Submitted:
14 May 2023
Posted:
15 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
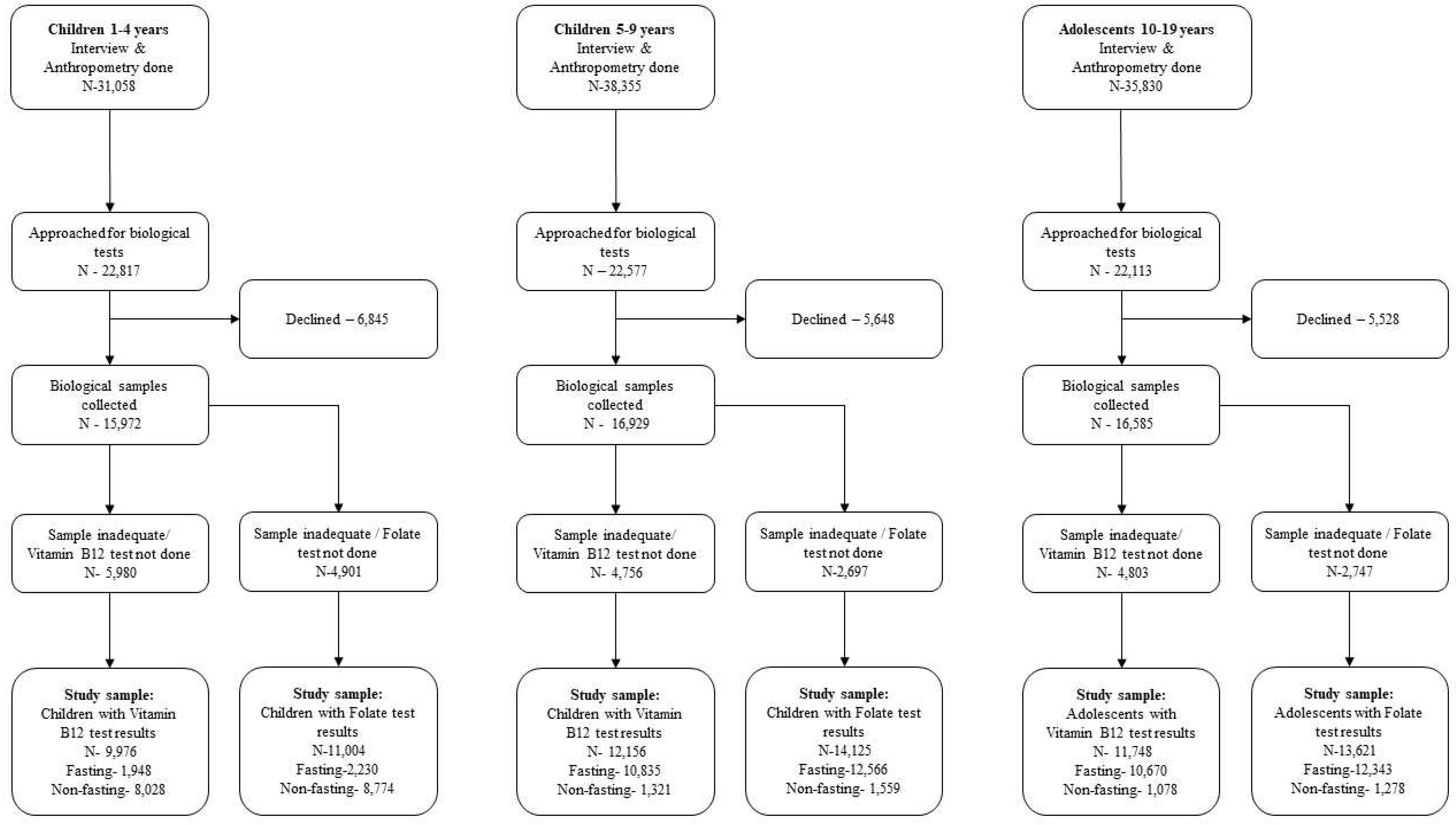
2.1. CNNS survey, serum B12 and erythrocyte FA analysis
2.2. Statistical analyses
3. Results
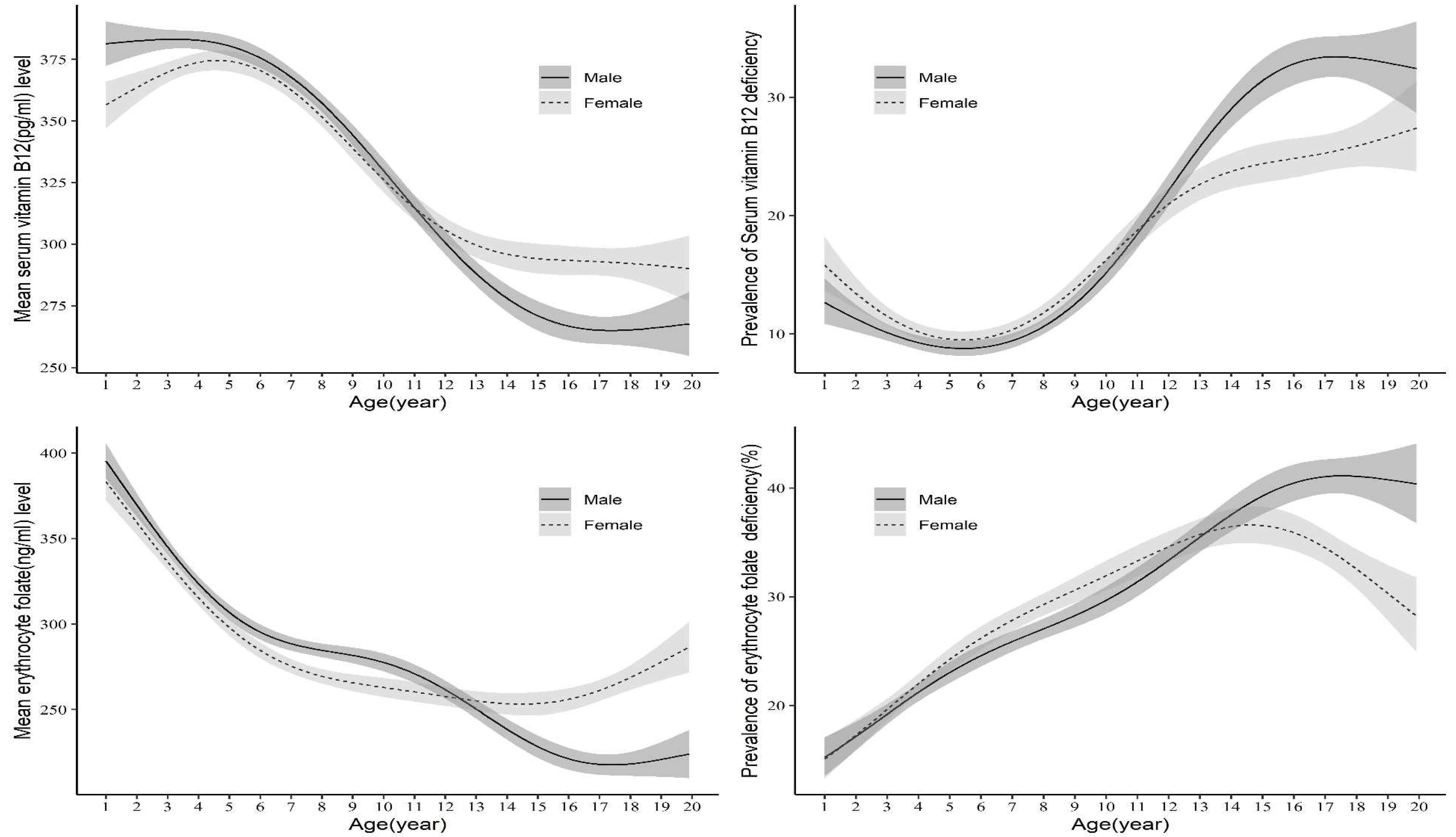
3.1. Serum B12 and erythrocyte FA concentration and prevalence of B12 and FA deficiency by age and sex
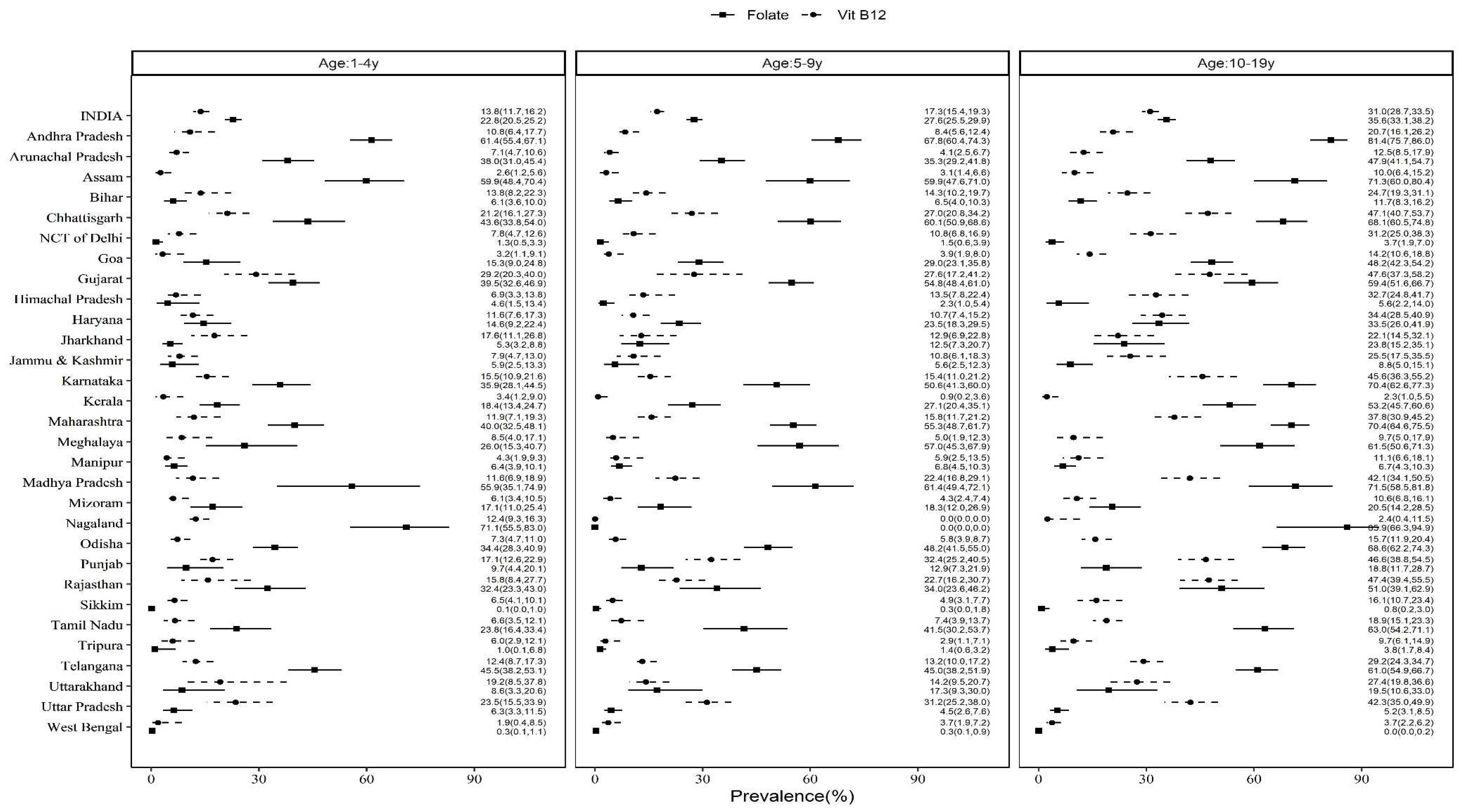
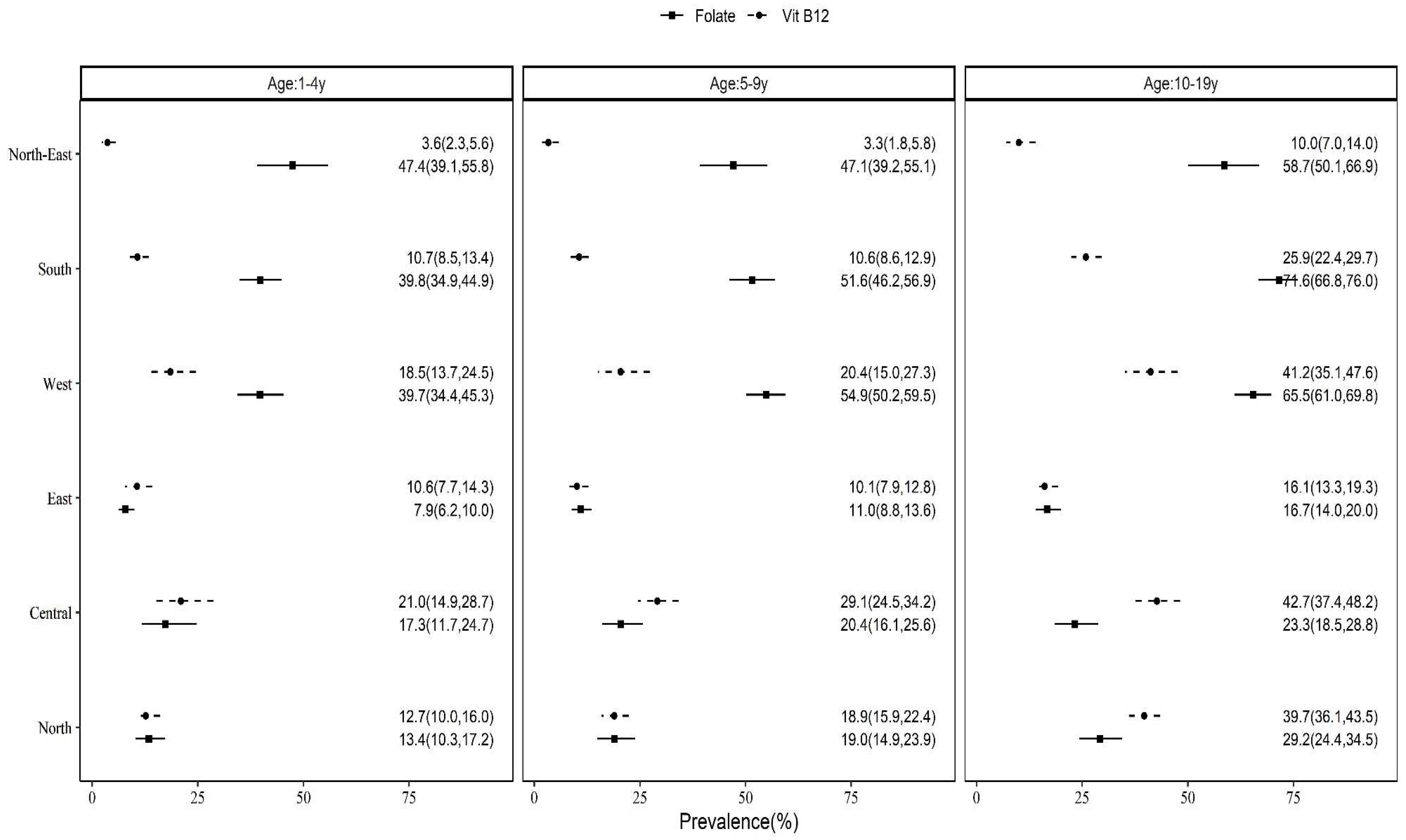
3.2. State-based, rural-urban and regional differences in prevalence of B12 and FA deficiencies
3.3. B12 and FA deficiency by socio-demography, WASH characteristics, undernutrition and morbidity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflict of Interest
Abbreviations
References
- Stover, P. J. Physiology of folate and vitamin B12 in health and disease. Nutr. Rev. 2004, 62(6 Pt 2), S3-12; discussion S13. [CrossRef]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, 3rd J.F.; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development-Folate Review. J. Nutr. 2015, 145, 1636S-1680S.
- Allen, L.H.; Miller, J.W.; Groot, L.D.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148(suppl_4), 1995S-2027S.
- Dwarkanath, P.; Barzilay, J.R.; Thomas, T.; Thomas, A.; Bhat, S.; Kurpad, A.V. High folate and low vitamin B-12 intakes during pregnancy are associated with small-for-gestational age infants in South Indian women: a prospective observational cohort study. Am. J. Clin. Nutr. 2013, 98, 1450-1458. [CrossRef]
- Puri, M.; Kaur, L.; Walia, G.K.; Mukhopadhhyay, R.; Sachdeva, M.P.; Trivedi, S.S.; Ghosh, P.K.; Saraswathy, K.N. MTHFR C677T polymorphism, folate, vitamin B12 and homocysteine in recurrent pregnancy losses: a case control study among North Indian women. J. Perinat. Med. 2013, 41, 549-554.
- Wong, E.; Molina-Cruz, R.; Rose, C.; Bailey, L.; Kauwell, G.P.A.; Rosenthal, J. Prevalence and Disparities in Folate and Vitamin B12 Deficiency Among Preschool Children in Guatemala. Matern. Child. Health. J. 2022, 26, 156-167. [CrossRef]
- Veena, S.R.; Krishnaveni, G.V.; Srinivasan, K.; Wills, A.K.; Muthayya, S.; Kurpad, A.V.; Yajnik, C.S.; Fall, C.H.D. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10- year-old children in South India. J. Nutr. 2010, 140, 1014-1022. [CrossRef]
- Sachdev, H.S.; Porwal, A.; Sarna, A.; Acharya, R.; Ramesh, S.; Kapil, U.; Kurpad, A.V. Intraindividual double-burden of anthropometric undernutrition and metabolic obesity in Indian children: a paradox that needs action. Eur. J. Clin. Nutr. 2021, 75, 1205-1217. [CrossRef]
- Gadgil, M.; Joshi, K.; Pandit, A.; Otiv, S.; Joshi, R.; Brenna, J.T.; Patwardhan, B. Imbalance of folic acid and vitamin B12 is associated with birth outcome: an Indian pregnant women study. Eur. J. Clin. Nutr. 2014, 68, 726-729. [CrossRef]
- Sivaprasad, M.; Shalini, T.; Balakrishna, N.; Sudarshan, M.; Lopamudra, P.; Suryanarayana, P.; Arlappa, N.; Ravikumar, B.P.; Radhika, M.S.; Reddy, G.B. Status of Vitamin B12 and Folate among the Urban Adult Population in South India. Ann. Nutr. Metab. 2016, 68, 94-102. [CrossRef]
- Sivaprasad, M.; Shalini, T.; Reddy, P.Y.; Seshacharyulu, M.; Madhavi, G.; Kumar, B.N.; Reddy, G.B. Prevalence of vitamin deficiencies in an apparently healthy urban adult population: assessed by subclinical status and dietary intakes. Nutrition. 2019, 63–64, 106–113. [CrossRef]
- Yajnik, C.S.; Deshpande, S.S.; Jackson, A.A.; Refsum, H.; Rao, S.; Fisher, D.J.; Bhat, D.S.; Naik, S.S.; Coyaji, K.J.; Joglekar, C.V.; et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008, 51, 29-38. [CrossRef]
- Strand, TA.; Taneja, S.; Ueland, P.M.; Refsum, H.; Bahl, R.; Schneede, J.; Sommerfelt, H.; Bhandari, N. Cobalamin and folate status predicts mental development scores in North Indian children 12-18 mo of age. Am. J. Clin. Nutr. 2013, 97, 310-317. [CrossRef]
- Kapil, U.; Sareen, N. Prevalence of ferritin, folate and vitamin B12 deficiencies amongst children in 5-18 years of age in Delhi. Indian. J. Pediatr. 2014, 8, 312.
- Shalini, T.; Sivaprasad, M.; Balakrishna, N.; Madhavi, G.; Radhika, M.S.; Boiroju, N.K.; Pullakhandam, R.; Reddy, G.B. Micronutrient intakes and status assessed by probability approach among the urban adult population of Hyderabad city in South India. Eur. J. Nutr. 2019, 58, 3147-3159. [CrossRef]
- Chakraborty, S.; Chopra, M.; Mani, K.; Giri, A.K.; Banerjee, P.; Sahni, N.S.; Siddhu, A.; Tandon, N.; Bharadwaj, D. Prevalence of vitamin B12 deficiency in healthy Indian school-going adolescents from rural and urban localities and its relationship with various anthropometric indices: a cross-sectional study. J. Hum. Nutr. Diet. 2018, 31, 513-522. [CrossRef]
- Comprehensive National Nutrition Survey (CNNS). National Report, Ministry of Health and Family Welfare (MoHFW): Government of India, New Delhi, 2019.
- National Family Health Survey (NFHS-4). International Institute for Population Sciences (IIPS) and ICF, Mumbai: IIPS, India, 2015-2016.
- World Health Organization (WHO)/ United Nations International Children's Emergency Fund (UNICEF). Joint Monitoring Programme (JMP) for Water Supply, Sanitation and Hygiene, JMP Methodology, 2018. Update & SDG Baselines. https:// washdata. org/ sites/ default/ files/documents/ reports/ 2018- 04/ JMP- 2017- update- methodology. pdf. Accessed 3 Mar 2021.
- World Health Organization (WHO). World Health Organization child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development; WHO: Geneva, Switzerland, 2006.
- Sachdev, H.S.; Porwal, A.; Acharya, R.; Ashraf, S.; Ramesh, S.; Khan, N.; Kapil, U.; Kurpad, A.V.; Sarna, A. Haemoglobin thresholds to define anaemia in a national sample of healthy children and adolescents aged 1-19 years in India: a population-based study. Lancet. Glob. Health. 2021, 9, e822-e831. [CrossRef]
- de Benoist, B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food. Nutr. Bull. 2008, 29(2 Suppl), S238-244.
- World Health Organisation (WHO). Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity; WHO: Geneva, Switzerland, 2011. Available from: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf?ua=1. Accessed 3 Mar 2021.
- Young, M.F.; Guo, J.; Williams, A.; Whitfield, K.C.; Nasrin, S.; Kancherla, V.; Suchdev, P.S.; Crider, K.S.; Pfeiffer, C.M.; Serdula, M. Interpretation of vitamin B-12 and folate concentrations in population-based surveys does not require adjustment for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2020, 111, 919-926. [CrossRef]
- Ulak, M.; Chandyo, R.K.; Adhikari, R.K; Sharma, P.R; Sommerfelt, H.; Refsum, H.; Strand, T.A. Cobalamin and folate status in 6 to 35 months old children presenting with acute diarrhea in Bhaktapur, Nepal. PLoS One. 2014, 9, e90079.
- Shahab-Ferdows, S.; Engle-Stone, R.; Hampel, D.; Ndjebayi, A.O.; Nankap, M.; Brown, K.H.; Allen, L.H. Regional, Socioeconomic, and Dietary Risk Factors for Vitamin B-12 Deficiency Differ from Those for Folate Deficiency in Cameroonian Women and Children. J. Nutr. 2015, 145, 2587-2595. [CrossRef]
- Ng'eno, B.N.; Perrine, C.G.; Whitehead, R.D.; Subedi, G.R.; Mebrahtu, S.; Dahal, P.; Jefferds, M.E.D. High Prevalence of Vitamin B12 Deficiency and No Folate Deficiency in Young Children in Nepal. Nutrients. 2017, 9, 72. [CrossRef]
- Awasthi, S.; Kumar, D.; Mahdi, A.A.; Agarwal, G.G.; Pandey, A.K.; Parveen, H.; Singh, S.; Awasthi, R.; Pande, H.; Anish, T.S.; et al. Prevalence of specific micronutrient deficiencies in urban school going children and adolescence of India: A multicenter cross-sectional study. PLoS One. 2022, 17, e0267003. [CrossRef]
- Cuevas-Nasu, L.; Mundo-Rosas, V.; Shamah-Levy, T.; Méndez-Gómez Humaran, I.; Avila-Arcos, M.A.; Rebollar-Campos, M.R.; Villalpando, S. Prevalence of folate and vitamin B12 deficiency in Mexican children aged 1 to 6 years in a population-based survey. Salud. Publica. Mex. 2012, 54, 116-124. [CrossRef]
- Garcia-Casal, M.N.; Osorio, C.; Landaeta, M.; Leets, I.; Matus, P.; Fazzino, F.; Marcos, E. High prevalence of folic acid and vitamin B12 deficiencies in infants, children, adolescents and pregnant women in Venezuela. Eur. J. Clin. Nutr. 2005, 59, 1064-1070. [CrossRef]
- McLean, E.; de Benoist, B.; Allen, L.H. (2008) Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull 29(2 Suppl): S38-51.
- Siekmann, J.H.; Allen, L.H.; Bwibo, N.O.; Demment, M.W.; Murphy, S.P.; Neumann, C.G. Kenyan school children have multiple micronutrient deficiencies, but increased plasma vitamin B-12 is the only detectable micronutrient response to meat or milk supplementation. J. Nutr. 2003, 133(11 Suppl 2), 3972S-3980S. [CrossRef]
- Herrmann, W.; Obeid, R. Causes and early diagnosis of vitamin B12 deficiency. Dtsch. Arztebl. Int. 2008, 105, 680-685.
- Hvas, A.M.; Nexo, E. Holotranscobalamin--a first choice assay for diagnosing early vitamin B deficiency? J. Intern. Med. 2005, 257, 289-298.
- Nexo, E.; Hoffmann-Lucke, E. Holotranscobalamin, a marker of vitamin B-12 status: analytical aspects and clinical utility. Am. J. Clin. Nutr. 2011, 94, 359S-365S. [CrossRef]
- Bawaskar, H. S.; Bawaksar, P.H. Profile of Vitamin B12 and Vitamin D in Rural School children in Raigad, India. Indian. Pediatr. 2020, 57, 871.
- Sarna, A.; Porwal, A.; Ramesh, S.; Agrawal, P.K.; Acharya, R.; Johnston, R.; Khan, N.; Sachdev, H.P.S.; Madhavan Nair, K.; Ramakrishnan, L.; et al. Characterisation of the types of anaemia prevalent among children and adolescents aged 1-19 years in India: a population-based study. Lancet. Child Adolesc. Health. 2020, 4, 515-525. [CrossRef]
- Scott, S.; Lahiri, A.; Sethi, V.; Wagt, A. de.; Menon, P.; Yadav, K.; Varghese, M.; Joe, W.; Vir, S.C.; Nguyen, P.H. Anaemia in Indians aged 10-19 years: Prevalence, burden and associated factors at national and regional levels. Matern. Child. Nutr. 2022, 18, e13391. [CrossRef]
- Villamor, E.; Mora-Plazas, M.; Forero, Y.; Lopez-Arana, S.; Baylin, A. Vitamin B-12 status is associated with socioeconomic level and adherence to an animal food dietary pattern in Colombian school children. J. Nutr. 2008, 138, 1391-8. [CrossRef]
| Characteristics | 1-4 years | 5-9 years | 10-19 years | ||||
|---|---|---|---|---|---|---|---|
| Vitamin B12 (n=9,976) % (95% CI) |
Folate (n=11,004) % (95% CI) |
Vitamin B12 (n=12,156) % (95% CI) |
Folate (n=14,125) % (95% CI) |
Vitamin B12 (n=11,748) % (95% CI) |
Folate (n=13,621) % (95% CI) |
||
| Age in years | Mean (95% CI) | 2.78 (2.74-2.83) | 2.78 (2.74-2.83) | 7.02 (6.97-7.06) | 7.02 (6.98-7.07) | 14.3 (14.2-14.4) | 14.3 (14.2-14.4) |
| Sex | Boys | 52.9 (50.3-55.4) | 52.5 (50.0-55.0) | 51.6 (49.8-53.3) | 51.2 (49.4-52.9) | 50.6 (48.6-52.5) | 50.6 (48.8-52.4) |
| Girls | 47.1 (44.6-49.7) | 47.5 (45.0-50.0) | 48.4 (46.7-50.2) | 48.8 (47.1-50.6) | 49.4 (47.5-51.4) | 49.4 (47.6-51.2) | |
| Residence | Urban | 24.9 (21.7-28.4) | 25.7 (22.5-29.1) | 23.6 (20.7-26.8) | 25.1 (22.1-28.2) | 25.2 (22.1-28.6) | 25.7 (22.6-29.0) |
| Rural | 75.1 (71.6-78.3) | 74.3 (70.9-77.5) | 76.4 (73.2-79.3) | 74.9 (71.8-77.9) | 74.8 (71.4-77.9) | 74.3 (71.0-77.4) | |
| Wealth Index | Poorest | 15.9 (13.8-18.3) | 15.8 (13.7-18.1) | 18.1 (16.2-20.3) | 18.0 (15.8-20.4) | 17.8 (15.5-20.4) | 18.3 (16.0-20.8) |
| Poor | 21.2 (18.5-24.1) | 20.5 (17.9-23.4) | 21.3 (19.4-23.3) | 20.2 (18.5-21.9) | 20.5 (18.8-22.3) | 20.6 (18.9-22.3) | |
| Middle | 22.3 (20.4-24.3) | 21.7 (19.9-23.5) | 21.5 (19.9-23.3) | 21.0 (19.4-22.5) | 21.4 (19.8-23.1) | 20.3 (18.8-21.9) | |
| Rich | 20.8 (18.9-22.9) | 21.7 (19.7-23.9) | 21.0 (19.4-22.7) | 21.6 (19.9-23.3) | 20.9 (19.3-22.7) | 21.0 (19.4-22.8) | |
| Richest | 19.8 (17.7-22.0) | 20.3 (18.3-22.5) | 18.0 (16.4-19.8) | 19.3 (17.6-21.1) | 19.3 (17.5-21.2) | 19.8 (17.9-21.8) | |
| Mother’s Schooling | Primary | 34.3 (31.7-36.9) | 34.6 (31.9-37.3) | 47.8 (45.4-50.3) | 47.9 (45.6-50.3) | 16.3 (14.3-18.4) | 15.9 (14.1-18.0) |
| Secondary | 44.3 (41.8-46.8) | 43.6 (41.3-46.0) | 40.1 (38.0-42.3) | 39.8 (37.8-41.8) | 68.7 (66.2-71.1) | 69.3 (67.0-71.5) | |
| Higher Secondary | 10.8 (9.3-12.5) | 11.4 (9.9-13.1) | 6.7 (6.0-7.6) | 6.9 (6.2-7.8) | 9.7 (7.7-12.1) | 9.5 (7.7-11.6) | |
| Graduation and above | 10.7 (9.2-12.4) | 10.4 (9.0-12.0) | 5.3 (4.7-6.1) | 5.4 (4.8-6.1) | 5.3 (4.4-6.3) | 5.3 (4.5-6.3) | |
| Father’s Occupation | Professional | 7.9 (6.8-9.2) | 8.5 (7.4-9.9) | 9.4 (8.1-10.8) | 9.5 (8.3-10.8) | 10.0 (8.5-11.7) | 9.4 (8.0-10.9) |
| Sales and services | 26.8 (24.6-29.1) | 28.0 (25.7-30.4) | 23.1 (21.4-25.0) | 24.2 (22.3-26.1) | 24.4 (22.7-26.2) | 24.1 (22.5-25.8) | |
| Manual, Agriculture | 51.5 (48.8-54.1) | 50.4 (47.6-53.2) | 54.8 (52.2-57.4) | 53.8 (51.4-56.2) | 51.3 (48.8-53.7) | 52.4 (50.0-54.7) | |
| Others | 13.8 (11.8-16.0) | 13.1 (11.3-15.1) | 12.7 (11.1-14.5) | 12.5 (11.0-14.2) | 14.3 (12.5-16.4) | 14.2 (12.4-16.1) | |
| Child schooling | Yes | - | - | 92.2 (91.1-93.2) | 92.1 (91.1-93.0) | 80.7 (78.9-82.4) | 80.9 (79.2-82.5) |
| No | - | - | 7.8 (6.8-8.9) | 7.9 (7.0-8.9) | 19.3 (17.6-21.1) | 19.1 (17.5-20.8) | |
| Stunting | No Stunting (HAZ < -2SD) | 64.4 (62.1-66.7) | 64.5 (62.3-66.7) | 79.3 (77.7-80.8) | 78.6 (76.9-80.3) | 73.0 (71.0-74.9) | 73.4 (71.6-75.2) |
| Moderate (HAZ: -3 to -2SD) | 24.0 (22.2-26.0) | 23.3 (21.6-25.0) | 15.5 (14.3-16.8) | 16.4 (15.1-17.8) | 21.3 (19.6-23.1) | 20.7 (19.2-22.3) | |
| Severe (HAZ < -3SD) | 11.5 (10.1-13.2) | 12.2 (10.7-13.9) | 5.1 (4.3-6.1) | 5.0 (4.2-5.8) | 5.7 (4.9-6.6) | 5.9 (5.1-6.7) | |
| Underweight | Not present (WAZ < -2SD) | 64.8 (62.0-67.6) | 65.1 (62.5-67.6) | - | - | - | - |
| Moderate (WAZ:-3 to -2SD) | 26.2 (23.7-28.8) | 26.7 (24.3-29.2) | - | - | - | - | |
| Severe (WAZ < -3SD) | 9.0 (7.8-10.3) | 8.2 (7.2-9.4) | - | - | - | - | |
| Wasting/Thinness | Not present (WHZ < -2SD) | 84.0 (82.1-85.6) | 85.0 (83.5-86.5) | 76.4 (74.8-78.0) | 76.6 (75.2-78.0) | 75.5 (73.7-77.2) | 75.7 (74.0-77.2) |
| Moderate (WHZ:-3 to -2SD) | 12.2 (10.8-13.8) | 11.6 (10.4-12.9) | 18.5 (17.1-19.9) | 18.4 (17.1-19.8) | 18.1 (16.6-19.7) | 17.9 (16.6-19.4) | |
| Severe (WHZ < -3SD) | 3.8 (3.0-4.8) | 3.4 (2.8-4.1) | 5.1 (4.3-6.0) | 5.0 (4.3-5.8) | 6.4 (5.7-7.3) | 6.4 (5.6-7.3) | |
| Drinking water source | Piped & Improved | 85.0 (82.4-87.3) | 85.2 (82.6-87.5) | 84.9 (82.5-87.0) | 85.2 (82.9-87.2) | 85.6 (83.5-87.5) | 86.2 (84.2-87.9) |
| Non-piped & Improved | 8.9 (7.0-11.2) | 9.2 (7.4-11.3) | 8.2 (6.6-10.2) | 8.1 (6.7-9.9) | 8.1 (6.8-9.6) | 7.7 (6.5-9.1) | |
| Unimproved | 6.1 (4.8-7.6) | 5.6 (4.3-7.4) | 7.0 (5.7-8.4) | 6.7 (5.3-8.4) | 6.3 (5.1-7.9) | 6.1 (5.0-7.6) | |
| Handwashing | Basic | 50.3 (47.5-53.1) | 52.4 (49.7-55.2) | 46.8 (44.1-49.5) | 49.3 (46.7-51.9) | 47.8 (45.3-50.3) | 48.7 (46.2-51.3) |
| Limited | 36.1 (33.4-38.8) | 33.8 (31.3-36.5) | 39.4 (36.6-42.3) | 37.2 (34.6-39.8) | 35.5 (33.1-38.0) | 34.7 (32.5-37.1) | |
| No facility | 13.6 (11.7-15.9) | 13.7 (11.8-15.9) | 13.8 (12.1-15.8) | 13.5 (11.8-15.4) | 16.7 (14.7-19.0) | 16.5 (14.4-18.8) | |
| Sanitation | Improved & Not shared | 43.8 (40.5-47.2) | 44.1 (41.0-47.1) | 39.7 (37.2-42.3) | 40.5 (38.0-43.0) | 47.8 (45.3-50.4) | 47.1 (44.5-49.7) |
| Improved & Shared | 12.2 (10.8-13.7) | 13.0 (11.6-14.6) | 12.3 (11.0-13.8) | 12.3 (11.0-13.8) | 8.7 (7.7-9.8) | 9.0 (8.0-10.1) | |
| Unimproved | 44.0 (40.1-48.0) | 43.0 (39.3-46.7) | 48.0 (44.8-51.2) | 47.2 (44.1-50.4) | 43.5 (40.6-46.4) | 43.9 (41.1-46.8) | |
| History of diarrhoea in the two weeks prior to survey | Yes | 15.0 (12.9-17.5) | 15.3 (13.3-17.6) | 9.4 (8.2-10.7) | 9.0 (8.0-10.2) | - | - |
| No | 85.0 (82.5-87.1) | 84.7 (82.4-86.7) | 90.6 (89.3-91.8) | 91.0 (89.8-92.0) | - | - | |
| History of fever in the two weeks prior to survey | Yes | 30.7 (28.3-33.2) | 31.5 (29.2-33.9) | 21.8 (19.8-24.0) | 22.2 (20.5-24.0) | - | - |
| No | 69.3 (66.8-71.7) | 68.5 (66.1-70.8) | 78.2 (76.0-80.2) | 77.8 (76.0-79.5) | - | - | |
| Sex | 1-4 years (n=9,976) | 5-9 years (n=12,156) | 10-19 years (n=11,748) | |||
|---|---|---|---|---|---|---|
| Vitamin B12 (pg/mL) Geometric mean (95% CI) |
Vitamin B12 deficiency % (95% CI) | Vitamin B12 (pg/mL) Geometric mean (95% CI) |
Vitamin B12 deficiency % (95% CI) | Vitamin B12 (pg/mL) Geometric mean (95% CI) |
Vitamin B12 deficiency % (95% CI) | |
| Boys | 310.3a (301.4-319.3) |
14.3a (11.4-17.7) |
297.3a (290.0-304.7) |
16.7a (14.7-18.9) |
241.6a (235.2-248.2) |
35.0a (31.8-38.3) |
| Girls | 313.3a (304.1-322.8) |
13.3a (10.9-16.2) |
291.7a (283.5-300.2) |
17.9a (15.3-20.8) |
257.1b (250.6-263.8) |
27.0b (24.4-29.7) |
| Total | 311.7* (305.0-318.5) |
13.8† (11.7-16.2) |
294.6# (288.2-301.0) |
17.3† (15.4-19.3) |
249.2$ (243.9-254.6) |
31.0‡ (28.7-33.5) |
| Sex | 1-4 years (n=11,004) | 5-9 years (n=14,125) | 10-19 years (n=13,621) | |||
|---|---|---|---|---|---|---|
| Folate (ng/mL) Geometric mean (95% CI) |
Folate deficiency % (95% CI) | Folate (ng/mL) Geometric mean (95% CI) |
Folate deficiency % (95% CI) | Folate (ng/mL) Geometric mean (95% CI) |
Folate deficiency % (95% CI) |
|
| Boys | 245.8a (233.4-258.9) |
22.6a (19.7-25.8) |
217.7a (208.0-228.0) |
27.7a (25.1-30.4) |
173.3a (164.4-182.6) |
38.3a (35.3-41.4) |
| Girls | 237.8a (225.1-251.1) |
22.9a (20.2-25.9) |
212.2a (201.8-223.2) |
27.6a (25.1-30.3) |
196.7b (186.6-207.3) |
32.9a (30.2-35.7) |
| Total | 241.9* (231.4-252.9) |
22.8† (20.5-25.2) |
215.0# (206.4-224.1) |
27.6‡ (25.5-29.9) |
184.5$ (176.3-193.0) |
35.6§ (33.1-38.2) |
| Characteristics | 1-4 years | 5-9 years | 10-19 years | ||||
|---|---|---|---|---|---|---|---|
| Vitamin B12 deficiency % (95% CI) | Folate deficiency % (95% CI) |
Vitamin B12 deficiency % (95% CI) | Folate deficiency % (95% CI) |
Vitamin B12 deficiency % (95% CI) | Folate deficiency % (95% CI) |
||
| Stunting | No Stunting (HAZ < -2SD) |
12.7a (10.2-15.8) |
22.9a (20.5-25.5) |
17.8a (15.8-20.0) |
28.1a (25.9-30.4) |
31.2a (28.8-33.7) |
37.2a (34.4-40.1) |
| Moderate (HAZ: -3 to -2SD) |
15.1a (12.2-18.5) |
23.5a (19.9-27.5) |
18.9a (15.2-23.2) |
27.2a (23.4-31.4) |
31.5a (26.3-37.3) |
31.8a (27.9-35.9) |
|
| Severe (HAZ < -3SD) |
18.9a (12.7-27.3) |
21.6a (16.8-27.4) |
8.2b (5.2-12.8) |
22.2a (16.7-28.8) |
27.6a (20.3-36.3) |
29.1a (23.5-35.5) |
|
| Wasting/Thinness | Not present (WAZ < -2SD) |
14.8a (12.3-17.6) |
22.8a (20.3-25.5) |
18.0a (15.9-20.3) |
27.4a (25.1-29.9) |
32.9a (30.0-35.9) |
35.8a (32.9-38.7) |
| Moderate (WAZ: -3 to -2SD) |
11.1a (8.2-14.8) |
21.5a (17.5-26.2) |
17.0a (13.7-20.8) |
29.1a (25.6-32.9) |
26.8ac (23.0-30.8) |
35.0a (31.1-39.1) |
|
| Severe (WAZ < -3SD) |
7.8a (4.7-12.7) |
24.0a (17.1-32.7) |
12.1a (8.0-18.0) |
26.4a (20.5-33.4) |
21.6bc (16.6-27.5) |
34.0a (28.4-40.1) |
|
| Underweight | Not present (WHZ < -2SD) |
13.6a (11.1-16.5) |
23.1a (20.5-25.9) |
- | - | - | - |
| Moderate (WHZ: -3 to -2SD) |
15.8a (12.2-20.2) |
22.6a (18.9-26.8) |
- | - | - | - | |
| Severe (WHZ < -3SD) |
11.8a (8.1-16.9) |
20.4a (16.5-24.9) |
- | - | - | - | |
| History of diarrhoea in the two weeks prior to survey | Yes | 19.1a (12.1-28.8) |
14.9a (11.5-19.1) |
23.8a (18.1-30.5) |
20.2a (15.8-25.5) |
- | - |
| No | 12.9a (11.1-15.0) |
24.2b (21.8-26.7) |
16.6a (14.8-18.6) |
28.4b (26.1-30.7) |
- | - | |
| History of fever in the two weeks prior to survey | Yes | 16.9a (11.6-23.9) |
19.6a (16.4-23.3) |
15.8a (12.5-19.8) |
22.8a (19.6-26.4) |
- | - |
| No | 12.5a (10.7-14.4) |
24.2a (21.5-27.1) |
17.7a (15.9-19.7) |
29.0b (26.7-31.4) |
- | - | |
| IFA | 1-4 years | 5-9 years | 10-19 years | |||
|---|---|---|---|---|---|---|
| Vitamin B12 deficiency % (95% CI) |
Folate deficiency % (95% CI) |
Vitamin B12 deficiency % (95% CI) |
Folate deficiency % (95% CI) |
Vitamin B12 deficiency % (95% CI) |
Folate deficiency % (95% CI) |
|
| Yes | 11.2a (7.2-16.9) |
14.6a (9.9-21.0) |
18.1a (13.8-23.2) |
28.9a (23.4-35.1) |
28.5a (23.6-34.0) |
38.9a (33.2-45.0) |
| No | 14.0a (11.7-16.7) |
23.3b (21.0-25.7) |
17.2a (15.4-19.2) |
27.5a (25.3-29.9) |
31.2a (28.7-33.7) |
35.5a (32.9-38.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





