Submitted:
15 May 2023
Posted:
16 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Management Strategies in Sows and Suckling Pigs to Increase Colostrum Intake
2.1. Pain Management in Sows
2.2. Split-Suckling
3. Nutritional Strategies in Suckling Pigs to Improve Growth and Intestinal Maturity at Weaning
3.1. Strategies to Help Maximize Dry Matter Intake in Piglets Prior to Weaning
3.1.1. Provision of Solid Creep Feed Pre-Weaning
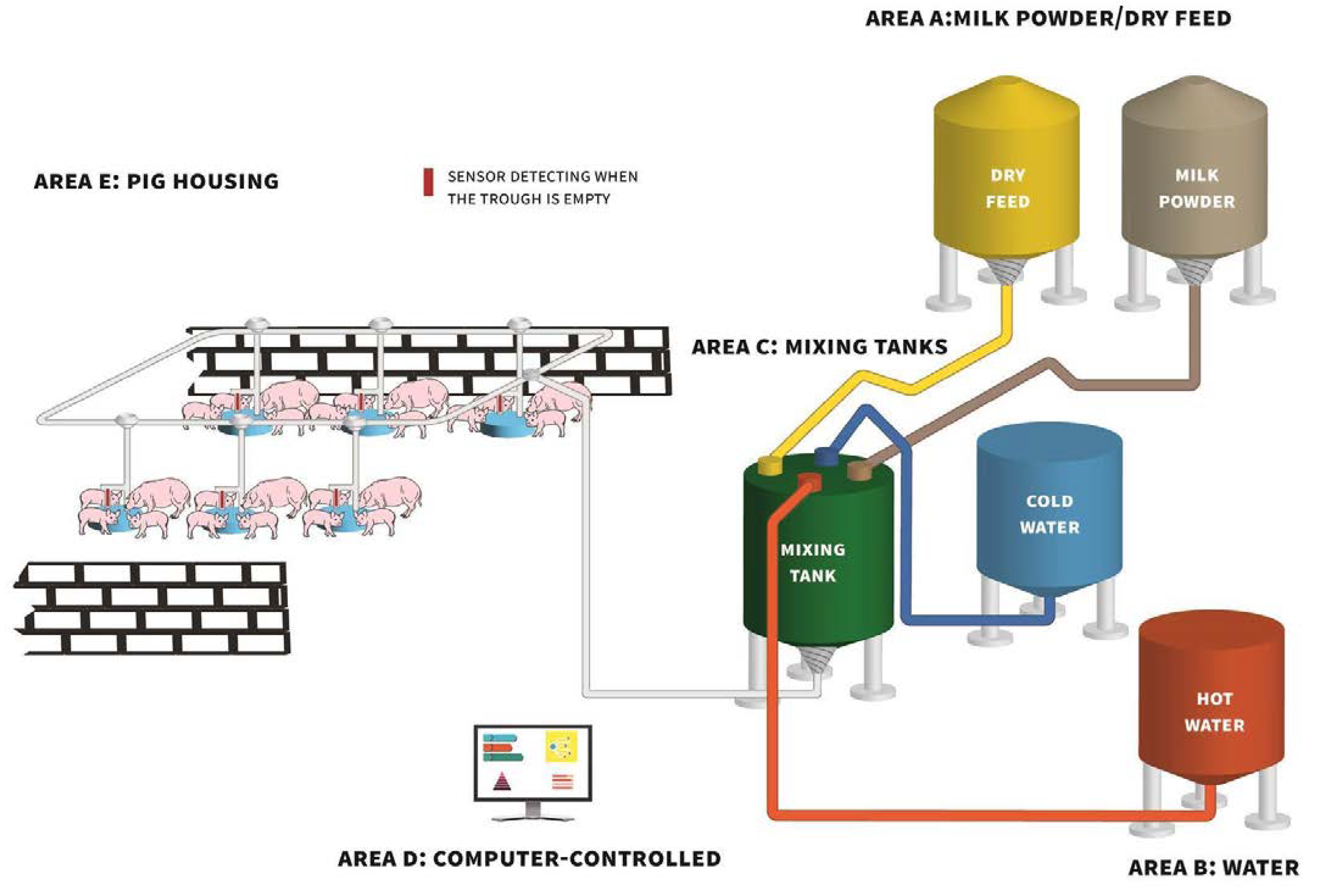
3.1.2. Provision of Supplemental Milk Pre-Weaning
| SA1 (days) | WA2 (days) | Pattern of Provision |
Pre-weaning effects (d0 = birth) | Post-weaning effects (d0 = weaning) | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Litter size | Supplemental Milk Intake | ADG3 | Weaning weight | Other | ADFI4 | ADG | FCR5 | Other | |||
| 1 | 21 | Ad libitum | 2.5 L of milk/pig (375 g DM6 cool season) 9.9 L of milk/pig (1.49 kg DM warm season) |
NA7 | ↑ | ↑ total litter weight= mortality↑ glucose, IGF-I8 and thyroxine in serum at weaning | NA | NA | NA | NA | [65] |
| 10.4 | |||||||||||
| 4 | 28 | Ad libitum | 4.76 L of cow’s milk/pig; 10.96 L artificial milk/pig (200 g total solids/ L) |
= from d0 to d14 ↑ from d14 to 28 ↑ from d0 to 28 |
↑ | NA | NA | NA | NA | NA | [71] |
| 12 | |||||||||||
| 10 | 20 | Ad libitum | 3.9 L of milk/pig (200 g of skim milk powder/ L) |
↑ | ↑ | NA | ↑ from d0 to d21 | ↑ from d0 to d21 | NA | ↑ weight at d21 | [67] |
| 12 | |||||||||||
| 3 | 21 | Ad libitum | 1000 g of milk powder /pig | = | ↑ | ↘ % mortality↗ number weaned | ↑ from 25 to 65 kg (grower period) | ↑ from 25 to 65 kg (grower period) | = | reached slaughter weight 3 days earlier | [63] |
| 12 | |||||||||||
| 3 | 26 | Ad libitum | 13.8 mL to 10.35 L of milk/ pig (winter); 43.7 mL to 17.25 L of milk/pig (summer) (150 g powder/L of water) |
= | ↑ | = % mortality= % medicated piglets | = from d0 to 42 | = from d0 to d42 | = from d0 to 42 | = % mortality= % medicated pigs | [72] |
| 10 to 11 | |||||||||||
| 4 | 21 | From 8:00 to 16:00 h daily |
NA (Trial 1 – late fall) 22 g of milk powder/pig (Trial 2 - summer) |
=(Trial 1) ↑(Trial 2) |
=(Trial 1) ↑(Trial 2) |
↘ % mortality (Trial 2) | NA | ↑ d21 to d54 (trial 1)= (trial 2) | NA | = carcass weight, back fat thickness, dressing percentage | [69] |
| 10 | |||||||||||
| 1 | 28 | Twice a day or as needed | 3.86 L/pig or 138 mL/pig/day (150 g of powder/L of water) |
= | = | = % mortality↑ antibiotic treatments | NA | = from d0 to d21, d21 to d72, d72 to 115 | NA | = % mortality | [66] |
| 11 to 12 | |||||||||||
| 2 | 28 | Ad libitum | 520 g of powder/pig (20 g/pig/day) |
= | = | ↑ number weaned↑ total litter weight= mortality, diarrhoea↓ treatment of facial lesions | NA | NA | NA | NA | [64] |
| 16.8 | |||||||||||
| 2 | 21 | Twice a day from 7:00 to 8:00 and from 15:00 to 16:00 h | From d0-d7: 75 g DM6 (litter/day) From d7-d14: 225 g DM (litter/day) From d14-21: 773 g DM (litter/day) |
NA | ↑ | ↑ IGF-18 gene expression at d21 in jejunum mucosa↑ small intestine weight at d21↑ crypt depth and ↓ villus height:crypt ratio in the ileum at d21↑ Volatile fatty acids in the colon at d21 | NA | NA | NA | NA | [62] |
| 13 to 14 | |||||||||||
| 22 | 27 | 200 ml/pig per day | 172.5 g of creep/pig | ↑ | ↑ | NA | ↑ from d0 to d14↑ from d14 to d28 | ↑ from d0 to d14↗ from d14 to d28 | = from d0 to d14↑ from d14 to d28 | NA | [68] |
| NA | |||||||||||
| 4 | 28 | Ad libitum | NA | = | NA | At d28 in colon:=bacterial diversity9=bacterial species richness10↑ VFA11↓ Lactobacillus, Clostridium XI, Blautia, Clostridium sensu stricto, Escherichia↑ Paraprevotella↗ Ruminococcus, Clostridium XIVa and IV, Succiniclasticum↑ TLR412 gene expression, ↓ IL-613 gene expression in mucosa | = from d0 to d7 | = from d0 to d7 | NA | ↘ diarrhea frequency | [75] |
| 8 | |||||||||||
| 4 | 28 | Ad libitum access, provision of fresh milk at 9:00 and 19:00h | NA | = | NA | = villus height, crypt depth in jejunum at d28↓ lactase activity and ↑ sucrase activity in jejunum | = | = | NA | In jejunum at d7:= villus height, crypt depth↑maltase activity↑ Lactobacillus↓ Streptococcus | [73] |
| 8 | |||||||||||
| 7 | 21 | Ad libitum access | NA | ↑ | ↑ | ↓ diarrheaAt d21, in jejunum:↑ bacterial species richness14= bacterial diversity↓ Romboutsia, Actinobacillus, Bacteroides and Lactobacillus | NA | NA | NA | NA | [74] |
| NA | |||||||||||
| 1 | 28 | From 15:00h on day 1 until weaning | For all piglets alive: From d1 to d12, 1.67 L/pig or 125 mL/pig/day) From d12 to d28, 3.2 L/pig or 200 mL/pig/day (150 g of powder/ L of water) |
NA | ↑ in litters of 17 piglets at d1 = in litters of 14 piglets at d1 |
↓ risk of piglets dying | NA | NA | NA | NA | [76] |
| 14 or 17 | |||||||||||
| 1 | 28 | From 15:00h on day 1 until weaning | NA | = | = | = body fat and body protein content | NA | NA | NA | NA | [77] |
| 14 or 17 | |||||||||||
3.1.3. Provision of Supplemental Liquid Feed Pre-Weaning
3.2. Other Pre-Weaning Strategies to Stimulate Earlier Enzyme Production
3.3. Other Pre-Weaning Strategies to Stimulate Gut Structure and Function
4. Conclusion
Acknowledgments
References
- Colson, V.; Martin, E.; Orgeur, P.; Prunier, A. Influence of housing and social changes on growth, behaviour and cortisol in piglets at weaning. Physiol Behav 2012, 107, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Klobasa, F.; Werhahn, E.; Butler, J.E. Composition of sow milk during lactation. Journal of Animal Science 1987, 64, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, P.G.; Gardiner, G.E.; Goodband, R.D. 10. Feeding the weaned piglets. In The suckling and weaned piglet, Farmer, C., Ed.; Wageningen Academic Publishers: 2020.
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr (Berl) 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front Immunol 2022, 13, 1042778. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends in Microbiology 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Canibe, N.; Højberg, O.; Kongsted, H.; Vodolazska, D.; Lauridsen, C.; Nielsen, T.S.; Schönherz, A.A. Review on Preventive Measures to Reduce Post-Weaning Diarrhoea in Piglets. Animals 2022, 12. [Google Scholar] [CrossRef]
- Bednorz, C.; Oelgeschläger, K.; Kinnemann, B.; Hartmann, S.; Neumann, K.; Pieper, R.; Bethe, A.; Semmler, T.; Tedin, K.; Schierack, P.; et al. The broader context of antibiotic resistance: zinc feed supplementation of piglets increases the proportion of multi-resistant Escherichia coli in vivo. Int J Med Microbiol 2013, 303, 396–403. [Google Scholar] [CrossRef]
- Iramiot, J.S.; Kajumbula, H.; Bazira, J.; Kansiime, C.; Asiimwe, B.B. Antimicrobial resistance at the human–animal interface in the Pastoralist Communities of Kasese District, South Western Uganda. Scientific Reports 2020, 10, 14737. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/4. REGULATION (EU) 2019/4 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 11 December 2018 on the manufacture, placing on the market and use of medicated feed, amending Regulation (EC) No 183/2005 of the European Parliament and of the Council and repealing Council Directive 90/167/EEC; THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION: Official Journal of the European Union, 2019. [Google Scholar]
- Zinc oxide Article-35 referral. Zinc oxide Article-35 referral - Annex I,II. EMEA/V/A/118, 2017.
- Oliviero, C. Offspring of hyper prolific sows: Immunity, birthweight, and heterogeneous litters. Mol Reprod Dev 2022. [Google Scholar] [CrossRef]
- King, R. Factors that influence milk production in well-fed sows. Journal of Animal Science 2000, 78. [Google Scholar] [CrossRef]
- Devillers, N.; Le Dividich, J.; Prunier, A. Influence of colostrum intake on piglet survival and immunity. Animal 2011, 5, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.L.; Pluske, J.R.; Morrison, R.S.; McDonald, T.N.; Smits, R.J.; Henman, D.J.; Stensland, I.; Dunshea, F.R. Post-weaning and whole-of-life performance of pigs is determined by live weight at weaning and the complexity of the diet fed after weaning. Animal nutrition 2017, 3, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Schoos, A.; Devreese, M.; Maes, D.G. Use of non-steroidal anti-inflammatory drugs in porcine health management. Vet Rec 2019, 185, 172. [Google Scholar] [CrossRef] [PubMed]
- Blavi, L.; Solà-Oriol, D.; Llonch, P.; López-Vergé, S.; Martín-Orúe, S.M.; Pérez, J.F. Management and Feeding Strategies in Early Life to Increase Piglet Performance and Welfare around Weaning: A Review. Animals (Basel) 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Edwards, S.A. Review: Improving the performance of neonatal piglets. animal 2022, 16, 100350. [Google Scholar] [CrossRef]
- Baxter, E.M.; Schmitt, O.; Pedersen, L.J. 3. Managing the litter from hyperprolific sows. In The suckling and weaned piglet, Farmer, C., Ed.; Wageningen Academic Publishers: 2020.
- Wensley, M.R.; Tokach, M.D.; Woodworth, J.C.; Goodband, R.D.; Gebhardt, J.T.; DeRouchey, J.M.; McKilligan, D. Maintaining continuity of nutrient intake after weaning. I. Review of pre-weaning strategies. Transl Anim Sci 2021, 5, txab021. [Google Scholar] [CrossRef] [PubMed]
- Middelkoop, A. Foraging in the farrowing room. PhD Thesis. Wageningen University, 2020.
- Huting, A.M.S.; Middelkoop, A.; Guan, X.; Molist, F. Using Nutritional Strategies to Shape the Gastro-Intestinal Tracts of Suckling and Weaned Piglets. Animals (Basel) 2021, 11. [Google Scholar] [CrossRef]
- Edwards, S.; Turpin, D.L.; Pluske, J. 9. Weaning age and its long-term influence on health and performance. 2020; pp. 225-250.
- De Vos, M.; Che, L.; Huygelen, V.; Willemen, S.; Michiels, J.; Van Cruchten, S.; Van Ginneken, C. Nutritional interventions to prevent and rear low-birthweight piglets. J Anim Physiol Anim Nutr (Berl) 2014, 98, 609–619. [Google Scholar] [CrossRef]
- Canibe, N.; O’Dea, M.; Abraham, S. Potential relevance of pig gut content transplantation for production and research. J Anim Sci Biotechnol 2019, 10, 55. [Google Scholar] [CrossRef]
- Tokach, M.; Scher Cemin, H.; Sulabo, R.; Goodband, R. Feeding the suckling pig: creep feeding. 2020; pp. 139-157.
- Rutherford, K.; Baxter, E.; D’Eath, R.; Turner, S.; Arnott, G.; Roehe, R.; Ask, B.; Sandøe, P.; Moustsen, V.; Thorup, F.; et al. The welfare implications of large litter size in the domestic pig I: Biologica factors. Animal Welfare 2013, 22, 199–218. [Google Scholar] [CrossRef]
- Hasan, S.; Orro, T.; Valros, A.; Junnikkala, S.; Peltoniemi, O.; Oliviero, C. Factors affecting sow colostrum yield and composition, and their impact on piglet growth and health. Livestock Science 2019, 227, 60–67. [Google Scholar] [CrossRef]
- Curtis, J.; Bourne, F.J. Immunoglobulin quantitation in sow serum, colostrum and milk and the serum of young pigs. Biochimica et Biophysica Acta 1971, 236, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Dividich, J.L.; Rooke, J.A.; Herpin, P. Nutritional and immunological importance of colostrum for the new-born pig. The Journal of Agricultural Science 2005, 143, 469–485. [Google Scholar] [CrossRef]
- Xu, R.J.; Sangild, P.T.; Zhang, Y.Q.; Zhang, S.H. Chapter 5 Bioactive compounds in porcine colostrum and milk and their effects on intestinal development in neonatal pigs11This work has been supported by the Research Grants Council of the Hong Kong Special Administrative Region, China (HKU 7234/98M). In Biology of Growing Animals, Zabielski, R., Gregory, P.C., Weström, B., Salek, E., Eds.; Elsevier: 2002; Volume 1, pp. 169-192.
- Quesnel, H.; Farmer, C.; Devillers, N. Colostrum intake: Influence on piglet performance and factors of variation. Livestock Science 2012, 146, 105–114. [Google Scholar] [CrossRef]
- Fraser, D. The role of behavior in swine production: A review of research. Applied Animal Ethology 1984, 11, 317–339. [Google Scholar] [CrossRef]
- Herskin, M.S.; Di Giminiani, P. 11 - Pain in pigs: Characterisation, mechanisms and indicators. In Advances in Pig Welfare, Špinka, M., Ed.; Woodhead Publishing: 2018; pp. 325-355.
- Kovac, G.; Tóthová, C.; Oskar, N.; H, S. Acute phase proteins during the reproductive cycle of sows. Acta veterinaria 2008, 58, 459–466. [Google Scholar] [CrossRef]
- European Medicines Agency - Science Medicines Health. Medicines. Available online: https://www.ema.europa.eu/en/medicines (accessed on 10/05/2023).
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-inflammatory Drugs (NSAIDs); Treasure Island (FL): StatPearls Publishing: 2021.
- Chaiamnuay, S.; Allison, J.J.; Curtis, J.R. Risks versus benefits of cyclooxygenase-2-selective nonsteroidal antiinflammatory drugs. Am J Health Syst Pharm 2006, 63, 1837–1851. [Google Scholar] [CrossRef]
- Mainau, E.; Temple, D.; Manteca, X. Experimental study on the effect of oral meloxicam administration in sows on pre-weaning mortality and growth and immunoglobulin G transfer to piglets. Preventive Veterinary Medicine 2016, 126, 48–53. [Google Scholar] [CrossRef]
- Navarro, E.; Mainau, E.; de Miguel, R.; Temple, D.; Salas, M.; Manteca, X. Oral Meloxicam Administration in Sows at Farrowing and Its Effects on Piglet Immunity Transfer and Growth. Frontiers in Veterinary Science 2021, 8, 574250. [Google Scholar] [CrossRef]
- Schoos, A.; Chantziaras, I.; Vandenabeele, J.; Biebaut, E.; Meyer, E.; Cools, A.; Devreese, M.; Maes, D. Prophylactic Use of Meloxicam and Paracetamol in Peripartal Sows Suffering From Postpartum Dysgalactia Syndrome. Frontiers in Veterinary Science 2020, 7, 603719. [Google Scholar] [CrossRef]
- Mainau, E.; Ruiz-de-la-Torre, J.L.; Dalmau, A.; Salleras, J.M.; Manteca, X. Effects of meloxicam (Metacam®) on post-farrowing sow behaviour and piglet performance. Animal 2012, 6, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Tenbergen, R.; Friendship, R.; Cassar, G.; Amezcua, M.; Haley, D. Investigation of the use of meloxicam post farrowing for improving sow performance and reducing pain. Journal of Swine Health and Production 2014, 22, 10–15. [Google Scholar]
- Tummaruk, P.; Sang-Gassanee, K. Effect of farrowing duration, parity number and the type of anti-inflammatory drug on postparturient disorders in sows: a clinical study. Trop Anim Health Prod 2013, 45, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Claeyé, E.; Beek, J.; Meyns, T.; Maes, D. Effect of ketoprofen treatment in the prevention of postpartum dysgalactia syndrome in sows. Vlaams Diergeneeskundig Tijdschrift 2015, 84, 127–132. [Google Scholar] [CrossRef]
- Viitasaari, E.; Hänninen, L.; Heinonen, M.; Raekallio, M.; Orro, T.; Peltoniemi, O.; Valros, A. Effects of post-partum administration of ketoprofen on sow health and piglet growth. Vet J 2013, 198, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Homedes, J.; Salichs, M.; Sabaté, D.; Sust, M.; Fabre, R. Effect of ketoprofen on pre-weaning piglet mortality on commercial farms. Vet J 2014, 201, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Ison, S.H.; Jarvis, S.; Hall, S.A.; Ashworth, C.J.; Rutherford, K.M.D. Periparturient Behavior and Physiology: Further Insight Into the Farrowing Process for Primiparous and Multiparous Sows. Front Vet Sci 2018, 5, 122. [Google Scholar] [CrossRef]
- Kuller, W.; Sietsma, S.; Hendriksen, S.; Sperling, D. Use of paracetamol in sows around farrowing: effect on health and condition of the sow, piglet mortality, piglet weight and piglet weight gain. Porcine Health Manag 2021, 7, 46. [Google Scholar] [CrossRef]
- Hirsch, A.C.; Philipp, H.; Kleemann, R. Investigation on the efficacy of meloxicam in sows with mastitis-metritis-agalactia syndrome. Journal of veterinary pharmacology and therapeutics 2003, 26, 355–360. [Google Scholar] [CrossRef]
- Arnaud, E.A.; Gardiner, G.E.; Halpin, K.M.; Ribas, C.; O’Doherty, J.V.; Sweeney, T.; Lawlor, P.G. Post-partum meloxicam administration to sows but not split-suckling increases piglet growth and reduces medicinal treatment of piglets. Unpublished 2023. [Google Scholar]
- Bandrick, M.; Pieters, M.; Pijoan, C.; Baidoo, S.K.; Molitor, T.W. Effect of cross-fostering on transfer of maternal immunity to Mycoplasma hyopneumoniae to piglets. Vet Rec 2011, 168, 100. [Google Scholar] [CrossRef] [PubMed]
- Donovan, T.S.; Dritz, S. Effects of split-nursing management on growth performance in nursing pigs. Kansas Agricultural Experiment Station Research Reports 1996. [Google Scholar] [CrossRef]
- Baxter, E.; Rutherford, K.; Arnott, G.; D’Eath, R.; Turner, S.; Jarvis, S.; Sandøe, P.; Moustsen, V.; Thorup, F.; Edwards, S.; et al. The welfare implications of large litter size in the domestic pig II: Management factors. Animal Welfare 2013, 22, 219–238. [Google Scholar] [CrossRef]
- Vandaele, M.; Van Kerschaver, C.; Degroote, J.; Van Ginneken, C.; Michiels, J. Piglet performance and colostrum intake in litters either or not split-suckled during the first day or during the first three days of life. Livestock Science 2020, 241, 104265. [Google Scholar] [CrossRef]
- Kyriazakis, I.; Edwards, S. The effect of “split-suckling” on behaviour and performance of piglets. Applied Animal Behaviour Science 1986, 16, 92. [Google Scholar] [CrossRef]
- Donovan, T.S.; Dritz, S.S. Effect of split nursing on variation in pig growth from birth to weaning. Journal of the American Veterinary Medical Association 2000, 217, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.M.; Langemeier, A.J.; Rathbun, T.J.; Davis, D.L. Immunocrit, colostrum intake, and preweaning body weight gain in piglets after split suckling based on birth weight or birth order. Translational Animal Science 2019, 3, 1460–1465. [Google Scholar] [CrossRef]
- Muns, R.; Manteca, X.; Gasa, J. Effect of different management techniques to enhance colostrum intake on piglets’ growth and mortality. Animal Welfare 2015, 24. [Google Scholar] [CrossRef]
- Galiot, L.; Lachance, I.; Laforest, J.-P.; Guay, F. Modelling piglet growth and mortality on commercial hog farms using variables describing individual animals, litters, sows and management factors. Animal reproduction science 2018, 188, 57–65. [Google Scholar] [CrossRef]
- Huser, J.; Plush, K.; Pitchford, W.; Kennett, T.; Lines, D. Neonatal split suckling improves survival of small piglets. Animal Production Science 2015, 55, 1477–1477. [Google Scholar] [CrossRef]
- De Greeff, A.; Resink, J.W.; van Hees, H.M.; Ruuls, L.; Klaassen, G.J.; Rouwers, S.M.; Stockhofe-Zurwieden, N. Supplementation of piglets with nutrient-dense complex milk replacer improves intestinal development and microbial fermentation. Journal of Animal Science 2016, 94, 1012–1019. [Google Scholar] [CrossRef]
- Wolter, B.F.; Ellis, M.; Corrigan, B.P.; DeDecker, J.M. The effect of birth weight and feeding of supplemental milk replacer to piglets during lactation on preweaning and postweaning growth performance and carcass characteristics. Journal of Animal Science 2002, 80, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Pustal, J.; Traulsen, I.; Preißler, R.; Müller, K.; Beilage, T.; Börries, U.; Kemper, N. Providing supplementary, artificial milk for large litters during lactation: Effects on performance and health of sows and piglets: A case study. Porcine Health Management 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Azain, M.J.; Tomkins, T.; Sowinski, J.S.; Arentson, R.A.; Jewell, D.E. Effect of supplemental pig milk replacer on litter performance: seasonal variation in response. J Anim Sci 1996, 74, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.L.; Edwards, S.A.; Kyriazakis, I. Management strategies to improve the performance of low birth weight pigs to weaning and their long-term consequences. Journal of Animal Science 2014, 92, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Dunshea, F.; Kerton, D.J.; Eason, P.; King, R.H. Supplemental skim milk before and after weaning improves growth performance of pigs. Crop and Pasture Science 1999, 50, 1165–1170. [Google Scholar] [CrossRef]
- Van Oostrum, M.; Lammers, A.; Molist, F. Providing artificial milk before and after weaning improves postweaning piglet performance. Journal of Animal Science 2016, 94, 429–432. [Google Scholar] [CrossRef]
- Park, B.C.; Ha, D.M.; Park, M.J.; Lee, C.Y. Effects of milk replacer and starter diet provided as creep feed for suckling pigs on pre- and post-weaning growth. Animal Science Journal 2014, 85, 872–878. [Google Scholar] [CrossRef]
- Kobek-Kjeldager, C.; Vodolazs’ka, D.; Lauridsen, C.; Canibe, N.; Pedersen, L.J. Impact of supplemental liquid feed pre-weaning and piglet weaning age on feed intake post-weaning. Livestock Science 2021, 252, 104680. [Google Scholar] [CrossRef]
- Dunshea, F.; Boyce, J.; King, R. Effect of supplemental nutrients on the growth performance of sucking pigs. Australian Journal of Agricultural Research - AUST J AGR RES 1998, 49. [Google Scholar] [CrossRef]
- Miller, Y.J.; Collins, A.M.; Smits, R.J.; Thomson, P.C.; Holyoake, P.K. Providing supplemental milk to piglets preweaning improves the growth but not survival of gilt progeny compared with sow progeny. J Anim Sci 2012, 90, 5078–5085. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Niu, Q.; Zhu, Y.; Shi, C.; Wang, J.; Zhu, W. Effects of early commercial milk supplement on the mucosal morphology, bacterial community and bacterial metabolites in jejunum of the pre- and post-weaning piglets. Asian-Australas Journal of Animal Sciences 2020, 33, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Jia, J.; Zhang, L.; Chen, Q.; Zhang, X.; Sun, W.; Ma, C.; Xu, F.; Zhan, S.; Ma, L.; et al. Jejunal inflammatory cytokines, barrier proteins and microbiome-metabolome responses to early supplementary feeding of Bamei suckling piglets. BMC Microbiology 2020, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhu, Y.; Niu, Q.; Wang, J.; Wang, J.; Zhu, W. The Changes of Colonic Bacterial Composition and Bacterial Metabolism Induced by an Early Food Introduction in a Neonatal Porcine Model. Curr Microbiol 2018, 75, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Kobek-Kjeldager, C.; Moustsen, V.A.; Theil, P.K.; Pedersen, L.J. Effect of litter size, milk replacer and housing on production results of hyper-prolific sows. Animal 2020, 14, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Kobek-Kjeldager, C.; Moustsen, V.A.; Pedersen, L.J.; Theil, P.K. Impact of litter size, supplementary milk replacer and housing on the body composition of piglets from hyper-prolific sows at weaning. Animal 2021, 15, 100007. [Google Scholar] [CrossRef]
- Lawlor, P.G.; Lynch, P.B.; Caffrey, P.J.; O’ Doherty, J.V. Effect of pre- and post-weaning management on subsequent pig performance to slaughter and carcass quality. Animal Science 2002, 75, 245–256. [Google Scholar] [CrossRef]
- Arnaud, E.A.; Gardiner, G.E.; Chombart, M.; O’Doherty, J.V.; Sweeney, T.; Lawlor, P.G. Effect of creep feeding solid starter diet, liquid milk and a liquid mixture of starter diet and milk to suckling pigs on their growth and medication usage Unpublished 2023.
- Byrgesen, N.; Madsen, J.G.; Larsen, C.; Kjeldsen, N.J.; Cilieborg, M.S.; Amdi, C. The Effect of Feeding Liquid or Dry Creep Feed on Growth Performance, Feed Disappearance, Enzyme Activity and Number of Eaters in Suckling Piglets. Animals (Basel) 2021, 11. [Google Scholar] [CrossRef]
- Martins, S.M.M.K.; Ferrin, M.O.; Poor, A.P.; Campos, G.A.; Torres, M.A.; Weigel, R.A.; Strefezzi, R.F.; Andrade, A.F.C. Gruel creep feed provided from 3 days of age did not affect the market weight and the sow’s catabolic state. Livestock Science 2020, 231, 103883. [Google Scholar] [CrossRef]
- Amdi, C.; Pedersen, M.L.M.; Klaaborg, J.; Myhill, L.J.; Engelsmann, M.N.; Williams, A.R.; Thymann, T. Pre-weaning adaptation responses in piglets fed milk replacer with gradually increasing amounts of wheat. Br J Nutr 2021, 126, 375–382. [Google Scholar] [CrossRef]
- Van den Borne, J.J.; Weström, B.R.; Kruszewska, D.; Botermans, J.A.; Svendsen, J.; Woliński, J.; Pierzynowski, S.G. Exocrine pancreatic secretion in pigs fed sow’s milk and milk replacer, and its relationship to growth performance. J Anim Sci 2007, 85, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Pierzynowski, S.G.; Weström, B.R.; Svendsen, J.; Karlsson, B.W. Development of exocrine pancreas function in chronically cannulated pigs during 1-13 weeks of postnatal life. J Pediatr Gastroenterol Nutr 1990, 10, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Pierzynowski, S.G.; Weström, B.R.; Erlanson-Albertsson, C.; Ahre’n, B.; Svendsen, J.; Karlsson, B.W. Induction of exocrine pancreas maturation at weaning in young developing pigs. J Pediatr Gastroenterol Nutr 1993, 16, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Słupecka, M.; Woliński, J.; Prykhodko, O.; Ochniewicz, P.; Gruijc, D.; Fedkiv, O.; Weström, B.R.; Pierzynowski, S.G. Stimulating effect of pancreatic-like enzymes on the development of the gastrointestinal tract in piglets. J Anim Sci 2012, 90 Suppl 4, 311–314. [Google Scholar] [CrossRef]
- Prykhodko, O.; Pierzynowski, S.G.; Nikpey, E.; Arevalo Sureda, E.; Fedkiv, O.; Weström, B.R. Pancreatic and pancreatic-like microbial proteases accelerate gut maturation in neonatal rats. PLoS One 2015, 10, e0116947. [Google Scholar] [CrossRef] [PubMed]
- Prykhodko, O.; Fedkiv, O.; Szwiec, K.; Botermans, J.; Weström, B.; Pierzynowski, S. Early treatment with pancreatic-like microbial-derived enzymes during the preweaning period promotes growth in growing–finishing pigs. Journal of Animal Science 2016, 94, 150–152. [Google Scholar] [CrossRef]
- Teixeira, A.; Nogueira, E.; Kutschenko, M.; Rostagno, H.; Lopes, D. Inclusion of glutamine associated with glutamic acid in the diet of piglets weaned at 21 days of age. Revista Brasileira de Saúde e Produção Animal 2014, 15, 881–896. [Google Scholar] [CrossRef]
- Wu, G.; Meier, S.A.; Knabe, D.A. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr 1996, 126, 2578–2584. [Google Scholar] [CrossRef]
- Domeneghini, C.; Di Giancamillo, A.; Bosi, G.; Arrighi, S. Can nutraceuticals affect the structure of intestinal mucosa? Qualitative and quantitative microanatomy in L-glutamine diet-supplemented weaning piglets. Vet Res Commun 2006, 30, 331–342. [Google Scholar] [CrossRef]
- Molino, J.; Donzele, J.; Oliveira, R.; Haese, D.; Fortes, E.; Souza, M.F.D.S. L-glutamine and L-glutamate in diets with different lactose levels for piglets weaned at 21 days of age. Revista Brasileira de Zootecnia 2012, 41, 98–105. [Google Scholar] [CrossRef]
- Rezaei, R.; Knabe, D.A.; Tekwe, C.D.; Dahanayaka, S.; Ficken, M.D.; Fielder, S.E.; Eide, S.J.; Lovering, S.L.; Wu, G. Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 2013, 44, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Knabe, D.A. Free and protein-bound amino acids in sow’s colostrum and milk. J Nutr 1994, 124, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Watford, M. Glutamine and glutamate: Nonessential or essential amino acids? Anim Nutr 2015, 1, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Haynes, T.E.; Li, P.; Li, X.; Shimotori, K.; Sato, H.; Flynn, N.E.; Wang, J.; Knabe, D.A.; Wu, G. L-Glutamine or L-alanyl-L-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 2009, 37, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, R.A.; Usry, J.L.; Arrellano, C.; Nogueira, E.T.; Kutschenko, M.; Moeser, A.J.; Odle, J. Effects of creep feeding and supplemental glutamine or glutamine plus glutamate (Aminogut) on pre- and post-weaning growth performance and intestinal health of piglets. J Anim Sci Biotechnol 2013, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livestock Production Science 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Ayuso, M.; Irwin, R.; Walsh, C.; Van Cruchten, S.; Van Ginneken, C. Low birth weight female piglets show altered intestinal development, gene expression, and epigenetic changes at key developmental loci. Faseb j 2021, 35, e21522. [Google Scholar] [CrossRef]
- Li, Z.; Sciascia, Q.L.; Görs, S.; Nguyen, N.; Rayatdoost Baghal, F.; Schregel, J.; Tuchscherer, A.; Zentek, J.; Metges, C.C. Glutamine supplementation moderately affects growth, plasma metabolite and free amino acid patterns in neonatal low birth weight piglets. British Journal of Nutrition 2022, 128, 2330–2340. [Google Scholar] [CrossRef]
- Schulze Holthausen, J.; Schregel, J.; Sciascia, Q.L.; Li, Z.; Tuchscherer, A.; Vahjen, W.; Metges, C.C.; Zentek, J. Effects of Oral Glutamine Supplementation, Birthweight and Age on Colonic Morphology and Microbiome Development in Male Suckling Piglets. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
- Rudar, M.; Fiorotto, M.L.; Davis, T.A. Regulation of Muscle Growth in Early Postnatal Life in a Swine Model. Annu Rev Anim Biosci 2019, 7, 309–335. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Johnson, G.A.; Knabe, D.A.; Burghardt, R.C.; Spencer, T.E.; Li, X.L.; Wang, J.J. TRIENNIAL GROWTH SYMPOSIUM: Important roles for L-glutamine in swine nutrition and production1,2. Journal of Animal Science 2011, 89, 2017–2030. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Albrecht, E.; Stange, K.; Li, Z.; Schregel, J.; Sciascia, Q.L.; Metges, C.C.; Maak, S. Glutamine supplementation stimulates cell proliferation in skeletal muscle and cultivated myogenic cells of low birth weight piglets. Sci Rep 2021, 11, 13432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Albrecht, E.; Sciascia, Q.L.; Li, Z.; Görs, S.; Schregel, J.; Metges, C.C.; Maak, S. Effects of Oral Glutamine Supplementation on Early Postnatal Muscle Morphology in Low and Normal Birth Weight Piglets. Animals (Basel) 2020, 10. [Google Scholar] [CrossRef] [PubMed]
| Area | Strategies | Review |
|---|---|---|
| Sow management |
|
[16], [17] |
|
[18] | |
|
[18], [19] | |
|
[18], [17], [19] | |
| Sow nutrition |
|
[20][17][17], [20][17][17][18] |
| Piglet management |
|
[18], [19], [20] |
|
[17], [18], [19], [20] | |
|
[18], [19] | |
|
[18][18][21] | |
|
[3], [20], [22], [23] | |
|
[24] | |
|
[20] | |
|
[18], [19] | |
|
[20] | |
| Piglet nutrition | Injection of glucose (energy booster) | [18] |
|
[22][17], [18][18][22][25] | |
|
[20], [22], [26][17], [22][17], [18], [19],[22] | |
|
[22][22][22][26][22][22][22] |
| Medication | Dose | Route of administration | Timing | Effects on sows | Effects on piglets | Reference |
|---|---|---|---|---|---|---|
| Meloxicam | 0.4 mg/kg BW1 | Intramuscular | ~90 min post-partum |
↓ time lying during day 3 post-partum = FI2 = RT3 |
= mortality ↑ ADG4 of low birth weight piglets (<1180 g) from multiparous sows |
[42] |
| Meloxicam | 0.4 mg/kg BW | Intramuscular | ~12 hours post-partum |
= RT | = mortality ↑ litter size at weaning ↗ weight gain in litter of 11-13 pigs |
[43] |
| Meloxicam | 0.4 mg/kg BW | Oral gavage | Beginning of farrowing |
NS5 | = mortality ↑ADG and weaning weight ↑ IgG6 in serum at day 1 and 2 |
[39] |
| Meloxicam/ paracetamol |
0.4/30 mg/kg BW | Oral gavage | Once a day for 7 days from day 113 of gestation (sows with PDS7) |
= RT paracetamol ↓ RT vs meloxicam |
= mortality = ADG |
[41] |
| Meloxicam | 0.4 mg/kg BW | Oral gavage | Beginning of farrowing |
↗ colostrum IgA8 and IgG | = mortality ↗ADG from day 9 to weaning ↑ IgA in serum at day 1 and 9 ↗ IL-29 and IL-49 in serum at day 9 |
[40] |
| Meloxicam | 0.4 mg/kg BW | Intramuscular | ~2 hours post-partum |
= back fat at weaning ↓ body weight at weaning |
↗ colostrum intake = mortality ↓ antibiotics/anti-inflammatories ↑ ADG and weaning weight ↑ slaughter weight |
[51] |
| Meloxicam/ Flunixin (no untreated sows) |
0.4 / 2 mg/kg BW | Intramuscular | 1.5–24 hours post-clinical PDS signs | FI: meloxicam = flunixin RT: meloxicam = flunixin |
Mortality: meloxicam < flunixin ADG: meloxicam = flunixin |
[50] |
| Flunixin/ Metamizole (no untreated sows) |
0.5/20 mg/kg BW | Intramuscular | End of parturition +24 hours later if needed | Flunixin: ↓ RT (day 1 vs day 3 post-partum) Metamizole: = RT (day 1 vs day 3 post-partum) |
NS | [44] |
| Paracetamol | 20 ml of paracetamol (400 mg/ml) | Over the feed divided over two meals |
6 days from 3 days before farrowing to 2 days post-partum | = RT ↑ back fat at weaning |
= mortality = ADG = IgG in serum at day 1 |
[49] |
| Ketoprofen | 3 mg/kg BW | Intramuscular | During 3 days post-partum | ↓ incidence of feed refusal ↑ back fat at week 2 ↓ constipation duration |
= ADG | [46] |
| Ketoprofen | 3 mg/kg BW | Intramuscular | Within 12 hours post-partum | NS | ↓ mortality ↑ litter size at weaning |
[47] |
| Ketoprofen | 1 mg/kg BW | Intramuscular | Within 12 hours post-partum | = back fat at weaning ↓ RT |
↘ mortality = weight gain per litter |
[45] |
| Ketoprofen | 3 mg/kg BW | Intramuscular | 1.5 hours post-partum | = putative pain behaviors = salivary cortisol = C-Reactive Protein = cytokines |
NS | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





