3.3. Characterization Data for Products 3
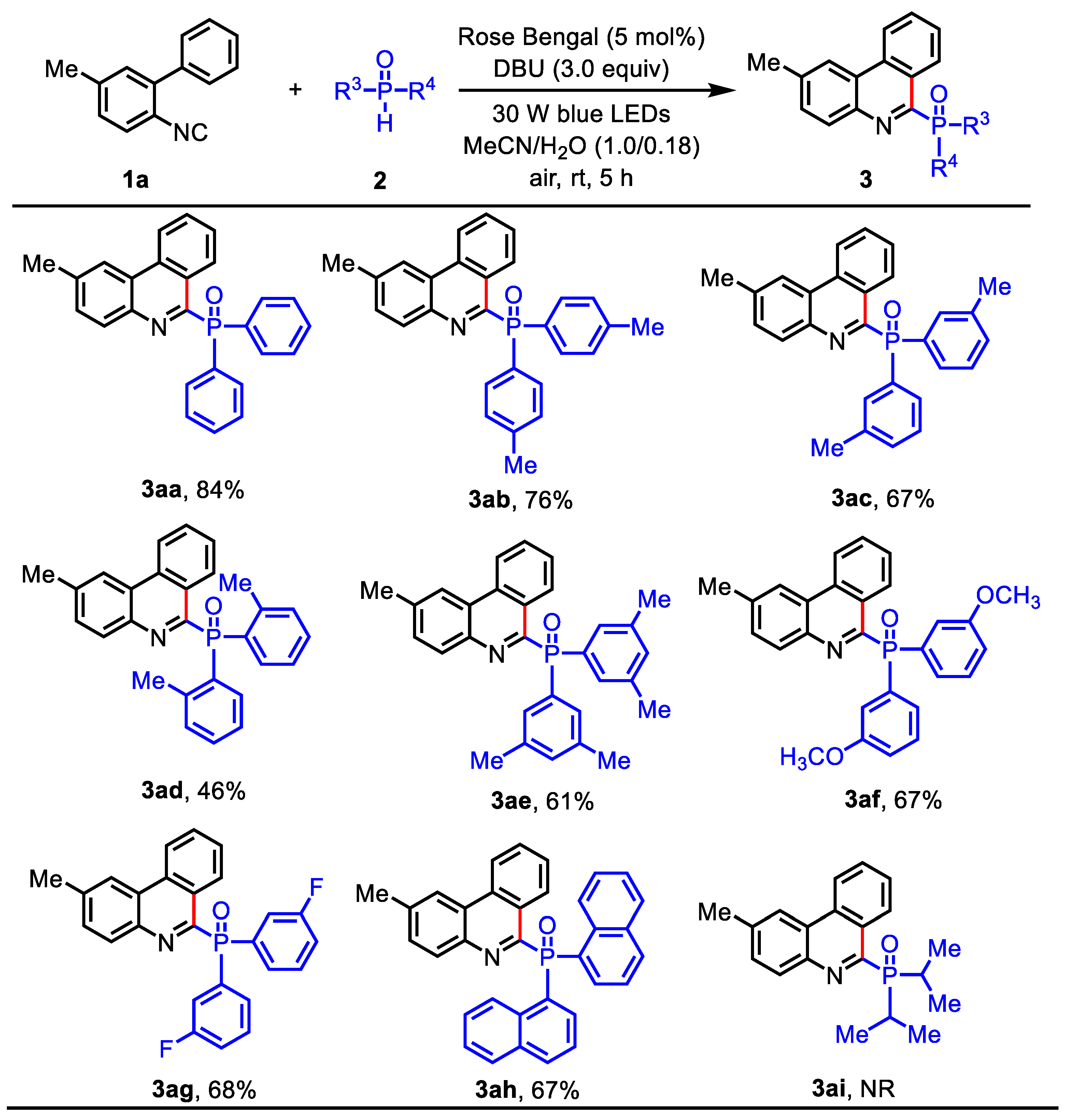
(2-Methylphenanthridin-6-yl)diphenylphosphine oxide (3aa): Isolated (Rf = 0.6, EtOAc – petroleum ether = 1:3) as a white solid (66.1 mg, 84% yield), mp: 222 – 223 oC. 1H NMR (400 MHz, CDCl3) δ 9.48 (d, J = 8.4 Hz, 1H), 8.57 (d, J = 8.4 Hz, 1H), 8.31 (s, 1H), 8.05 – 7.84 (m, 5H), 7.76 (t, J = 7.6 Hz, 1H), 7.63 (t, J = 7.6 Hz, 1H), 7.52 – 7.37 (m, 7H), 2.57 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 155.6 (d, JC-P = 128.7 Hz), 141.3 (d, JC-P = 23.3 Hz), 139.1, 133.1 (d, JC-P = 104.0 Hz), 132.4, 132.3 (d, JC-P = 9.1 Hz), 131.6 (d, JC-P = 2.5 Hz), 130.9, 130.8, 130.5, 128.5, 128.2 (d, JC-P = 12.1 Hz), 127.9, 127.7, 124.2 (d, JC-P = 2.4 Hz), 122.1, 121.7, 22.2. 31P NMR (162 MHz, CDCl3) δ 28.2. HRMS (ESI): m/z [M + H]+ calcd for C26H21NOP: 394.1355, found: 394.1358.
(2-Methylphenanthridin-6-yl)di-p-tolylphosphine oxide (3ab): Isolated (Rf = 0.4, EtOAc – petroleum ether = 1:3) as a white solid (64.1 mg, 76% yield), mp: 249 – 251 oC. 1H NMR (400 MHz, CDCl3) δ 9.45 (d, J = 8.0 Hz, 1H), 8.63 (d, J = 8.4 Hz, 1H), 8.37 (s, 1H), 7.95 (d, J = 8.4 Hz, 1H), 7.87 – 7.73 (m, 5H), 7.65 (t, J = 7.6 Hz, 1H), 7.52 (dd, J = 8.4, 0.8 Hz, 1H), 7.26 – 7.21 (m, 4H), 2.63 (s, 3H), 2.37 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 156.0 (d, JC-P = 128.4 Hz), 141.9 (d, JC-P = 2.7 Hz), 141.2 (d, JC-P = 23.4 Hz), 138.9, 132.3 (d, JC-P = 9.5 Hz), 130.9, 130.4, 130.0 (d, JC-P = 106.4 Hz), 128.9 (d, JC-P = 12.5 Hz), 128.6, 127.9 (d, JC-P = 23.0 Hz), 127.7, 124.2, 122.0, 121.6, 22.2, 21.6. 31P NMR (162 MHz, CDCl3) δ 28.8. HRMS (ESI): m/z [M + H]+ calcd for C28H25NOP: 422.1668, found: 422.1670.
(2-Methylphenanthridin-6-yl)di-m-tolylphosphine oxide (3ac): Isolated (Rf = 0.4, EtOAc – petroleum ether = 1:3) as a white solid (56.5 mg, 67% yield), mp: 237 – 239 oC. 1H NMR (400 MHz, CDCl3) δ 9.38 (d, J = 8.4 Hz, 1H), 8.50 (d, J = 8.4 Hz, 1H), 8.24 (s, 1H), 7.85 (d, J = 8.4 Hz, 1H), 7.76 – 7.64 (m, 3H), 7.62 – 7.51 (m, 3H), 7.41 (d, J = 8.3 Hz, 1H), 7.23 – 7.17 (m, 4H), 2.50 (s, 3H), 2.25 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 155.8 (d, JC-P = 128.1 Hz), 141.3 (d, JC-P = 23.2 Hz), 139.0, 138.0 (d, JC-P = 11.9 Hz), 133.0 (d, JC-P = 104.4 Hz), 132.6 (d, JC-P = 8.9 Hz), 132.4 (d, JC-P = 2.7 Hz), 132.3 (d, JC-P = 6.8 Hz), 130.9, 130.7, 130.4, 129.5 (d, JC-P = 9.3 Hz), 128.6, 128.1, 128.0 (d, JC-P = 12.8 Hz), 127.7, 124.2 (d, JC-P = 2.4 Hz), 122.1, 121.7, 22.2, 21.5. 31P NMR (162 MHz, CDCl3) δ 28.6. HRMS (ESI): m/z [M + H]+ calcd for C28H25NOP: 422.1668, found: 422.1671.
(2-Methylphenanthridin-6-yl)di-o-tolylphosphine oxide (3ad): Isolated (Rf = 0.4, EtOAc – petroleum ether = 1:3) as a white solid (38.8 mg, 46% yield), mp: 228 – 230 oC. 1H NMR (400 MHz, CDCl3) δ 9.11 (d, J = 8.4 Hz, 1H), 8.58 (d, J = 8.4 Hz, 1H), 8.31 (s, 1H), 7.73 (d, J = 8.0 Hz, 2H), 7.54 (t, J = 7.6 Hz, 1H), 7.42 (d, J = 8.0 Hz, 1H), 7.36 – 7.26 (m, 4H), 7.24 – 7.18 (m, 2H), 7.07 (t, J = 7.2 Hz, 2H), 2.55 (s, 3H), 2.36 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 156.1 (d, JC-P = 128.3 Hz), 143.4 (d, JC-P = 7.8 Hz), 141.3 (d, JC-P = 23.5 Hz), 139.1, 133.1 (d, JC-P = 11.9 Hz), 132.4 (d, JC-P = 6.5 Hz), 131.8 (d, JC-P = 2.5 Hz), 131.6, 131.5, 131.1, 130.7, 130.5, 130.4, 128.8, 127.7, 127.6 (d, JC-P = 23.0 Hz), 125.3 (d, JC-P = 12.8 Hz), 124.3, 122.2, 121.6, 22.2, 22.0, 22.0. 31P NMR (162 MHz, CDCl3) δ 38.2. HRMS (ESI): m/z [M + H]+ calcd for C28H25NOP: 422.1668, found: 422.1673.
Bis(3,5-dimethylphenyl)(2-methylphenanthridin-6-yl)phosphine oxide (3ae): Isolated (Rf = 0.5, EtOAc – petroleum ether = 1:3) as a white solid (54.8 mg, 61% yield). mp: 265 – 268 oC. 1H NMR (400 MHz, CDCl3) δ 9.46 (d, J = 8.4 Hz, 1H), 8.62 (d, J = 8.4 Hz, 1H), 8.36 (s, 1H), 7.97 (d, J = 8.4 Hz, 1H), 7.80 (t, J = 7.4 Hz, 1H), 7.65 (t, J = 7.4 Hz, 1H), 7.55-7.50 (m, 5H), 7.12 (s, 2H), 2.62 (s, 3H), 2.30 (s, 12H). 13C NMR (100 MHz, CDCl3) δ 156.0 (d, JC-P = 127.6 Hz), 141.2 (d, JC-P = 23.2 Hz), 138.9, 137.7 (d, JC-P = 12.6 Hz), 133.4 (d, JC-P = 2.8 Hz), 132.9 (d, JC-P = 104.5 Hz), 132.3 (d, JC-P = 6.8 Hz), 131.0, 130.5 (d, JC-P = 30.6 Hz), 129.9 (d, JC-P = 9.2 Hz), 128.2 (d, JC-P = 100.6 Hz), 128.0 (d, JC-P = 23.0 Hz), 124.2 (d, JC-P = 2.4 Hz), 122.0, 121.6, 22.2, 21.4. 31P NMR (162 MHz, CDCl3) δ 29.0. HRMS (ESI): m/z [M + H]+ calcd for C30H29NOP: 450.1981, found: 450.1986.
Bis(3-methoxyphenyl)(2-methylphenanthridin-6-yl)phosphine oxide (3af): Isolated (Rf = 0.6, EtOAc – petroleum ether = 1:3) as a yellow solid (60.7 mg, 67% yield), mp: 241 – 243 oC. 1H NMR (400 MHz, CDCl3) δ 9.33 (d, J = 8.0 Hz, 1H), 8.51 (d, J = 8.2 Hz, 1H), 8.25 (s, 1H), 7.85 (d, J = 8.4 Hz, 1H), 7.69 (t, J = 7.6 Hz, 1H), 7.55 (t, J = 7.6 Hz, 1H), 7.49 – 7.40 (m, 3H), 7.36 (d, J = 7.6 Hz, 1H), 7.33 (d, J = 8.0 Hz, 1H), 7.27 – 7.18 (m, 2H), 6.93 (d, J = 7.6 Hz, 2H), 3.68 (s, 6H), 2.51 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 159.3 (d, JC-P = 15.0 Hz), 155.4 (d, JC-P = 129.3 Hz), 141.2 (d, JC-P = 23.5 Hz), 139.1, 134.2 (d, JC-P = 103.4 Hz), 132.3 (d, JC-P = 6.8 Hz), 130.9, 130.8, 130.5, 129.3 (d, JC-P = 14.3 Hz), 128.4, 127.9 (d, JC-P = 23.5 Hz), 127.7, 124.7 (d, JC-P = 9.2 Hz), 124.3, 122.1, 121.7, 118.0 (d, JC-P = 2.5 Hz), 117.0 (d, JC-P = 10.1 Hz), 55.4, 22.2. 31P NMR (162 MHz, CDCl3) δ 28.2. HRMS (ESI): m/z [M + H]+ calcd for C28H25NO3P: 454.1567, found: 454.1568.
Bis(3-fluorophenyl)(2-methylphenanthridin-6-yl)phosphine oxide (3ag): Isolated (Rf = 0.5, EtOAc – petroleum ether = 1:3) as a yellow solid (58.4 mg, 68% yield), mp: 257 – 259 oC. 1H NMR (400 MHz, CDCl3) δ 9.44 (d, J = 8.4 Hz, 1H), 8.67 (d, J = 8.4 Hz, 1H), 8.40 (s, 1H), 7.98 (d, J = 8.4 Hz, 1H), 7.86 (t, J = 7.6 Hz, 1H), 7.77 – 7.62 (m, 5H), 7.58 (d, J = 8.0 Hz, 1H), 7.48 – 7.40 (m, 2H), 7.22 (td, J = 8.4, 2.0 Hz, 2H), 2.65 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.4 (dd, JC-F = 247.9, JC-P = 17.0 Hz Hz), 160.0, 153.4 (d, JC-P = 131.6 Hz), 140.1 (d, JC-F = 23.9 Hz), 138.5, 134.4 (dd, JC-P = 103.5, JC-F = 5.6 Hz Hz), 131.4 (d, JC-P = 7.1 Hz), 130.0, 129.8, 129.7, 129.2 (d, JC-F = 7.3 Hz), 129.1 (d, JC-F = 7.3 Hz), 128.3, 128.1, 127.1, 127.0 (d, JC-P = 3.0 Hz), 126.9, 126.8, 123.3, 121.2, 120.7, 118.3 (d, JC-F = 9.9 Hz), 118.1, 118.0 (d, JC-F = 9.1 Hz), 117. 9 (d, JC-P = 2.6 Hz), 21.2. 31P NMR (162 MHz, CDCl3) δ 24.9. HRMS (ESI): m/z [M + H]+ calcd for C26H19F2NOP: 430.1167, found: 430.1169.
(2-Methylphenanthridin-6-yl)di(naphthalen-1-yl)phosphine oxide (3ah): Isolated (Rf = 0.5, EtOAc – petroleum ether = 1:3) as a yellow solid (66.1 mg, 67% yield), mp: 293 – 296°C. 1H NMR (400 MHz, CDCl3) δ 9.41 (dd, J = 8.2, 2.0 Hz, 1H), 8.49-8.44 (m, 3H), 8.20 (s, 1H), 7.88 – 7.79 (m, 3H), 7.75-7.72 (m, 4H), 7.69 (d, J = 8.0 Hz, 2H), 7.64 (t, J = 7.6 Hz, 1H), 7.51 (t, J = 7.6 Hz, 1H), 7.41 – 7.31 (m, 5H), 2.45 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 155.7 (d, JC-P = 129.5 Hz), 141.3 (d, JC-P = 23.4 Hz), 139.2, 134.7 (d, JC-P = 2.3 Hz), 134.0 (d, JC-P = 8.9 Hz), 132.5 (d, JC-P = 7.3 Hz), 132.4 (d, JC-P = 6.9 Hz), 130.9, 130.8, 130.5, 130.4 (d, JC-P = 103.8 Hz), 129.1, 128.5, 128.1, 127.9, 127.8 (2C), 127.7, 127.6, 126.7, 124.3, 122.2, 121.7, 22.2. 31P NMR (162 MHz, CDCl3) δ 28.9. HRMS (ESI): m/z [M + H]+ calcd for C34H25NOP: 494.1668, found: 494.1672.
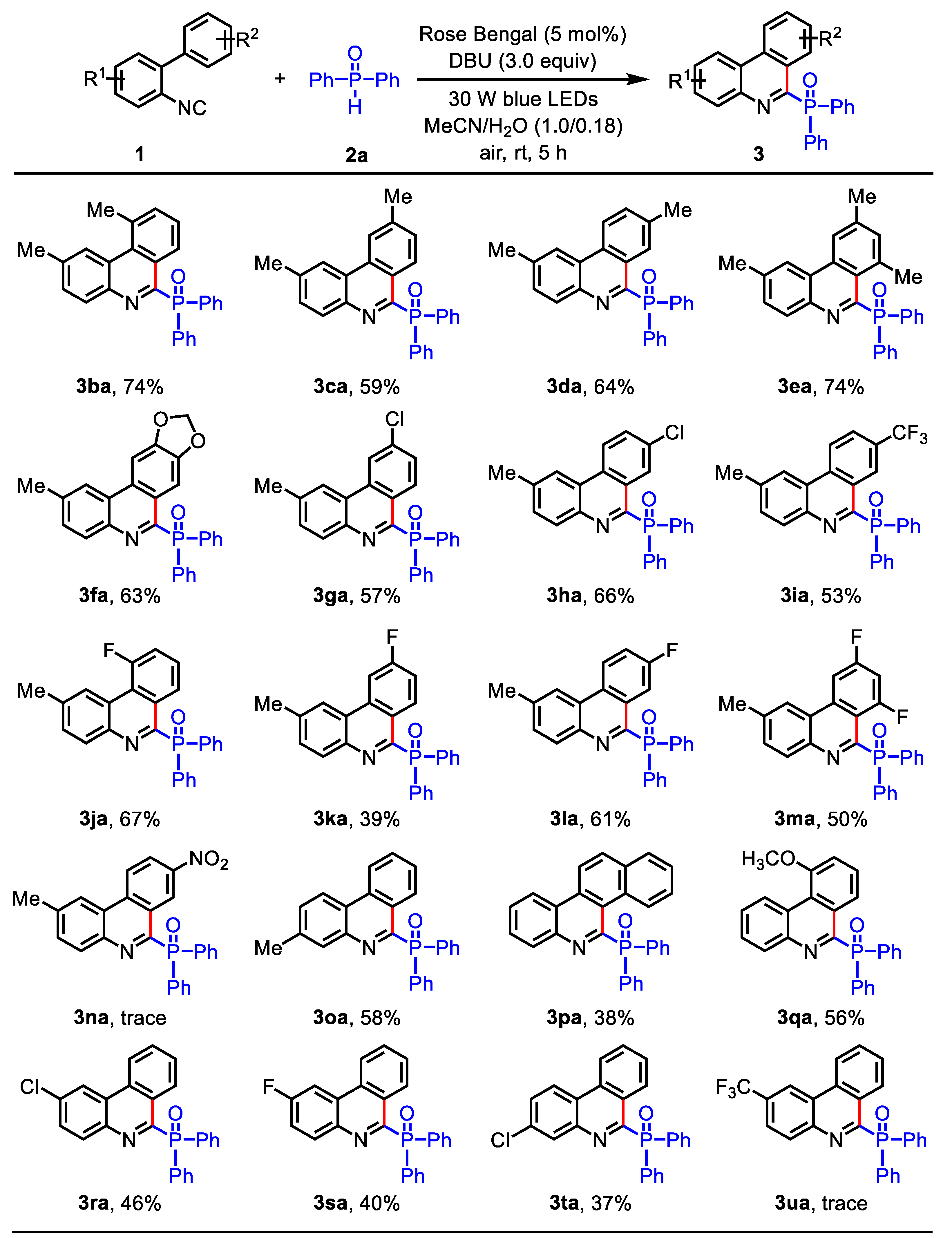
(2,10-Dimethylphenanthridin-6-yl)diphenylphosphine oxide (3ba): Isolated (Rf = 0.5, EtOAc – petroleum ether = 1:3) as a white solid (60.3 mg, 74% yield), mp: 222 – 225 oC. 1H NMR (400 MHz, CDCl3) δ 9.32 (d, J = 8.0 Hz, 1H), 8.52 (s, 1H), 7.89 – 7.73 (m, 5H), 7.55 (d, J = 7.2 Hz, 1H), 7.46 (t, J = 8.0 Hz, 1H), 7.43 – 7.37 (m, 3H), 7.37-7.30 (m, 4H), 3.01 (s, 3H), 2.52 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 156. 1 (d, JC-P = 129.0 Hz), 142.3 (d, JC-P = 23.8 Hz), 138.0, 135.3, 135.0, 133.2 (d, JC-P = 104.3 Hz), 132.3 (d, JC-P = 9.1 Hz), 131.9 (d, JC-P = 6.7 Hz), 131.6 (d, JC-P = 2.5 Hz), 131.2, 129.5, 129.3 (d, JC-P = 23.4 Hz), 128.1 (d, JC-P = 12.1 Hz), 127.2, 127.0, 126.4, 125.7 (d, JC-P= 2.4 Hz), 27.1, 22.5. 31P NMR (162 MHz, CDCl3) δ 29.4. HRMS (ESI): m/z [M + H]+ calcd for C27H23NOP: 408.1512, found: 408.1515.
(2,8-Dimethylphenanthridin-6-yl)diphenylphosphine oxide (3da): Isolated (Rf = 0.5, EtOAc – petroleum ether = 1:3) as a white solid (48.1 mg, 64% yield), mp: 231 – 233 oC. 1H NMR (400 MHz, CDCl3) δ 9.28 (s, 1H), 8.48 (dd, J = 8.4, 1.2 Hz, 1H), 8.29 (s, 1H), 7.97 – 7.87 (m, 5H), 7.61 (dd, J = 8.4, 1.2 Hz, 1H), 7.53 – 7.36 (m, 7H), 2.58 (s, 3H), 2.53 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 155.0 (d, JC-P = 129.0 Hz), 140.9 (d, JC-P = 23.4 Hz), 139.0, 137.9, 133.6 (d, JC-P = 104.0 Hz), 132.7, 132.4 (d, JC-P = 9.1 Hz), 131.5 (d, JC-P = 2.6 Hz), 130.8, 130.2 (d, JC-P = 6.9 Hz), 130.0, 128.4, 128.1 (d, JC-P = 12.0 Hz), 127.6, 124.3 (d, JC-P = 2.5 Hz), 122.0, 121.5, 22.2, 21.9. 31P NMR (162 MHz, CDCl3) δ 28.0. HRMS (ESI): m/z [M + H]+ calcd for C27H23NOP: 408.1512, found: 408.1516.
Diphenyl(2,7,9-trimethylphenanthridin-6-yl)phosphine oxide (3ea): Isolated (Rf = 0.5, EtOAc – petroleum ether = 1:3) as a white solid (62.3 mg, 74% yield), mp: 255 – 257 oC. 1H NMR (400 MHz, CDCl3) δ 8.21 (s, 2H), 7.72 – 7.65 (m, 4H), 7.51 (d, J = 8.0 Hz, 1H), 7.40-7.35 (m, 2H), 7.34 – 7.27 (m, 5H), 7.17 (s, 1H), 2.84 (s, 3H), 2.48 (s, 3H), 2.43 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 155.4 (d, JC-P = 129.9 Hz), 140.5, 139.8 (d, JC-P = 24.1 Hz), 138.9, 137.4, 135.1 (d, JC-P = 106.9 Hz), 134.2 (d, JC-P = 6.7 Hz), 133.3, 132.0 (d, JC-P = 8.9 Hz), 131.1 (d, JC-P = 2.5 Hz), 130.3, 130.2, 128.0 (d, JC-P = 12.1 Hz), 125.7 (d, JC-P = 23.7 Hz), 124.1 (d, JC-P = 2.6 Hz), 121.8, 120.0, 25.0, 22.2, 21.9. 31P NMR (162 MHz, CDCl3) δ 36.2. HRMS (ESI): m/z [M + H]+ calcd for C28H25NOP: 422.1668, found: 422.1672.
(2-Methyl-[
1,
3]
dioxolo[4,5-j]phenanthridin-6-yl)diphenylphosphine oxide (3fa): Isolated (R
f = 0.4, EtOAc – petroleum ether = 1:1) as a white solid (53.3 mg, 63% yield), mp: 262 – 265
oC.
1H NMR (400 MHz, CDCl
3) δ 8.98 (s, 1H), 8.11 (s, 1H), 7.98 – 7.84 (m, 6H), 7.54 – 7.38 (m, 7H), 6.08 (s, 2H), 2.58 (s, 3H).
13C NMR (101 MHz, CDCl
3) δ 153.4 (d,
JC-P = 131.3 Hz), 151.1, 148.2, 141.0 (d,
JC-P = 23.2 Hz), 138.5, 133.8, 132.2 (d,
JC-P = 103.9 Hz), 132.3 (d,
JC-P = 9.1 Hz), 131.6, 130.7 130.5 (d,
JC-P = 7.4 Hz), 129.9, 128.1 (d,
JC-P = 12.0 Hz), 125.2 (d,
JC-P = 23.4 Hz), 124.4, 121.4, 105.6, 102.0, 99.8, 22.1.
31P NMR (162 MHz, CDCl
3) δ 28.1. HRMS (ESI):
m/z [M + H]
+ calcd for C
27H
21NO
3P: 4038.1254, found: 438.1257.
(9-Chloro-2-methylphenanthridin-6-yl)diphenylphosphine oxide (3ga): Isolated (Rf = 0.4, EtOAc – petroleum ether = 1:3) as a white solid (48.8 mg, 57% yield), mp: 211 – 214 oC. 1H NMR (400 MHz, CDCl3) δ 9.50 (d, J = 8.8 Hz, 1H), 8.57 (s, 1H), 8.27 (s, 1H), 7.97 – 7.86 (m, 5H), 7.60 (dd, J = 8.8, 1.6 Hz, 1H), 7.58 – 7.48 (m, 3H), 7.47-7.41 (m, 4H), 2.62 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 155.3 (d, JC-P = 130.1 Hz), 141.5 (d, JC-P = 22.9 Hz), 139.5, 137.4, 133.8 (d, JC-P = 6.7 Hz), 132.8 (d, JC-P = 104.4 Hz), 132.3 (d, JC-P = 9.2 Hz), 131.8, 131.2, 130.9, 130.2, 128.4, 128.2 (d, JC-P = 12.0 Hz), 126.3 (d, JC-P = 23.4 Hz), 123.2, 121.7 (d, JC-P = 12.1 Hz), 21.2. 31P NMR (162 MHz, CDCl3) δ 27.9. HRMS (ESI): m/z [M + H]+ calcd for C26H20ClNOP: 428.0966, found: 428.0972.
(8-Chloro-2-methylphenanthridin-6-yl)diphenylphosphine oxide (3ha): Isolated (Rf = 0.5, EtOAc – petroleum ether = 1:3) as a white solid (56.5 mg, 66% yield), mp: 229 – 231 oC. 1H NMR (400 MHz, CDCl3) δ 9.62 (d, J = 2.0 Hz, 1H), 8.46 (dd, J = 8.8, 1.2 Hz, 1H), 8.23 (s, 1H), 8.06 – 7.90 (m, 5H), 7.69 (dd, J = 8.8, 2.0 Hz, 1H), 7.57 – 7.47 (m, 3H), 7.46 – 7.39 (m, 4H), 2.58 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 154. 5 (d, JC-P = 128.6 Hz), 141.1 (d, JC-P = 22.7 Hz), 139.7, 133.8, 132.9 (d, JC-P = 104.6 Hz), 132. 3 (d, JC-P = 9.1 Hz), 131.8 (d, JC-P = 2.6 Hz), 131.5, 130.9, 130.8, 130.7 (d, JC-P = 6.8 Hz), 128.8 (d, JC-P = 23.1Hz), 128.2 (d, JC-P = 12.1 Hz), 127.5, 123.7, 123.6 (d, JC-P = 2.4 Hz), 121.5, 22.2. 31P NMR (162 MHz, CDCl3) δ 27.2. HRMS (ESI): m/z [M + H]+ calcd for C26H20ClNOP: 428.0966, found: 428.0969.
(2-Methyl-8-(trifluoromethyl)phenanthridin-6-yl)diphenylphosphine oxide (3ia): Isolated (Rf = 0.6, EtOAc – petroleum ether = 1:3) as a yellow solid (48.9 mg, 53% yield), mp: 199 – 201 oC.1H NMR (400 MHz, CDCl3) δ 10.00 (s, 1H), 8.70 (d, J = 8.8 Hz, 1H), 8.35 (s, 1H), 8.05 – 7.95 (m, 6H), 7.60 (d, J = 8.4 Hz, 1H), 7.55 – 7.48 (m, 2H), 7.47 – 7.41 (m, 4H), 2.63 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 155.8 (d, JC-P = 127.7 Hz), 141.8 (d, JC-P = 12.5 Hz), 139.9, 134.4 (d, JC-P = 6.2 Hz), 132.8 (d, JC-P = 105.4 Hz), 132.3 (d, JC-P = 9.1 Hz), 131.8 (d, JC-P = 2.6 Hz), 131.7, 131.0, 129.4 (q, JC-F = 32.6 Hz), 128.3 (d, JC-P = 12.1 Hz), 127.3 (d, JC-P = 22.9 Hz), 126.6 (q, 1JC-F= 3.1 Hz), 126.2 (d, JC-P = 4.3 Hz), 123.9 (q, JC-F = 270.8 Hz), 123.2 (d, JC-P = 2.2 Hz), 123.1, 122.0, 22.2. 31P NMR (162 MHz, CDCl3) δ 26.8. HRMS (ESI): m/z [M + H]+ calcd for C27H20F3NOP: 462.1229, found: 462.1232.
(10-Fluoro-2-methylphenanthridin-6-yl)diphenylphosphine oxide (3ja): Isolated (Rf = 0.3, EtOAc – petroleum ether = 1:3) as a white solid (55.1 mg, 67% yield), mp: 192 – 194 oC. 1H NMR (400 MHz, CDCl3) δ 9.35 (d, J = 8.2 Hz, 1H), 8.78 (s, 1H), 7.96 – 7.86 (m, 5H), 7.65 – 7.58 (m, 1H), 7.57 – 7.48 (m, 4H), 7.47 – 7.42 (m, 4H), 2.61 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 160.5 (d, 1JC-F = 253.5 Hz), 155.0 (d, JC-P = 129.1 Hz), 141.5 (d, JC-P = 23.0 Hz), 139.8, 132.8 (d, JC-P = 104.8 Hz), 132.3 (d, JC-P = 9.1 Hz), 131.7 (d, JC-P = 2.5 Hz), 130.7 (d, JC-F = 17.7 Hz), 129. 9 (d, JC-F = 24.3), 128.2 (d, JC-P = 12.1 Hz), 128.1 (d, JC-P = 8.9 Hz), 126.6 (d, 2JC-F = 23.0 Hz), 124.6 (d, JC-P = 4.0 Hz), 121.8 (m), 117.3 (d, 2JC-F = 23.2 Hz), 22.4. 31P NMR (162 MHz, CDCl3) δ 28.6. HRMS (ESI): m/z [M + H]+ calcd for C26H20FNOP: 412.1261, found: 412.1267.
(9-Fluoro-2-methylphenanthridin-6-yl)diphenylphosphine oxide (3ka): Isolated (Rf = 0.4, EtOAc – petroleum ether = 1:3) as a white solid (32.1 mg, 39% yield), mp: 205 – 208 oC. 1H NMR (400 MHz, CDCl3) δ 9.60 (dd, J = 9.2, 6.0 Hz, 1H), 8.23 (s, 1H), 8.21 (d, J = 9.2 Hz, 1H), 7.98 – 7.89 (m, 5H), 7.57 – 7.49 (m, 3H), 7.47 – 7.36 (m, 5H), 2.62 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 163.8 (d, 1JC-F = 251.6 Hz), 155.1 (d, 2JC-P = 128.9 Hz), 141.3 (d, 2JC-F= 23.2 Hz), 139.2, 135.0 (d, JC-P = 9.3 Hz), 134.9 (d, JC-P = 9.3 Hz), 132.9 (d, JC-P = 104.4 Hz), 132.4, 132.3, 131.7 (d, JC-P = 2.7 Hz), 131.6, 131.2, 130.9, 128.2 (d, JC-P = 12.1 Hz), 125.1 (d, 2JC-P = 24.3 Hz), 123.8, 121.8, 116.9 (d, JC-F = 23.3 Hz), 107.2 (d, JC-F = 22.2 Hz), 22.1.31P NMR (162 MHz, CDCl3) δ 27.9. HRMS (ESI): m/z [M + H]+ calcd for C26H20FNOP: 412.1261, found: 412.1265.
(8-Fluoro-2-methylphenanthridin-6-yl)diphenylphosphine oxide (3la): Isolated (Rf = 0.5, EtOAc – petroleum ether = 1:3) as a yellow solid (50.2 mg, 61% yield), mp: 216 – 219 oC. 1H NMR (400 MHz, CDCl3) δ 9.29 (dd, J = 10.2, 2.6 Hz, 1H), 8.61 – 8.54 (m, 1H), 8.27 (s, 1H), 7.98 – 7.90 (m, 5H), 7.56 – 7.47 (m, 4H), 7.46 – 7.40 (m, 4H), 2.60 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.2 (d, 1JC-F = 247.4 Hz), 154.6 (d, JC-P = 129.2 Hz), 140.9 (d, 2JC-F = 23.5 Hz), 132.8 (d, JC-P = 104.3 Hz), 132.3 (d, JC-P = 9.1 Hz), 131.7 (d, JC-P = 2.5 Hz), 130.9, 130.4, 129.3 (d, 3JC-F = 9.3 Hz), 129.0 (d, 3JC-F = 8.8 Hz), 128.2 (d, JC-P = 12.1 Hz), 124.6 (d, JC-P = 8.5 Hz), 123.9, 121.4 120.3 (d, JC-F = 24.2 Hz), 113.1 (d, 2JC-F= 23.1 Hz), 22.2. 31P NMR (162 MHz, CDCl3) δ 27.4. HRMS (ESI): m/z [M + H]+ calcd for C26H20FNOP: 412.1261, found: 412.1263.
(7,9-Difluoro-2-methylphenanthridin-6-yl)diphenylphosphine oxide (3ma): Isolated (Rf = 0.5, EtOAc – petroleum ether = 1:1) as a white solid (42.9 mg, 50% yield), mp: 245 – 247 oC. 1H NMR (400 MHz, CDCl3) δ 8.20 (s, 1H), 8.08 (d, J = 9.6 Hz, 1H), 7.80 – 7.70 (m, 5H), 7.56-7.51 (m, 3H), 7.48 – 7.43 (m, 4H), 7.04 (t, J = 9.6 Hz, 1H), 2.62 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 163.6 (d, 1JC-F= 252.5 Hz), 160.2 (d, 1JC-F = 246.6 Hz), 141.2, 140.1, 136.1, 133.1 (d, JC-P = 109.2 Hz), 131.9, 131.8, 131.4, 131.3, 130.9, 128.8, 128.0 (d, JC-P = 12.2 Hz), 122.5, 121.9, 104.3 (d, JC-F = 27.2 Hz), 103.9, 22.2. 31P NMR (162 MHz, CDCl3) δ 34.8 (d, J = 4.1 Hz). HRMS (ESI): m/z [M + H]+ calcd for C26H19F2NOP: 430.1167, found: 430.1171.
(3-Methylphenanthridin-6-yl)diphenylphosphine oxide (3oa): Isolated (Rf = 0.4, EtOAc – petroleum ether = 1:3) as a white solid (45.6 mg, 58% yield), mp: 197 – 200 oC. 1H NMR (400 MHz, CDCl3) δ 9.39 (d, J = 8.0 Hz, 1H), 8.46 (d, J = 8.4 Hz, 1H), 8.32 (dd, J = 8.2, 2.4 Hz, 1H), 7.88-7.80 (m, 4H), 7.72 (s, 1H), 7.68 (t, J = 7.2 Hz, 1H), 7.53 (t, J = 7.6 Hz, 1H), 7.42 – 7.37 (m, 3H), 7.36 – 7.30 (m, 4H), 2.42 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 156.7 (d, JC-P = 128.2 Hz), 142.9 (d, JC-P = 23.1 Hz), 139.0, 133.1 (d, JC-P = 104.1 Hz), 132.7 (d, JC-P = 6.9 Hz), 132.3 (d, JC-P = 9.1 Hz), 131.6 (d, JC-P = 2.6 Hz), 130.9, 130.6, 130.5, 128.5, 128.2 (d, JC-P = 12.1 Hz), 127.5 (d, JC-P = 12.6 Hz), 127.4, 122.0 (d, JC-P = 2.5 Hz), 121.9 (d, JC-P = 5.0 Hz), 21.4. 31P NMR (162 MHz, CDCl3) δ 28.1. HRMS (ESI): m/z [M + H]+ calcd for C26H21NOP: 394.1355, found: 394.1358.
Benzo[i]phenanthridin-5-yldiphenylphosphine oxide (3pa): Isolated (Rf = 0.5, EtOAc – petroleum ether = 1:1) as a white solid (32.6 mg, 38% yield), mp: 221 – 223 oC. 1H NMR (400 MHz, CDCl3) δ 9.40 (d, J = 8.8 Hz, 1H), 9.09-9.06 (m, 1H), 9.05 – 9.00 (m, 1H), 8.16 – 8.12 (m, 1H), 8.04-8.00 (m, 1H), 7.98 – 7.90 (m, 5H), 7.77– 7.66 (m, 4H), 7.54 – 7.40 (m, 6H). 13C NMR (100 MHz, CDCl3) δ 155.3 (d, JC-P = 128.2 Hz), 144.8 (d, JC-P = 23.3 Hz), 133.1 (d, JC-P = 104.6 Hz), 132.4 (d, JC-P = 9.1 Hz), 131.9 (d, JC-P = 6.8 Hz), 131.7, 131.6, 130.9, 128.9, 128.8, 128.7, 128.5, 128.4 (d, JC-P = 2.7 Hz), 128.2 (d, JC-P = 12.0 Hz), 128.1, 127.4 (d, JC-P = 22.6 Hz), 127.2, 126.7, 124.6, 124.0. 31P NMR (162 MHz, CDCl3) δ 29.1. HRMS (ESI): m/z [M + H]+ calcd for C29H21NOP: 430.1355, found: 430.1359.
(10-Methoxyphenanthridin-6-yl)diphenylphosphine oxide (3qa): Isolated (Rf = 0.3, EtOAc – petroleum ether = 1:1) as a white solid (45.9 mg, 56% yield), mp: 244 – 246 oC. 1H NMR (400 MHz, CDCl3) δ 9.58 – 9.45 (m, 1H), 9.14 (d, J = 8.2 Hz, 1H), 8.03 – 7.98 (m, 1H), 7.93-7.86 (m, 4H), 7.72 – 7.64 (m, 2H), 7.62 (t, J = 8.0 Hz, 1H), 7.53 – 7.47 (m, 2H), 7.46-7.40 (m, 4H), 7.30 (d, J = 8.0 Hz, 1H), 4.10 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 158.1 (d, JC-P = 2.7 Hz), 156.4 (d, JC-P = 129.1 Hz), 143.3 (d, JC-P = 23.2 Hz), 133.1 (d, JC-P = 104.5 Hz), 132.3 (d, JC-P = 104.5 Hz), 132.3 (d, JC-P = 9.1 Hz), 131.6 (d, JC-P = 2.4 Hz), 130.9, 129.7 (d, JC-P = 23.8 Hz), 128.7, 128.2, 128.1 (d, JC-P = 12.1 Hz), 128.0 (d, JC-P = 5.6 Hz), 124.2, 123.1 (d, JC-P = 7.0 Hz), 120.8, 112.1, 55.8. 31P NMR (162 MHz, CDCl3) δ 29.2. HRMS (ESI): m/z [M + H]+ calcd for C26H21NO2P: 410.1304, found: 410.1309.
(2-Chlorophenanthridin-6-yl)diphenylphosphine oxide (3ra): Isolated (Rf = 0.4, EtOAc – petroleum ether = 1:3) as a white solid (38.1 mg, 46% yield), mp: 241 – 243 oC. 1H NMR (400 MHz, CDCl3) δ 9.41 (d, J = 8.0 Hz, 1H), 8.48 (d, J = 8.0 Hz, 1H), 8.47 (s), 7.89 (d, J = 8.8 Hz, 1H), 7.86 – 7.80 (m, 4H), 7.77 (d, J = 8.0 Hz, 1H), 7.64 (t, J = 7.6 Hz, 1H), 7.56 (dd, J = 8.8, 2.0 Hz, 1H), 7.47-7.42 (m, 2H), 7.40-7.34 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 156.3 (d, JC-P = 128.2 Hz), 140.1 (d, JC-P = 23.4 Hz), 138.2, 133.9, 131.6 (d, JC-P = 104.7 Hz), 131.5, 131.3, 131.2, 130.8 (d, JC-P = 2.6 Hz), 130.3, 128.3, 127.7, 127.5, 127.2 (d, JC-P = 12.2 Hz), 124.4, 121.1, 120.7, 113.0. 31P NMR (162 MHz, CDCl3) δ 28.5. HRMS (ESI): m/z [M + H]+ calcd for C25H18ClNOP: 414.0809, found: 414.0813.
(2-Fluorophenanthridin-6-yl)diphenylphosphine oxide (3sa): Isolated (Rf = 0.3, EtOAc – petroleum ether = 1:3) as a yellow solid (31.8 mg, 40% yield), mp: 235 – 237 oC. 1H NMR (400 MHz, CDCl3) δ 9.41 (d, J = 8.4 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 8.13 – 8.09 (m, 1H), 7.99 – 7.93 (m, 1H), 7.86 – 7.76 (m, 5H), 7.64 (t, J = 7.6 Hz, 1H), 7.48 – 7.33 (m, 7H). 13C NMR (100 MHz, CDCl3) δ 161.4 (d, JC-F = 248.9 Hz), 155.1 (d, JC-P = 131.7 Hz), 138.6 (d, JC-P = 23.1 Hz), 132.5 (d, JC-P = 9.3 Hz), 131.7 (d, JC-P = 104.3 Hz), 131.2 (d, JC-P = 9.1 Hz), 130.7, 130.1, 127.6 (d, JC-F = 16.3 Hz), 127.2 (d, JC-P = 12.1 Hz), 126.6, 124.9, 121.3, 116.8 (d, JC-F = 24.5 Hz), 106.1 (d, JC-F = 23.2 Hz). 31P NMR (162 MHz, CDCl3) δ 28.5. HRMS (ESI): m/z [M + H]+ calcd for C25H18FNOP: 398.1105, found: 398.1112.
(3-Chlorophenanthridin-6-yl)diphenylphosphine oxide (3ta): Isolated (Rf = 0.4, EtOAc – petroleum ether = 1:3) as a white solid (30.6 mg, 37% yield), mp: 194 – 197 oC. 1H NMR (400 MHz, CDCl3) δ 9.44 (d, J = 8.4 Hz, 1H), 8.48 (d, J = 8.0 Hz, 1H), 8.40 (d, J = 8.8 Hz, 1H), 7.95 (d, J = 1.6 Hz, 1H), 7.87-7.80 (m, 4H), 7.76 (t, J = 7.6 Hz, 1H), 7.64 – 7.54 (m, 2H), 7.47 – 7.41 (m, 2H), 7.41 – 7.32 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 158.5 (d, JC-P = 125.7 Hz), 143.2 (d, JC-P = 23.4 Hz), 134.5, 132.6 (d, JC-P = 104.5 Hz), 132.2 (d, JC-P = 9.1 Hz), 131.9, 131.5, 130.1, 129.3, 128.8, 128.3 (d, JC-P = 12.3 Hz), 127.8 (d, JC-P = 22.7 Hz), 123.6, 122.9 (d, JC-P = 2.4 Hz), 122.0. 31P NMR (162 MHz, CDCl3) δ 28.3. HRMS (ESI): m/z [M + H]+ calcd for C25H18ClNOP: 414.0809, found: 414.0816.