Submitted:
15 May 2023
Posted:
16 May 2023
You are already at the latest version
Abstract
Keywords:
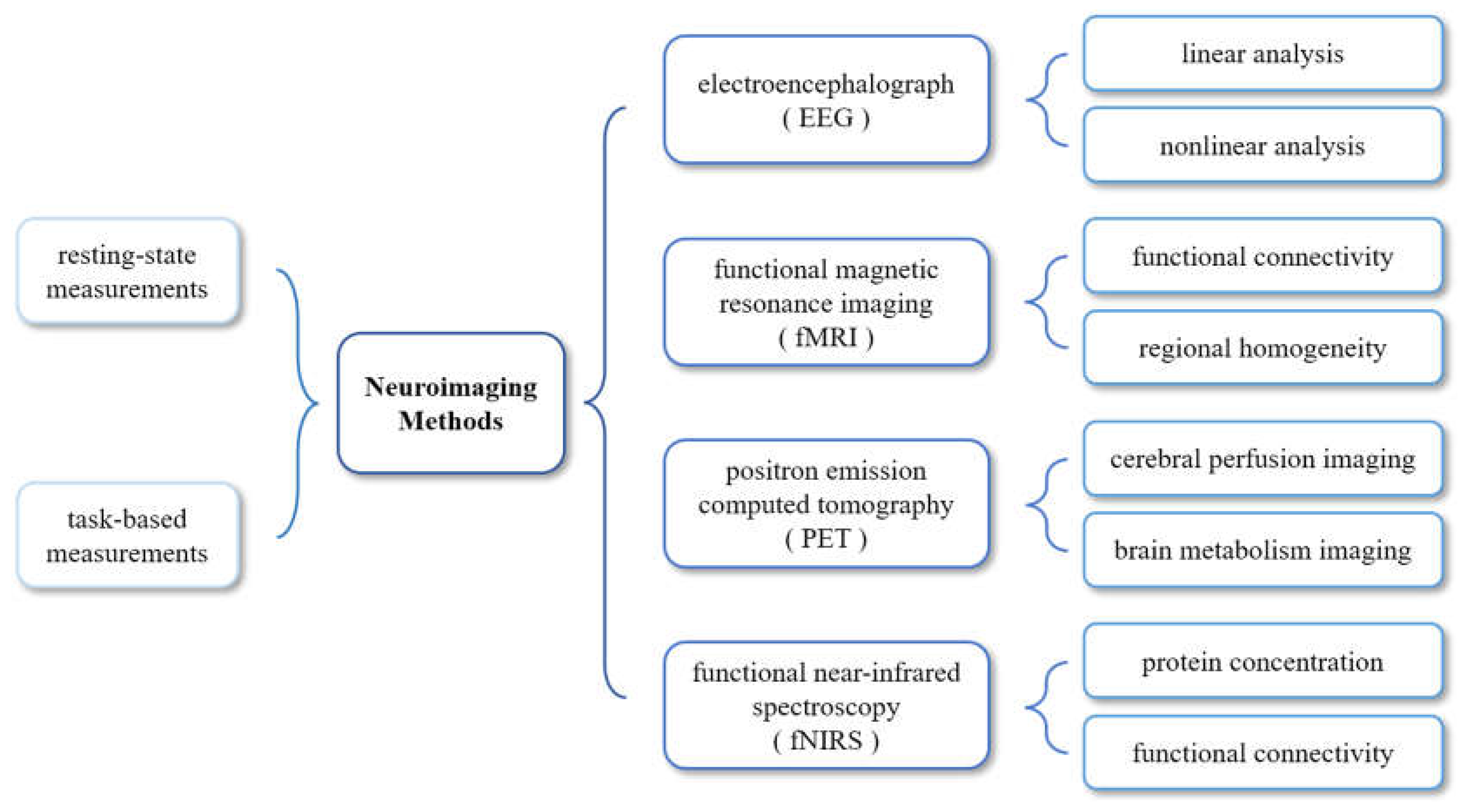
1. Introduction
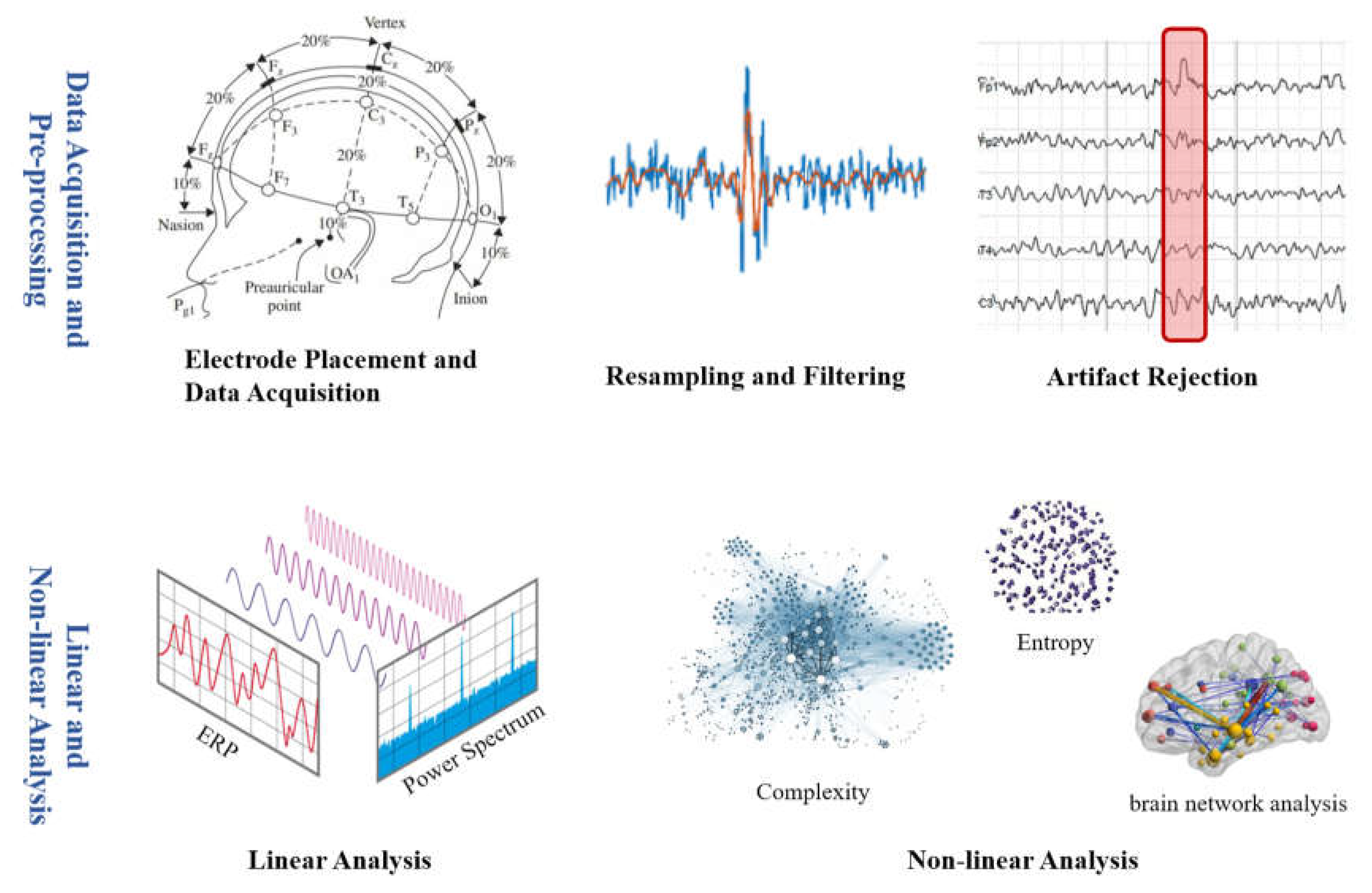
2. EEG Used in DoC Studies
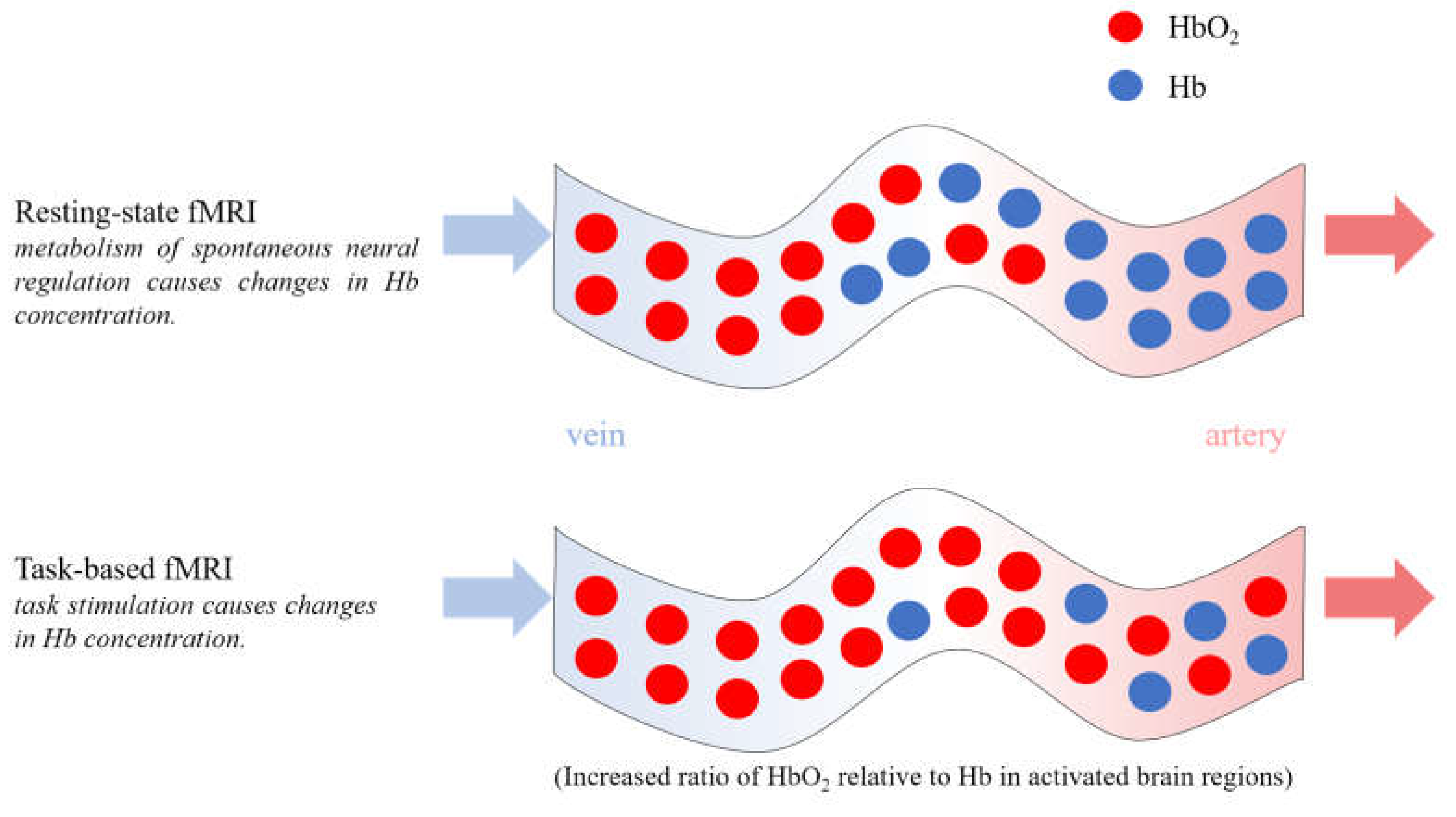
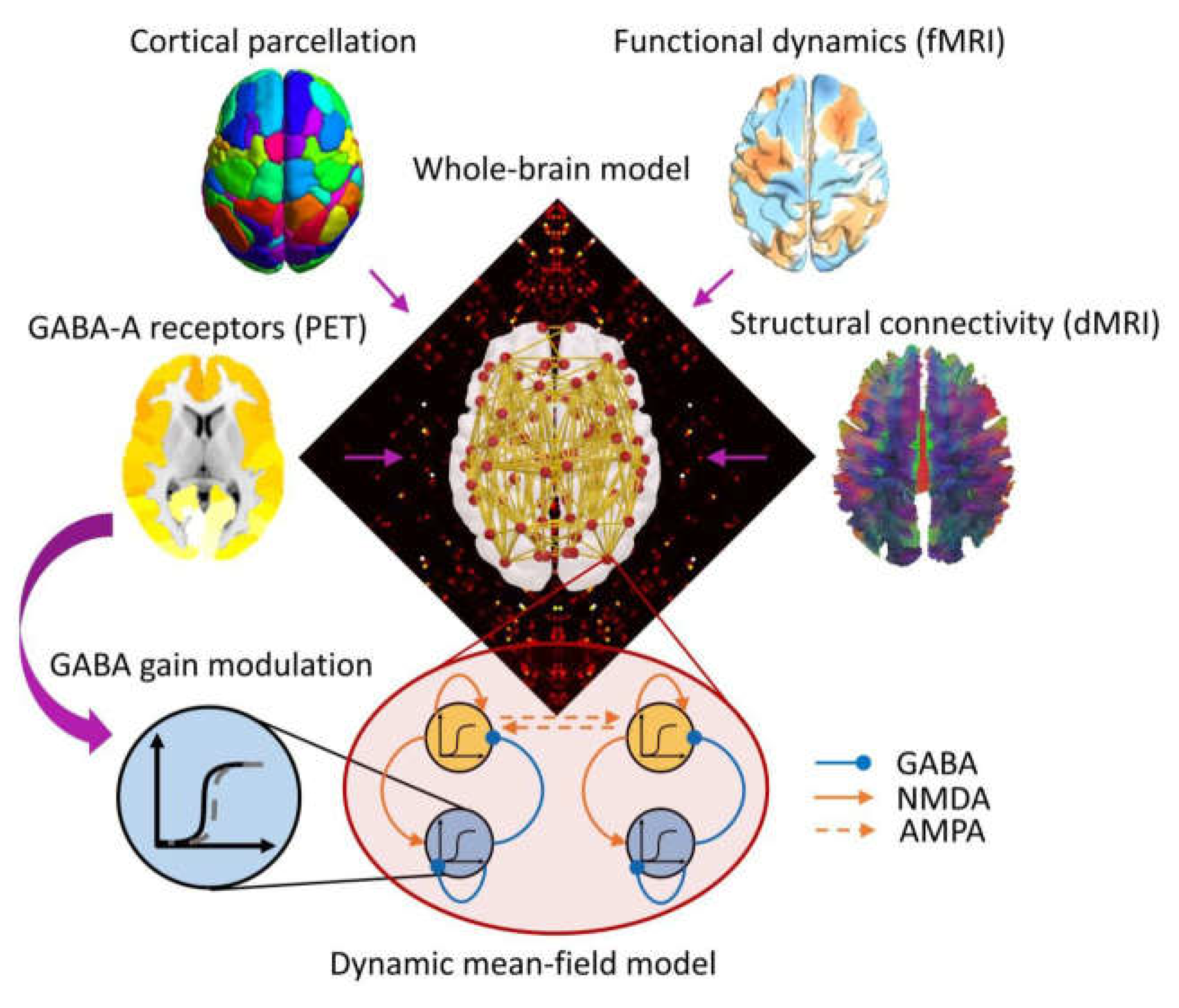
4. PET Used in DoC Studies
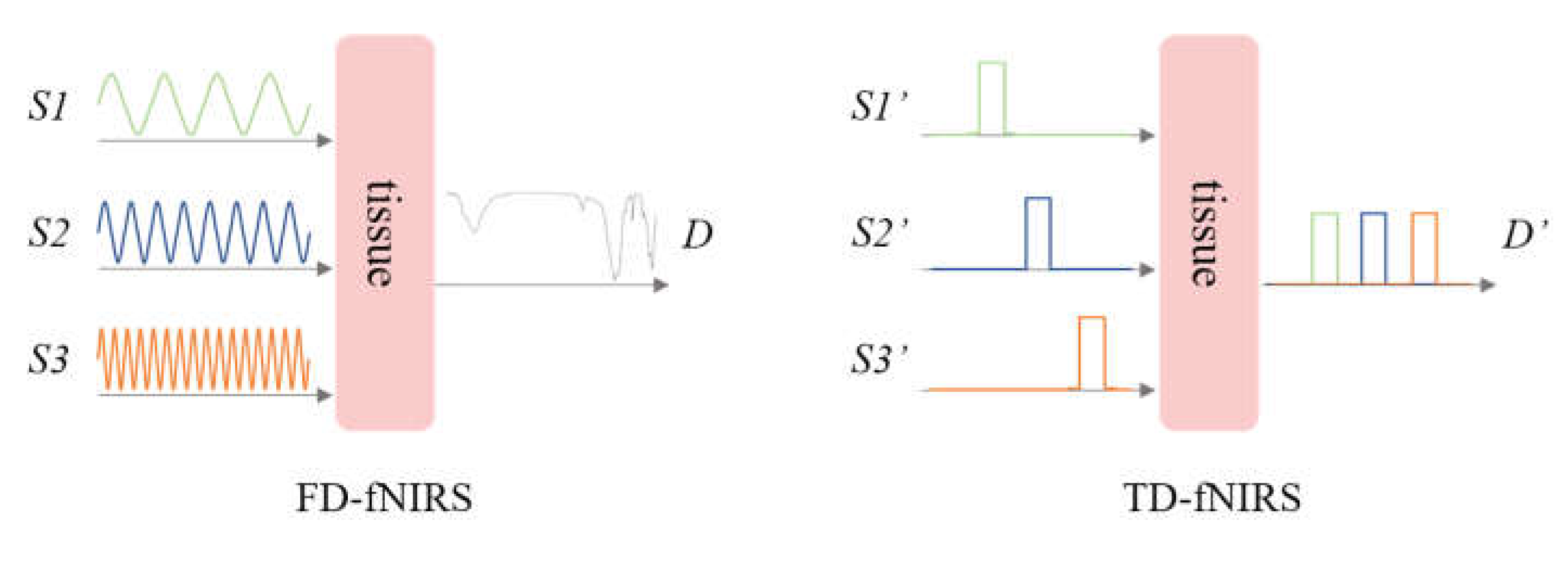
5. fNIRS Used in DoC Studies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, J.; Yan, Y.; Zhou, W.; Lin, Y.; Shen, Z.; Mou, X.; Ren, Y.; Hu, X.; Di, H. Clinical Research: Auditory Stimulation in the Disorders of Consciousness. Front. Hum. Neurosci. 2019, 13, 324. [Google Scholar] [CrossRef] [PubMed]
- Laureys, S.; Celesia, G. G.; Cohadon, F.; Lavrijsen, J.; Leon-Carrion, J.; Sannita, W. G.; Sazbon, L.; Schmutzhard, E.; von Wild, K. R.; Zeman, A.; Dolce, G. Unresponsive Wakefulness Syndrome: A New Name for the Vegetative State or Apallic Syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J. T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement Characteristics and Diagnostic Utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef]
- Arico, I.; Naro, A.; Pisani, L. R.; Leo, A.; Muscara, N.; De Salvo, S.; Silvestri, R.; Bramanti, P.; Calabro, R. S. Could Combined Sleep and Pain Evaluation Be Useful in the Diagnosis of Disorders of Consciousness (DOC)? Preliminary Findings. Brain Inj. 2016, 30, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Kondziella, D.; Friberg, C. K.; Frokjaer, V. G.; Fabricius, M.; Moller, K. Preserved Consciousness in Vegetative and Minimal Conscious States: Systematic Review and Meta-Analysis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 485–492. [Google Scholar] [CrossRef]
- Thibaut, A.; Panda, R.; Annen, J.; Sanz, L. R. D.; Naccache, L.; Martial, C.; Chatelle, C.; Aubinet, C.; Bonin, E. A. C.; Barra, A.; Briand, M.-M.; Cecconi, B.; Wannez, S.; Stender, J.; Laureys, S.; Gosseries, O. Preservation of Brain Ac-tivity in Unresponsive Patients Identifies MCS Star. Ann. Neurol. 2021, 90, 89–100. [Google Scholar] [CrossRef]
- Scolding, N.; Owen, A. M.; Keown, J. Prolonged Disorders of Consciousness: A Critical Evaluation of the New UK Guidelines. Brain 2021, 144, 1655–1660. [Google Scholar] [CrossRef]
- Ballanti, S.; Campagnini, S.; Liuzzi, P.; Hakiki, B.; Scarpino, M.; Macchi, C.; Oddo, C. M.; Carrozza, M. C.; Grippo, A.; Mannini, A. EEG-Based Methods for Recovery Prognosis of Patients with Disorders of Consciousness: A Systematic Review. Clinical Neurophysiology 2022, 144, 98–114. [Google Scholar] [CrossRef]
- Wu, L.; Wang, X.-Q.; Yang, Y.; Dong, T.-F.; Lei, L.; Cheng, Q.-Q.; Li, S.-X. Spatio-Temporal Dynamics of EEG Features during Sleep in Major Depressive Disorder after Treatment with Escitalopram: A Pilot Study. BMC Psychiatry 2020, 20, 124. [Google Scholar] [CrossRef]
- Theiler, J.; Eubank, S.; Longtin, A.; Galdrikian, B.; Doyne Farmer, J. Testing for Nonlinearity in Time Series: The Method of Surrogate Data. Physica D: Nonlinear Phenomena 1992, 58, 77–94. [Google Scholar] [CrossRef]
- Schorr, B.; Schlee, W.; Arndt, M.; Bender, A. Coherence in Resting-State EEG as a Predictor for the Recovery from Unresponsive Wakefulness Syndrome. J Neurol 2016, 263, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Lehembre, R.; Bruno, M.-A.; Vanhaudenhuyse, A.; Chatelle, C.; Cologan, V.; Leclercq, Y.; Soddu, A.; Macq, B.; Laureys, S.; Noirhomme, Q. Resting-State EEG Study of Comatose Patients: A Connectivity and Frequency Analysis to Find Differences between Vegetative and Minimally Conscious States. Funct Neurol 2012, 27, 41–47. [Google Scholar] [PubMed]
- Naro, A.; Bramanti, A.; Leo, A.; Cacciola, A.; Manuli, A.; Bramanti, P.; Calabro, R. S. Shedding New Light on Disorders of Consciousness Diagnosis: The Dynamic Functional Connectivity. Cortex 2018, 103, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Stefan, S.; Schorr, B.; Lopez-Rolon, A.; Kolassa, I.-T.; Shock, J. P.; Rosenfelder, M.; Heck, S.; Bender, A. Conscious-ness Indexing and Outcome Prediction with Resting-State EEG in Severe Disorders of Consciousness. Brain Topogr 2018, 31, 848–862. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, D.; Rowan, M.; Walsh, J. B.; Coakley, D. P300 as a Predictor of Recovery from Coma. Lancet 1990, 336, 1265–1266. [Google Scholar] [CrossRef] [PubMed]
- Kempny, A. M.; James, L.; Yelden, K.; Duport, S.; Farmer, S. F.; Playford, E. D.; Leff, A. P. Patients with a Severe Prolonged Disorder of Consciousness Can Show Classical EEG Responses to Their Own Name Compared with Others’ Names. NeuroImage-Clin. 2018, 19, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Bao, W.-X.; Zhang, J.; Hu, Y.-F.; Gao, J.; Luo, B.-Y. Effect of Acoustic Stimuli in Patients with Disorders of Consciousness: A Quantitative Electroencephalography Study. Neural Regen. Res. 2018, 13, 1900–1906. [Google Scholar] [CrossRef]
- Li, J.; Shen, J.; Liu, S.; Chauvel, M.; Yang, W.; Mei, J.; Lei, L.; Wu, L.; Gao, J.; Yang, Y. Responses of Patients with Disorders of Consciousness to Habit Stimulation: A Quantitative EEG Study. Neurosci. Bull. 2018, 34, 691–699. [Google Scholar] [CrossRef]
- Sinitsyn, D. O.; Poydasheva, A. G.; Bakulin, I. S.; Legostaeva, L. A.; Iazeva, E. G.; Sergeev, D. V.; Sergeeva, A. N.; Kremneva, E. I.; Morozova, S. N.; Lagoda, D. Y.; Casarotto, S.; Comanducci, A.; Ryabinkina, Y. V.; Suponeva, N. A.; Piradov, M. A. Detecting the Potential for Consciousness in Unresponsive Patients Using the Perturbational Complexity Index. Brain Sci. 2020, 10, 917. [Google Scholar] [CrossRef]
- Burle, B.; Spieser, L.; Roger, C.; Casini, L.; Hasbroucq, T.; Vidal, F. Spatial and Temporal Resolutions of EEG: Is It Really Black and White? A Scalp Current Density View. International Journal of Psychophysiology 2015, 97, 210–220. [Google Scholar] [CrossRef]
- Dong, L.; Gong, D.; Valdes-Sosa, P. A.; Xia, Y.; Luo, C.; Xu, P.; Yao, D. Simultaneous EEG-FMRI: Trial Level Spa-tio-Temporal Fusion for Hierarchically Reliable Information Discovery. Neuroimage 2014, 99, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Ramakrishnan, A. G. Electrophysiological and Neuroimaging Studies – During Resting State and Sensory Stimulation in Disorders of Consciousness: A Review. Front. Neurosci. 2020, 14, 555093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, R.; Guo, Y.; Zhao, D.; Li, S.; Chen, M.; Shi, L.; Yao, D.; Gao, J.; Wang, X.; Hu, Y. Assessing Residual Motor Function in Patients with Disorders of Consciousness by Brain Network Properties of Task-State EEG. Cogn. Neurodynamics 2022, 16, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, F.; La Porta, F.; Petrone, V.; Battaglia, S.; Orlandi, S.; Ippolito, G.; Romei, V.; Piperno, R.; Lullini, G. Accuracy of EEG Biomarkers in the Detection of Clinical Outcome in Disorders of Consciousness after Severe Acquired Brain Injury: Preliminary Results of a Pilot Study Using a Machine Learning Approach. Biomedicines 2022, 10, 1897. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, Z.; Xia, X.; Bai, Y.; Liang, Z.; He, J.; Li, X. Application of Fast Perturbational Complexity Index to the Diagnosis and Prognosis for Disorders of Consciousness. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Z.; Bai, Y. Frontal and Parietal Lobes Play Crucial Roles in Understanding the Disorder of Consciousness: A Perspective from Electroencephalogram Studies. Front. Neurosci. 2023, 16, 1024278. [Google Scholar] [CrossRef]
- Power, J. D.; Cohen, A. L.; Nelson, S. M.; Wig, G. S.; Barnes, K. A.; Church, J. A.; Vogel, A. C.; Laumann, T. O.; Miezin, F. M.; Schlaggar, B. L.; Petersen, S. E. Functional Network Organization of the Human Brain. Neuron 2011, 72, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Di, H. B.; Yu, S. M.; Weng, X. C.; Laureys, S.; Yu, D.; Li, J. Q.; Qin, P. M.; Zhu, Y. H.; Zhang, S. Z.; Chen, Y. Z. Cerebral Response to Patient’s Own Name in the Vegetative and Minimally Conscious States. Neurology 2007, 68, 895–899. [Google Scholar] [CrossRef]
- Fernandez-Espejo, D.; Junque, C.; Vendrell, P.; Bernabeu, M.; Roig, T.; Bargallo, N.; Mercader, J. M. Cerebral Re-sponse to Speech in Vegetative and Minimally Conscious States after Traumatic Brain Injury. Brain Inj. 2008, 22, 882–890. [Google Scholar] [CrossRef]
- Okumura, Y.; Asano, Y.; Takenaka, S.; Fukuyama, S.; Yonezawa, S.; Kasuya, Y.; Shinoda, J. Brain Activation by Music in Patients in a Vegetative or Minimally Conscious State Following Diffuse Brain Injury. Brain Injury 2014, 28, 944–950. [Google Scholar] [CrossRef]
- Wang, F.; Di, H.; Hu, X.; Jing, S.; Thibaut, A.; Di Perri, C.; Huang, W.; Nie, Y.; Schnakers, C.; Laureys, S. Cerebral Response to Subject’s Own Name Showed High Prognostic Value in Traumatic Vegetative State. BMC Medicine 2015, 13, 83. [Google Scholar] [CrossRef]
- Boltzmann, M.; Schmidt, S. B.; Gutenbrunner, C.; Krauss, J. K.; Stangel, M.; Höglinger, G. U.; Wallesch, C.-W.; Münte, T. F.; Rollnik, J. D. Auditory Stimulation Modulates Resting-State Functional Connectivity in Unresponsive Wakefulness Syndrome Patients. Frontiers in Neuroscience 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Kuhlmann, L.; Johnston, L. A.; Grayden, D. B.; Vogrin, S.; Crossley, R.; Fuller, K.; Lourensz, M.; Cook, M. J. Extending Communication for Patients with Disorders of Consciousness. Journal of Neuroimaging 2014, 24, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hu, N.; Hu, X.; Jing, S.; Heine, L.; Thibaut, A.; Huang, W.; Yan, Y.; Wang, J.; Schnakers, C.; Laureys, S.; Di, H. Detecting Brain Activity Following a Verbal Command in Patients With Disorders of Consciousness. Frontiers in Neuroscience 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Bodien, Y. G.; Giacino, J. T.; Edlow, B. L. Functional MRI Motor Imagery Tasks to Detect Command Following in Traumatic Disorders of Consciousness. Frontiers in Neurology 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Luppi, A. I.; Craig, M. M.; Pappas, I.; Finoia, P.; Williams, G. B.; Allanson, J.; Pickard, J. D.; Owen, A. M.; Naci, L.; Menon, D. K.; Stamatakis, E. A. Consciousness-Specific Dynamic Interactions of Brain Integration and Functional Diversity. Nat Commun 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Vanhaudenhuyse, A.; Noirhomme, Q.; Tshibanda, L. J.-F.; Bruno, M.-A.; Boveroux, P.; Schnakers, C.; Soddu, A.; Perlbarg, V.; Ledoux, D.; Brichant, J.-F.; Moonen, G.; Maquet, P.; Greicius, M. D.; Laureys, S.; Boly, M. Default Network Connectivity Reflects the Level of Consciousness in Non-Communicative Brain-Damaged Patients. Brain 2010, 133, 161–171. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Wu, J.; Mashour, G. A.; Hudetz, A. G. Temporal Circuit of Macroscale Dynamic Brain Activity Supports Human Consciousness. Science Advances 2020, 6, eaaz0087. [Google Scholar] [CrossRef]
- Qin, P.; Wu, X.; Wu, C.; Wu, H.; Zhang, J.; Huang, Z.; Weng, X.; Zang, D.; Qi, Z.; Tang, W.; Hiromi, T.; Tan, J.; Tanabe, S.; Fogel, S.; Hudetz, A. G.; Yang, Y.; Stamatakis, E. A.; Mao, Y.; Northoff, G. Higher-Order Sensorimotor Circuit of the Brain’s Global Network Supports Human Consciousness. NeuroImage 2021, 231, 117850. [Google Scholar] [CrossRef]
- Xia, M.; He, Y. Functional Connectomics from a “Big Data” Perspective. NeuroImage 2017, 160, 152–167. [Google Scholar] [CrossRef]
- Li, H.; Smith, S. M.; Gruber, S.; Lukas, S. E.; Silveri, M. M.; Hill, K. P.; Killgore, W. D. S.; Nickerson, L. D. Denoising Scanner Effects from Multimodal MRI Data Using Linked Independent Component Analysis. NeuroImage 2020, 208, 116388. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.T.; Jamison, K.; Glasser, M. F.; Smith, S. M.; Coalson, T.; Moeller, S.; Auerbach, E. J.; Uğurbil, K.; Yacoub, E. Tradeoffs in Pushing the Spatial Resolution of FMRI for the 7T Human Connectome Project. NeuroImage 2017, 154, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, R.; Giacino, J. T. The Vegetative and Minimally Conscious States: Diagnosis, Prognosis and Treatment. Neurologic Clinics 2011, 29, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Jox, R. J.; Bernat, J. L.; Laureys, S.; Racine, E. Disorders of Consciousness: Responding to Requests for Novel Di-agnostic and Therapeutic Interventions. The Lancet Neurology 2012, 11, 732–738. [Google Scholar] [CrossRef]
- de Jong, B. M.; Willemsen, A. T. M.; Paans, A. M. J. Regional Cerebral Blood Flow Changes Related to Affective Speech Presentation in Persistent Vegetative State. Clinical Neurology and Neurosurgery 1997, 99, 213–216. [Google Scholar] [CrossRef]
- Menon, D. K.; Owen, A. M.; Boniface, S. J.; Pickard, J. D. Cortical Processing in Persistent Vegetative State. The Lancet 1998, 352, 1148–1149. [Google Scholar] [CrossRef]
- Laureys, S.; Faymonville, M. E.; Peigneux, P.; Damas, P.; Lambermont, B.; Del Fiore, G.; Degueldre, C.; Aerts, J.; Luxen, A.; Franck, G.; Lamy, M.; Moonen, G.; Maquet, P. Cortical Processing of Noxious Somatosensory Stimuli in the Persistent Vegetative State. NeuroImage 2002, 17, 732–741. [Google Scholar] [CrossRef]
- Bruno, M.-A.; Vanhaudenhuyse, A.; Schnakers, C.; Boly, M.; Gosseries, O.; Demertzi, A.; Majerus, S.; Moonen, G.; Hustinx, R.; Laureys, S. Visual Fixation in the Vegetative State: An Observational Case Series PET Study. BMC Neurol 2010, 10, 35. [Google Scholar] [CrossRef]
- Schiff, N. D.; Ribary, U.; Moreno, D. R.; Beattie, B.; Kronberg, E.; Blasberg, R.; Giacino, J.; McCagg, C.; Fins, J. J.; Llinás, R.; Plum, F. Residual Cerebral Activity and Behavioural Fragments Can Remain in the Persistently Vegetative Brain. Brain 2002, 125, 1210–1234. [Google Scholar] [CrossRef]
- Rudolf, J.; Sobesky, J.; Ghaemi, M.; Heiss, W.-D. The Correlation between Cerebral Glucose Metabolism and Benzodiazepine Receptor Density in the Acute Vegetative State. European Journal of Neurology 2002, 9, 671–677. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, J.; Wang, X.; Yu, R.; Xie, Q.; Zhang, J.; Ouyang, X.; Liu, W. Assessment of the brain function with 18F-FDG PET/CT in patients with disorders of consciousness. Chinese Journal of Nuclear Medicine and Molecular Imaging 2018, 38, 97–100. [Google Scholar]
- Usami, N.; Asano, Y.; Ikegame, Y.; Takei, H.; Yamada, Y.; Yano, H.; Shinoda, J. Cerebral Glucose Metabolism in Patients with Chronic Disorders of Consciousness. Canadian Journal of Neurological Sciences 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, T.; Hatakeyama, N.; Murayama, T.; Funakura, M.; Hara, T.; Onodera, S.; Ito, D.; Yakufujiang, M.; Odaki, M.; Oka, N.; Kobayashi, S. Prediction of Voluntary Movements of the Upper Extremities by Resting State-Brain Regional Glucose Metabolism in Patients with Chronic Severe Brain Injury: A Pilot Study. Hum. Brain Mapp. [CrossRef] [PubMed]
- Hattori, N.; Huang, S.-C.; Wu, H.-M.; Yeh, E.; Glenn, T. C.; Vespa, P. M.; McArthur, D.; Phelps, M. E.; Hovda, D. A.; Bergsneider, M. Correlation of Regional Metabolic Rates of Glucose with Glasgow Coma Scale After Traumatic Brain Injury. Journal of Nuclear Medicine 2003, 44, 1709–1716. [Google Scholar] [PubMed]
- Hermann, B.; Stender, J.; Habert, M.-O.; Kas, A.; Denis-Valente, M.; Raimondo, F.; Pérez, P.; Rohaut, B.; Sitt, J. D.; Naccache, L. Multimodal FDG-PET and EEG Assessment Improves Diagnosis and Prognostication of Disorders of Consciousness. NeuroImage: Clinical 2021, 30, 102601. [Google Scholar] [CrossRef] [PubMed]
- Luppi, A. I.; Mediano, P. A. M.; Rosas, F. E.; Allanson, J.; Pickard, J. D.; Williams, G. B.; Craig, M. M.; Finoia, P.; Peattie, A. R. D.; Coppola, P.; Owen, A. M.; Naci, L.; Menon, D. K.; Bor, D.; Stamatakis, E. A. Whole-Brain Model-ling Identifies Distinct but Convergent Paths to Unconsciousness in Anaesthesia and Disorders of Consciousness. Commun Biol 2022, 5, 384. [Google Scholar] [CrossRef]
- Ekkekakis, P. Illuminating the Black Box: Investigating Prefrontal Cortical Hemodynamics During Exercise With Near-Infrared Spectroscopy. J. Sport Exerc. Psychol. 2009, 31, 505–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kang, X.; Chen, B.; Song, C.; Liu, Y.; Hao, J.; Yuan, F.; Jiang, W. Detecting Residual Brain Networks in Disorders of Consciousness: A Resting-State FNIRS Study. Brain Res. 2023, 1798, 148162. [Google Scholar] [CrossRef]
- Shu, Z.; Wu, J.; Li, H.; Liu, J.; Lu, J.; Lin, J.; Liang, S.; Wu, J.; Han, J.; Yu, N. FNIRS-Based Functional Connectivity Signifies Recovery in Patients with Disorders of Consciousness after DBS Treatment. Clin. Neurophysiol. 2023, 147, 60–68. [Google Scholar] [CrossRef]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P. W. The Present and Future Use of Functional Near-Infrared Spectroscopy (FNIRS) for Cognitive Neuroscience. Ann. N.Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef]
- Kempny, A. M.; James, L.; Yelden, K.; Duport, S.; Farmer, S.; Playford, E. D.; Leff, A. P. Functional near Infrared Spectroscopy as a Probe of Brain Function in People with Prolonged Disorders of Consciousness. NeuroImage-Clin. 2016, 12, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, Y.; Zhang, Y.; Gao, Y.; Jing, R.; Dang, Y.; Chen, X.; He, J.; Si, J. Detecting Residual Awareness in Patients With Prolonged Disorders of Consciousness: An FNIRS Study. Front. Neurol. 2021, 12, 618055. [Google Scholar] [CrossRef] [PubMed]
- Kurz, E.-M.; Wood, G.; Kober, S. E.; Schippinger, W.; Pichler, G.; Mueller-Putz, G.; Bauernfeind, G. Towards Using FNIRS Recordings of Mental Arithmetic for the Detection of Residual Cognitive Activity in Patients with Disorders of Consciousness (DOC). Brain Cogn. 2018, 125, 78–87. [Google Scholar] [CrossRef]
- Si, J.; Dang, Y.; Zhang, Y.; Li, Y.; Zhang, W.; Yang, Y.; Cui, Y.; Lou, X.; He, J.; Jiang, T. Spinal Cord Stimulation Frequency Influences the Hemodynamic Response in Patients with Disorders of Consciousness. Neurosci. Bull. 2018, 34, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Si, J.; Xia, X.; He, J.; Jiang, T. Influence of Inter-Stimulus Interval of Spinal Cord Stimulation in Patients with Disorders of Consciousness: A Preliminary Functional near-Infrared Spectroscopy Study. NeuroImage: Clinical 2018, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bicciato, G.; Keller, E.; Wolf, M.; Brandi, G.; Schulthess, S.; Friedl, S. G.; Willms, J. F.; Narula, G. Increase in Low-Frequency Oscillations in FNIRS as Cerebral Response to Auditory Stimulation with Familiar Music. Brain Sci. 2022, 12, 42. [Google Scholar] [CrossRef]
- Abdalmalak, A.; Milej, D.; Norton, L.; Debicki, D. B.; Owen, A. M.; Lawrence, K. St. The Potential Role of FNIRS in Evaluating Levels of Consciousness. Front. Hum. Neurosci. 2021, 15, 703405. [Google Scholar] [CrossRef]
- Blumenfeld, H. Brain Mechanisms of Conscious Awareness: Detect, Pulse, Switch, and Wave. Neuroscientist 2023, 29, 9–18. [Google Scholar] [CrossRef]
- Kondziella, D.; Bender, A.; Diserens, K.; van Erp, W.; Estraneo, A.; Formisano, R.; Laureys, S.; Naccache, L.; Ozturk, S.; Rohaut, B.; Sitt, J. D.; Stender, J.; Tiainen, M.; Rossetti, A. O.; Gosseries, O.; Chatelle, C. European Academy of Neurology Guideline on the Diagnosis of Coma and Other Disorders of Consciousness. European Journal of Neurology 2020, 27, 741–756. [Google Scholar] [CrossRef]
- Huang, H.; Xie, Q.; Pan, J.; He, Y.; Wen, Z.; Yu, R.; Li, Y. An EEG-Based Brain Computer Interface for Emotion Recognition and Its Application in Patients with Disorder of Consciousness. IEEE Trans. Affective Comput. 2021, 12, 832–842. [Google Scholar] [CrossRef]
- Song, M.; Zhang, Y.; Cui, Y.; Yang, Y.; Jiang, T. Brain Network Studies in Chronic Disorders of Consciousness: Advances and Perspectives. Neurosci. Bull. 2018, 34, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.; Kandeepan, S.; Aiello, M.; Ribeiro de Paula, D.; Marchitelli, R.; Fiorenza, S.; Orsini, M.; Trojano, L.; Masotta, O.; St. Lawrence, K.; Loreto, V.; Chronik, B. A.; Nicolai, E.; Soddu, A.; Estraneo, A. Multimodal Neu-roimaging Approach to Variability of Functional Connectivity in Disorders of Consciousness: A PET/MRI Pilot Study. Front. Neurol. 2018, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Heiss, W.-D. Hybrid PET/MR Imaging in Neurology: Present Applications and Prospects for the Future. J. Nucl. Med. 2016, 57, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Chatzichristos, C.; Kofidis, E.; Van Paesschen, W.; De Lathauwer, L.; Theodoridis, S.; Van Huffel, S. Early Soft and Flexible Fusion of Electroencephalography and Functional Magnetic Resonance Imaging via Double Coupled Matrix Tensor Factorization for Multisubject Group Analysis. Hum. Brain Mapp. 2022, 43, 1231–1255. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Fisher, P. M.; Raimondo, F.; Sidaros, A.; Cacic Hribljan, M.; Othman, M. H.; Zibrandtsen, I.; Albrechtsen, S. S.; Bergdal, O.; Hansen, A. E.; Hassager, C.; Højgaard, J. L. S.; Jakobsen, E. W.; Jensen, H. R.; Møller, J.; Nersesjan, V.; Nikolic, M.; Olsen, M. H.; Sigurdsson, S. T.; Sitt, J. D.; Sølling, C.; Welling, K. L.; Willumsen, L. M.; Hauerberg, J.; Larsen, V. A.; Fabricius, M.; Knudsen, G. M.; Kjaergaard, J.; Møller, K.; Kondziella, D. Multimodal Prediction of Residual Consciousness in the Intensive Care Unit: The CONNECT-ME Study. Brain 2023, 146, 50–64. [Google Scholar] [CrossRef]
- Othman, M. H.; Bhattacharya, M.; Moller, K.; Kjeldsen, S.; Grand, J.; Kjaergaard, J.; Dutta, A.; Kondziella, D. Resting-State NIRS-EEG in Unresponsive Patients with Acute Brain Injury: A Proof-of-Concept Study. Neurocrit. Care 2021, 34, 31–44. [Google Scholar] [CrossRef]
- Rupawala, M.; Dehghani, H.; Lucas, S. J. E.; Tino, P.; Cruse, D. Shining a Light on Awareness: A Review of Func-tional Near-Infrared Spectroscopy for Prolonged Disorders of Consciousness. Front. Neurol. 2018, 9, 350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




