolescence is associated with a high resistance to vaginal
Candida infection despite the fact that sexual activity in this age group predisposes to a higher vaginal fungal load and that the age range with the highest number of infected is between 25 and 34 years of age. [
10,
12,
13,
18,
19,
23,
24].
Even though the Center for Disease Control (CDC) recommendations for first-line treatment for vulvovaginal candidiasis is fluconazole, in our study,
C. glabrata species were not sensitive to this antifungal.[
7]
Finally, as a secondary analysis, the number of patients who tested positive for bacteria (54.2%) draws attention; this will be the subject of another study, considering the same perinatal complications, since it is described that the presence of
C. albicans in the vagina can generate dysbiosis due to the presence of virulence factors, as well as the production of proteolytic enzymes that alter vaginal immunity [
1,
18,
23,
29].
The limitation of this study for the recruitment of a greater number of patients is related to the fact that the hospital, due to its level of complexity, can only monitor high-risk pregnant women.
5. Conclusions
No association was found between colonization by Candida spp. and the number of pregnancies, gestational age, body mass index or perinatal results. However, it was found that age (adolescence) may be a risk factor for the development of vulvovaginitis during pregnancy. Fluconazole is a good therapeutic option for vulvovaginal candidiasis caused by C. albicans and not for those caused by C. glabrata, in our study population.
Author Contributions
Conceptualization: T. R.-L; Methodology: T. R.-L; R.P.-A.; C.E.P.-S.; X.R.-M.; E. C.-T.; E.G.-S.; Formal Analysis: T. R.-L; E.G.-S.; Data curation: R.P.-A.; Writing – original draft; T. R.-L ; G.A.-A.; E. C.-T.; C.E.P.-S.; X.R.-M.; Project administration: T. R.-L.;. G.A.-A.;
Funding
This research did not receive external funding.
Institutional Review Board Statement
Project approved by the Teaching and Research Committee of Hospital Regional de Alta Especialidad de Ixtapaluca.
Data Availability Statement
The patient database is available at the Research Unit of the Hospital Regional de Alta Especialidad de Ixtapaluca and in the digital clinical record.
Acknowledgments
Professor Alejandro Vázquez Ubaldo for the translation of the manuscript.
Conflicts of Interest
None.
References
- Chee WJY, Chew SY, Than LTL. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Wells JS, Chandler R, Dunn A, Brewster G. The Vaginal Microbiome in U.S. Black Women: A Systematic Review. J Womens Health (Larchmt). 2020, 29, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Hotkani ZG, Ghaedmohammadi S, Mozdoori N. Meta-analysis of race and age influence on the vaginal microbiome in pregnant and nonpregnant healthy women. Future Microbiol. 2022, 17, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Chen X, Lu Y, Chen T, Li R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front Cell Infect Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Bagga R, Arora P. Genital Micro-Organisms in Pregnancy. Front Public Health. 2020, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Nyirjesy P, Brookhart C, Lazenby G, Schwebke J, Sobel JD. Vulvovaginal Candidiasis: A Review of the Evidence for the 2021 Centers for Disease Control and Prevention of Sexually Transmitted Infections Treatment Guidelines. Clin Infect Dis. 2022, 74 (Suppl. 2), S162–S168. [Google Scholar] [CrossRef] [PubMed]
- Juliana NCA, Suiters MJM, Al-Nasiry S, Morré SA, Peters RPH, Ambrosino E. The Association Between Vaginal Microbiota Dysbiosis, Bacterial Vaginosis, and Aerobic Vaginitis, and Adverse Pregnancy Outcomes of Women Living in Sub-Saharan Africa: A Systematic Review. Front Public Health. 2020, 8, 567885. [Google Scholar] [CrossRef]
- Disha T, Haque F. Prevalence and Risk Factors of Vulvovaginal Candidosis during Pregnancy: A Review. Infect Dis Obstet Gynecol. 2022, 2022, 6195712. [Google Scholar] [CrossRef]
- Schuster HJ, de Jonghe BA, Limpens J, Budding AE, Painter RC. Asymptomatic vaginal Candida colonization and adverse pregnancy outcomes including preterm birth: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020, 2, 100163. [Google Scholar] [CrossRef] [PubMed]
- Maki Y, Fujisaki M, Sato Y, Sameshima H. Candida Chorioamnionitis Leads to Preterm Birth and Adverse Fetal-Neonatal Outcome. Infect Dis Obstet Gynecol. 2017, 2017, 9060138. [Google Scholar] [CrossRef]
- Farr A, Effendy I, Frey Tirri B, Hof H, Mayser P, Petricevic L, Ruhnke M, Schaller M, Schaefer APA, Sustr V, Willinger B, Mendling W. Guideline: Vulvovaginal candidosis (AWMF 015/072, level S2k). Mycoses. 2021, 64, 583–602. [Google Scholar] [CrossRef] [PubMed]
- He Y, Tang R, Deng J, Cai T, He P, Wu J, Cao Y. Effects of oestrogen on vulvovaginal candidosis. Mycoses. 2022, 65, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Donders G, Sziller IO, Paavonen J, Hay P, de Seta F, Bohbot JM, Kotarski J, Vives JA, Szabo B, Cepuliené R, Mendling W. Management of recurrent vulvovaginal candidosis: Narrative review of the literature and European expert panel opinion. Front Cell Infect Microbiol. 2022, 12, 934353. [Google Scholar] [CrossRef]
- Zheng N, Guo R, Wang J, Zhou W, Ling Z. Contribution of Lactobacillus iners to Vaginal Health and Diseases: A Systematic Review. Front Cell Infect Microbiol. 2021, 11, 792787. [Google Scholar] [CrossRef]
- Peelen MJ, Luef BM, Lamont RF, de Milliano I, Jensen JS, Limpens J, Hajenius PJ, Jørgensen JS, Menon R; PREBIC Biomarker Working Group 2014–2018. The influence of the vaginal microbiota on preterm birth: A systematic review and recommendations for a minimum dataset for future research. Placenta. 2019, 79, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Gudnadottir U, Debelius JW, Du J, Hugerth LW, Danielsson H, Schuppe-Koistinen I, Fransson E, Brusselaers N. The vaginal microbiome and the risk of preterm birth: a systematic review and network meta-analysis. Sci Rep. 2022, 12, 7926. [Google Scholar] [CrossRef]
- Sustr V, Foessleitner P, Kiss H, Farr A. Vulvovaginal Candidosis: Current Concepts, Challenges and Perspectives. J Fungi (Basel). 2020, 6, 267. [Google Scholar] [CrossRef]
- Chatzivasileiou P, Vyzantiadis TA. Vaginal yeast colonisation: From a potential harmless condition to clinical implications and management approaches-A literature review. Mycoses. 2019, 62, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Solís-Arias MP, Moreno-Morales M, Dávalos-Tanaka M, Fernández-Martánez RF, Díaz Flores O, Arenas-Guzmán R. Colonización vaginal por Candida spp. Frecuencia y descripción de las especies aisladas en mujeres asintomáticas [Vaginal colonization by Candida spp. Frequency and description of the species isolated in asymptomatic women]. Ginecol Obstet Mex. 2014, 82, 1–8 Spanish PMID: 24701855. [Google Scholar] [PubMed]
-
List of Azole antifungals. (n.d.). Drugs.com. Retrieved May 3, 2023, from https://www.drugs.com/drug-class/azole-antifungals.html.
- Rotem R, Fishman B, Daniel S, Koren G, Lunenfeld E, Levy A. Risk of major congenital malformations following first-trimester exposure to vaginal azoles used for treating vulvovaginal candidiasis: a population-based retrospective cohort study. BJOG. 2018, 125, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Sasani E, Rafat Z, Ashrafi K, Salimi Y, Zandi M, Soltani S, Hashemi F, Hashemi SJ. Vulvovaginal candidiasis in Iran: A systematic review and meta-analysis on the epidemiology, clinical manifestations, demographic characteristics, risk factors, etiologic agents and laboratory diagnosis. Microb Pathog. 2021, 154, 104802. [Google Scholar] [CrossRef] [PubMed]
- Bender RA, Çalışkan Ş, Önal B, Aslancan R, Çalışkan E. Treatment methods for vulvovaginal candidiasis in pregnancy. J Mycol Med. 2021, 31, 101138. [Google Scholar] [CrossRef] [PubMed]
- Waikhom ND, Afeke I, Kwawu GS, Mbroh HK, Osei GY, Louis B, Deku JG, Kasu ES, Mensah P, Agede CY, Dodoo C, Asiamah EA, Tampuori J, Korbuvi J, Opintan JA. Prevalence of vulvovaginal candidiasis among pregnant women in the Ho municipality, Ghana: species identification and antifungal susceptibility of Candida isolates. BMC Pregnancy Childbirth. 2020, 20, 266. [Google Scholar] [CrossRef]
- López-Moreno A, Aguilera M. Vaginal Probiotics for Reproductive Health and Related Dysbiosis: Systematic Review and Meta-Analysis. J Clin Med. 2021, 10, 1461. [Google Scholar] [CrossRef] [PubMed]
- García-Salazar E, Acosta-Altamirano G, Betancourt-Cisneros P, Reyes-Montes MDR, Rosas-De-Paz E, Duarte-Escalante E, Sánchez-Conejo AR, Ocharan Hernández E, Frías-De-León MG. Detection and Molecular Identification of Eight Candida Species in Clinical Samples by Simplex PCR. Microorganisms. 2022, 10, 374. [Google Scholar] [CrossRef]
- Osman Mohamed A, Suliman Mohamed M, Hussain Mallhi T, Abdelrahman Hussain M, Ali Jalloh M, Ali Omar K, Omar Alhaj M, Makki Mohamed Ali AA. c. Prevalence of vulvovaginal candidiasis among pregnant women in Africa: A systematic review and meta-analysis. J Infect Dev Ctries. 2022, 16, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Lozada, T., Espinosa-Hernández, V.M., Frías-De-León, M.G. et al. Update of Vulvovaginal Candidiasis in Pregnant and Non-pregnant Patients. Curr Fungal Infect Rep. 2019, 13, 181–190. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).