1. Introduction
The development of new packaging solutions is a result of how packaging technology develops in response to consumer expectations for food products that are fresh, safe, and of an exceptional quality [
1,
2]. Bioactive substances, such as antimicrobials [
3] and antioxidants [
4], are present in active food packaging materials to increase shelf life, preserve the quality, and stabilize food-packaged commodities [
5]. The goal of intelligent food packaging solutions is to monitor the environment or the conditions of the food, identify any physical, chemical, or biological changes, and take appropriate action. The outcome is intended to provide a quick evaluation of food quality [
6,
7,
8].
According to the variables that can be controlled, intelligent sensor-based packaging materials can be divided into time-temperature, gas, and freshness indicators to track improper temperature changes along the supply chain, variations in the gas composition in the headspace of the food package, particularly in modified atmosphere packaging, and freshness decay, through alterations in the amount of metabolites indicators of microbial growth and, consequently, altering the freshness of food [
2,
9]. The most popular freshness indicators are made consisting of a solid support and a dye that reacts to pH changes by changing color and giving a visual response to the environment inside the package [
2,
6]. Despite the fact that several synthetic dyes have been investigated as pH indicators in numerous research [
10,
11,
12], leaching of the dye and consumer knowledge of the negative effects induced by chemically produced dyes raises concerns due to their toxicity and bioaccumulation [
2,
6,
13]. Due to their biodegradability, lack of toxicity, lack of carcinogenicity, and ecologically benign manufacture, natural dyes derived from a variety of sources seem to be a potential option [
14,
15]. Anthocyanins from barberry (
Berberis vulgaris L.), black carrots (
Daucus carota L.), saffron (
Crocus sativus L.), red cabbage (
Brassica oleraceae), and the red naphthoquinone pigment shikonin from the root of gromwell are a few examples of natural pigments used in colorimetric indicator systems [
7,
8,
16,
17].
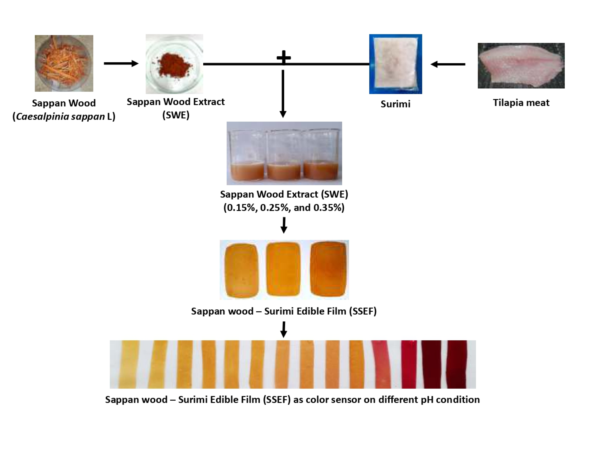
Sappan wood (
Caesalpinia sappan L.) is naturally present across Asia, including China, Japan, and Thailand. This wood plant is now grown in a number of other parts of the world, including Africa, Europe, North America, and South America, due to its many beneficial uses. The wood has the potential to be used as a natural red dye as opposed to a synthetic dye since it is cheap and lacks a distinctive flavor [
18]. Brazilein, a white phenolic compound with two aromatic rings, one pyrone, and one five-membered ring, is the primary active component of sappan. However, the hydroxyl group in the brazilein structure is easily oxidized and can convert into a carbonyl group, leading to a structural change and the creation of brazilein, a colorful molecule. Being a polyphenolic substance, brazilein is expected to change color as a result of changes in pH that impact the hydroxyl group in its molecule [
19,
20]. In addition to brazilein,
C. sappan is also thought to be a possible source of anthocyanins, natural substances that might be candidates to replace synthetic dyes because of their attractive, vivid colors (orange, red, and purple), which exhibit rapid color fading when exposed to light, oxygen, hot temperatures, pH, salt stress, and enzymes [
21].
The incorporation of biopolymers in active packaging has attracted the attention of numerous researchers. These biopolymers include the units created by a covalent peptide link, proteins [
22]. Many crucial sources of protein can be obtained in various plant or animal sources. Researchers began to extract polypeptides from a wide range of vegetable and animal products or by-products due to the abundance of resources inside these fundamental products [
23,
24]. Surimi, a by-product prepared from minced, deboned fish meat, contains concentrated myofibrillar fish proteins [
25]. The attributes of myofibrillar protein-based films are slightly superior to those of known protein films, and myofibrillar protein exhibited outstanding film-forming capacities in both acidic and alkaline environments [
26,
27,
28].
To the best of our knowledge, no study has yet been conducted on the changes on physical properties and color of myofibrillar protein-based edible film incorporated with sappan wood extract at varied pH and soaking time in different condition. Thus, the aim of the present research is to investigate the physical properties of the myofibrillar protein-based edible film added with different concentration of sappan wood extract. Additionally, the color of the film at different pH and soaking condition also evaluated.
2. Materials and Methods
2.1. Raw materials
The surimi was obtained from the processing of Nile tilapia (Orechromis niloticus) were bought from a supermarket (local market, West Java, Indonesia), with the process was done following the method described by Shiku et al. (2004) with modification. The HCl (PubChem CID 313), and NaOH (PubChem CID 14798) were procured from LOBA CHEMIE PVT. LTD., India. All chemicals were analytical grade.
2.2. Preparation of sappan wood surimi edible film
The preparation of sappan wood-surimi edible film (SSEF) was done by following the method described by [
29] with slight modification. Frozen surimi was thawed for 20 min and the thawed surimi (10% w/w) was mixed with distilled water (150 mL) and 1M HCl until the pH was 3. The mixture was homogenized using a homogenizer (PRO250 Homogenizer, Thomas 1204B63, Thomas Scientific, USA) for 30 min at 55°C with the addition of 50% glycerol from the weight of the surimi (w/w) and sieved using 150 mesh nylon sieve. The sappan wood extract (SWE) was added into the film mixture with different concentration (0.15%, 0.25%, and 0.35% w/v) as natural dye. The SSEF mixture was homogenized, poured into a glass plate (20 x 20 cm), and dried in a hot air oven (Binder, Binder GmbH, Germany) at 50°C for 48 h. After the drying process, the SSEF film was packed in a polyethylene bag and put in a desiccator for further use.
2.3. Physical properties analysis
2.3.1. Thickness
The thickness of the SSFEs was measured by using a digital micrometer (Mitutoyo, Tokyo, Japan). The measurement was done from different areas of the films. The thickness measurement was done in triplicate
2.3.2. Transparency
The film transparency (%) was measured using a UV spectrophotometer (model UV-160, Shimadzu, Kyoto, Japan) at 600 nm, according to the method of [
30]. The transparency value of the film was calculated by the following equation:
where T
600 is the fractional transmittance at 600 nm and x is the thickness of the film (mm).
2.3.3. Mechanical properties
The tensile strength (TS) and elongation at break of the SSEFs were measured according to the standard protocols [
31]. Films were cut into 1 cm x 10 cm strips and kept in a desiccator containing NaBr solution with RH of 57% for 72 h prior to the test. The measurement was done using a texture analyzer (SMT5, Santam, Tehran, Iran) equipped with 100 N load cell, 10 cm distance between grips, and the crosshead speed of 10 mm/min. The measurement of the color values was conducted in quintuplet.
2.4. Color values at different pH and soaking time at different condition
The color (L*, a* and b*) values of the SSFEs at different pH (1 to 14) using a buffer solution at respective pH and soaking time (0 - 20 min, observed periodically every 2 min) at different condition (acid, neutral, and alkaline) were measured using a Colorimeter (ColorFlex, Hunter Lab Inc., USA), with the L* represents the dark-light spectrum, the a* represents red intensity, and the b* represents yellow intensity [
32]. The measurement of the color values was conducted in triplicate. The total color difference (ΔE) was calculated as follows:
where ΔL*, Δa*, and Δb* were the differences between color parameters of control sample and the color parameters obtained at different pH and soaking time at different condition.
2.5. Statistical analysis
All obtained results were analyzed and reported as mean ± SD (standard deviation). The statistical analysis was conducted using SPSS software version 17 (SPSS Inc., Chicago, IL, USA) using Duncan’s Multiple Range Test (DMRT) with the significance level determined at 95% confidence limit.
3. Results
3.1. Physical properties
The results on the physical properties of the SSEFs are shown in
Table 1. The thickness of the SSEFs was in the range of 0.17 - 0.22 mm. Based on the results, the transparency of the SSEFs was in the range of 0.84 - 2.16. The determination of the TS and the elongation at break of the SSEF revealed that the values of these properties were in the range of 1.70 - 10.15 MPa and 12.68 - 15.70%, respectively. The addition of SWE at different concentration in the formulation of SSEF significantly (
p < 0.05) affected the properties of the resulted film.
3.2. Color values of SSEF at different condition
3.2.1. Color values at different pH
The sensitivity of the SSEF with different SWE concentration to pH changes was evaluated through immersion in buffer solutions with pH values ranging from 1 to 14 (
Table 2). Based on the color measurement of SSFE with different SWE concentration at same pH value, the L* of the SSEF with 0.15% SWE exhibited significantly (
p < 0.05) higher value (65.86 - 74.97) than the film with 0.25% (64.75 - 73.56) and 0.35% (44.35 - 60.96) SWE. Conversely, the a* and b* value of the SSEF containing the highest concentration of SWE showed significantly (
p < 0.05) higher value (16.72 - 58.25 and 25.14 - 43.58, respectively) than the SSEF with 0.25% (7.68 - 50.22 and 15.65 - 29.87, respectively) and 0.15% (5.43 - 48.87 and 11.75 - 22.15, respectively) extract (
Table 1). Consequently, the total color difference (ΔE) of the SSEF with the addition of 0.15%, 0.25%, and 0.35% SWE were increased significantly (2.30 - 44.20, 8.37 - 43.90, and 3.77 - 45.86, respectively;
p < 0.05)
3.2.2. Color values at different soaking time in different condition
The SSEF with different SWE concentration also subjected to different soaking time at various condition (
Table 3,
Table 4 and
Table 5). Based on the color measurement of the SSEF at different soaking time in acidic condition (
Table 3), the L* of the SSEF with 0.15% SWE exhibited significantly (
p < 0.05) higher value (73.09 - 73.41) than the film with 0.25% (67.56 - 68.74) and 0.35% (59.75 - 60.62) SWE. Conversely, the a* and b* value of the SSEF containing the highest concentration of SWE showed significantly (
p < 0.05) higher value (15.31 - 19.96 and 39.42 - 45.07, respectively) than the SSEF with 0.25% (10.22 - 10.81 and 28.95 - 34.79, respectively) and 0.15% (5.65 - 6.29 and 19.61 - 21.15, respectively) extract. The total color difference (ΔE) of the SSEF with the addition of 0.15%, 0.25%, and 0.35% SWE were increased significantly (0.29 - 1.69, 0.26 - 5.99, and 1.49 - 7.37, respectively).
In neutral condition (
Table 4), the L* of the SSEF with 0.15% SWE also exhibited significantly (
p < 0.05) higher value (71.61 - 73.06) than the film with 0.25% (65.26 - 67.56) and 0.35% (56.81 - 59.76) SWE. Conversely, the a* and b* value of the SSEF containing the highest concentration of SWE showed significantly (
p < 0.05) higher value (19.96 - 21.08 and 38.04 - 45.07, respectively) than the SSEF with 0.25% (10.81 - 11.69 and 30.21 - 34.79, respectively) and 0.15% (6.29 - 7.59 and 18.91 - 21.15, respectively) extract. The total color difference (ΔE) of the SSEF with the addition of 0.15%, 0.25%, and 0.35% SWE were increased significantly (0.54 - 2.98, 0.46 - 5.20, and 1.09 - 7.70, respectively).
The L* of the SSEF soaked in alkaline condition with 0.15% SWE also exhibited significantly (p < 0.05) higher value (60.73 - 73.09) than the film with 0.25% (54.02 - 67.55) and 0.35% (51.40 - 59.75) SWE. In contrast, the a* and b* value of the SSEF with the highest SWE concentration showed significantly (p < 0.05) higher value (19.96 - 42.21 and 32.71 - 45.07, respectively) than the SSEF with 0.25% (10.81 - 32.50 and 26.83 - 34.79, respectively) and 0.15% (6.29 - 24.79 and 13.85 - 21.15, respectively) extract. The total color difference (ΔE) of the SSEF with the addition of 0.15%, 0.25%, and 0.35% SWE were increased significantly (5.16 - 23.42, 2.71 - 26.78, and 4.60 - 26.79, respectively).
4. Discussion
4.1. Physical properties
The strength of the material and the ability of the edible films to retain the integrity of the packed food are determined by their mechanical properties [
33]. The increasing concentration of SWE increased the thickness of the edible film. According to reports, natural films made for food packaging range in thickness from 0.05 to 0.2 mm [
34]. The results showed that the thickness of the films were within the acceptable range. Although the casting solutions have the same weight, this thickness variation can also be related to the varied film drying kinetics, which have an impact on the resulting thickness and structure, as has previously been noted in the literature [
35,
36,
37]. Conversely, the increasing of the SWE concentration decreased the transparency of the film. The transparency of the film offers details about the size distribution of the particles in the matrix [
38]. Based on the results, the highest TS value was obtained by the SSEF with the highest concentration of SWE.
4.2. Color values of SSEF at different condition
4.2.1. Color values at different pH
The increasing concentration of SWE at same pH condition decreased the L* value of the film, while the a* and b* value was increased. Considering the pH value to the color of the SSEF, the increasing SWE concentration contributed to the increasing darkness, redness, and yellowness of the SSFE. Shown by the results on the color measurement, the values of the a*, which indicates the redness of the sample, was increased by the increasing pH values. Moreover, the yellowness, indicated by the b* value, was decreased with the increasing of the pH value. Brazilein showed a yellow color when the environment was acidic; the color changed to red as the pH rose to an alkaline condition. The lowering L* value also reflects the increased darkness, which was caused by raising the pH to the alkaline region. The protonation and deprotonation of the hydroxyl (OH) group of polyphenolic compounds (such as anthocyanins and other flavonoids), which frequently occurred upon pH shift, resulted in alterations in the molecular structure of brazilein and caused changes in the color value [
19].
4.2.2. Color values at different soaking time in different condition
The increasing of SWE concentration increased the a* value of the film, while the L* and b* value was decreased. Prolonged soaking duration also shown to significantly (p < 0.05) affected the color values of the SSEF. The increased soaking duration in neutral condition increased the a* value, while decreased the L* and b* value. Different with the neutral condition, the acidic and alkaline environment resulting in similar results. The L* value was increased with the increasing of the SWE concentration, while the a* and b* value was decreased. Extended soaking time also shown to significantly (p < 0.05) affected the color values of the SSEF. The prolonged soaking duration in acidic and alkaline condition increased the a* value, while decreased the L* and b* value.
As can be seen from the results, the gap of the color values between soaking period were getting wider with the increasing of the pH of the soaking condition, With the highest gap was observed at the SSEF soaked at alkaline condition. According to [
19], brazilein exhibits stronger stability characteristics at lower pH values and reduced stability at higher pH values. These color variations are caused by the protonation and deprotonation of the hydroxyl (OH) group of brazilein. Similar results were seen for other polyphenolic pigments discovered by different researchers. According to studies by [
39,
40,
41,
42], the flavylium cation, the most common form of anthocyanins under an acidic condition (pH 3), had higher stability than the other forms, which exist at higher pH (e.g., quinoidal base, carbinol pseudobase and chalcone). In the case of phenolic compounds, decreased stability at higher pH is observed to be caused by the creation of quinone intermediate, which is quickly damaged through oxidation process [
19]. Anthocyanin also play roles in the color change of the SSEF. Anthocyanins are sensitive to pH fluctuations since they start to lose their color at pH levels greater than 3.0. At pH levels below 3, anthocyanins mostly reside in the form of the extremely stable red flavylium cation. The quick hydration of the flavylium cation causes the colored carbinol pseudobase to be produced when the pH rises from 4 to 5. At pH 6-7, a neutral quinonoidal base (purple to violet in color) results from the deprotonation of the flavylium cation; at pH 7-8, an anionic quinonoidal base is produced (blue color) [
43,
44,
45,
46,
47].
5. Conclusions
It can be concluded that edible film made from surimi with the addition of SWE can be used as a bio-based color sensor that sensitive to pH changes. Based on the results, different concentration of the SWE significantly (p < 0.05) affected the physical properties of the film. The pH, soaking time, and soaking condition, also significantly (p < 0.05) affected the color values of the film. With the increasing of pH values, the L* and b* values were decreased, while the a* value was increased. Based on the evaluation of the SSEF with different soaking condition at prolonged soaking duration, the color changes of the film in the acidic condition was more stable than in neutral and alkaline condition, while the acidic and alkaline environment resulting in similar results, which were increasing L* value and decreasing a* and b* value, correlated to the stability of the bioactive compound exhibited in the extract of the sappan wood.
Author Contributions
Conceptualization, I.R. and J.; methodology, I.R. and E.W.; software, I.R.; validation, I.R., J. and E.W.; formal analysis, I.R.; investigation, I.R.; resources, I.R., J. and E.W.; data curation, I.R.; writing—original draft preparation, I.R.; writing—review and editing, I.R.; visualization, I.R.; supervision, I.R., J. and E.W. All authors have read and agreed to the published version of the manuscript.
Funding
Universitas Padjadjaran through the International Open Access Program (IOAP)
Acknowledgments
The authors would like to thank the Ministry of Research, Technology and Higher Education of the Republic of Indonesia for the Domestic Postgraduate Education Scholarships (BPPDN) 2019 and the Faculty of Fisheries and Marine Science for supporting laboratory equipment for this research.
Conflicts of Interest
The authors declare that there are no conflict of interest regarding the role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rai. M.; Ingle,A.P.; Gupta.I.; Pandit.R.; Paralikar.P.; Gade.A.; Chaud.M.V.; dos Santos.C.A. Smart Nanopackaging for the Enhancement of Food Shelf Life. Environmental Chemistry Letters 2019, 17, 277–290. [Google Scholar]
- Balbinot-Alfaro. E.; Craveiro.D.V.; Lima.K.O.; Costa.H.L.G.; Lopes.D.R.; Prentice.C. Intelligent Packaging with PH Indicator Potential. Food Engineering Reviews 2019, 11, 235–244. [Google Scholar] [CrossRef]
- Bahrami. A.; Delshadi.R.; Assadpour.E.; Jafari.S.M.; Williams.L. Antimicrobial-Loaded Nanocarriers for Food Packaging Applications. Advances in Colloid and Interface Science 2020, 278, 102140. [Google Scholar] [CrossRef] [PubMed]
- Tran. T. Active Antioxidant Additives in Sustainable Food Packaging. In Sustainable Food Packaging Technology; Wiley: Hoboken, NJ, USA, 2021; pp. 349–367. [Google Scholar]
- Almasi. H.; Jahanbakhsh Oskouie.M.; Saleh.A.A. Review on Techniques Utilized for Design of Controlled Release Food Active Packaging. Critical Reviews in Food Science and Nutrition 2020, 61, 2601–2621. [Google Scholar]
- Terra. A.L.M.; Moreira.J.B.; Costa.J.A.V.; de Morais.M.G. Development of Time-PH Indicator Nanofibers from Natural Pigments: An Emerging Processing Technology to Monitor the Quality of Foods. LWT – Food Science and Technology 2021, 142, 111020. [Google Scholar] [CrossRef]
- Alizadeh-Sani. M.; Tavassoli.M.; McClements.D.J.; Hamishehkar.H. Multifunctional Halochromic Packaging Materials: Saffron Petal Anthocyanin Loaded-Chitosan Nanofiber/Methyl Cellulose Matrices. Food Hydrocolloids 2021, 111, 106237. [Google Scholar] [CrossRef]
- Alizadeh-Sani. M.; Tavassoli.M.; Mohammadian.E.; Ehsani.A.; Khaniki.G.J.; Priyadarshi.R.; Rhim.J.-W. PH-Responsive Color Indicator Films Based on Methylcellulose/Chitosan Nanofiber and Barberry Anthocyanins for Real-Time Monitoring of Meat Freshness. International Journal of Biological Macromolecules 2021, 166, 741–750. [Google Scholar] [CrossRef]
- Drago. E.; Campardelli.R.; Pettinato.M.; Perego.P. Innovations in Smart Packaging Concepts for Food: An Extensive Review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef]
- Koxmak. S.; Yimamumaimaiti.T.; Abdukeremu.H.; Nizamidin.P.; Yimit.A. Detection of Amines in Lamb Spoilage by Optical Waveguide Sensor Based on Bromophenol Blue-Silicon Composite Film. Chemical Research in Chinese Universities 2019, 35, 193–199. [Google Scholar] [CrossRef]
- Morsy. M.K.; Zór.K.; Kostesha.N.; Alstrøm.T.S.; Heiskanen.A.; El-Tanahi.H.; Sharoba.A.; Papkovsky.D.; Larsen.J.; Khalaf.H.; et al. Development and Validation of a Colorimetric Sensor Array for Fish Spoilage Monitoring. Food Control 2016, 60, 346–352. [Google Scholar]
- Pacquit. A.; Lau.K.T.; McLaughlin.H.; Frisby.J.; Quilty.B.; Diamond.D. Development of a Volatile Amine Sensor for the Monitoring of Fish Spoilage. Talanta 2006, 69, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi. B.; Jayus; Restyana.A.; Abdullah.A.; Heng.L.Y.; Ahmad.M. A Novel Colorimetric Food Package Label for Fish Spoilage Based on Polyaniline Film. Food Control 2012, 25, 184–189. [Google Scholar] [CrossRef]
- Yusuf. M.; Shabbir.M.; Mohammad.F. Natural Colorants: Historical, Processing and Sustainable Prospects. Natural Products and Bioprospecting 2017, 7, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Wang. S.; Xu.F.; Zhan.J. Introduction of Natural Pigments from Microorganisms. In Bio-Pigmentation and Biotechnological Implementations; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–22. [Google Scholar]
- Pourjavaher. S.; Almasi.H.; Meshkini.S.; Pirsa.S.; Parandi.E. Development of a Colorimetric PH Indicator Based on Bacterial Cellulose Nanofibers and Red Cabbage (Brassica oleraceae) Extract. Carbohydrate Polymers 2017, 156, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ezati. P.; Bang.Y.-J.; Rhim.J.-W. Preparation of a Shikonin-Based PH-Sensitive Color Indicator for Monitoring the Freshness of Fish and Pork. Food Chemistry 2021, 337, 127995. [Google Scholar] [CrossRef] [PubMed]
- Jin. S.-K.; Ha.S.-R.; Choi.J.-S. Effect of Caesalpinia sappan L. extract on physico-chemical properties of emulsion-type pork sausage during cold storage. Meat Science 2015, 110, 245–252. [Google Scholar] [CrossRef]
- Ngamwonglumlert. L.; Devahastin.S.; Chiewchan.M.; Raghavan.G.S.V. Color and molecular structure alterations of brazilein extracted from Caesalpinia sappan L. under diferent pH and heating conditions. Scientific Reports 2020, 10, 12386. [Google Scholar] [CrossRef]
- de Oliveira. L.F.C.; Edwards.H.G.M.; Velozo.E.S.; Nesbitt.M. Vibrational spectroscopic study of brazilin and brazilein, the main constituents of brazilwood from Brazil. Vibrational Spectroscopy 2002, 28, 243–249. [Google Scholar] [CrossRef]
- Azman. E.M.; Yusof.N.; Chatzifragkou.A.; Charalampopoulos.D. Stability Enhancement of Anthocyanins from Blackcurrant (Ribes Nigrum L.) Pomace through Intermolecular Copigmentation. Molecules 2022, 27, 5489. [Google Scholar] [CrossRef]
- Hanani. Z.N.; Roos.Y.; Kerry.J. Use and application of gelatin as potential biodegradable packaging materials for food products. International Journal of Biological Macromolecules 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Parimi. N.S.; Singh.M.; Kastner.J.R.; Das.K.C.; Forsberg.L.S.; Azadi.P. Optimization of Protein Extraction from Spirulina platensis to Generate a Potential Co-Product and a Biofuel Feedstock with Reduced Nitrogen Content. Frontiers in Energy Research 2015, 3, 30. [Google Scholar]
- Soto-Sierra. L.; Stoykova.P.; Nikolov.Z.L. Extraction and fractionation of microalgae-based protein products. Algal Research 2018, 36, 175–192. [Google Scholar] [CrossRef]
- Yingchutrakul. M.; Wasinnitwing.N.; Benjakul.S.; Singh.A.; Zheng.Y.; Mubango.E.; Luo.Y.; Tan.Y.; Hong.H. Asian Carp, an Alternative Material for Surimi Production: Progress and Future. Foods 2022, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- Chinabhark. S.; Benjakul.S.; Prodpran.T. Effect of pH on the properties of protein-based film from bigeye snapper (Priacanthus tayenus) surimi. Bioresource Technology 2007, 98, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Shiku. Y.; Hamaguchi.P.Y.; Benjakul.S.; Visessanguan.W.; Tanaka.M. Effect of surimi quality on properties of edible films based on Alaska Pollack. Food Chemistry 2004, 86, 493–499. [Google Scholar] [CrossRef]
- Nie. X.; Gong.Y.; Wang.N.; Meng.X. Preparation and characterization of edible myofibrillar protein-based film incorporated with grape seed procyanidins and green tea polyphenol. LWT – Food Science and Technology 2015, 64, 1042–1046. [Google Scholar] [CrossRef]
- Shiku. Y.; Hamaguchi.P.Y.; Tanaka,.M. Effect of pH on the preparation of edible films based on fish myofibrillar proteins. Fisheries Science 2003, 69, 1026–1032. [Google Scholar] [CrossRef]
- Jongjareonrak. A.; Benjakul.S.; Visessanguan.W.; Tanaka.M. Antioxidative activity and properties of fish skin gelatin films incorporated with BHT and atocopherol. Food Hydrocolloids 2011, 22, 449–458. [Google Scholar]
- American Society for Testing and Materials. Standard test method for tensile properties of thin plastic sheeting-D882-02. In Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 2002; pp. 1–9. [Google Scholar]
- Renaldi. G.; Junsara.K.; Jannu.T.; Sirinupong.N.; Samakradhamrongthai.R.S. Physicochemical, textural, and sensory qualities of pectin/gelatin gummy jelly incorporated with Garcinia atroviridis and its consumer acceptability. International Journal of Gastronomy and Food Science 2022, 28, 100505. [Google Scholar] [CrossRef]
- Aldana. D.S.; Contreras-Esquivel.J.C.; Nevárez-Moorillón.G.V.; Aguilar.C.N. Characterization of edible films from pectic extracts and essential oil from Mexican lime. CyTA - Journal of Food 2014, 13, 17–25. [Google Scholar]
- Garavand. F.; Rouhi.M.; Razavi.S.H.; Cacciotti.I.; Mohammadi.R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. International Journal of Biological Macromolecules 2017, 104, 687–707. [Google Scholar] [CrossRef] [PubMed]
- Kokoszka. S.; Debeaufort.F.; Lenart.A.; Voilley.A. Water vapour permeability, thermal and wetting properties of whey protein isolate based edible films. International Dairy Journal 2010, 20, 53–60. [Google Scholar] [CrossRef]
- Karbowiok. T.; Debeaufort.F.; Voilley.A. Influence of thermal process on structure and functional properties of emulsion-based edible films. Food Hydrocolloids 2007, 21, 879–888. [Google Scholar] [CrossRef]
- Chakravartula. S.S.N.; Soccio.M.; Lotti.N.; Balestra.F.; Rosa.M.D.; Siracusa.V. Characterization of Composite Edible Films Based on Pectin/Alinate/Whey Protein Concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef] [PubMed]
- Kampeerapappun. P.; Aht-Ong.D.; Pentrakon.D.; Srikulkit.K. Preparation of Cassava Starch/Montmorillonite Composite Film. Carbohydrate Polymers 2007, 67, 155–163. [Google Scholar] [CrossRef]
- Fossen. T.; Cabrita.L.; Andersen.Ø.M. Colour and stability of pure anthocyanins influenced by pH including the alkaline region. Food Chemistry 1998, 63, 435–440. [Google Scholar] [CrossRef]
- Reyes. L.; Cisneros-Zevallos.L. Degradation kinetics and colour of anthocyanins in aqueous extracts of purple- and red-flesh potatoes (Solanum tuberosum L.). Food Chemistry 2007, 100, 885–894. [Google Scholar] [CrossRef]
- Hurtado. N.H.; Morales.A.L.; González-Miret.M.L.; Escudero-Gilete.M.L.; Heredia.F.J. Colour, pH stability and antioxidant activity of anthocyanin rutinosides isolated from tamarillo fruit (Solanum betaceum Cav.). Food Chemistry 2009, 117, 88–93. [Google Scholar] [CrossRef]
- Calogero. G.; Bartolotta.A.; Marco.G.D.; Carlo.A.D.; Bonaccorso.F. Vegetable-based dye-sensitized solar cells. Chemical Society Reviews 2015, 44, 3244–3294. [Google Scholar] [CrossRef]
- He. J.; Giusti.M.M. Anthocyanins: Natural colorants with health-promoting properties. Annual Review of Food Science and Technology 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Wrolstad. R.E.; Culver.C.A. Alternatives to those artificial FD&C food colorants. Annual Review of Food Science and Technology 2012, 3, 59–77. [Google Scholar]
- Rose. P.M.; Cantrill.V.; Benohoud.M.; Tidder.A.; Rayner.C.M.; Blackburn.R.S. Application of anthocyanins from blackcurrant (Ribes nigrum L.) fruit waste as renewable hair dyes. Journal of Agricultural and Food Chemistry 2018, 66, 6790–6798. [Google Scholar] [CrossRef] [PubMed]
- Trouillas. P.; Sancho-García.J.C.; De Freitas.V.; Gierschner.J.; Otyepka.M.; Dangles.O. Stabilizing and modulating colour by copigmentation: Insights from theory and experiment. Chemical Reviews 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [PubMed]
- Houghton. A.; Appelhagen.I.; Martin.C. Natural blues: Structure meets function in anthocyanins. Plants 2021, 10, 726. [Google Scholar] [CrossRef]
Table 1.
Physical properties of SSEF with different SWE concentration.
Table 1.
Physical properties of SSEF with different SWE concentration.
| Treatment(%) |
Thickness(mm) |
Transparency |
Tensile strength (mPa) |
Elongation at break (%) |
| 0.15 |
0.17 ± 0.01c
|
2.16 ± 0.13a
|
10.15 ± 1.1a
|
12.68 ± 1.17b
|
| 0.25 |
0.19 ± 0.01b
|
1.26 ± 0.03b
|
8.48 ± 1.0b
|
14.80 ± 0.96a
|
| 0.35 |
0.22 ± 0.01a
|
0.84 ± 0.04c
|
7.70 ± 0.7b
|
15.70 ± 1.26a
|
Table 2.
Apparent color and colorimetric parameters (L*, a*, and b*) of SSEF with different SWE concentration at different pH values (1 to 14).
Table 2.
Apparent color and colorimetric parameters (L*, a*, and b*) of SSEF with different SWE concentration at different pH values (1 to 14).
Table 3.
Apparent color and colorimetric parameters (L*, a*, and b*) of the SSEF with different SWE concentration and soaking time at acidic condition.
Table 3.
Apparent color and colorimetric parameters (L*, a*, and b*) of the SSEF with different SWE concentration and soaking time at acidic condition.
Table 4.
Apparent color and colorimetric parameters (L*, a*, and b*) of the SSEF with different SWE concentration and soaking time at neutral condition.
Table 4.
Apparent color and colorimetric parameters (L*, a*, and b*) of the SSEF with different SWE concentration and soaking time at neutral condition.
Table 5.
Apparent color and colorimetric parameters (L*, a*, and b*) of the SSEF with different SWE concentration and soaking time at alkaline condition.
Table 5.
Apparent color and colorimetric parameters (L*, a*, and b*) of the SSEF with different SWE concentration and soaking time at alkaline condition.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).