1. Introduction
Chitosan has gained significant attention as a promising biomaterial for a wide range of biomedical applications. Its biocompatibility, biodegradability, and bioactivity make it an attractive candidate in the field. Researchers have extensively explored the use of chitosan in textiles, focusing on properties such as antimicrobial activity, water absorption, and tensile strength, which play crucial roles in these applications1–5. Chitosan, a biopolymer, possesses a range of valuable physicochemical properties such as solubility, reactivity, adsorption, and crystallinity. Additionally, it exhibits various biological properties including biodegradability, antimicrobial activity, cytocompatibility, non-toxicity, and fungicidal effects. Moreover, its beneficial characteristics like anti-cholesteremic and antioxidant activities, macrophage activation, anti-inflammatory effects, stimulation of angiogenesis, mucoadhesion, antitumor properties, promotion of granulation and scar formation, hemostatic action, and facilitation of wound healing, position it as an exceptionally promising material for a multitude of applications in the field of biomedicine6–8. Chitosan fibers have found application in tissue engineering for the fabrication and reinforcement of scaffolds. In recent studies, chitosan fibers have been employed as a method of reinforcement to enhance the mechanical properties of scaffolds. The mechanical properties of the fibers play a vital role in determining the overall mechanical characteristics of the scaffold. Notably, chitosan scaffolds reinforced with high-mechanical-property chitosan fibers exhibited a doubling in strength and a fivefold increase in stiffness compared to scaffolds reinforced with fibers possessing lower mechanical properties 9–12. Chemical cross-linking refers to intermolecular or intramolecular joining of two or more molecules by a covalent bond. The reagents that are used for the purpose are referred to as 'cross-linking reagents' or 'cross-linkers'. Physical and chemical cross-linking agents are the two main categories of cross-linking agents. For chitosan, glutaraldehyde, formaldehyde, tripolyphosphate, and polyaspartic acid sodium salt are the most commonly used as cross-linkers. However, at certain concentrations, glutaraldehyde and formaldehyde are considered toxic and raise health concerns and cause undesirable side effects. To overcome this problem such non-toxic cross-linkers as genipin or TPP are used13. Chitosan's distinctive properties make it suitable for a wide range of applications. Its ability to establish cross-linked networks is an essential aspect of its functionality. Chitosan can be cross-linked using a variety of cross-linkers or cross-linking agents. Tripolyphosphate (TPP) also sodium triphosphate (STP) or sodium tripolyphosphate (STPP) is an inorganic compound it is the sodium salt of the polyphosphate penta-anion, which is the conjugate base of triphosphoric acid. Some chitosan cross-linkers commonly used are: glutaraldehyde14, tricarboxylic acid15, genipin16, tripolyphosphate (TPP)17, epoxy compounds18. The aim of this study is to enhance the mechanical properties of chitosan fibers through wet impregnation using tripolyphosphate (TPP) as a cross-linker. The study evaluates the physical, chemical, and mechanical characteristics of the treated fibers.
2. Experimental
In this section is shown the procedure to modify the surface of chitosan fibres with TPP by wet impregnation method.
2.1. Reagents and Materials
- -
Deionized water
- -
Tripolyphosphate (TPP) (Na5P3O10), Assay (unspecified): 90% min , Alfa Aesar.
- -
Chitosan fibres, concentration 7%, prepared at the Institute of Materials Science of Textiles and Polymer Composites, Lodz University of Technology from chitosan powder commercial product of Sigma-Aldrich; molecular weight 60 kDa; degree of deacetylation (DDA) 96%.
2.2. Fibre Impregnation Procedure
A solution of tripolyphosphate was prepared by dissolving TPP in distilled water at a concentration of 1% w/v. In order to get the chitosan fibres ready for the crosslinking process, five separate specimens of chitosan fibres were cut to the same weight of 1.0 g each. After that, each specimen was then immersed individually in 100 cm3 of TPP solution at room temperature for varying immersion times of 1, 2, 4, 6, and 8 hours respectively. After each immersion period, the specimens were carefully removed from the TPP solution and rinsed with distilled water to remove any excess TPP solution. This process was repeated for each of the five specimens, and the resulting samples were further characterized and evaluated for their physical, chemical, and mechanical properties by means of FTIR, SEM and dynamometry in order to assess the effectiveness of the wet impregnation method using TPP as a cross-linker.
3. Characterization of Fibres and Results
3.1. FTIR Spectroscopy
The infrared transmission and reflectance spectra were recorded in the range from 4000 to 600 cm-1 with a resolution 4 cm-1 and 32 scans.
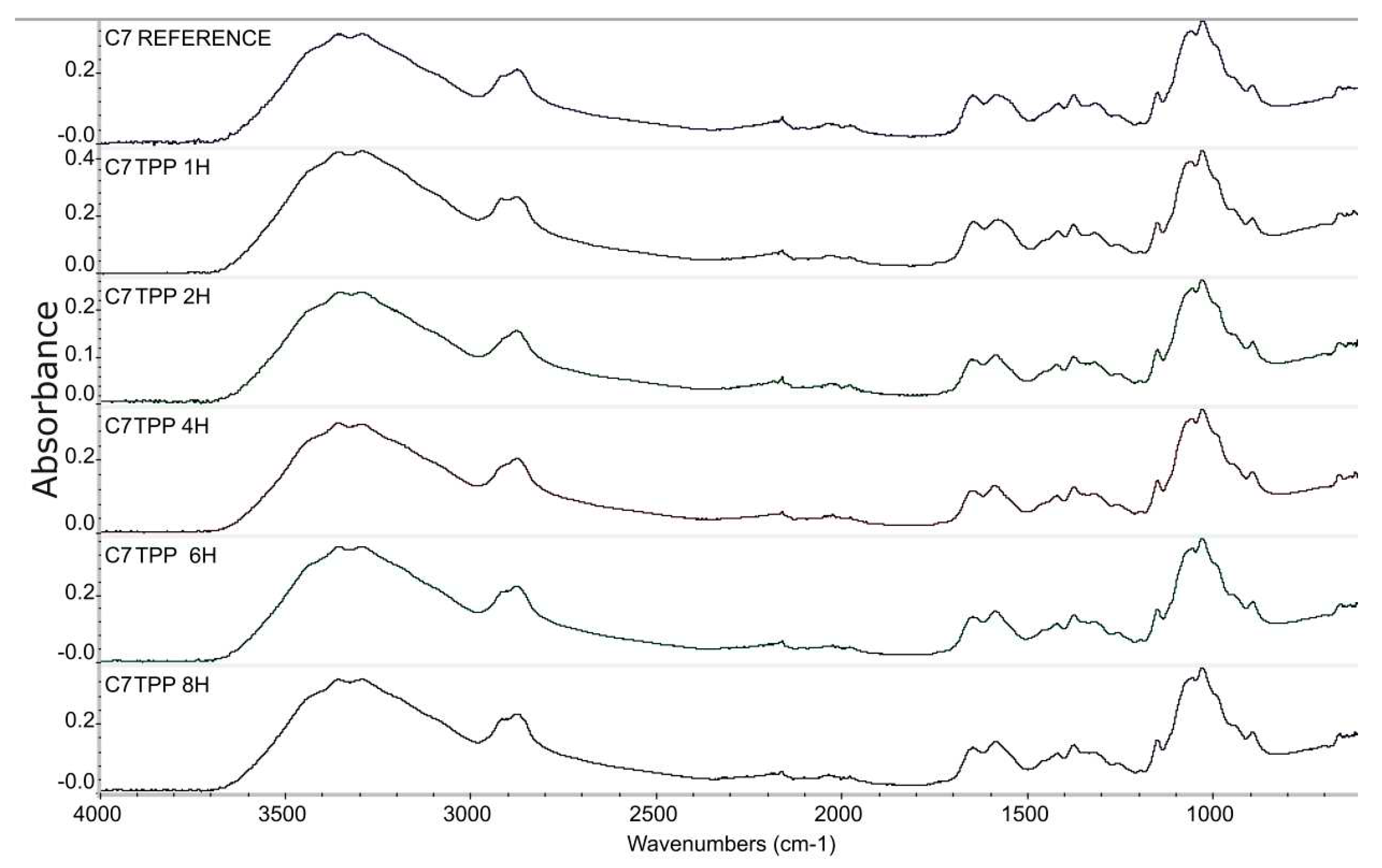
Figure 1.
Infrared spectroscopy spectra graph, fibres samples 7% chitosan reference, 7% chitosan impregnated with TPP 1, 2, 4, 6, and 8 hours.
Figure 1.
Infrared spectroscopy spectra graph, fibres samples 7% chitosan reference, 7% chitosan impregnated with TPP 1, 2, 4, 6, and 8 hours.
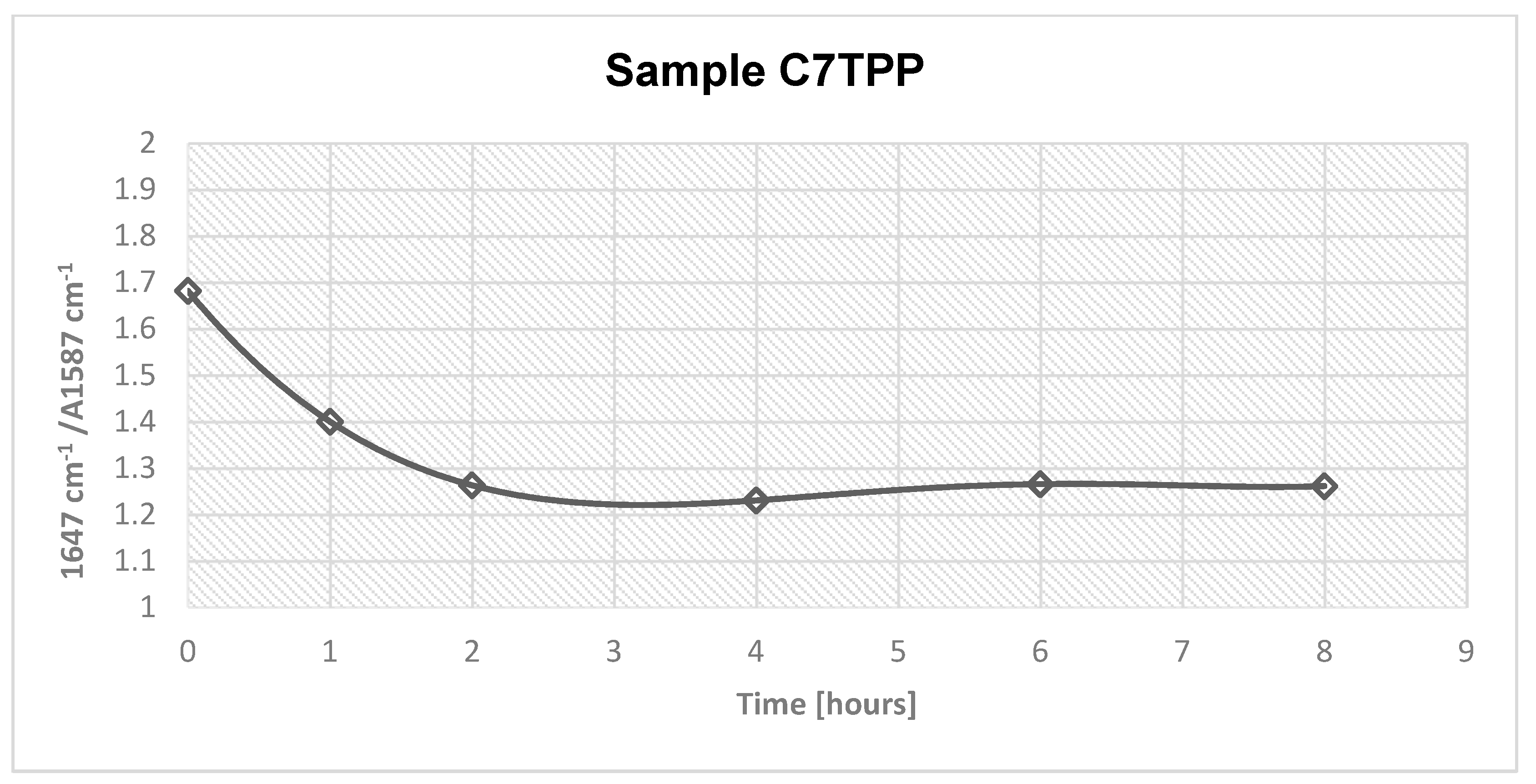
Figure 2.
Absorbance area, amide/amine ratio for samples C7TPP 0, 1, 2, 4, 6 and 8 hours.
Figure 2.
Absorbance area, amide/amine ratio for samples C7TPP 0, 1, 2, 4, 6 and 8 hours.
Based on the obtained results, the absorbance ratio at 1647 cm-1 / 1587 cm-1 of samples C7TPP at different time set points (0, 1, 2, 4, 6, and 8 hours) suggests that the crosslinking reaction between chitosan fibres and TPP ions continued up to 2 hours of immersion, as indicated by the decreasing absorbance ratio. However, after 2 hours of immersion, the absorbance ratio remained relatively constant, indicating that the reaction had reached completion. This suggests that the reaction had utilized most of the available protonated amine groups for cross-linking, and there were no more significant changes in the chemical structure of the sample during the remaining immersion time. Generally, the FTIR results suggest that protonated amine's interaction with TPP ions was successful on the surface of chitosan fibres.
Following, the fibre’s morphology was studied and the mechanical properties were tested to analyze if the cross-linking process improved the fibres’ tensile strength.
3.2. Scanning Electron Microscope Images
The surface structure of the obtained fibres was analyzed using microscopy obtained by means Scanning electron microscope Nova Nanosem 230.
The
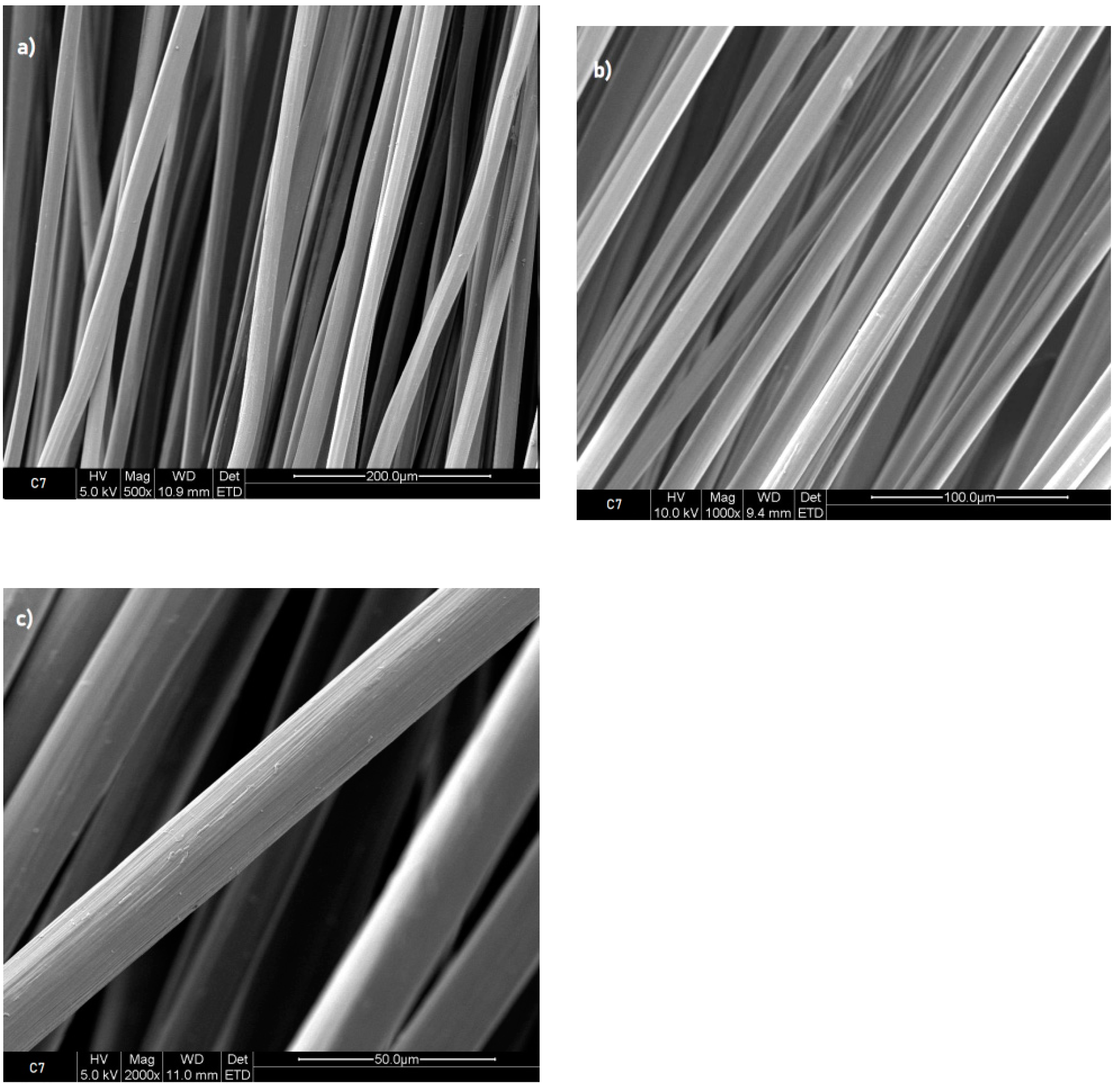
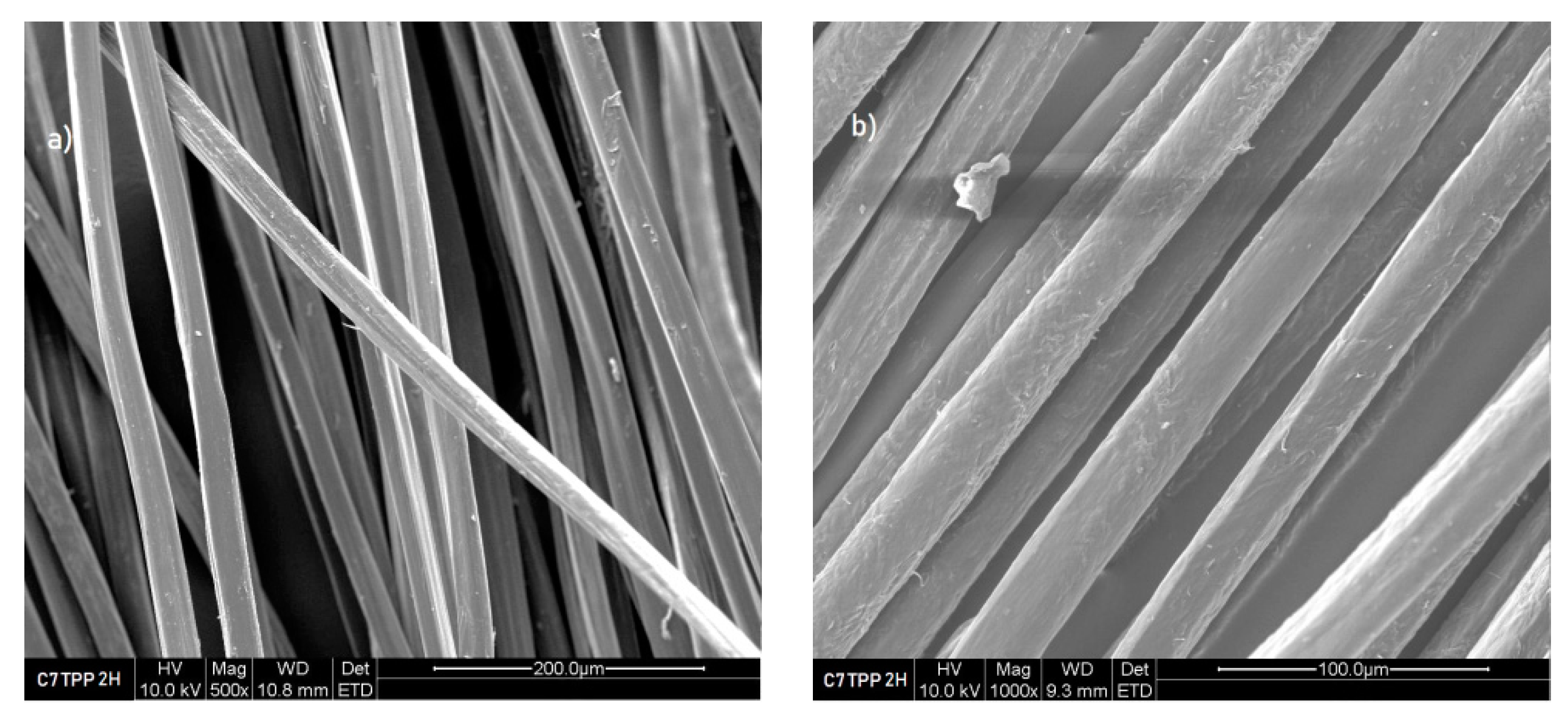
Figure 4 shows that after 2 hours of immersion C7TPP has a rougher surface than C7 (without TPP treatment) see (
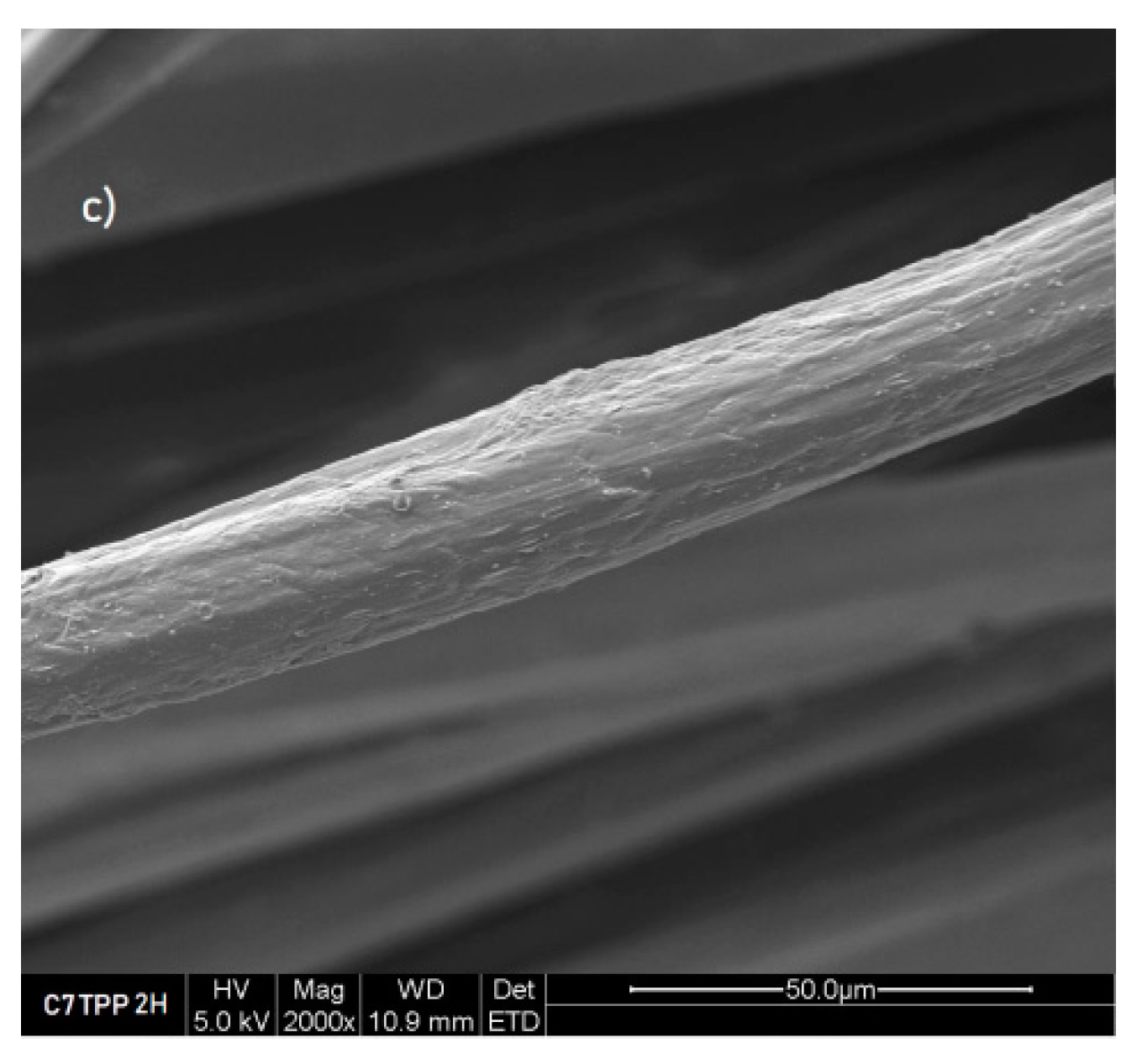
Figure 3); this suggests that the crosslinking reaction between chitosan and TPP ions might have caused some roughness on the surface of the fibres. It could be due to the formation of new chemical bonds between the chitosan and TPP ions, which can cause some deformation in the surface’s morphology of the fibres. The pictures captured from the samples immersed for 4, 6, and 8 hours exhibited a similarity to the images obtained from the sample after 2 hours of immersion.
Figure 3.
Fibres 7% chitosan reference, SEM images a) 500x, b) 1000x and c) 2000x.
Figure 3.
Fibres 7% chitosan reference, SEM images a) 500x, b) 1000x and c) 2000x.
Figure 4.
Fibres 7% chitosan impregnated with TPP 2 hours, SEM images a) 500x, b) 1000x and c) 2000x.
Figure 4.
Fibres 7% chitosan impregnated with TPP 2 hours, SEM images a) 500x, b) 1000x and c) 2000x.
3.3. Tensile Strength Test
This test was performed according the International Standard ISO 2062:2009; Textiles — Yarns from packages — Determination of single-end breaking force and elongation at break using constant rate of extension (CRE) tester.
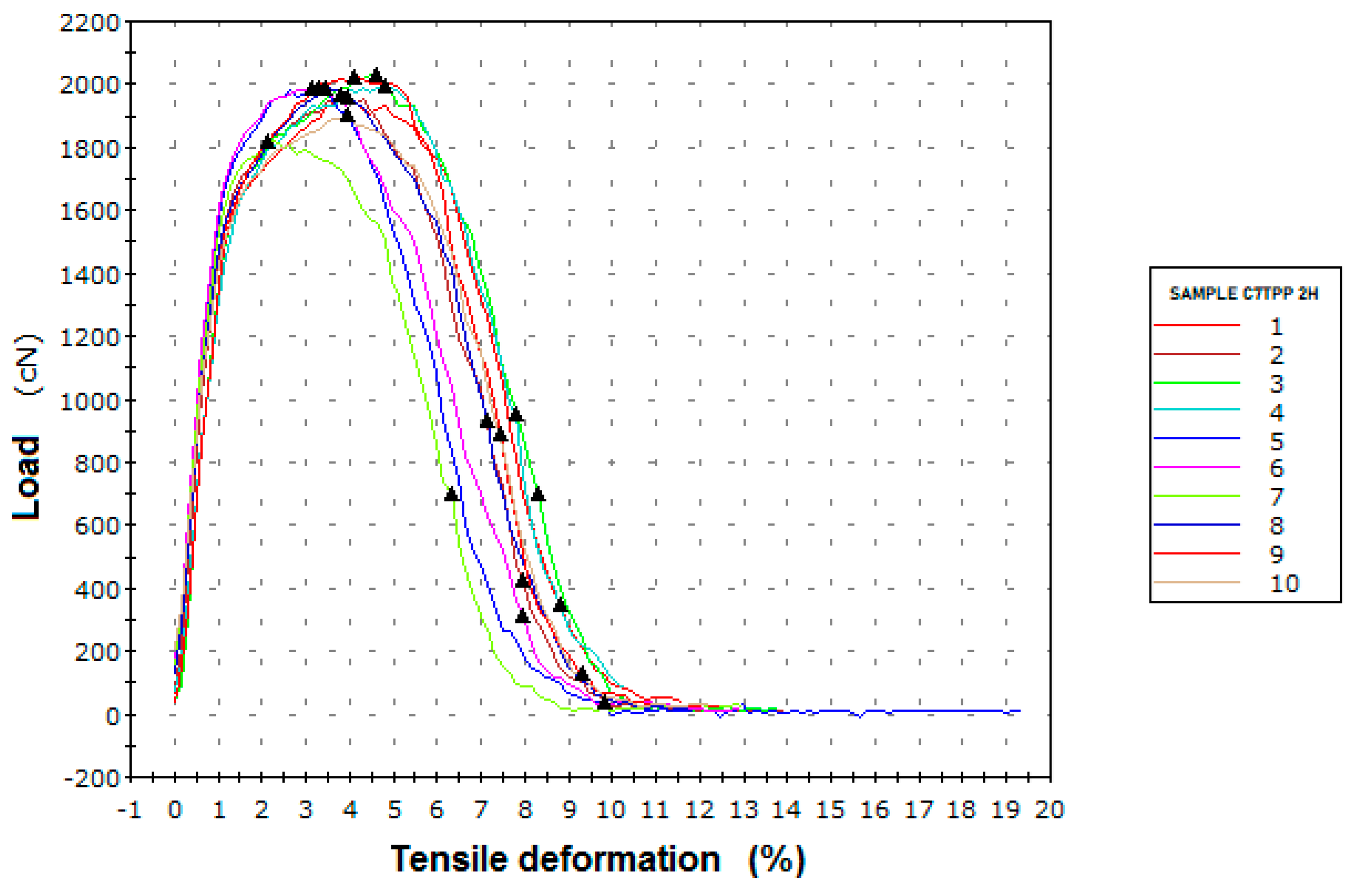
Figure 5.
Tensile deformation graphic, sample C7TPP after 2 hours of immersion.
Figure 5.
Tensile deformation graphic, sample C7TPP after 2 hours of immersion.
Table 1.
Tensile strength results, sample C7AATPP after 2 hours of immersion.
Table 1.
Tensile strength results, sample C7AATPP after 2 hours of immersion.
Number of
specimens tested
10
|
Specific strength at
maximum force
(cN/tex)
Average
|
Relative elongation at maximum force(%)
Average
|
| 14.25 |
3.94 |
| Standard deviation |
0.56 |
0.74 |
| Coefficient of variation |
2.63 |
10.89 |
Table 2.
Comparison of results before and after TPP impregnation.
Table 2.
Comparison of results before and after TPP impregnation.
| Sample ID |
Specific strength at
maximum force
(cN/tex)
Average |
Relative elongation at maximum force (%)
Average |
Fibre’s strength improvement (%) |
Fibre’s elongation improvement (%) |
| C7AA |
12.39 |
3.59 |
|
|
| C7AATPP 2 hours |
14.25 |
3.94 |
15.01 |
9.75 |
| C7AATPP 4 hours |
14.20 |
3.89 |
14.60 |
8.35 |
| C7AATPP 6 hours |
14.22 |
3.87 |
14.76 |
7.80 |
| C7AATPP 8 hours |
14.17 |
3.90 |
14.36 |
8.63 |
There was a significant improvement in the strength of the sample C7TPP after two hours of immersion, compared to the strength of the C7 sample that did not undergo the wet impregnation treatment. This leads one to assume that the fibres were successfully strengthened by the crosslinking reaction that occurred between chitosan and TPP. The increase in strength can be attributed to the formation of a strong ionic bond between the positively charged amino groups of chitosan and the negatively charged TPP ions, which creates a crosslinked network within the fibres. Additionally, the increase in relative elongation at maximum force between the two samples suggests that the crosslinking reaction has also made the fibres more ductile.
4. Conclusions
The surface of chitosan fibers can be modified using the wet impregnation method and TPP through a cross-linking reaction. This modification leads to improved mechanical properties, including up to 15% increase in tensile strength and up to 9.75% increase in relative elongation. These enhanced fibers demonstrate promising potential for applications in wound dressings or scaffolds, offering improved antibacterial and mechanical properties.
References
- Albanna, M.Z.; Bou-Akl, T.H.; Blowytsky, O.; Walters, H.L.; Matthew, H.W.T. Chitosan Fibers with Improved Biological and Mechanical Properties for Tissue Engineering Applications. J. Mech. Behav. Biomed. Mater. 2013, 20, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Antaby, E.; Klinkhammer, K.; Sabantina, L. Electrospinning of Chitosan for Antibacterial Applications—Current Trends. Appl. Sci. 2021, 11, 11937. [Google Scholar] [CrossRef]
- Fan, M.; Hu, Q. Super Absorption Behavior of Chitosan by Freeze-Blasting in Different Alkaline Solvents. J. Renew. Mater. 1970, 6, 457–463. [Google Scholar] [CrossRef]
- Geng, L.; Lin, Y.; Chen, S.; Shi, S.; Cai, Y.; Li, L.; Peng, X. Superior Strength and Toughness of Graphene/Chitosan Fibers Reinforced by Interfacial Complexation. Compos. Sci. Technol. 2020, 194, 108174. [Google Scholar] [CrossRef]
- Sikorski, D.; Bauer, M.; Frączyk, J.; Draczyński, Z. Antibacterial and Antifungal Properties of Modified Chitosan Nonwovens. Polymers 2022, 14, 1690. [Google Scholar] [CrossRef] [PubMed]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Fundamentals of Chitosan for Biomedical Applications. In Chitosan Based Biomaterials Volume 1; Elsevier, 2017; pp 3–30. [CrossRef]
- Perez, J.; Becherán, L.; Bocourt, M.; Peniche, C. Chitosan in Biomedicine. From Gels to Nanoparticles. 2014, XIV, 217–224. [Google Scholar]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as Biomaterial in Drug Delivery and Tissue Engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, Q.; Liu, X.; Dong, W.; Cui, F. Collagen-Based Implants Reinforced by Chitin Fibres in a Goat Shank Bone Defect Model. Biomaterials 2006, 27, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Dong, W.; Feng, Q.; Cui, F.; Uo, M.; Akasaka, T.; Watari, F. In Vitro Evaluation of Porous Poly(L-Lactic Acid) Scaffold Reinforced by Chitin Fibers. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Fabrication of nano-fibrous PLLA scaffold reinforced with chitosan fibers - PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19874673/ (accessed on 16 May 2023).
- Albanna, M.Z.; Bou-Akl, T.H.; Walters, H.L.; Matthew, H.W.T. Improving the Mechanical Properties of Chitosan-Based Heart Valve Scaffolds Using Chitosan Fibers. J. Mech. Behav. Biomed. Mater. 2012, 5, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Arora, B.; Tandon, R.; Attri, P.; Bhatia, R. Chemical Crosslinking: Role in Protein and Peptide Science. Curr. Protein Pept. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of Chitosan Membranes Using Glutaraldehyde: Effect on Ion Permeability and Water Absorption. J. Membr. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, G.; Murray, P.; Zhang, H. Porous Chitosan by Crosslinking with Tricarboxylic Acid and Tuneable Release. SN Appl. Sci. 2020, 2, 435. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef] [PubMed]
- Pati; Adhikari, B.; Dhara, S. Development of Chitosan–Tripolyphosphate Fibers through PH Dependent Ionotropic Gelation. Carbohydr. Res. 2011, 346, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, S.; Saeed, S.; Kausar, A.; Muhammad, B.; Gul, S.; Farooq, D.-M. Influence of Chitosan and Epoxy Cross Linking on Physical Properties of Binary Blends. Int. J. Polym. Anal. Charact. 2015, 21. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).