1. Introduction
The importance and the role of adipose tissue has been greatly re-evaluated after the discovery, in 2006, that adipose tissue is the largest endocrine organ interacting with all major organs through the production of a wide range of hormones and cytokines [
1]. Adipose tissue is constituted by different cell populations such as mature adipocytes and their progenitors, Endothelial Cells (ECs) and Endothelial Progenitor Cells (EPCs) constituting the vascular system of the tissue, immune cells, and Mesenchymal Stem Cells (MSCs) [
2,
3]. MSCs were firstly isolated by Friedenstein and colleagues from the bone marrow and later defined as multipotent stem cells with both self-renewal and differentiation ability [
4,
6]. First isolated bone marrow derived MSCs when cultured, showed an intrinsic ability to form colonies with a great cellular heterogeneity in terms of progenitors and undifferentiated stem cells, able to regenerate bone rudiments. Thanks to their multipotency, it was proved that they were able to differentiate in three different cell types: osteoblasts, chondrocytes, and adipocytes [
4]. MSCs are found in many tissues, including bone marrow, umbilical cord, placental tissue, and adipose tissue. The component of stem cells in adipose tissue is identified also, as Adipose-derived Stem Cells (ASCs), first isolated from adipose tissue by Zuk and colleagues in 2001 [
2]. ASCs are plastic-adherent, multipotent, proliferative stem cells which could remain undifferentiated, self-renew, or undergo to multilineage differentiation [
7,
8]. Since their first isolation more than 20 years ago, they have raised increasing interests in the field of regenerative and aesthetic medicine thanks to their regenerative potential outlined by several groups which showed that these cells can differentiate along multiple pathways [
9].
Historically, the first use of ASCs was in reconstructive surgery: autologous fat transplant was performed for aesthetical purposes but due to long-term problems of volume maintenance, additional transplant over time was required. Nowadays, to overcome this problem, Stromal Vascular Fraction cells (SVF) are cryopreserved and thawed secondarily to treat again the patients. This procedure represents, from a pharmaceutical perspective, a consistent step forward in trying to standardize a cellular product. This trend in the use of adipose tissue for regenerative purposes may thus facilitate the introduction of clinically translatable protocols not only for aesthetic purposes but also for other medical indications like orthopedics or neurodegenerative diseases [
10,
11] in view of the easier way to isolate ASCs rather than bone marrow derived MSCs, that could constitute a potential new vehicle in the development of Advanced Therapy Medicinal Products (ATMPs). Few works have been reported on SVF process standardization in the last 10 years [
12,
13] but the sensitivity of using ATMPs as drugs for regenerative medicine and the number of new candidate-drugs containing SVF or only ASCs for therapeutical use are increasing especially in orthopedics (www.clinical trials.gov, [
14]. With this purpose, we published a collaborative work where we standardized the Good Manufacturing Practices (GMP) - compliant isolation and analysis processes in two different and distant clean rooms [
15].
Here, we present data of our clean room facility in Switzerland 302 samples processed following GMP rules with the aim to further improve the standardization process considering also upstream variability in operators collecting adipose tissue, biological patient intra-variability, anatomical site of collection and finally transportation conditions. In our knowledge, these variables have not yet been considered in peer-reviewed papers, despite they might be relevant for this study. The data reported in this study are the outcome of an effort of standardization at the GMP level considering transport of samples, processing in clean room and their analysis in terms of cellular composition.
2. Results
Producing ATMPs following GMP guidelines ensures a standardized protocol, normally reviewed, and authorized by the local regulatory authority and pharmaceutical quality in terms of sterility of the product, number of delivered cells and finally a solid tracking system in case of recalls, complaint and withdraws. In the last 10 years we made significant improvements trying to standardize the analysis protocol of cells extracted from the samples arriving at our GMP compliant facility. Indeed, we recently published a collaborative study with the University of Marseille, grouping 364 patients processed in both GMP facilities, relating on the possibility to harmonize two slightly different analytical methods to obtain a common protocol for adipose-derived mesenchymal stem cells analysis [
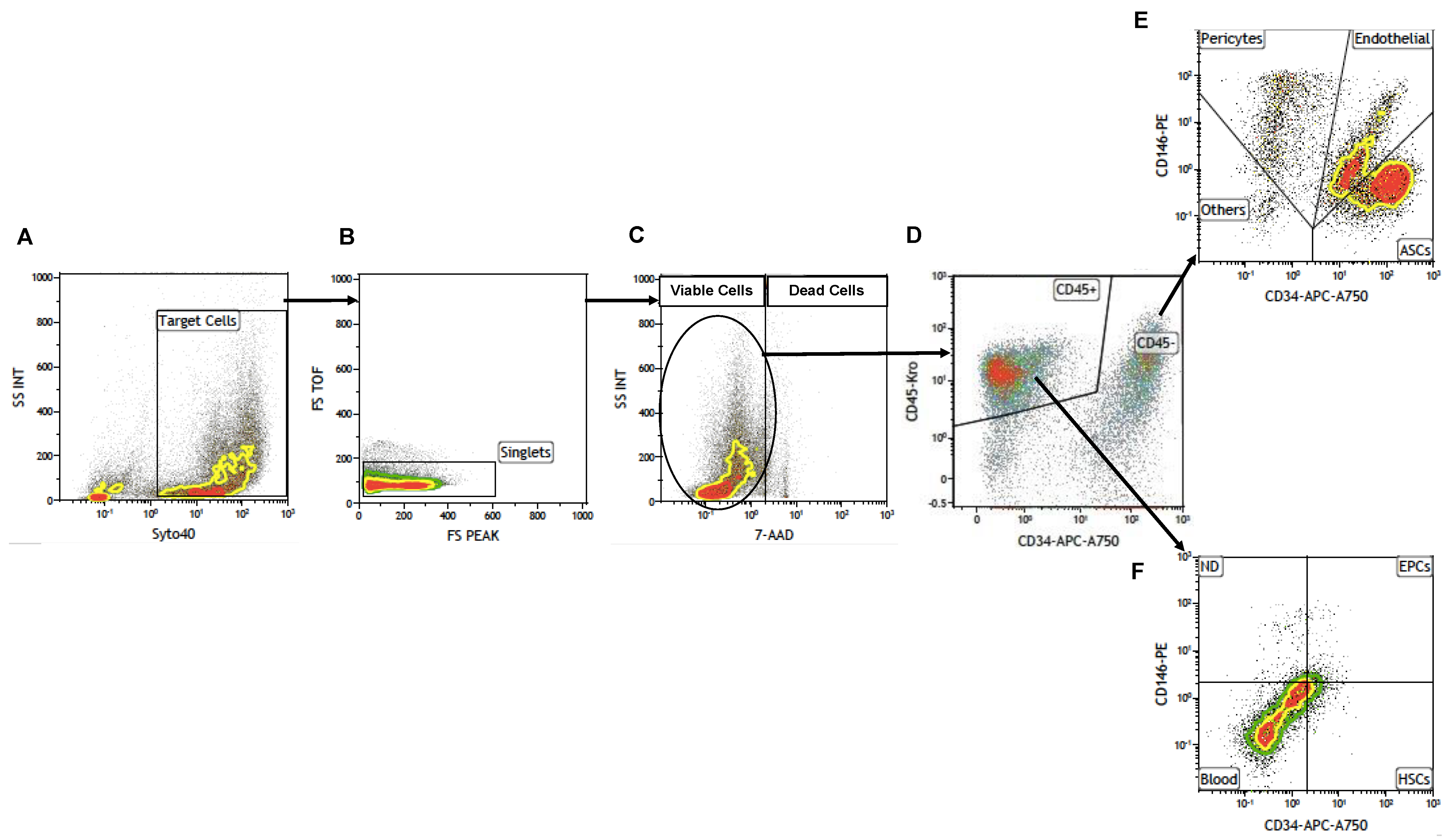
15]. This standardization process led us to develop a cytofluorimetric gating strategy to obtain adipose-derived cell sub-populations and characterize them as shown in
Figure 1.
SVF cells were extracted from adipose tissue as previously reported in our paper in 2014 [
20] and sorted through cytofluorimetric analysis first by selecting nucleated cells with Syto40 (
Figure 1A) and, after removing cellular aggregates (
Figure 1B), discriminating viable cells from Total Nucleated Cells (TNCs), with death marker 7-Amino-Actinomycin D (7-AAD) (
Figure 1C). Then, gated living cells were selected, first for CD45 expression (
Figure 1D) discriminating hematopoietic CD45+ cells from CD45- cells which were successively analyzed for CD34 and CD146 expression (
Figure 1E), giving rise to three different cell sub-populations: ASCs,(
Figure 1E, lower right panel), EPCs (
Figure 1E, upper right panel) and pericytes (
Figure 1E, upper left panel). Other CD45-positive cell sub-populations could be observed in
Figure 1F as previously described [
15,
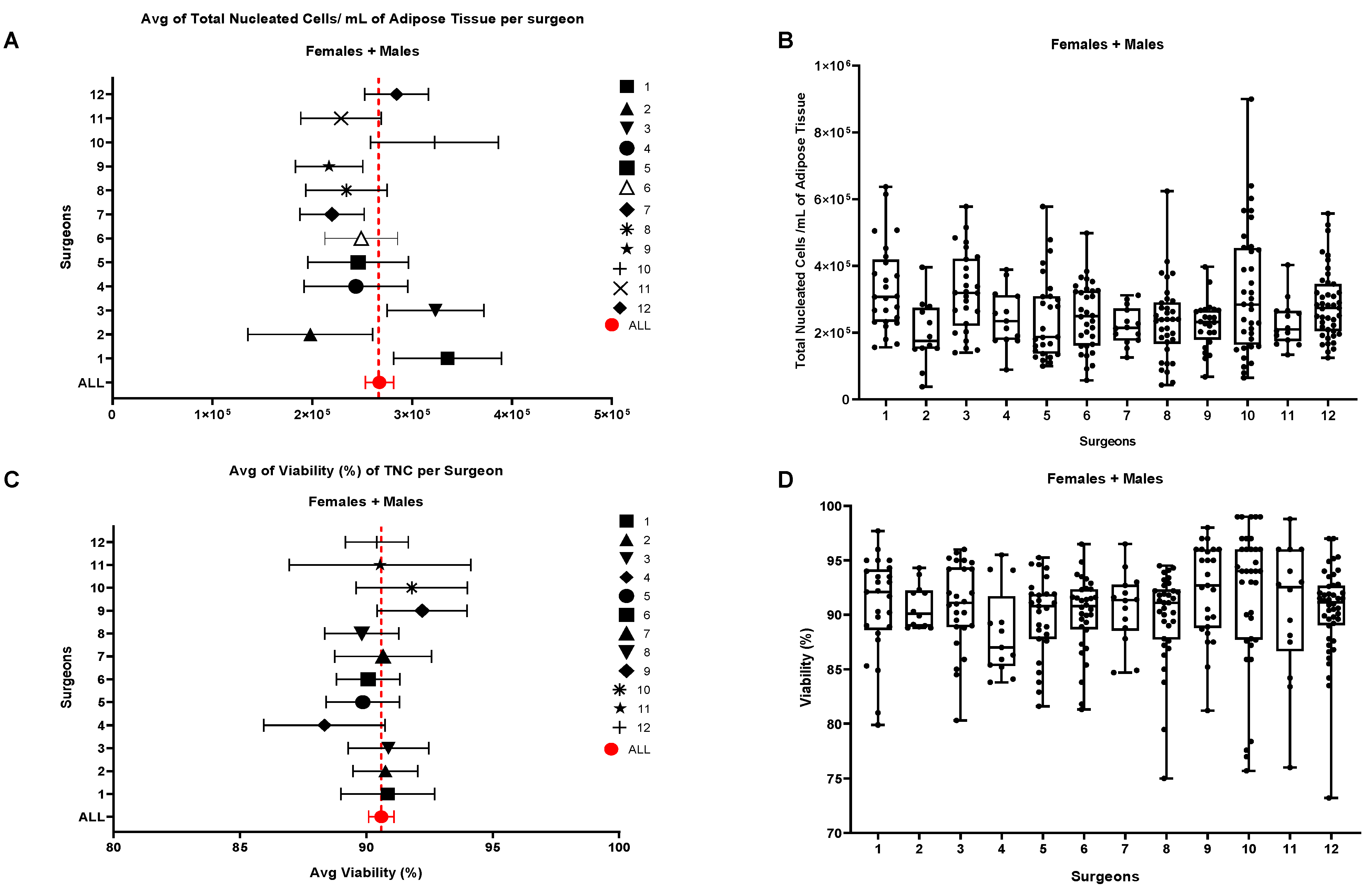
16]. This gating strategy was applied to all 302 samples to standardize data production. With this purpose, we first tried to analyze some potential influencing factors like harvesting operators and anatomical sites of collection, which are sources of variability independent from our control. Each surgeon had a preferred collection technique which represents the major hurdle in biodiversity biases which make difficult to completely standardize the method of adipose tissue collection. We thus grouped the patients based on the surgeon who performed the liposuction, obtaining 12 different groups of patients, each with N > 10 patients. For each group the average number of TNCs per mL of adipose tissue and the average viability were compared to the average of the whole cohort of 302 samples.
Results are shown in
Figure 2 where we couldn’t find any significant statistical difference for both parameters in the group of 12 surgeons meaning that, the surgeon is not a relevant factor influencing TNCs and cell viability. Deeper analysis by splitting every patient cohort following gender did not sort any additional statistically significant effect (
Figure S1), neither by splitting data for cell sub-populations like ASCs, EPCs and pericytes (
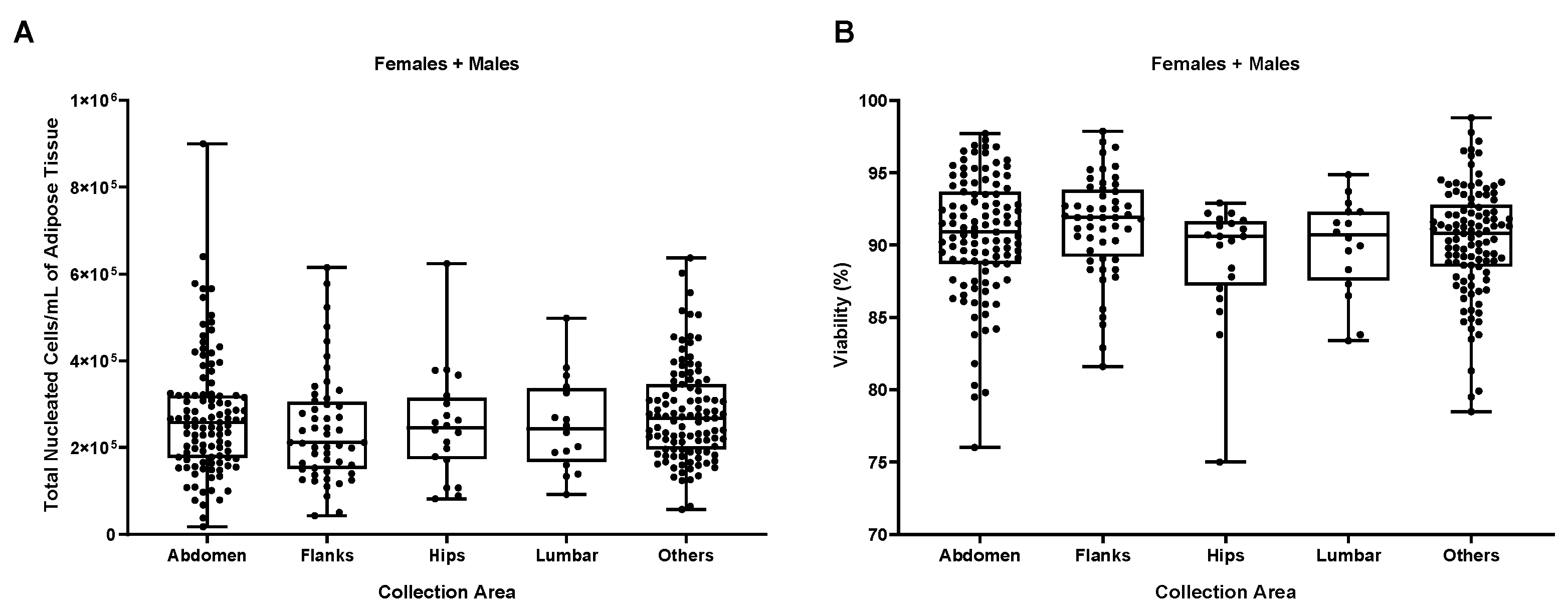
Figure S2). The fact that we couldn’t find any difference between surgeons encouraged us to check for another possible influencing factor, the anatomical site of collection. For this purpose, we divided the 302 samples in 5 groups, based on the most representative anatomical collections sites, Abdomen, Flanks, Hips, Lumbar and Others (where all the different sites of collection together with unknown and multiple areas were included) and, in a first instance, we analyzed them against TNCs per mL of adipose tissue and viability.
As shown in
Figure 3, no statistically significant differences were found comparing the different anatomical areas, suggesting that they do not have any impact on the two biological parameters TNCs per mL of adipose tissue and percentage of viable cells which are considered the most important parameters for cell therapies. The scheme adopted for analysis of data from site collection was the same as for Surgeons, we evaluated TNCs/ml, viability and all the different cell types/ml of our characterized sub-populations splitting each group following the gender and again, no differences could be detected (
Figures S3-S4).
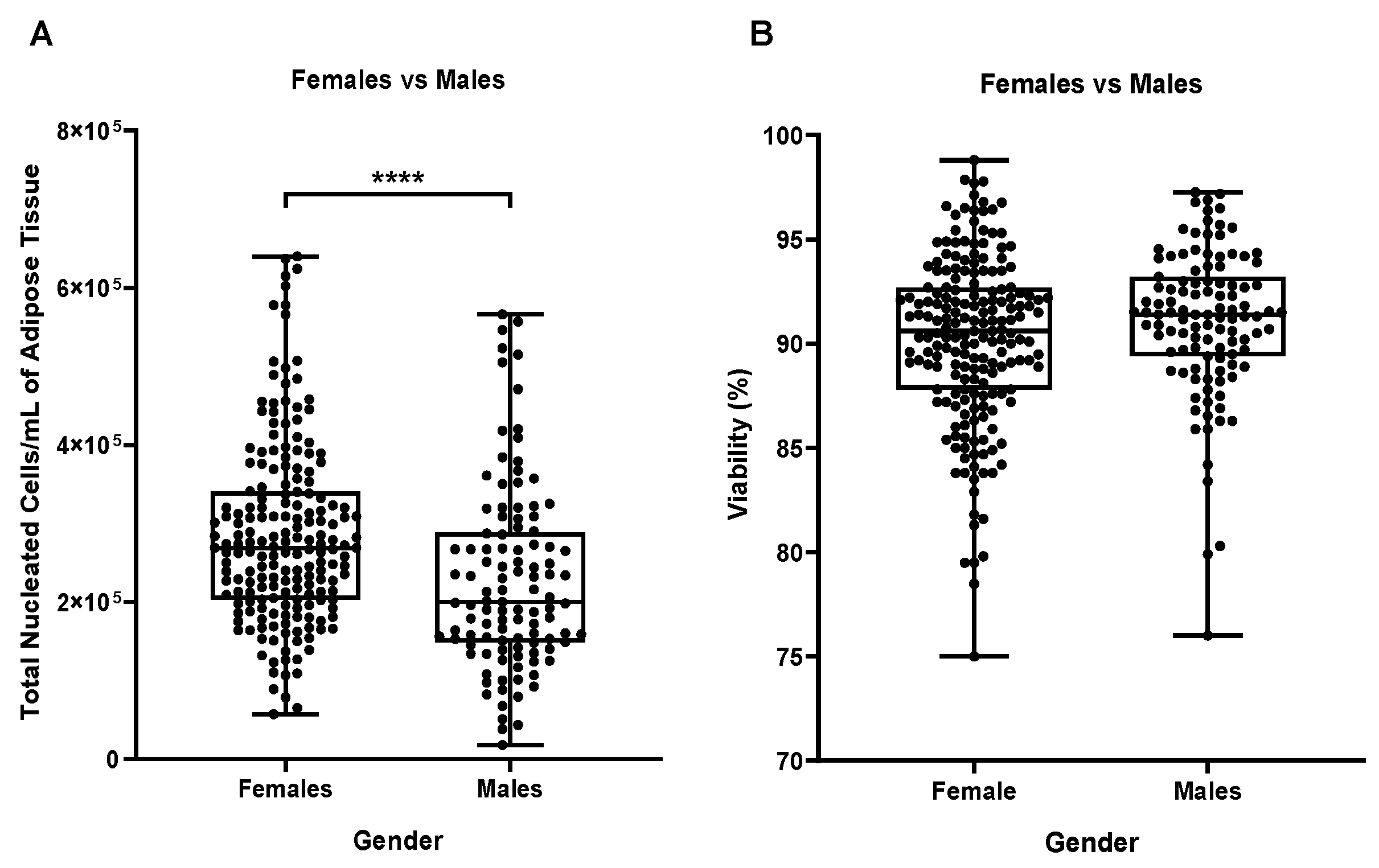
At this point, we could eliminate two potential influencing factors, surgeon and anatomical site of collection and proceed to the inter-analysis based on two biological parameters, gender, and age of the patients. Comparison of TNCs per mL of adipose tissue by gender surprisingly outlined a high statistically significant difference between male and female samples as shown in
Figure 4A (p≤0.001) with female samples containing higher numbers of TNCs. On the other hand, when comparing female vs males’ cells viability, no statistical differences were detectable (
Figure 4B).
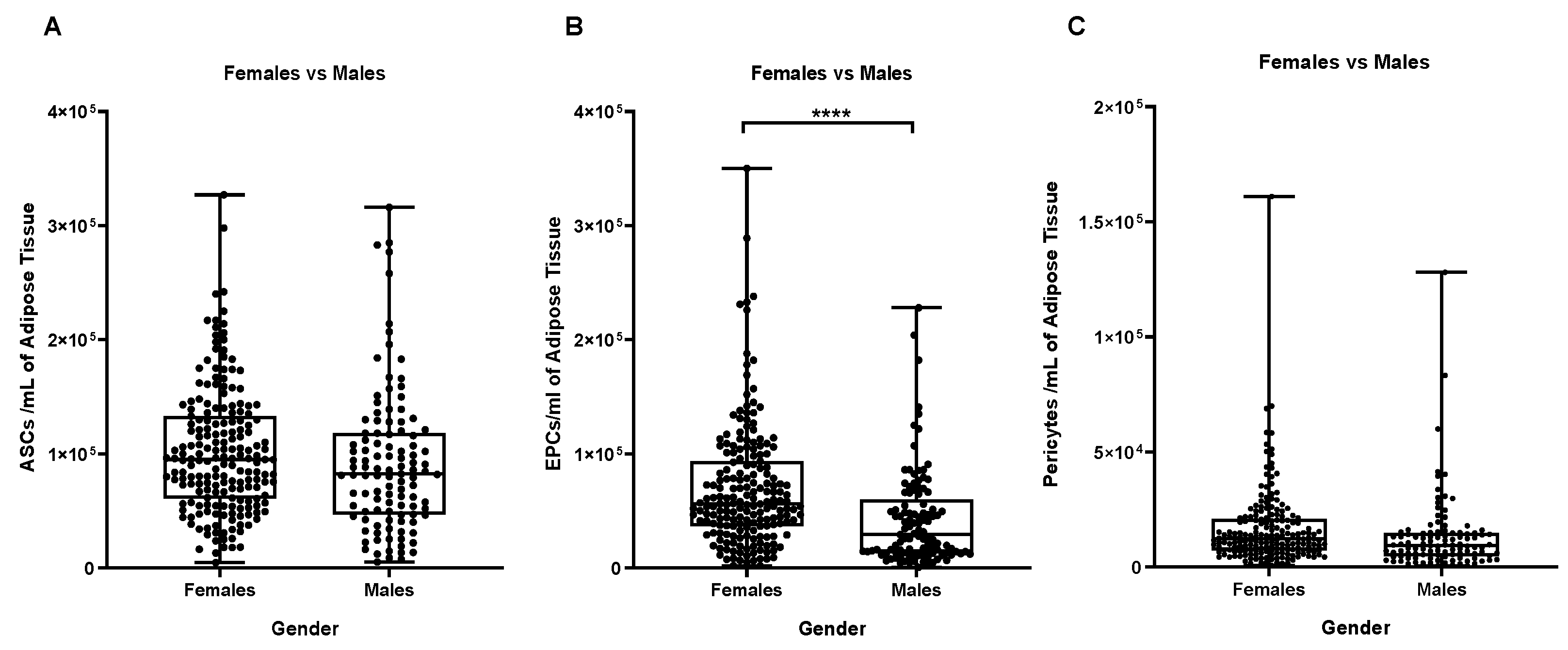
Stromal vascular cells divided into their main cell sub-populations (ASCs, endothelial precursors and pericytes) allowed us to better characterize the difference highlighted between male and female samples in
Figure 5, showing that female samples contain more EPCs than male ones (p≤0.0001,
Figure 5B).
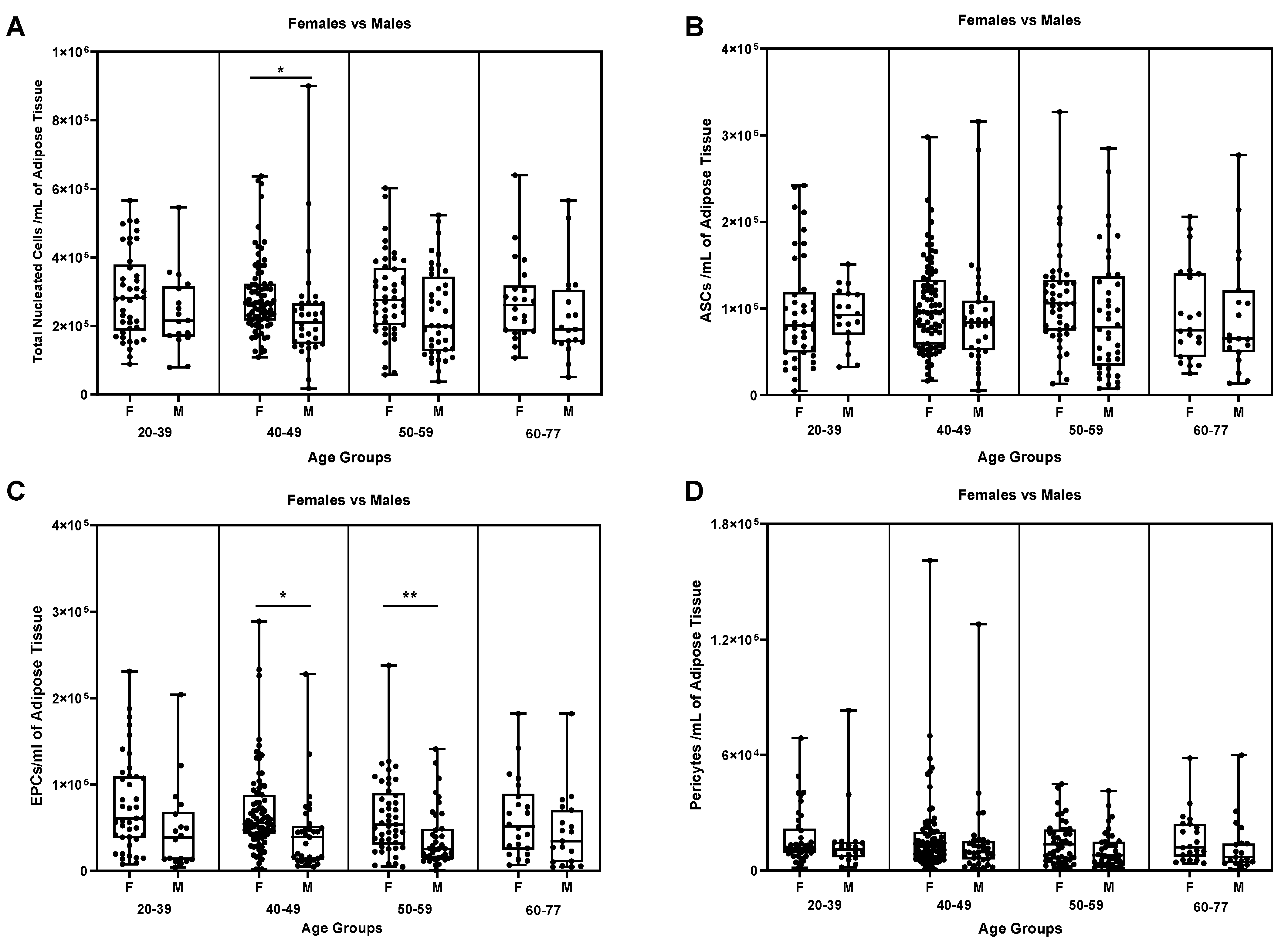
We then considered another biological factor, the age of the donors. So, samples were divided following 4 age groups respectively of 20-39, 40-49, 50-59 and 60-77 and compared the previous mentioned parameters for male and female samples. Hence, we could spot a statistically significant difference in the age group of 40-49 (
Figure 6A, p ≤ 0.05) in the number of TNCs per mL of adipose tissue.
The same difference was detectable in the EPCs sub-groups of 40-49 (
Figure 6C, p ≤ 0.05) and 50-59 (
Figure 6C, p ≤ 0.001).
Besides these different concentrations, no other statistically significant difference could be observed neither for ASCs nor for pericytes in the same age sub-groups (
Figure 6B and D).
Further analysis on age-split groups did not show any statistically significant difference neither in cellular viability in “female + male” samples cohort, nor in the two separated groups (
Figure S5).
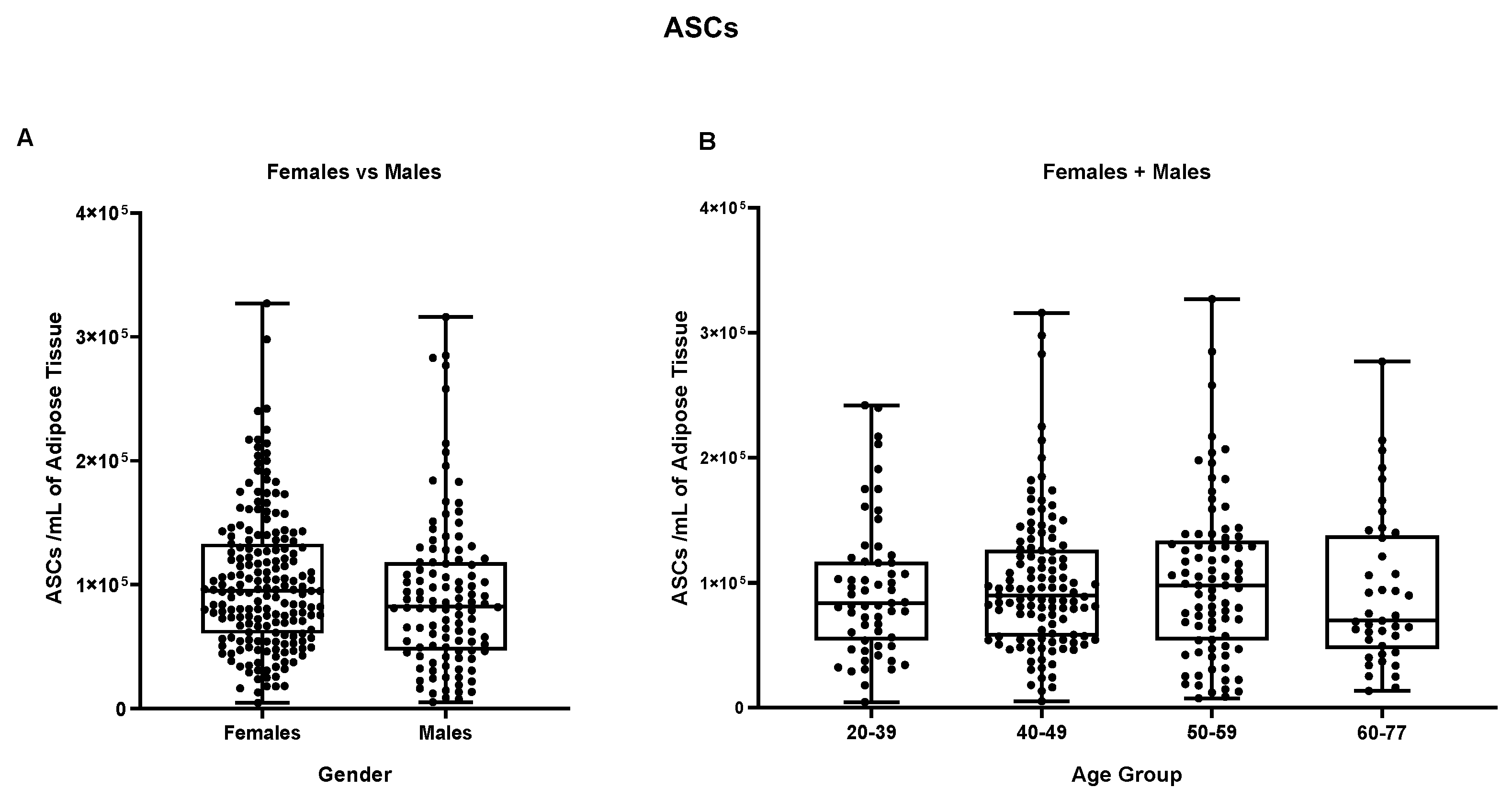
The main sub-populations reported in the stromal vascular fraction, i.e. ASCs, endothelial precursors and pericytes were then, also compared based on gender and age sub-groups. As reported in
Figure 7, no statistically significant difference was detectable in the ASCs group between male and female samples comparing both female and male groups with mean values of respectively 1,02 x 10
5 ASCs per ml and 9,2 x 10
4 ASCs per ml of adipose tissue. These results were also confirmed by comparing both “female + male” cohort following age sub-groups and female/male cohort taken separately (
Figure S6).
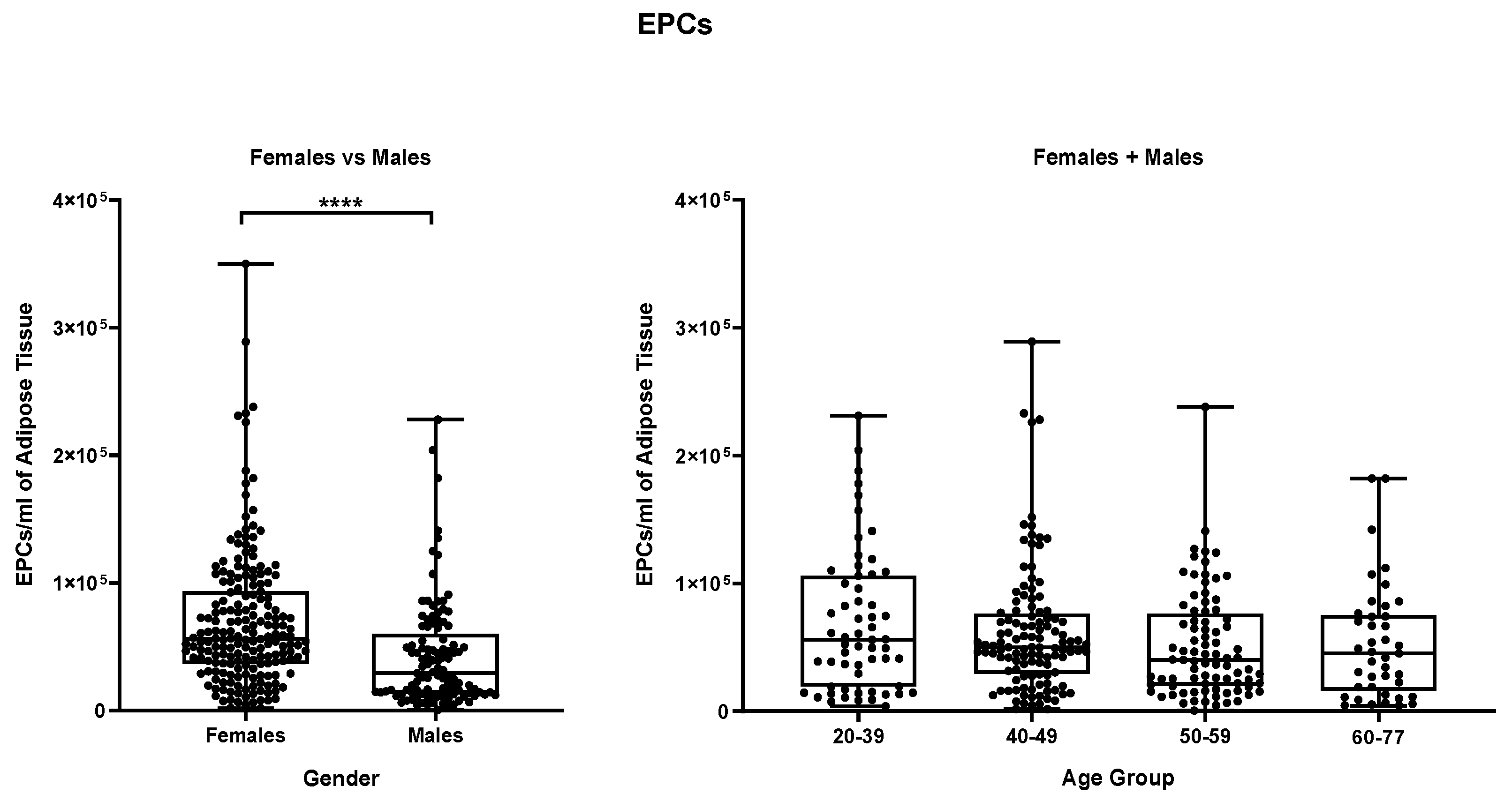
Figure 8 shows the same data analyzed for endothelial precursors where a statistically significant difference is evidenced (p ≤ 0,0001) between female and the male samples group. Female samples contain a mean of 6,96 x 10
4 endothelial precursors cells per mL while male samples which have a mean value of 4,26 x 10
4 cells per ml.
Splitting samples by age groups do not highlight any further statistically significant difference, neither in the “female + male” group nor in the separated female and male groups (
Figure S7). Finally, in
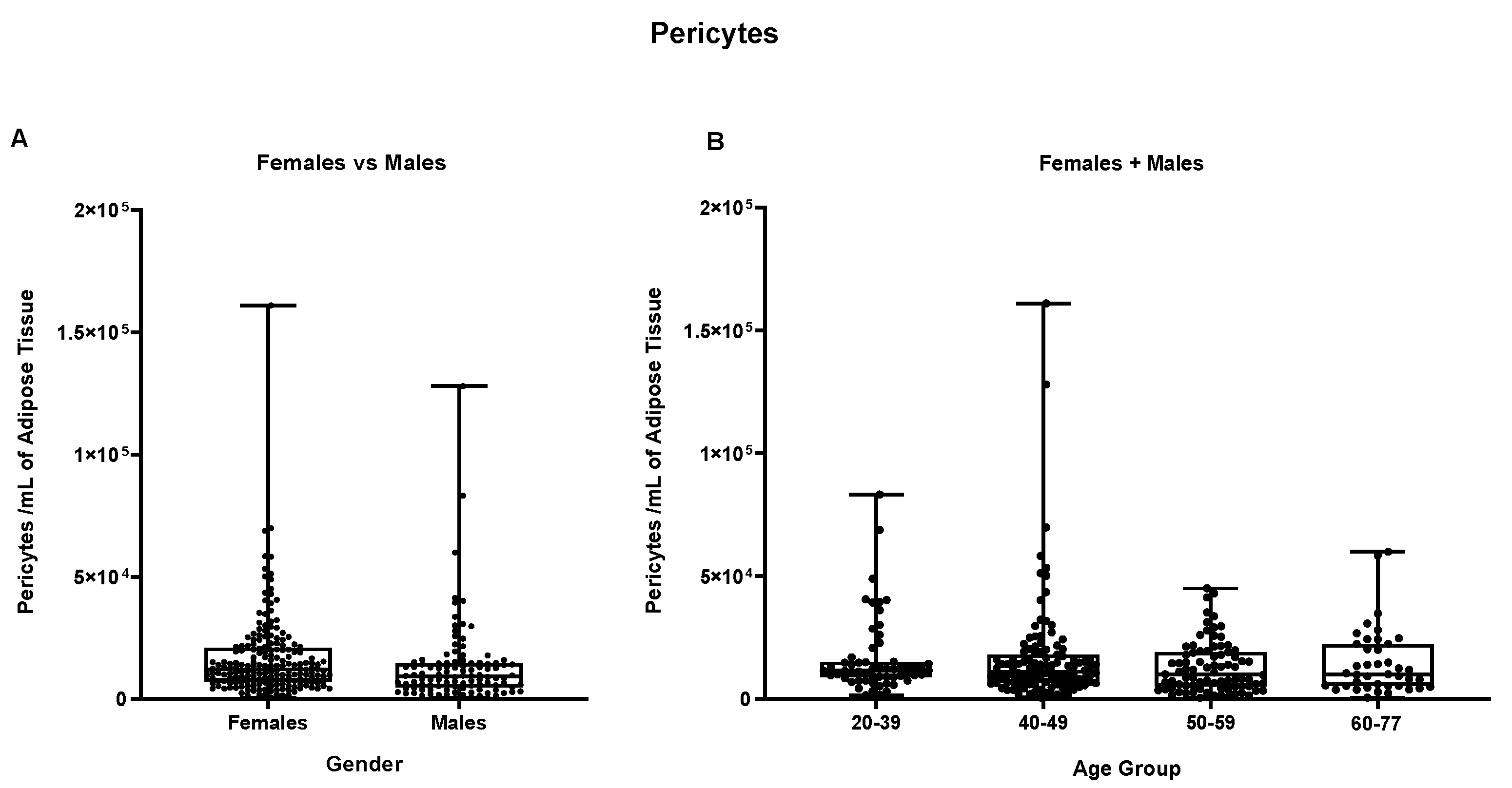
Figure 9, pericytes cells in both female and male groups show mean values of respectively 1,66 x 10
4 pericytes per mL of adipose tissue in males and 1,31 x 10
4 pericytes per mL of adipose tissue in female samples but not statistically different, results confirmed also by the splitting of samples by age groups (
Figure S8).
Overall, our results suggest that TNC, cell viability and the distribution of SVF subpopulations are not influenced by surgeons’ technique of liposuction nor by the anatomical site of collection. Nevertheless, from the intra-sample comparison emerged that the only statistically significant different parameters on 302 samples processed in compliance with GMP procedures, are the concentration of total nucleated cells and, consequently, the number of EPCs per mL in female samples compared to male ones.
3. Discussion
The objective of this study was to investigate the biological variability in terms of number of nucleated cells, viability and characterized cell sub-populations of more than 300 samples of adipose tissue processed with a standardized, GMP- compliant protocol in our facilities with the declared aim to improve the use of SVF as an ATMP and thus, open its use in cell therapies. We showed that despite each surgeon has a preferable technique of adipose tissue harvesting, implying the use of specific cannulas and liposuction devices, together with a specific anatomical site of collection, these two parameters do not have any significative influence on biological characteristics of the tissue. This could be due to fact that all the surgeons participating in our Swiss network are trained and follow specific guidelines for adipose tissue collection. Our resulted number of TNCs/ml and ASCs/ml when compared for the five different anatomical sites of collection are in contrast with previous findings, reporting a higher number of TNCs and ASCs in inner thighs [
21] and a higher yield of ASCs in abdomen samples [
22]. These contradictory findings may be explained by the different number of samples analyzed and, by the gender of the donors.
Once excluded these two variables, we addressed to the gender and the age of the donors, two parameters which have been investigated mainly in terms of ASCs proliferative capacity, differentiation ability and, more recently, at a transcriptomic level [
23]. In fact, gender dimorphism of adipose tissue in humans has been widely studied also, from an anatomical and physiological point of view, in terms of differential accumulation of fat depots [
24] a phenomenon strictly related to hormonal influence [
25,
26] i.e., estrogens and androgens fluctuation during the entire life influence the metabolic balance. This gender variability has been confirmed by our analysis: comparing the number of total nucleated cells per ml of tissue among females and males and resulted to be significatively higher in females. We then further analyzed whether this difference in TNCs is due to a prevalence of a specific cellular sub-population and found EPCs to be consistently outnumbered in females aged 40-59 years. This outcome may be explained, as mentioned above, by the larger subcutaneous adipose tissue depots found in females which require, consequently, a wider vascular network mainly formed by EPCs interacting with other cell types. The neovascularization process is stimulated by the action of sexual hormones, i.e., estrogens and androgens [
25,
26,
27], as they promote the secretion of leptin, a typical adipose-tissue secreted hormone. Leptin stimulates the expression of VEGF by ASCs and endothelial progenitors, which constitute, in concert with pericytes through several molecular interactions, a new vascular network [
28,
29,
30]. EPCs are defined as circulating cells expressing multiple cell surface markers, such as CD34, CD31, vWF (Von-Willebrand Factor), CD146 and CD144 [
31,
32] and were demonstrated to provide building blocks for the formation of a new vascular system [
33]. As opposed to EPCs cells, number of ASCs and pericytes/ml of tissue compared on patients’ gender and age didn’t show any statistical difference. ASCs represent the most interesting population in the field of regenerative medicine as they are involved in the maintenance of the tissue metabolic balance, in angiogenesis, wound healing and scar reparation [
34,
35,
36,
37,
38] by also contributing, in synergy with pericytes and EPCs, in sustaining the capillaries vascular network [
36,
39]. Pericytes or perivascular cells, retain a strong differentiation and angiogenic potential as recently discovered by Ahmed and colleagues [
40] since they display similar characteristics to MSCs and are crucial for ECs survival, migration and sustain [
41,
42,
43]. No other variations in terms of cellular concentrations nor cellular viability are reported in this work, suggesting that processing samples with a standardized GMP compliant protocol, could better characterize the biological parameters important for further use of these cells as ATMPs as we also previously shown in a collaborative study [
15]. Moreover, this characterization could help in defining new cellular standards for ATMPs use in cell therapy as we indeed established for GMP-prepared stromal vascular fraction cell release criteria with defined cell parameters.
Figure 1.
Illustration of the gating strategy. ASCs: Adipose-derived Stem Cells; Endothelial: Endothelial Cells; FS: Forward Scatter; Pericytes: Pericytes; SS: Side Scatter; EPCs: Endothelial Progenitor Cells; HSCs: Hematopoietic Stem Cells; Blood: Blood Cells.
Figure 1.
Illustration of the gating strategy. ASCs: Adipose-derived Stem Cells; Endothelial: Endothelial Cells; FS: Forward Scatter; Pericytes: Pericytes; SS: Side Scatter; EPCs: Endothelial Progenitor Cells; HSCs: Hematopoietic Stem Cells; Blood: Blood Cells.
Figure 2.
Influence of the Surgeon on the number of TNCs per mL of adipose tissue and on the cellular viability. (A, B) Comparison of the number of TNCs/mL between samples harvested by different Surgeons. (B, C) Cellular viability. Single groups average compared with the total average of the 302 samples. Females + Males cohort N= 302; Females N=191, Males N=111.
Figure 2.
Influence of the Surgeon on the number of TNCs per mL of adipose tissue and on the cellular viability. (A, B) Comparison of the number of TNCs/mL between samples harvested by different Surgeons. (B, C) Cellular viability. Single groups average compared with the total average of the 302 samples. Females + Males cohort N= 302; Females N=191, Males N=111.
Figure 3.
(A) Influence of the anatomical area of collection on the number of TNCs per mL of adipose tissue and on the cellular viability. (B) Influence of different anatomical areas of collection on the different cellular sub-populations. Females + Males N=302, Abdomen N=107, Flanks N=52, Hips N=20, Lumbar N=16, Others N=107.
Figure 3.
(A) Influence of the anatomical area of collection on the number of TNCs per mL of adipose tissue and on the cellular viability. (B) Influence of different anatomical areas of collection on the different cellular sub-populations. Females + Males N=302, Abdomen N=107, Flanks N=52, Hips N=20, Lumbar N=16, Others N=107.
Figure 4.
(A) Influence of the gender on the number of total nucleated cells (TNCs) per mL of adipose tissue and (B) on the cellular viability of N=191 females and N=111 males. ****p≤0,0001.
Figure 4.
(A) Influence of the gender on the number of total nucleated cells (TNCs) per mL of adipose tissue and (B) on the cellular viability of N=191 females and N=111 males. ****p≤0,0001.
Figure 5.
Influence of the Gender on the number of a specific cell sub-type per ml of adipose tissue. (A) ASCs/ml, (B) EPCs/ml and (C) Pericytes/ml. **** p ≤ 0,0001.
Figure 5.
Influence of the Gender on the number of a specific cell sub-type per ml of adipose tissue. (A) ASCs/ml, (B) EPCs/ml and (C) Pericytes/ml. **** p ≤ 0,0001.
Figure 6.
Inter-comparison within the same group of age among Females and Males. * p ≤ 0,05; ** p ≤ 0,005. Comparison of (A)TNCs/ml, (B) ASCs/ml, (C) EPCs/ml and (D) Pericytes/ml among females and males of the same age group. N for each group: Females (20-39) = 41, Males: (20-39) = 18; Females (40-49) = 83, Males (40-49) = 34; Females (50-59) = 45, Males (50-59) = 40; Females (60-77) = 22, Males (60-77) = 19.
Figure 6.
Inter-comparison within the same group of age among Females and Males. * p ≤ 0,05; ** p ≤ 0,005. Comparison of (A)TNCs/ml, (B) ASCs/ml, (C) EPCs/ml and (D) Pericytes/ml among females and males of the same age group. N for each group: Females (20-39) = 41, Males: (20-39) = 18; Females (40-49) = 83, Males (40-49) = 34; Females (50-59) = 45, Males (50-59) = 40; Females (60-77) = 22, Males (60-77) = 19.
Figure 7.
ASCs/ mL of adipose tissue. (A) Comparison between Females and Males, and (B) between different age groups (Females + Males cohorts).
Figure 7.
ASCs/ mL of adipose tissue. (A) Comparison between Females and Males, and (B) between different age groups (Females + Males cohorts).
Figure 8.
EPCs per mL of adipose tissue. (A) Comparison between Females and Males, (B) comparison between different age groups (Females + Males cohorts). **** p≤0,0001.
Figure 8.
EPCs per mL of adipose tissue. (A) Comparison between Females and Males, (B) comparison between different age groups (Females + Males cohorts). **** p≤0,0001.
Figure 9.
Pericytes per mL of adipose tissue. (A) Comparison between Females vs Males, (B) comparison between different age groups (Females + Males cohorts).
Figure 9.
Pericytes per mL of adipose tissue. (A) Comparison between Females vs Males, (B) comparison between different age groups (Females + Males cohorts).