1. Introduction
The larynx is a part of the upper respiratory system [
1] and protects the respiratory tract during swallowing, respiration and voice production, and provides a mechanism for organisms to seal the airways, and hold their breath [
1,
2]. The larynx is important for communication between members of the same species and evolutionary studies, as in the case of anuran species [
2,
3]. Voice production occurs as a result of vibrations of the vocal folds [
1,
4]. Although there are descriptions on the form and constitution of the cartilaginous muscle of the larynx of animals, there are few studies on signs of sexual dimorphism in the larynx in domestic or wild mammals [
5].Vocal fold vibration facilitates the production of sounds that exhibit reduced environmental attenuation [
6]. “The size and composition of vocal folds” appears to contribute to species specific acoustic properties [
7,
8]. Little is known about size and morphology of cattle larynx. Vocal organ morphology represents a “complex shape and the absence of homologous landmarks pose major challenges in its geometrical analysis” [
9]. In older females, vocal cord contact increases, while in males it decreases with age [
10,
11].
The association between vocal fold length, elasticity, and body size has been described in several mammal species, such as human, red deer, horses, and Rocky Mountain elk [
4,
12]. Moreover, in humans the larynx is sexually dimorphic [
13,
14] as well as in rats [
14]. Despite this observed sexual dimorphism, little is known about the sexual dimorphism of the thyroarytenoid, the main muscle of the vocal fold [
14]. The literature suggests that the number of muscle fibers decreases in the thyroarytenoid, and differences between the thyroarytenoid muscles of male and female rats in old age have been found [
15]. We therefore hypothesized that there would be noticeable shape changes in the vocal folds associated with functional vocalizations differences among genders in cattle.
Although geometric morphometrics (GM) is not a novel technique for the study of form in structures, it has been scarcely applied to the study of soft tissues [
16]. Nor has it been applied to the study of soft tissue sexual dimorphism [
17]. So, its application to the analysis of shape variation for vocal folds is totally novel. In this research, we apply GM techniques to the study of vocal folds in a sample of meat calves, with the purpose to study form (size + shape) of both sides of vocal folds, and test the following research questions:
Although the GM method is not new in analyzing shape variation in geometry morphometric, its application to the analysis of shape variation for vocal folds is novel.
2. Materials and Methods
2.1. Sample
A sample of 14 larynges from calves belonging to “Bruna dels Pirineus” breed and its F1 crosses was obtained in an abattoir during the first semester of 2021. “Bruna dels Pirineus” is a breed from Catalonia (NE Spain) with meat purposes and managed under semi-extensive conditions mainly on the Pyrenean and Prepyrenean area. Sample included 6 females and 8 males in an age range 335 to 625 days, which is the commercial age demanded by the Catalan market. No animal presented anomalies on
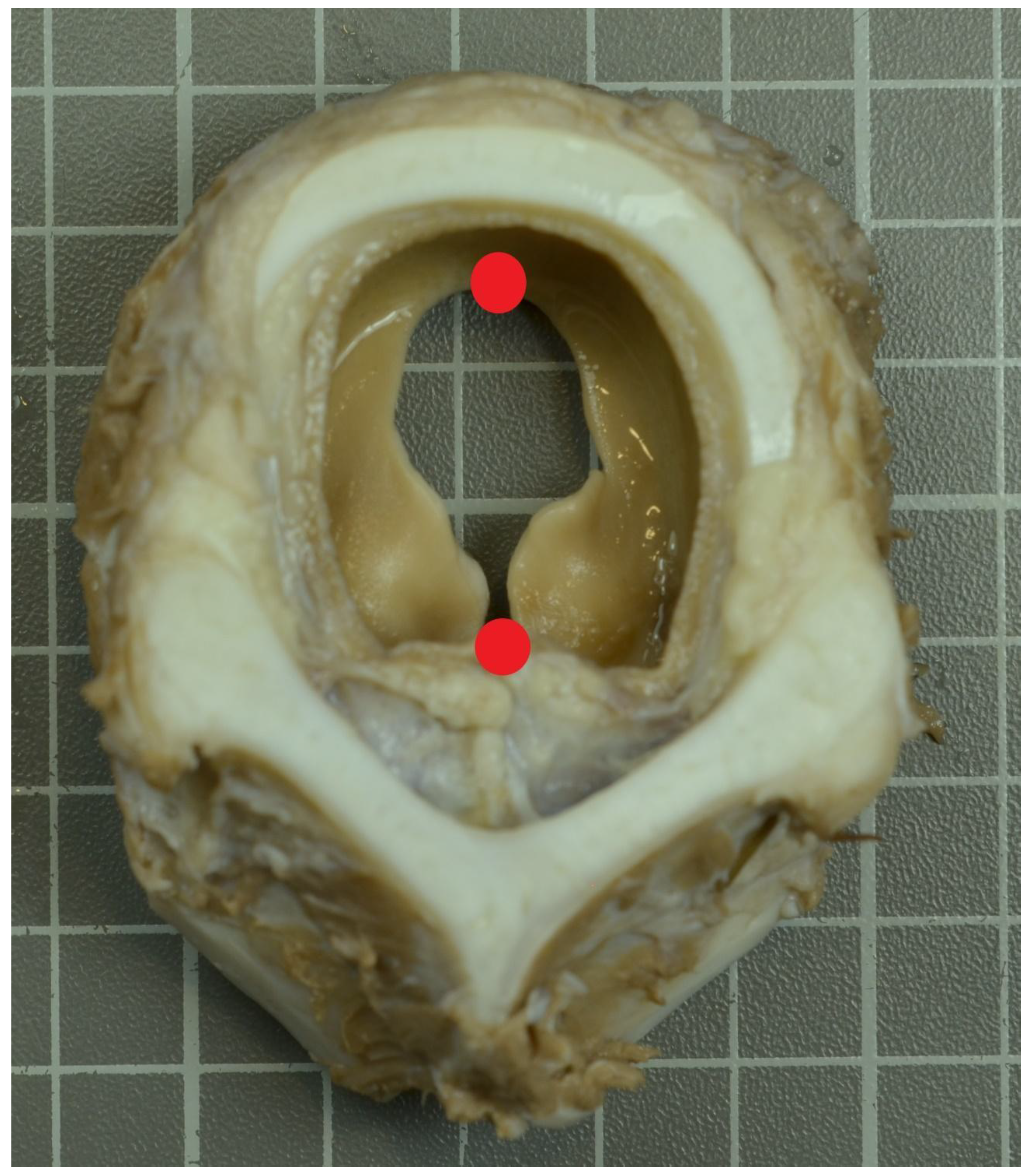
antemortem inspection. Larynx samples were obtained from fresh carcasses. Hot carcass weight was registered. At the laboratory, larynges were dissected and were preserved in buffered formaldehyde 4% until they were photographed. A transversal cut at the level of vocal fold was obtained and fixed in a standard plane and pictures were then taken using a digital camera (Nikon D1500) equipped with a lens (Nikon DX de 18-105 mm). A scale was included and placed parallel to the face plane (
Figure 1).
2.2. Comparison between Sexes
Age and hot carcass weight between sexes were compared using the Mann-Whitney U test.
2.3. Geometric Morphometrics
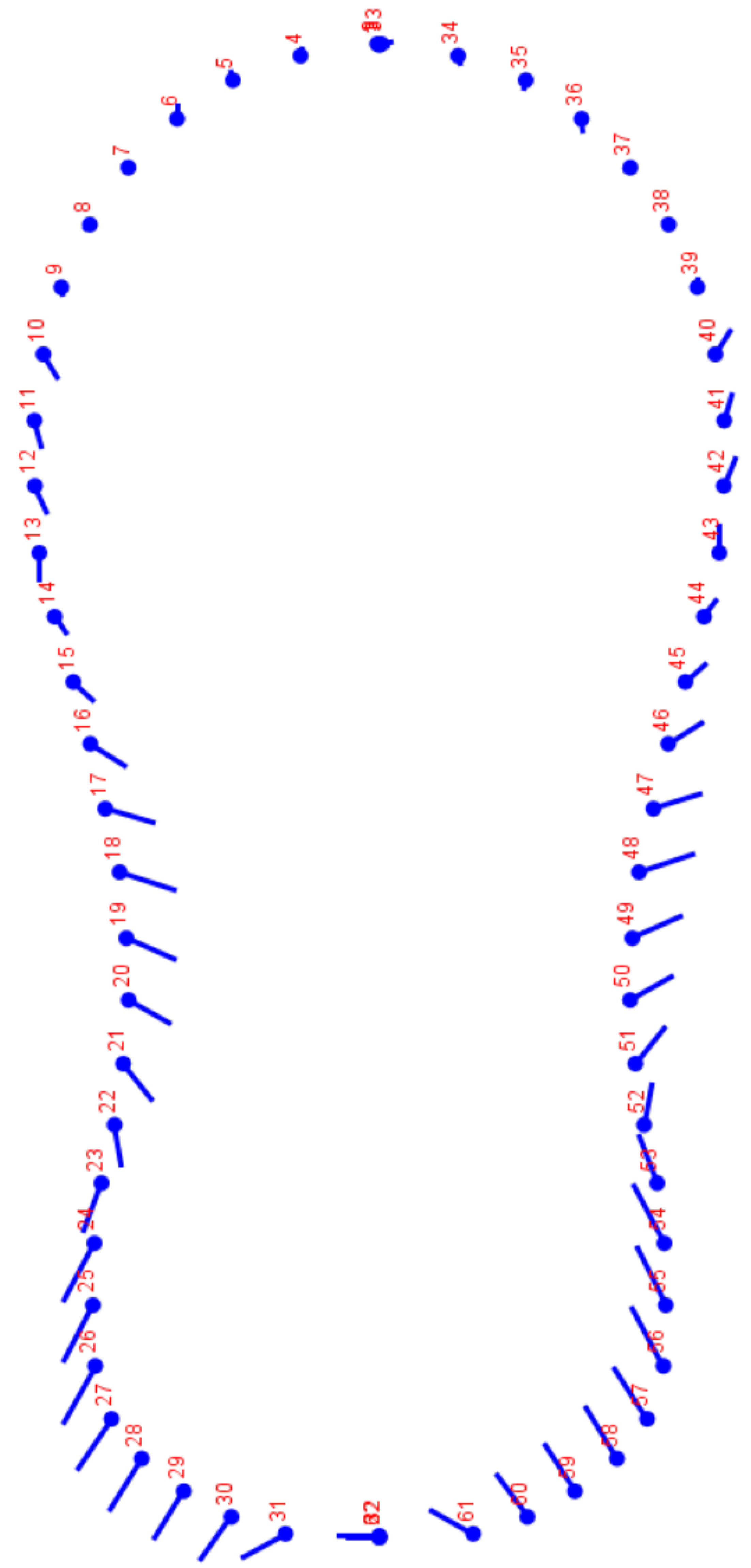
A set 2 midsagittal landmarks and 30 semi-landmarks per side were selected. Location and digitalization were performed with TpsDig v. 2.16 [
18]. Digitalization was done in two separate sessions. Semi-landmarks are points on curves for which their exact position cannot be determined using anatomical criteria. They were ulteriorly transformed to landmarks with the program TpsUtility v. 1.70 [
18].
All coordinates were then aligned using a Procrustes superimposition, thus eliminating non-shape information. Size (expressed as Centroid Size, CS) was estimated as “the square root of the sum of squared distances from the centroid of a landmark configuration” [
19]. The influence of size on shape was estimated by regressing the shape scores on the CS log transformed. It has been noted “that measurement error is a confounding factor in the assessment of fluctuating asymmetry” [
20,
21,
22].
Therefore, all pictures were digitized twice to estimate inter-observer error. A shape Procrustes ANOVA to analyze the variation in total shape and to examine the mean squares proportion of measurement error to the overall variation was performed. The symmetric component is the variation among individuals in the consensus. “The asymmetric component is the squared distances between the original configurations from this symmetric component” [
21,
22]. Since Procrustes ANOVA assumes an isotropic variation around the landmark configuration, a Multivariate Analysis of Variance (MANOVA) test with a Pillai’s trace and the associated parametric
p value to assess the statistical significance was performed. Finally, the asymmetry between sexes was assessed by means of a Canonical Variate Analysis (CVA) on regression residuals of asymmetric components using the Mahalanobis distance, which assumes a lack of isotropy.
Symmetry was considered as object symmetry for example, as a single structure which is identical according to a mid-sagittal plane [
21]. To analyze the total shape variation of the entire sample into components of symmetric variation ("Individuals"), directional asymmetry ("Side"), and fluctuating asymmetry ("Individual by Side interaction"), a Procrustes ANOVA was calculated. Fluctuating asymmetry is the variation of the individual asymmetries, whereas directional asymmetry is the average asymmetry.
Analysis was carried out with MorphoJ v. 1.07a software [
23]. A
p value of <0.05 was considered statistically significant.
4. Results
4.1. Comparison between Sexes
Carcass weight was statistically higher (U = 42; p = 0.00123) among males (220.2 ± 46.7 kg) than among females (177.8 ± 42.4 kg), but age did not differ between sexes (U = 116; p = 0.664).
4.2. Allometry
The contribution of global size variation was 17.52% (p = 0.0008; 10,000 randomization rounds). A clear correlation between centroid size and carcass weight was found (r2 = 0.527; p = 0.0019, data log-transformed).
4.3. Analysis of Variance
Measurement error was 4.5% (
Table 1). ANOVA also showed a significant effect for DA (47.6%) and FA (9.5%, mainly on the former (
Table 1).
4.4. Shape Changes
Deformation grids helped visualize the shifts in aligned coordinates from one shape to another and it depicted a clear right-biased basal and left-biased central cartilage (
Figure 2). Significant differences in the morphometric profile were found between the right and left vocal folds, and between the sexes.
4.5. Canonical Variate Analysis
The asymmetric components were significantly different among sexes (p < 0.0001; 10,000 permutation rounds).
5. Discussion
In this study, significant differences were found between the sexes and in the morphometric profile between the left and right vocal folds. Laryngeal sexual dimorphism is recognized in humans [
24] and reindeer [
25] but has not been found in dogs [
26], in horses [
27] or pigs [
28]. In the larynx of adult rats, researchers have also found sexual dimorphism in function and structure, with lower frequency of vocalization in males [
14].
At the onset of normal vocal fold vibration, the vocal folds are adducted [
29] (their displacement is away from the midline in symmetric movements), so our results signal an asymmetric vibration, with a possible lack of equivalence with the shape properties of the vocal folds. In fact, it has been presumed “that laryngeal asymmetry (either in mass or tension) causes irregular vibrations” [
30]. The laryngeal tissue, in particular the cartilages is sensitive to testosterone leading to a larger overall growth in males, and this in turn leads to larynx size sexual dimorphism in some species [
4], and this would explain the detected sexual dimorphism, as males were larger than females. Asymmetry was correlated with larynx size, which in turn appeared to be correlated with body weight (e.g., more asymmetry with body weight). Other studies report that the higher the body height, “the laryngeal measurements were statistically significant”, and higher for males [
31]. Variations in laryngeal structure may explain much of the interpopulation signal differences [
32].
The main limitation of the present study is that the sample size was limited to 14 larynges. As formaldehyde fixation can induce shrinkage, the question remains how different a fixed vocal fold is from a fresh one? Our results do not necessarily correspond to the situation of living cattle. But, we have just intended an approximation of comparison between sides, so detected asymmetry could be found in fresh structures as well.
In conclusion, this study can be considered the first to detect vocal folds asymmetries in bovine livestock. Further study involving a larger sample size is needed to validate this information in a larger population. Regarding other limitations, the present study has not sampled adult animals, so it would be worth investigating if results are similar among full-grown animals.
Authors’ contributions: Larynx obtention at abattoir, P.M.P.-C.; Writing—original draft preparation, methodology, formal analysis, P.M.P.-C., A.S-C.; writing—review and editing, writing—original draft preparation, conceptualization, A.S-C., P.M.P.-C., and O.M.V-T.; conceptualization, N.I.M-O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Committee-CONADI of the Universidad Cooperativa de Colombia.
Institutional Review Board Statement
Not applicable; the study did not involve humans or animals. Animals were sacrificed for commercial purposes (meat production), and the inedible parts (larynx) were used postmortem.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon reasonable request to the second author.
Acknowledgments
We thank MAFRISEU SA for providing access to the larynges and the individual information for each animal. Its official veterinary inspector, kindly supported us in the obtention of material. We are also grateful to anonymous reviewers for their insightful recommendations.
Conflicts of Interest
The authors declare that they have no conflicts of interest that may have influenced this research.
References
- Riede:, T.; Coyne, M.; Tafoya, B.; Baab, K. L. Postnatal Development of the Mouse Larynx: Negative Allometry, Age-Dependent Shape Changes, Morphological Integration, and a Size-Dependent Spectral Feature. J. Speech Lang. Hear.Res. 2020, 63, 2680–2694. [Google Scholar] [CrossRef]
- Joshi, M. M.; Joshi, S. S.; Joshi, S. D. The morphological study of adult human larynx in a Western Indian population. J. Laryngol. Voice 2011, 1, 50–54. [Google Scholar] [CrossRef]
- de La Vega, B.; Pombal, J. J. P.; Hepp, F. Description and evolution of the larynx of the Physalaemus olfersii species group, with remarks on the laryngeal anatomy of the P. cuvieri clade (Amphibia: Anura: Leiuperinae). J. Anat. 2021, 239, 557–582. [Google Scholar] [PubMed]
- Riede, T.; Brown, C. Size, Vocal Fold Length, and Fundamental Frequency - Implications for Mammal Vocal Communication. Nova Acta Leopoldina NF 2013, 111, 295–314. [Google Scholar]
- Souza Junior, P.; Carvalho, N. C.; Mattos, K.; Anjos, B. L.; Santos, A. L. Q. Morfologia da laringe em Cerdocyon thous (Linnaeus, 1766). Pesq. Vet. Bras. 2016, 36, 45–54. [Google Scholar] [CrossRef]
- Wahlberg, M.; Larsen, O. N. Propagation of sound. In Comparative Bioacoustics: An Overview; Brown, C., Riede, T., Eds.; Bentham Science Publishers: Sharjah, UAE, 2017. [Google Scholar]
- Sacchi, R.; Galeotti, P.; Fasola, M.; Gerzeli, G. Larynx morphology and sound production in three species of Testudinidae. J. Morphol. 2004, 261, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Riede, T.; Kobrina, A.; Bone, L.; Darwaiz, T.; Pasch, B. Mechanisms of sound production in deer mice (Peromyscus spp. ). J. Expl. Biol. 2022, 225, 1–12. [Google Scholar] [CrossRef]
- Borgard, H. L.; Baab, K.; Pasch, B.; Riede, T. The Shape of Sound: a Geometric Morphometrics Approach. J. Mammal. Evol. 2020, 27, 577–590. [Google Scholar] [CrossRef]
- Linville, S. E. Source characteristics of aged voice assessed from long-term average spectra. J. Voice 2002, 16, 472–479. [Google Scholar] [CrossRef]
- Ma, E. P.; Love, A. L. Electroglottographic evaluation of age and gender effects during sustained phonation and connected speech. J. Voice 2010, 24, 146–152. [Google Scholar] [CrossRef]
- Riede, T.; Titze, I. R. Vocal fold elasticity of the Rocky Mountain elk (Cervus elaphus nelsoni) - producing high fundamental frequency vocalization with a very long vocal fold. J Exp. Biol. 2008, 211, 2144–2154. [Google Scholar] [CrossRef] [PubMed]
- Titze, I. R. Physiologic and Acoustic Differences between Male and Female Voices. J. Acoust. Soc. Am. 1989, 85, 1699–1707. [Google Scholar] [CrossRef]
- Lenell, C.; Johnson, A. M. Sexual dimorphism in laryngeal muscle fibers and ultrasonic vocalizations in the adult rat. Laryngoscope 2017, 127, E270–E276. [Google Scholar] [CrossRef]
- Nishida, N.; Taguchi, A.; Motoyoshi, K.; Hyodo, M.; Gyo, K.; Desaki, J. Age-related changes in rat intrinsic laryngeal muscles: analysis of muscle fibers, muscle fiber proteins, and subneural apparatuses. Eur. Arch. Otorhinolaryngol. 2013, 270, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Sumruayphol, S.; Siribat, P.; Dujardin, J. P.; Dujardin, S.; Komalamisra, C.; Thaenkham, U. Fasciola gigantica, F. Hepatica and Fasciola intermediate forms: Geometric morphometrics and an artificial neural network to help morphological identification. PeerJ 2020, 8, e8598.
- Agbolade, O.; Nazri, A.; Yaakob, R.; Ghani, A. A.; Cheah, Y. K. Morphometric Analysis of 3D Soft-Tissue for Sexual Dimorphism in Human Face. Int. J. Morphol. 2020, 38, 367–373. [Google Scholar] [CrossRef]
- Rohlf, F. J. The Tps Series of Software. Hystrix, Ital. J. Mamm. 2015, 26, 9–12. [Google Scholar] [CrossRef]
- Adams, D. C.; Rohlf, F. J.; Slice, D. E. A field comes of age: Geometric morphometrics in the 21st century. Hystrix 2013, 24, 7–14. [Google Scholar] [CrossRef]
- Palmer, A. R.; Strobeck, C. Fluctuanting asymmetry: Measurement, Analysis, Patterns. Ann. Rev. Ecol. Syst. 1986, 17, 391–421. [Google Scholar] [CrossRef]
- Klingenberg, C. P.; Barluenga, M.; Meyer, A. Shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolution 2002, 56, 1909–1920. [Google Scholar]
- Chovalopoulou, M. E.; Papageorgopoulou, C.; Bertsatos, A. Cranium asymmetry in a modern Greek population sample of known age and sex. Int. J. Legal Med. 2017, 131, 803–812. [Google Scholar] [CrossRef]
- Klingenberg, C. P. MorphoJ: An Integrated Software Package for Geometric Morphometrics. Mol. Ecol. Res. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, J.; Kielska, E.; Orszulak, P.; Reymond, J. Measurements of pre- and postpubertal human larynx: a cadaver study. Surg. Radiol. Anat. 2008, 30, 191–199. [Google Scholar] [CrossRef]
- Frey, R.; Gebler, A.; Fritsch, G.; Nygrén, K.; Weissengruber, G. E. Nordic rattle: the hoarse vocalization and the inflatable laryngeal air sac of reindeer (Rangifer tarandus). J. Anat. 2007, 210, 131–159. [Google Scholar] [CrossRef] [PubMed]
- Tayama, N.; Kaga, K.; Chan, R. W.; Titze, I. R. Geometric characterization of the laryngeal cartilage framework for the purpose of biomechanical modeling. Ann. Otorhinolaryngol 2001, 110, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Charuta, A.; Dzierzecka, M.; Wysocki, J. Evaluation of sexual dimorphism in horses on the basis of the morphology and morphometry of the larynx. Bull. Vet. Inst. Pulawy 2009, 53, 477–486. [Google Scholar]
- Wysocki, J.; Kielska, E.; Janiuk, I.; Charuta, A. Analysis of larynx measurements and proportions in young and adult domestic pigs (Sus scropha domestica). Turk. J. Vet. Anim. Sci. 2010, 34, 339–347. [Google Scholar] [CrossRef]
- Kendal, K. A.; Leonardo, R. J. 15 Normal Vocal Fold Symmetry and Phase Characteristics. In Laryngeal Evaluation, 2010; pp 1-3.
- Eysholdt, U.; Rosanowski, F.; Hoppe, U. Vocal fold vibration irregularities caused by different types of laryngeal asymmetry. Eur. Arch. Otorhinolaryngol. 2003, 260, 412–417. [Google Scholar] [CrossRef]
- Enver, N.; Doruk, C.; Kara, E.; Kaşali, K.; Asliyuksek, H.; Basaran, B. Does Size Matter in Laryngology? Relation Between Body Height and Laryngeal Morphometry. J. Voice 2021, 35, 291–299. [Google Scholar] [CrossRef]
- López, C.; Quispe, M.; Villalón, A.; Concha, M. L.; Penna, M.; Velásquez, N. A. Geographic variation in the laryngeal morphology of a widely distributed South-American anuran: behavioural and evolutionary implications. Zool. J. Linnean Soc. 2020, 190, 140–148. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).