Submitted:
11 May 2023
Posted:
16 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction:
2. Materials and Methods
2.1. Study population
2.2. Pollutant and Meteorological Data
2.3. Statistical analyses
3. Results
3.1. Air pollutants and meteorological results
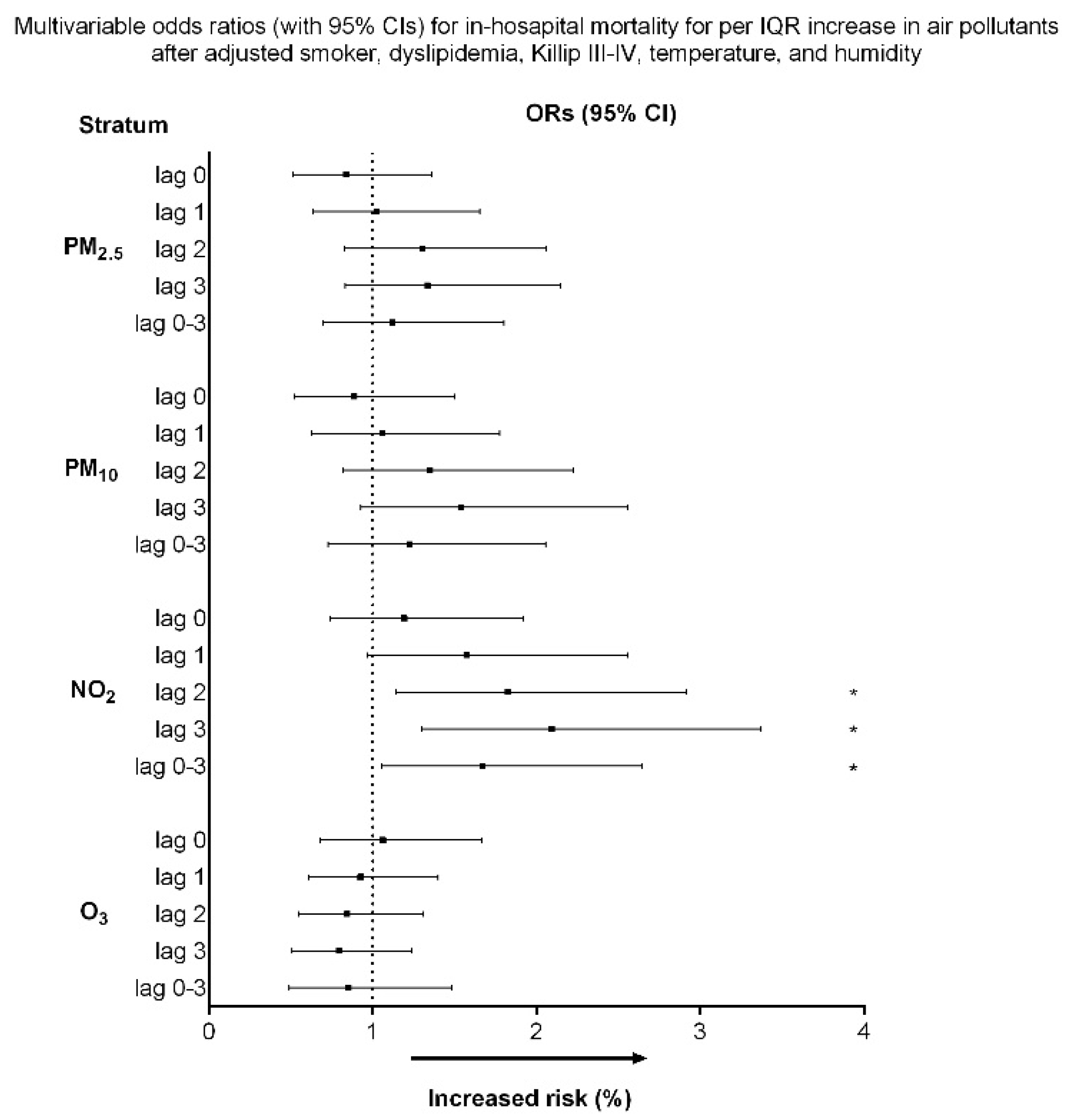
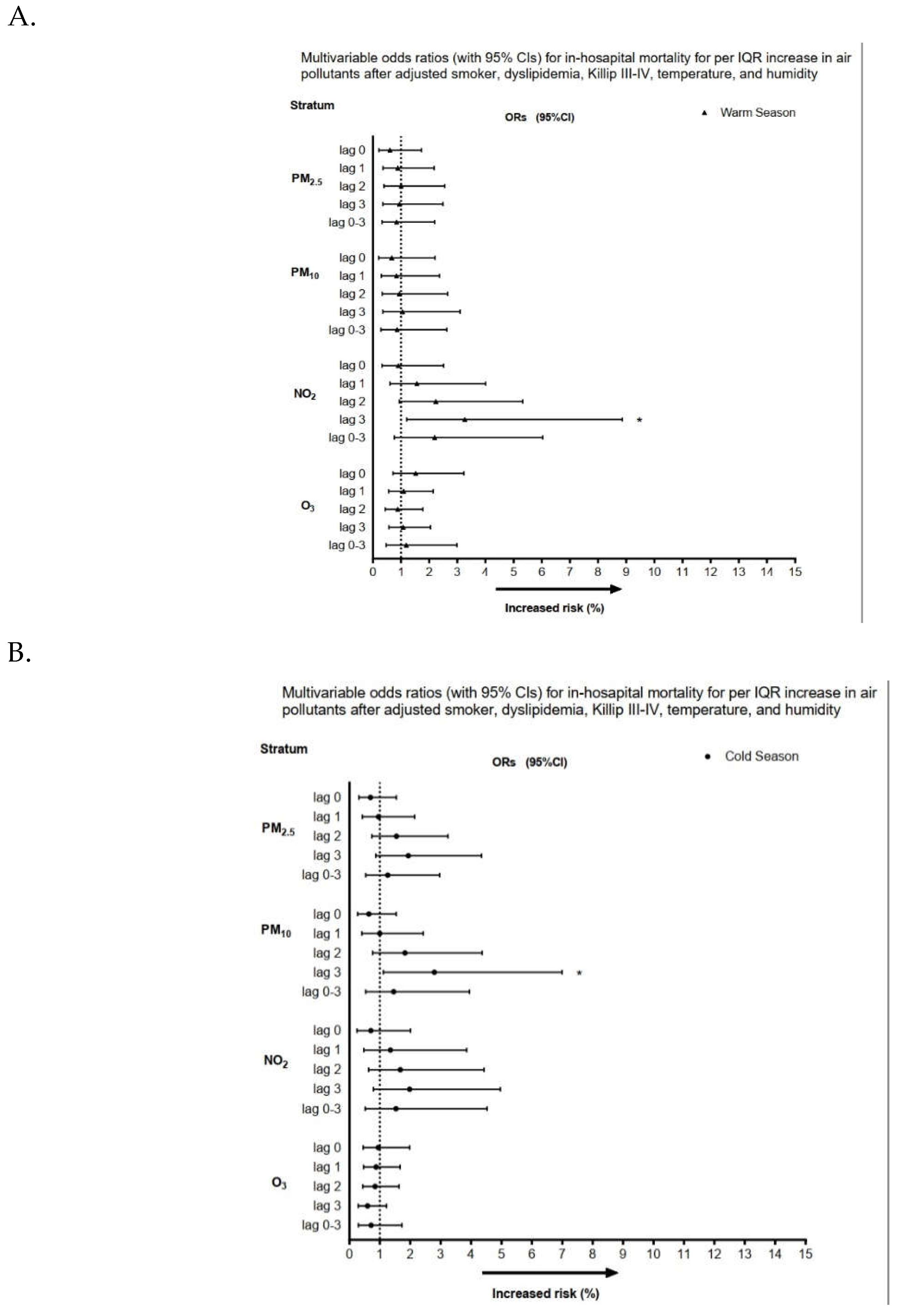
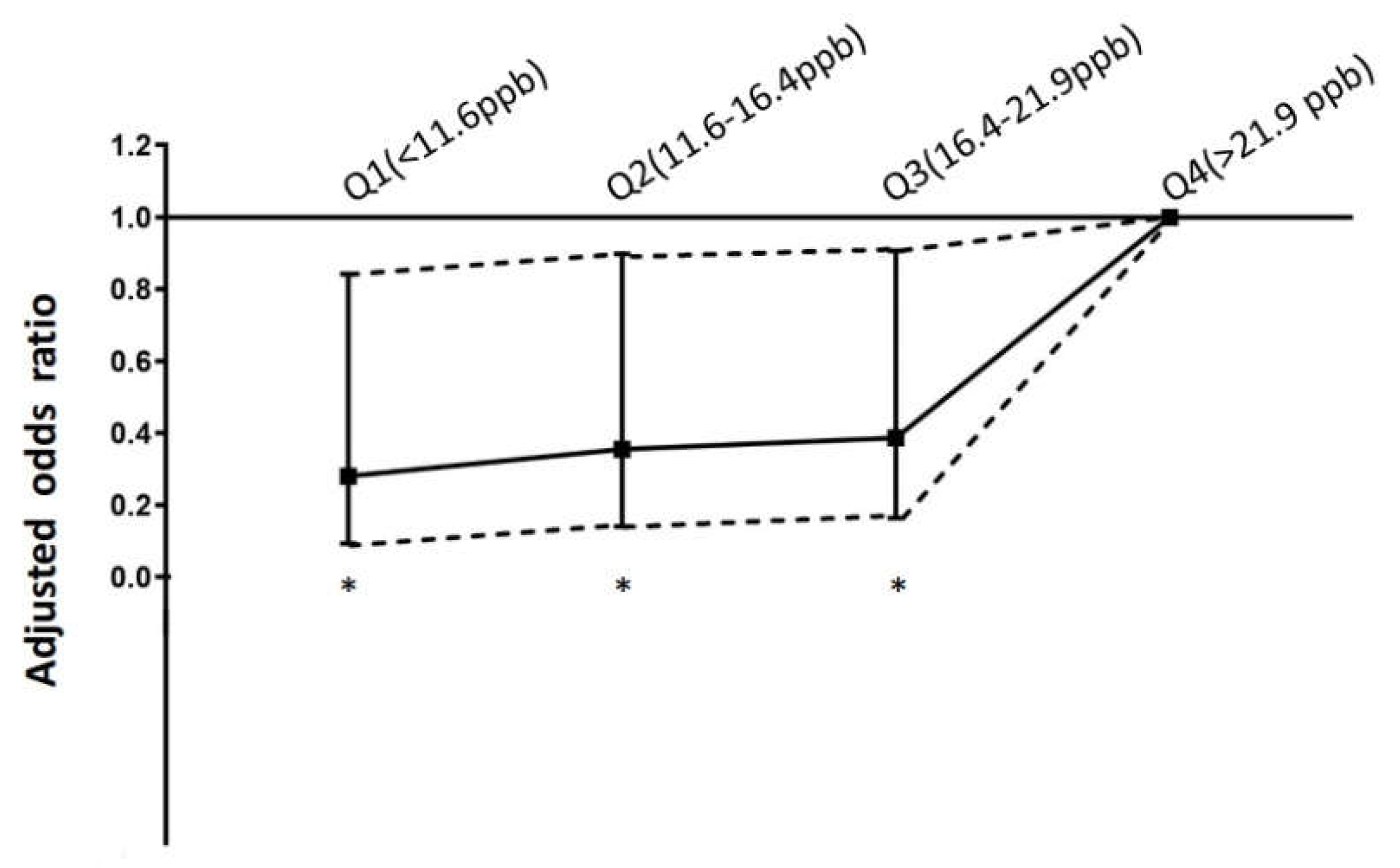
3.2. Association between air pollutants exposure and in-hospital mortality for STEMI
4. Discussion
4.1. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ho, Y.N.; et al. Fine particulate matter constituents associated with emergency room visits for pediatric asthma: a time-stratified case-crossover study in an urban area. BMC Public Health 2021, 21, 1593. [Google Scholar] [CrossRef] [PubMed]
- Baneras, J.; et al. Short-term exposure to air pollutants increases the risk of ST elevation myocardial infarction and of infarct-related ventricular arrhythmias and mortality. Int J Cardiol 2018, 250, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Rhinehart, Z.J.; et al. Association of Fine Particulate Matter and Risk of Stroke in Patients With Atrial Fibrillation. JAMA Netw Open 2020, 3, e2011760. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; et al. Association between Ambient Air Pollution and Emergency Room Visits for Pediatric Respiratory Diseases: The Impact of COVID-19 Pandemic. Toxics 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- de Bont, J.; et al. Ambient air pollution and cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. J Intern Med 2022, 291, 779–800. [Google Scholar] [CrossRef]
- Liang, S.; et al. Repeat dose exposure of PM2.5 triggers the disseminated intravascular coagulation (DIC) in SD rats. Sci Total Environ 2019, 663, 245–253. [Google Scholar] [CrossRef]
- Maciejczyk, P.; et al. Association of Cardiovascular Responses in Mice with Source-apportioned PM2.5 Air Pollution in Beijing. Aerosol and Air Quality Research 2018, 18, 1839–1852. [Google Scholar] [CrossRef]
- Lin, C.I.; et al. Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice. Int J Biol Sci 2018, 14, 253–265. [Google Scholar] [CrossRef]
- Aztatzi-Aguilar, O.G.; et al. Early kidney damage induced by subchronic exposure to PM2. 5 in rats. Part Fibre Toxicol 2016, 13, 68. [Google Scholar] [CrossRef]
- Go, A.S.; et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014, 129, 399–410. [Google Scholar] [CrossRef]
- Ghaffari, S.; et al. Air pollution and admissions due to ST elevation myocardial infarction-a time-series study from northwest of Iran. Environ Sci Pollut Res Int 2017, 24, 27469–27475. [Google Scholar] [CrossRef]
- Liu, Y.; et al. Short-Term Exposure to Ambient Air Pollution and Mortality From Myocardial Infarction. J Am Coll Cardiol 2021, 77, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; et al. Association between Particulate Matter Exposure and Short-term Prognosis in Patients with Pneumonia. Aerosol and Air Quality Research 2020, 20, 89–96. [Google Scholar] [CrossRef]
- Ishii, M.; et al. Association of short-term exposure to air pollution with myocardial infarction with and without obstructive coronary artery disease. Eur J Prev Cardiol 2021, 28, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; et al. Ambient air pollution is associated with pediatric pneumonia: a time-stratified case–crossover study in an urban area. Environmental Health 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; et al. Ambient Air Pollution and Risk for Stroke Hospitalization: Impact on Susceptible Groups. Toxics 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Kuzma, L.; et al. Exposure to air pollution-a trigger for myocardial infarction? A nine-year study in Bialystok-the capital of the Green Lungs of Poland (BIA-ACS registry). Int J Hyg Environ Health 2020, 229, 113578. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; et al. PM2.5 Induces the Expression of Inflammatory Cytokines via the Wnt5a/Ror2 Pathway in Human Bronchial Epithelial Cells. Int J Chron Obstruct Pulmon Dis 2020, 15, 2653–2662. [Google Scholar] [CrossRef]
- Chen, R.; et al. Fine Particulate Air Pollution and the Expression of microRNAs and Circulating Cytokines Relevant to Inflammation, Coagulation, and Vasoconstriction. Environ Health Perspect 2018, 126, 017007. [Google Scholar] [CrossRef]
- Xu, X.; et al. TP53-dependent autophagy links the ATR-CHEK1 axis activation to proinflammatory VEGFA production in human bronchial epithelial cells exposed to fine particulate matter (PM2.5). Autophagy 2016, 12, 1832–1848. [Google Scholar] [CrossRef]
- Dadvand, P.; et al. Air pollution and biomarkers of systemic inflammation and tissue repair in COPD patients. Eur Respir J 2014, 44, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; et al. Urban Particulate Matter Induces Changes in Gene Expression in Vascular Endothelial Cells that Are Associated with Altered Clot Structure In Vitro. Thromb Haemost 2018, 118, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Hassanvand, M.S.; et al. Short-term effects of particle size fractions on circulating biomarkers of inflammation in a panel of elderly subjects and healthy young adults. Environ Pollut 2017, 223, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Lane, K.J.; et al. Association of modeled long-term personal exposure to ultrafine particles with inflammatory and coagulation biomarkers. Environ Int 2016, 92–93, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Pálková, L.; et al. The aryl hydrocarbon receptor-mediated and genotoxic effects of fractionated extract of standard reference diesel exhaust particle material in pulmonary, liver and prostate cells. Toxicol In Vitro. 2015, 29, 438–448. [Google Scholar] [CrossRef]
- Zhu, N.; et al. Environmental nitrogen dioxide (NO2) exposure influences development and progression of ischemic stroke. Toxicol Lett 2012, 214, 120–130. [Google Scholar] [CrossRef]
- Yuan, C.S.; et al. Kidney damage induced by repeated fine particulate matter exposure: Effects of different components. Sci Total Environ 2022, 847, 157528. [Google Scholar] [CrossRef]
- Hsu, W.H.; et al. Seasonal and temperature modifications of the association between fine particulate air pollution and cardiovascular hospitalization in New York state. Sci Total Environ 2017, 578, 626–632. [Google Scholar] [CrossRef]
- Huang, Y.T.; et al. Short-Term Effects of Particulate Matter and Its Constituents on Emergency Room Visits for Chronic Obstructive Pulmonary Disease: A Time-Stratified Case-Crossover Study in an Urban Area. Int J Environ Res Public Health 2021, 18. [Google Scholar] [CrossRef]
- Grivas, G.; et al. Elemental Composition and Source Apportionment of Fine and Coarse Particles at Traffic and Urban Background Locations in Athens, Greece. Aerosol and Air Quality Research 2018, 18, 1642–1659. [Google Scholar] [CrossRef]
- Altemose, B.; et al. Association of air pollution sources and aldehydes with biomarkers of blood coagulation, pulmonary inflammation, and systemic oxidative stress. J Expo Sci Environ Epidemiol 2017, 27, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.L.; et al. Effects of fine particulate matter and its constituents on emergency room visits for asthma in southern Taiwan during 2008-2010: a population-based study. Environ Sci Pollut Res Int 2017, 24, 15012–15021. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.-S.; et al. Repeated exposure to fine particulate matter constituents lead to liver inflammation and proliferative response in mice. Ecotoxicology and Environmental Safety 2021, 224, 112636. [Google Scholar] [CrossRef]
- Xiao, Q.; et al. Pediatric emergency department visits and ambient Air pollution in the U.S. State of Georgia: a case-crossover study. Environ Health 2016, 15, 115. [Google Scholar] [CrossRef]
- Imaizumi, Y.; Eguchi, K.; Kario, K. Coexistence of PM2.5 and low temperature is associated with morning hypertension in hypertensives. Clin Exp Hypertens 2015, 37, 468–472. [Google Scholar] [CrossRef]
| Survival to discharge | In-hospital mortality | ||
|---|---|---|---|
| Characteristics | N = 947 | N = 56 | P |
| Male | 789 | 46 | 0.819 |
| Age | 60.3±12.7 | 60.1 ± 12.9 | 0.652 |
| Diabetes | 359 | 24 | 0.459 |
| Hypertension | 595 | 34 | 0.75 |
| Current smoker | 531 | 17 | <0.001 |
| Dyslipidemia | 696 | 32 | 0.008 |
| Killip III to IV | 193 | 38 | <0.001 |
| Body mass index | 25.4 ± 3.7 | 24.6 ± 5.1 | 0.192 |
| History of coronary atery disease | 53 | 4 | 0.627 |
| PM2.5, μg/m3 | |||
| lag 0 | 34.3 ± 19.6 | 31.9 ± 18.3 | 0.363 |
| lag 1 | 33.9 ± 19.7 | 34.1 ± 17.7 | 0.954 |
| lag 2 | 33.6 ± 19.2 | 36.6 ± 19.6 | 0.261 |
| lag 3 | 33.7 ± 19.0 | 36.2 ±21.3 | 0.345 |
| lag 0–3 | 33.8 ± 17.7 | 34.7 ± 17.7 | 0.724 |
| PM10,μg/m3 | |||
| lag 0 | 65.3 ± 29.8 | 63.1 ± 28.5 | 0.595 |
| lag 1 | 65.0 ± 30.1 | 66.2 ± 26.7 | 0.766 |
| lag 2 | 64.8 ± 29.3 | 69.8 ±30.3 | 0.217 |
| lag 3 | 64.8 ± 29.2 | 70.7 ± 32.9 | 0.145 |
| lag 0–3 | 65.2 ± 26.9 | 67.9 ± 26.9 | 0.456 |
| NO2, ppb | |||
| lag 0 | 17.7 ± 6.5 | 18.7 ± 7.3 | 0.252 |
| lag 1 | 17.6 ± 6.6 | 19.3 ± 7.1 | 0.075 |
| lag 2 | 17.6 ± 6.7 | 19.9 ± 7.9 | 0.017 |
| lag 3 | 17.8 ± 6.7 | 20.4 ± 7.8 | 0.005 |
| lag 0–3 | 17.7 ± 6.1 | 19.6 ± 7.0 | 0.027 |
| O3, ppb | |||
| lag 0 | 28.5 ± 12.2 | 28.3 ± 12.2 | 0.883 |
| lag 1 | 28.6 ± 12.7 | 27.9 ± 14.8 | 0.677 |
| lag 2 | 28.2 ± 12.5 | 27.1 ± 11.8 | 0.5 |
| lag 3 | 28.3 ± 12.7 | 26.8 ± 11.4 | 0.403 |
| lag 0–3 | 28.4 ± 10.7 | 27.5 ± 10.4 | 0.543 |
| Minimum | Percentiles | Maximum | Mean | |||
|---|---|---|---|---|---|---|
| 25% | 50% | 75% | ||||
| PM2.5 μg/m3 | 1.6 | 16.1 | 29.9 | 44.1 | 120.8 | 31.3± 17.8 |
| PM10 μg/m3 | 16.1 | 37.0 | 61.0 | 84.7 | 181.0 | 63.5± 28.8 |
| NO2 (ppb) | 4.8 | 11.6 | 16.4 | 21.9 | 35.0 | 17.1± 7.4 |
| O3 (ppb) | 3.5 | 18.6 | 27.1 | 36.6 | 61.7 | 28.4±12.4 |
| Temperature (°C) | 7.1 | 22.5 | 26.5 | 29.0 | 32.1 | 25.5±4.2 |
| Humidity (%) | 35.3 | 70.4 | 73.8 | 77.4 | 94.4 | 74.0 ± 6.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





