Submitted:
17 May 2023
Posted:
18 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
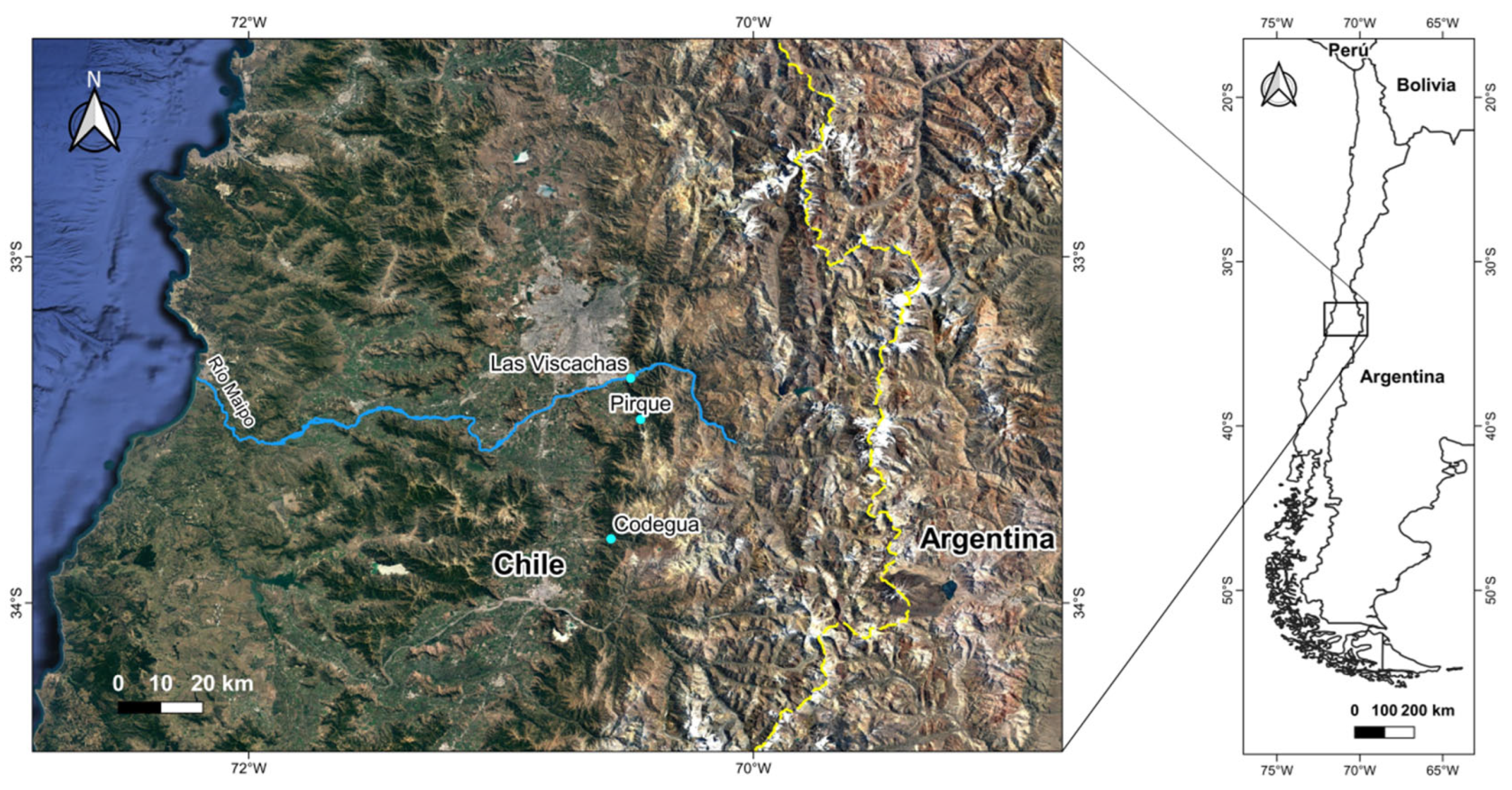
2.1. Experiment I. Recognition between homo- vs. heterotypic competitors
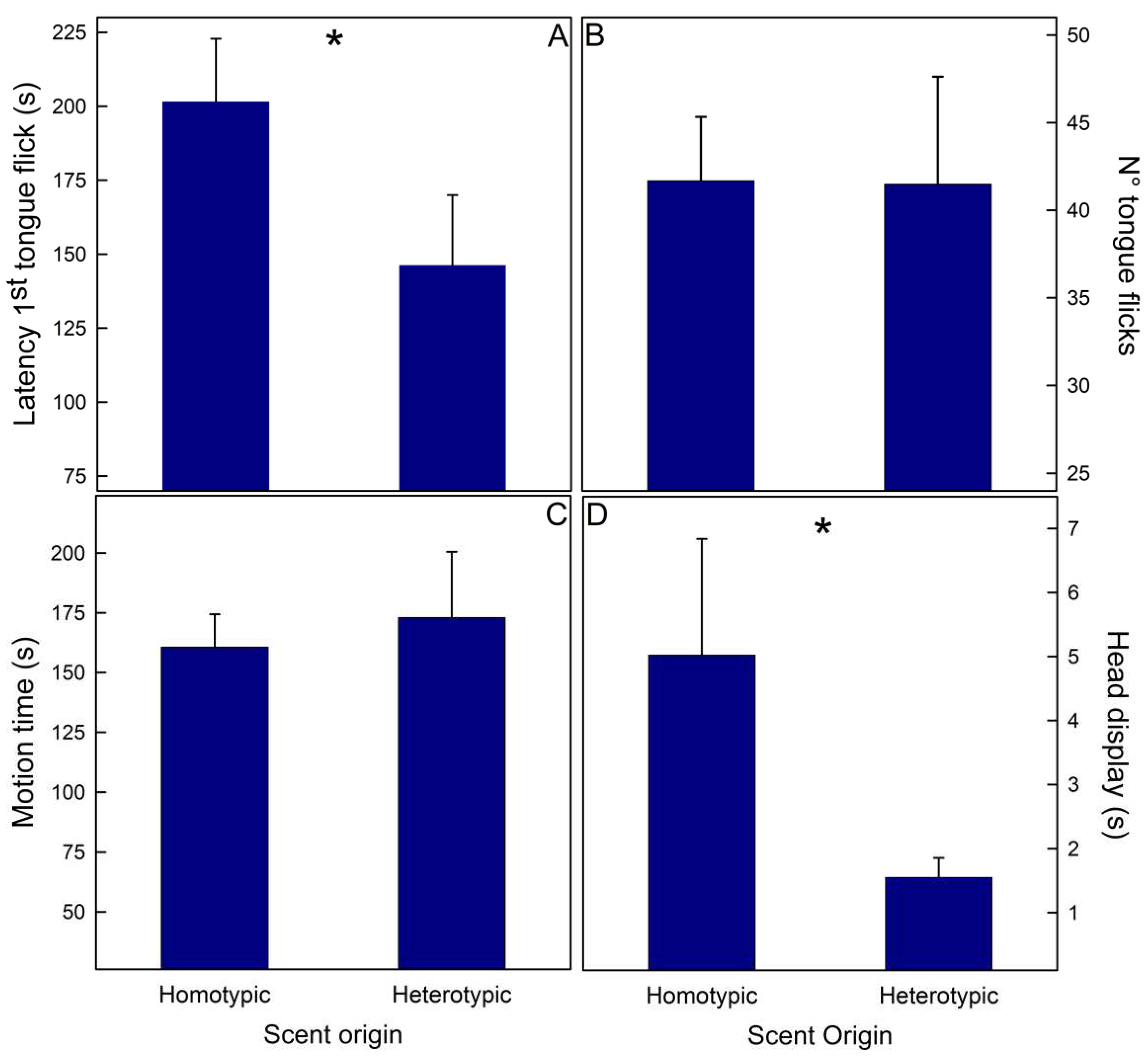
2.2. Experiment II. Recognition between homo- vs. heterotypic potential sexual partners
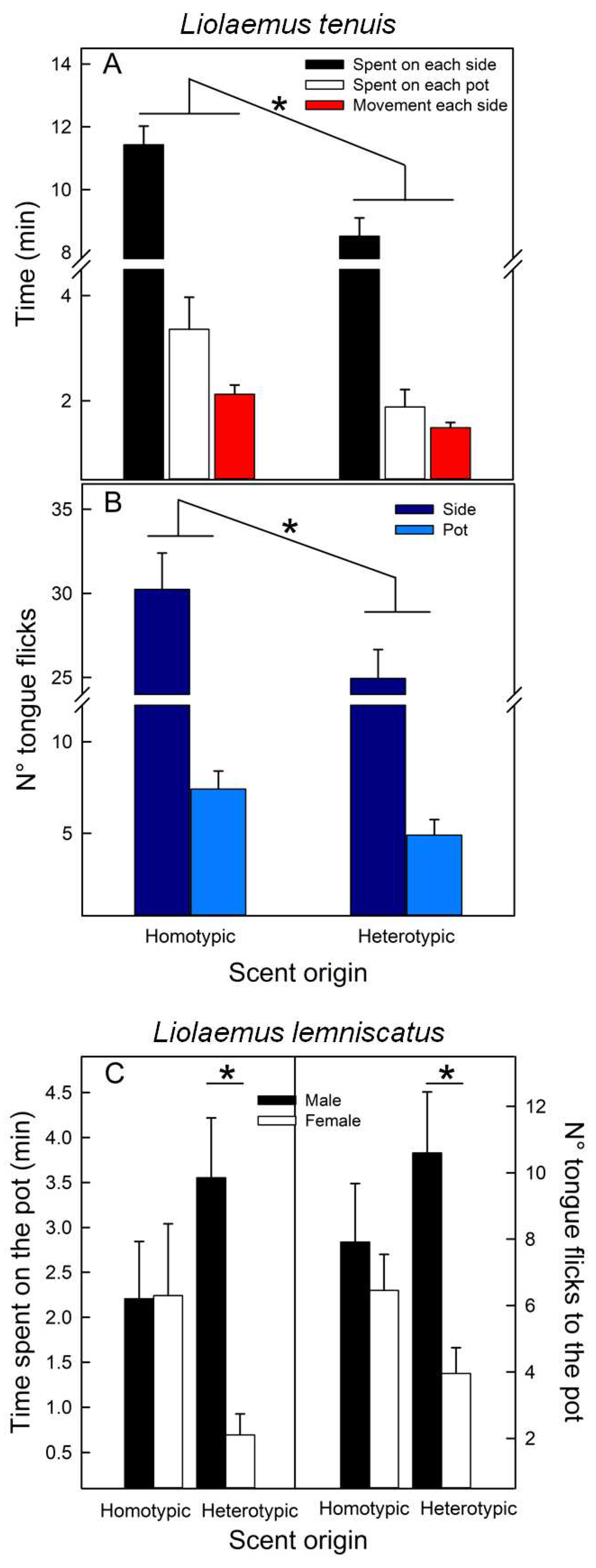
2.4. Experiment III. Scent preference for homo- vs. heterotypic potential sexual partners
2.5. Statistics
3. Results
3.1. Experiment I. Recognition between homo- vs. heterotypic competitors
3.2. Experiment II Recognition between homo- vs. heterotypic potential sexual partners
3.3. Experiment III. Scent preference for homo- vs. heterotypic potential sexual partners
4. Discussion
5. Conclusions
Funding
Acknowledgments
References
- Matute, D.R.; Cooper, B.S. Comparative studies on speciation: 30 years since Coyne and Orr. Evolution, 2021, 75, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Coyne, J.A.; Orr, H.A. Speciation. Sinauer Associates: Sunderland, 2004.
- Mayr, E. Systematics and the Origin of Species. Columbia University Press New York, 1942.
- Gavrilets, S.; Vose, A. Dynamic patterns of adaptive radiation: Evolution of mating preferences. In: Butlin, R.K., Bridle, J.R., Schluter, D., eds. Speciation and Patterns of Diversity. Cambridge, United Kingdom: Cambridge University Press, 2009; pp. 102-126.
- Butlin, R.K.; Ritchie, M.G. Behaviour and speciation. In: Slater, P.J.B., Halliday, T.R., Barrett, P., eds. Behaviour and Evolution New York: Cambridge University Press, 1994; pp. 43-79.
- Panhuis, T.M.; Butlin, R.K.; Zuk, M.; Tregenza, T. Sexual selection and speciation. Trends Ecol. Evol., 2001, 16, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, T.C.; Safran, R.J. Speciation by sexual selection: 20 years of progress. Trends Ecol. Evol., 2021, 36, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- West-Eberhard, M.J. Sexual selection, social competition, and speciation. Q. Rev. Biol., 1983, 58, 155–183. [Google Scholar] [CrossRef]
- Roriz, A.K.P.; Japyassú, H.F.; Cáceres, C.; Vera, M.T.; Joachim-Bravo, I.S. Pheromone emission patterns and courtship sequences across distinct populations within Anastrepha fraterculus (Diptera-Tephritidae) cryptic species complex. Bull. Entomol. Res., 2019, 109, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.S.; Mendelson, T.C. Identifying female phenotypes that promote behavioral isolation in a sexually dimorphic species of fish Etheostoma zonale. Curr. Zool., 2021, 67, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Uy, J.A.C.; Irwin, D.E.; Webster, M.S. Behavioral isolation and incipient speciation in birds. Annual Rev. Ecol. Evol. System., 2018, 49, 1–24. [Google Scholar] [CrossRef]
- Lipshutz, S.E.; Overcast, I.A.; Hickerson, M.J.; Brumfield, R.T.; Derryberry, E.P. Behavioural response to song and genetic divergence in two subspecies of white-crowned sparrows (Zonotrichia leucophrys). Mol. Ecol., 2017, 26, 3011–3027. [Google Scholar] [CrossRef]
- Mason, N.A.; Burns, K.J.; Tobias, J.A.; Claramunt, S.; Seddon, N.; Derryberry, E.P. Song evolution, speciation, and vocal learning in passerine birds. Evolution, 2017, 71, 786–796. [Google Scholar] [CrossRef]
- Mendelson, T.C.; Shaw, K.L. Rapid speciation in an arthropod. Rapid speciation in an arthropod, 2005, 433, 375–376. [Google Scholar] [CrossRef]
- Knight, M.E.; Turner, G.F. Laboratory mating trials indicate incipient speciation by sexual selection among populations of the cichlid fish Pseudotropheus zebra from Lake Malawi. P. Roy. Soc. Lond. B Bio., 2004, 271, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Maan, M.E.; Seehausen, O.; Söderberg, L.; Johnson, L.; Ripmeester, E.A.P.; Mrosso, H.D.J.; Taylor, M.I.; van Dooren, T.J.M.; van Alphen, J.J.M. Intraspecific sexual selection on a speciation trait, male coloration, in the Lake Victoria cichlid Pundamilia nyererei. P Roy. Soc. Lond. B Bio., 2004, 271, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Uetz, P.; Freed, P.; Aguilar, R.; Hošek, J. (2023). The Reptile Database. Access 15-06-2023.
- Abdala, C.; Laspiur, A.; Scrocchi, G.; Semhan, R.; Lobo, F.; Valladares, P. Las Lagartijas de la Familia Liolaemidae. Sistemática, Distribución e Historia Natural de una de las Familias de Vertebrados más Diversas del Cono Sur de Sudamérica. V1, V2. RIL Editores, Universidad de Tarapacá: Santiago, 2021.
- Esquerré, D.; Brennan, I.G.; Catullo, R.A.; Torres-Pérez, F.; Keogh, J.S. How mountains shape biodiversity: The role of the Andes in biogeography, diversification, and reproductive biology in South America’s most species-rich lizard radiation (Squamata: Liolaemidae). Evolution, 2019, 73, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Esquerré, D.; Ramírez-Álvarez, D.; Pavón-Vázquez, C.J.; Troncoso-Palacios, J.; Garín, C.F.; Keogh, J.S.; Leaché, A.D. Speciation across mountains: Phylogenomics, species delimitation and taxonomy of the Liolaemus leopardinus clade (Squamata, Liolaemidae). Mol. Phylogenet. Evol., 2019, 139 106524. [CrossRef]
- Torres-Pérez, F.; Mendez, M.A.; Benavides, E.; Moreno, R.A.; Lamborot, M.; Palma, R.E.; Ortiz, J.C. Systematics and evolutionary relationships of the mountain lizard Liolaemus monticola (Liolaemini): How morphological and molecular evidence contributes to reveal hidden species diversity. Biol. J. Linnean Soc., 2009, 96, 635–650. [Google Scholar] [CrossRef]
- Muñoz-Mendoza, C.; D'Elía, G.; Panzera, A.; Méndez T, M.A.; Villalobos-Leiva, A.; Sites, J.W.; Victoriano, P.F. Geography and past climate changes have shaped the evolution of a widespread lizard from the Chilean hotspot. Mol. Phylogenet. Evol., 2017, 116, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, F.; Yáñez, R.P.; Eduardo, R.E.; Garin, C.F.; Torres-Pérez, F. Deep divergences within Liolaemus nigroviridis (Squamata, Liolaemidae) lineages associated with sky islands in central Chile. Zootaxa, 2013, 3619. [CrossRef]
- Boschman, L.M.; Condamine, F.L. Mountain radiations are not only rapid and recent: Ancient diversification of South American frog and lizard families related to Paleogene Andean orogeny and Cenozoic climate variations. Global Planet. Change, 2022, 208, 103704. [Google Scholar] [CrossRef]
- Vallejos Garrido, P.; Pino, K.; Espinoza-Aravena, N.; Pari, A.; Inostroza-Michael, O.; Toledo-Muñoz, M.; Castillo-Ravanal, B.; Romero-Alarcón, V.; Hernández, C.; Palma, R.; Rodríguez-Serrano, E. The importance of the Andes in the evolutionary radiation of Sigmodontinae (Rodentia, Cricetidae), the most diverse group of mammals in the Neotropics. Sci. Rep., 2023, 13. [CrossRef]
- Chaves, J.A.; Weir, J.T.; Smith, T.B. Diversification in Adelomyia hummingbirds follows Andean uplift. Mol. Ecol., 2011, 20, 4564–4576. [Google Scholar] [CrossRef]
- Singhal, S.; Colli, G.R.; Grundler, M.R.; Costa, G.C.; Prates, I.; Rabosky, D.L. No link between population isolation and speciation rate in squamate reptiles. P. Natl. Acad. Sci. -Biol., 2022, 119, e2113388119. [Google Scholar] [CrossRef]
- Barker, M.J.; Wilson, R.A. Cohesion, gene flow, and the nature of species. J. Philos., 2010, 107, 61–79 http://www.jstor.org/stable/25700483. [Google Scholar] [CrossRef]
- Smadja, C.; Butlin, R.K. On the scent of speciation: The chemosensory system and its role in premating isolation. Heredity, 2009, 102, 77–97. [Google Scholar] [CrossRef]
- Labra, A. Multi-contextual use of chemosignals by Liolaemus lizards. In: Hurst, J.L., Beynon, R.J., Roberts, S.C., Wyatt, T.D., eds. Chemical Signals in Vertebrates XI. New York, USA: SpringerLink, 2008; pp. 357-365.
- Labra, A. Comunicación, el mediador de las interacciones sociales en animales. In: Ebensperger, L.A., Labra, A., eds. Comportamiento Social de la Fauna Nativa de Chile. Santiago, Chile: Ediciones UC, 2020; pp. 263-273.
- Labra, A. Chemoreception and the assessment of fighting abilities in the lizard Liolaemus monticola. Ethology, 2006, 112, 993–999. [Google Scholar] [CrossRef]
- Labra, A. Chemical stimuli and species recognition in Liolaemus lizards. J. Zool., 2011, 285, 215–221. [Google Scholar] [CrossRef]
- Labra, A. The chemical-speciation hypothesis in Liolaemus: A response to Pincheira-Donoso. J. Zool., 2012, 288, 234–236. [Google Scholar] [CrossRef]
- Valdecantos, S.; Labra, A. Testing the functionality of precloacal secretions from both sexes in the South American lizard, Liolaemus chiliensis. Amphibia-Reptilia, 2017, 38, 209–216. [Google Scholar] [CrossRef]
- Labra, A. Sistemas de comunicación en reptiles. In: Vidal, M.A., Labra, A., eds. Herpetología de Chile. Santiago, Chile: Science Verlag, 2008; pp. 547-577.
- Labra, A.; Brann, J.H.; Fadool, D.A. Heterogeneity of voltage-and chemosignal-activated response profiles in vomeronasal sensory neurons. J. Neurophysiol., 2005, 94, 2535–2548. [Google Scholar] [CrossRef] [PubMed]
- García-Roa, R.; Carreira, S.; López, P.; Martín, J. Genders matters: Sexual differences in chemical signals of Liolaemus wiegmannii lizards (Iguania, Liolaemidae). Biochem. Syst. Ecol., 2016, 69, 108–114. [Google Scholar] [CrossRef]
- Escobar, C.A.; Labra, A.; Niemeyer, H.M. Chemical composition of precloacal secretions of Liolaemus lizards. J. Chem. Ecol., 2001, 27, 1677–1690. [Google Scholar] [CrossRef]
- Escobar, C.; Escobar, C.A.; Labra, A.; Niemeyer, H.M. Chemical composition of precloacal secretions of two Liolaemus fabiani populations: Are they different? J. Chem. Ecol., 2003, 29, 629–638. [Google Scholar] [CrossRef]
- Runemark, A.; Gabirot, M.; Svensson, E. Population divergence in chemical signals and the potential for premating isolation between islet-and mainland populations of the Skyros wall lizard (Podarcis gaigeae). J. Evol. Biol., 2011, 24, 795–809. [Google Scholar] [CrossRef]
- Raya-García, E.; Suazo-Ortuño, I.; Campos-García, J.; Martín, J.; Alvarado-Díaz, J.; Mendoza-Ramírez, E. Chemical signal divergence among populations influences behavioral discrimination in the whiptail lizard Aspidoscelis lineattissimus (Squamata: Teiidae). Behav. Ecol. Sociobiol., 2020, 74, 144. 10.1007/s00265–020. [Google Scholar] [CrossRef]
- Victoriano, P.F.; Ortiz, J.C.; Benavides, E.; Adams, B.J.; Sites, J.W. Comparative phylogeography of codistributed species of Chilean Liolaemus (Squamata: Tropiduridae) from the central-southern Andean range. Mol. Ecol., 2008, 17, 2397–2416. [Google Scholar] [CrossRef] [PubMed]
- Baeckens, S.; Martín, J.; García-Roa, R.; Pafilis, P.; Huyghe, K.; Van Damme, R. Environmental conditions shape the chemical signal design of lizards. Funct. Ecol., 2018, 32, 566–580. [Google Scholar] [CrossRef]
- Zozaya, S.M.; Teasdale, L.C.; Moritz, C.; Higgie, M.; Hoskin, C.J. Composition of a chemical signalling trait varies with phylogeny and precipitation across an Australian lizard radiation. J. Evol. Biol., 2022, 35, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.M.; Pruett, J.A.; Soini, H.A.; Zúñiga-Vega, J.J.; Goldberg, J.K.; Vital-García, C.; Hews, D.K.; Novotny, M.V.; Martins, E.P. Volatile fatty acid and aldehyde abundances evolve with behavior and habitat temperature in Sceloporus lizards. Behav. Ecol., 2020, 31, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Victoriano, P.F. Phylogeography of Chilean lizards: Histories of genetic diversification on the western slope of southern Andes. In: Morando, M., Avila, L.J., eds. Lizards of Patagonia. Diversity, Systematics, Biogeography and Biology of the Reptiles at the End of the World. Switzerland: Springer, 2020; pp. 255-291.
- Lamborot, M.; Alvarez-Sarret, E. Karyotypic variation within and between populations of Liolaemus monticola (Tropiduridae) separated by the Maipo River in the Coastal Range of Central Chile. Herpetologica, 1993, 49, 435–449 http://wwwjstororg/stable/3892638. [Google Scholar]
- Lamborot, M.; Eaton, L. The Maipo River as a biogeographical barrier to Liolaemus monticola (Tropiduridae) in the mountain ranges of central Chile. J. Zool. Syst. Evol. Res., 1997, 35, 105–111. [Google Scholar] [CrossRef]
- Labra, A.; Niemeyer, H.M. Variability in the assessment of snake predation risk by Liolaemus lizards. Ethology, 2004, 110, 649–662. [Google Scholar] [CrossRef]
- Labra, A.; Pienaar, J.; Hansen, T.F. Evolution of thermal physiology in Liolaemus lizards: Adaptation, phylogenetic inertia, and niche tracking. Am. Nat., 2009, 174, 204–220. [Google Scholar] [CrossRef]
- Halpern, M.; Martínez-Marcos, A. Structure and function of the vomeronasal system: an update. Prog. Neurobiol., 2003, 70, 245–318. [Google Scholar] [CrossRef]
- Price, T. Sexual selection and natural selection in bird speciation. Philos. T. Roy. Soc. B, 1998, 353, 251–260. [Google Scholar] [CrossRef]
- Weir, J.T.; Price, T.D. Song playbacks demonstrate slower evolution of song discrimination in birds from Amazonia than from temperate North America. PLoS Biol., 2019, 17, e3000478. [Google Scholar] [CrossRef] [PubMed]
- Labra, A.; Escobar, C.A.; Niemeyer, H.M. Chemical discrimination in Liolaemus lizards: Comparison of behavioral and chemical data. In: Marchelewska-Koj, A., Lepri, J.J., Müller-Schwarze, D., eds. Chemical Signals in Vertebrates IX. New York, USA: Kluwer Academic/Plenum Publishers, 2001; pp. 439-444.
- Wyatt, T. Pheromones and signature mixtures: Defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J. Comp. Physiol. A, 2010, 196, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Panzera, A.; Leaché, A.D.; D'Elía, G.; Victoriano, P.F. Phylogenomic analysis of the Chilean clade of Liolaemus lizards (Squamata: Liolaemidae) based on sequence capture data. PeerJ 2017, 5, e3941. [Google Scholar] [CrossRef] [PubMed]
- Zozaya, S.M.; Higgie, M.; Moritz, C.; Hoskin, C.J. Are pheromones key to unlocking cryptic lizard diversity? Am. Nat., 2019, 194, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Mangiacotti, M.; Fumagalli, M.; Scali, S.; Zuffi, M.A.; Cagnone, M.; Salvini, R.; Sacchi, R. Inter-and intra-population variability of the protein content of femoral gland secretions from a lacertid lizard. Curr. Zool., 2016, 63, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.; Martín, J.; Crochet, P.A.; López, P.; Clobert, J. Seasonal and interpopulational phenotypic variation in morphology and sexual signals of Podarcis liolepis lizards. PLoS ONE, 2019, 14, e0211686. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, A.; Menke, M.; Quezada, G. Gustavo Jiménez-Uzcátegui; Schulz, S.; Steinfartz, S. Diversity of compounds in femoral secretions of Galápagos iguanas (genera: Amblyrhynchus and Conolophus), and their potential role in sexual communication in lek-mating marine iguanas (Amblyrhynchus cristatus). PeerJ, 2017, 5:e3689. [Google Scholar] [CrossRef]
- Gabirot, M.; López, P.; Martín, J. Differences in chemical sexual signals may promote reproductive isolation and cryptic speciation between Iberian wall lizard populations. Intern. J. Evol. Biol., 2012, 2012, ID 698520. [Google Scholar] [CrossRef]
- MacGregor, H.E.A.; Lewandowsky, R.A.M.; d'Ettorre, P.; Leroy, C.; Davies, N.W.; While, G.M.; Uller, T. Chemical communication, sexual selection, and introgression in wall lizards. Evolution, 2017, 71, 2327–2343. [Google Scholar] [CrossRef]
- Martín, J.; López, P. Interpopulational differences in chemical composition and chemosensory recognition of femoral gland secretions of male lizards Podarcis hispanica: Implications for sexual isolation in a species complex. Chemoecology, 2006, 16, 31–38. [Google Scholar] [CrossRef]
- Martín, J.; López, P.; Iraeta, P.; Díaz, J.A.; Salvador, A. Differences in males’ chemical signals between genetic lineages of the lizard Psammodromus algirus promote male intrasexual recognition and aggression but not female mate preferences. Behav. Ecol. Sociobiol., 2016, 70, 1657–1668. [Google Scholar] [CrossRef]
- Barley, A.J.; Nieto-Montes de Oca, A.; Reeder, T.W.; Manríquez-Morán, N.L.; Monroy, J.C.; Hernández-Gallegos, O.; Thomson, R.C. Complex patterns of hybridization and introgression across evolutionary timescales in Mexican whiptail lizards (Aspidoscelis). Mol. Phyl. Evol., 2019, 132, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Butlin, R.; Debelle, A.; Kerth, C.; Snook, R.R.; Beukeboom, L.W.; Cajas, R.C.; Diao, W.; Maan, M.E.; Paolucci, S.; Weissing, F.J. What do we need to know about speciation? Trends Ecol. Evol., 2012, 27, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Esquerré, D.; Keogh, J.S.; Demangel, D.; Morando, M.; Avila, L.J.; Sites Jr, J.W.; Ferri-Yáñez, F.; Leaché, A.D. Rapid radiation and rampant reticulation: Phylogenomics of South American Liolaemus lizards. Syst. Biol., 2022, 71, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Morando, M.; Avila, L.J.; Baker, J.; Sites, J.W. Phylogeny and phylogeography of the Liolaemus darwinii complex (Squamata : Liolaemidae): Evidence for introgression and incomplete lineage sorting. Evolution, 2004, 58, 842–861. [Google Scholar] [CrossRef]
- Morando, M.; C.D., M.; Minoli, I.; Pérez, C.H.F.; Sites, J.W.J.; Avila, L.J. Diversification and evolutionary histories of Patagonian steppe lizards. In: Morando, M., Avila, L.J., eds. Lizards of Patagonia. Diversity, Systematics, Biogeography and Biology of the Reptiles at the End of the World. Cham, Switzerland: SpringerNature Switzerland, 2020; pp. 217–254.
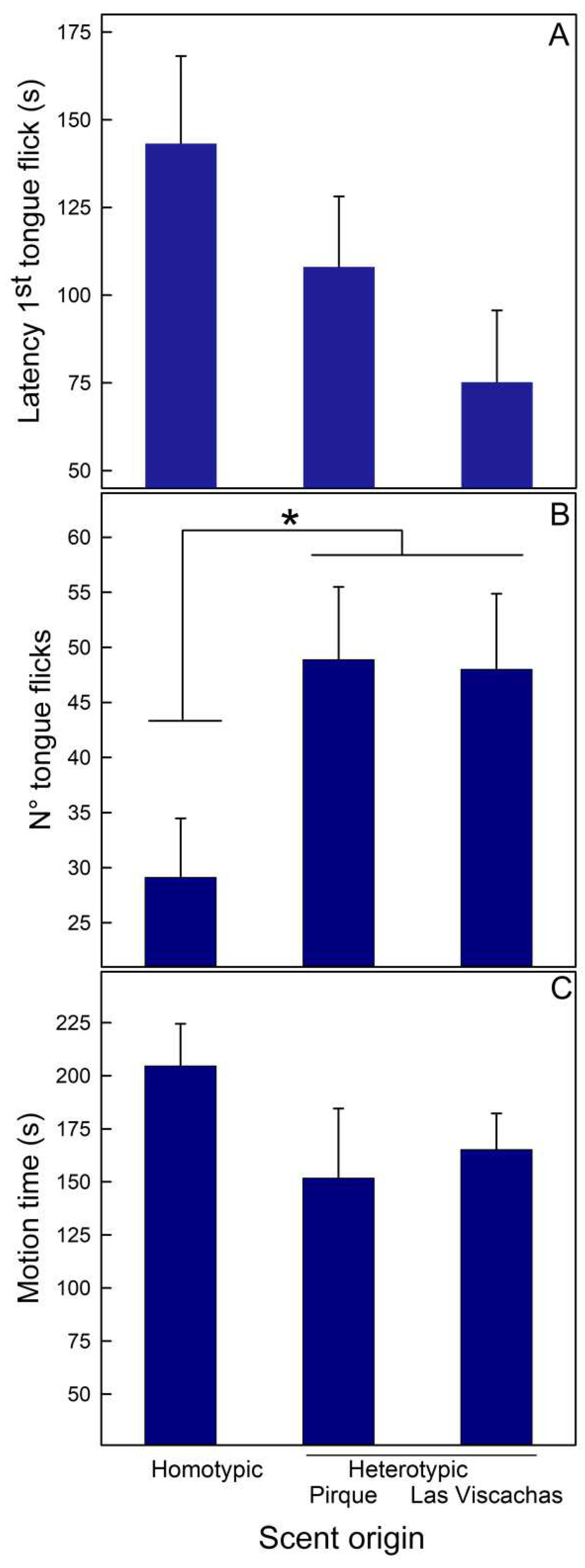
| A- Liolaemus tenuis F[2,16] (p) | ||||||
| Variable | Scent origin | |||||
| Latency 1st tongue flick (s) | 1.909 (0.181) | |||||
| N° tongue flicks | 4.757 (0.024) | |||||
| Motion time (s) | 1.048 (0.374) | |||||
| B- Liolaemus lemniscatus F[1,14](p) | ||||||
| Variable | Scent origin | Population | Scent origin*Population | |||
| Latency 1st tongue flick (s) | 5.147 (0.039) | 0.098 (0.756) | 0.476 (0.502) | |||
| N° tongue flicks | 0.001 (0.978) | 0.321 (0.580) | 1.778 (0.204) | |||
| Motion time (s) | 0.311 (0.586) | 0.186 (0.673) | 1.872 (0.193) | |||
| Head displays (s) | 6.494 (0.023) | 1.284 (0.276) | 1.984 (0.181) | |||
| A- Liolaemus tenuis F[1,38](p) | ||||||
| Scent origin | Population | Sex | Scent origin*Pop. | Scent origin*Sex | ||
| Time on each side (min) | 5.968 (0.019) | 0.432 (0.515) | 3.304 (0.077) | 0.001 (0.982) | 0.386 (0.538) | |
| Time on each pot (min) | 4.261 (0.046) | 0.501(0.483) | 3.578(0.066) | 0.029 (0866) | 0.371 (0.546) | |
| Tongue flicks each side | 4.110 (0.050) | 0.522(0.474) | 0.247(0.622) | 0.456 (0.504) | 0.754 (0.391) | |
| Tongue flicks each pot | 4.902 (0.033) | 0.306 (0.583) | 0.000 (0.980) | 0.523 (0.474) | 0.247 (0.622) | |
| Motion each side (min) | 12.854 (<0.001) | 4.472 (0.041) | 5.485 (0.025) | 0.649 (0.425) | 0.242 (0.626) | |
| B- Liolaemus lemniscatus F[1,54](p) | |||||
| Scent origin | Population | Sex | Scent origin*Pop. | Scent origin*Sex | |
| Time on each side (min) | 0.373 (0.544) | 1.049 (0.310) | 0.119 (0.731) | 0.086 (0.771) | 1.583 (0.214) |
| Time on each pot (min) | 0.103 (0.750) | 0.570(0.453) | 4.454 (0.039) | 0.428 (0.516) | 4.566 (0.037) |
| Tongue flicks each side | 0.738 (0.394) | 1.223 (0.274) | 0.601 (0.442) | 0.186 (0.668) | 1.127 (0.293) |
| Tongue flicks each pot | 0.005 (0.945) | 0.633 (0.430) | 3.871 (0.054) | 0.001 (0.971) | 5.233 (0.026) |
| Motion each side (min) | 0.130 (0.719) | 0.785 (0.380) | 1.535 (0.221) | 0.485 (0.489) | 0.551 (0.461) |
| Variables | Liolaemus tenuis F[1,26](p) |
Liolaemus lemniscatus F[1,26](p) |
|---|---|---|
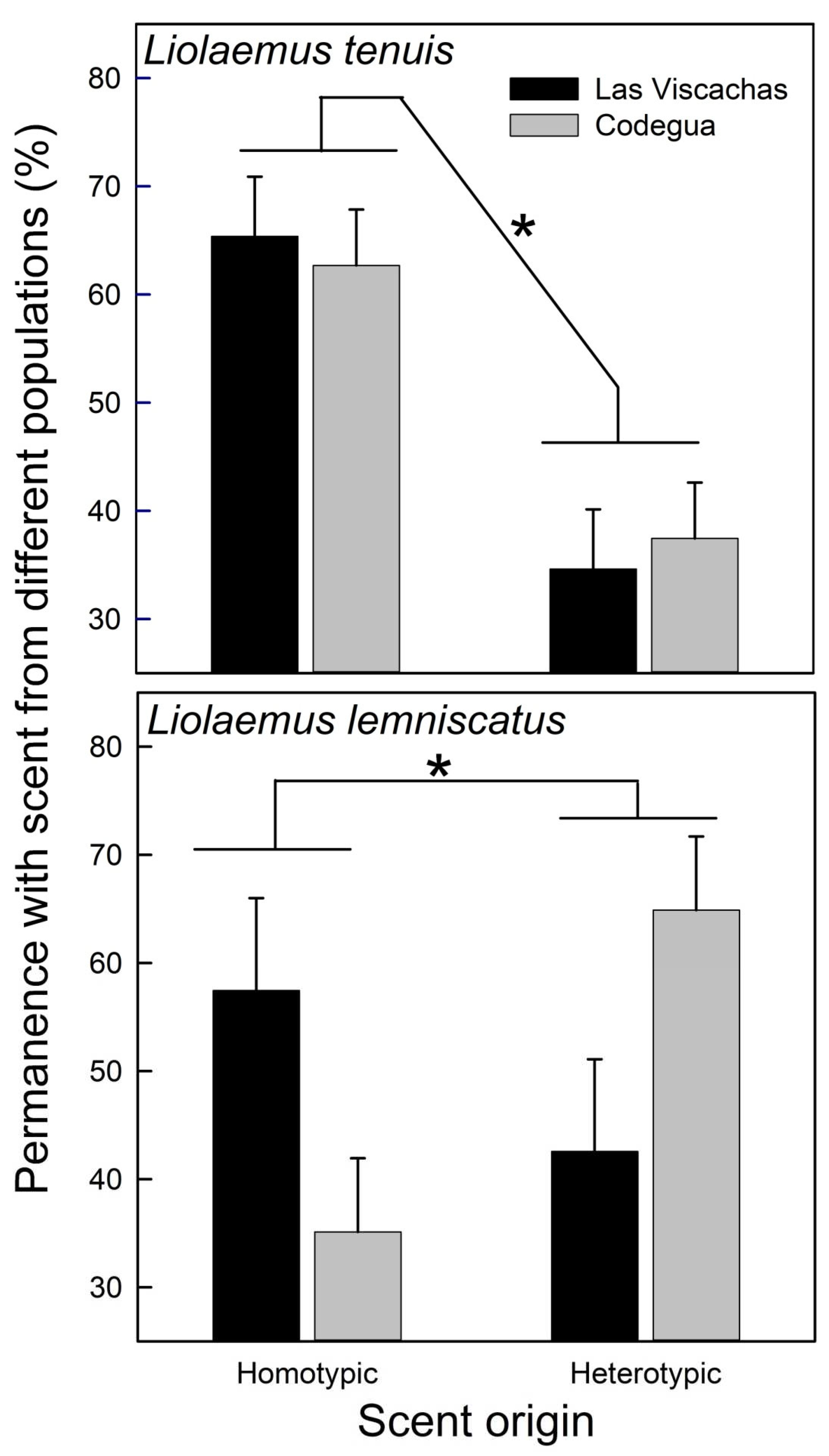
| Scent origin | 11.953 (0.002) | 0.262(0.613) |
| Population | 1.934 (0.176) | 0.000 (1.000) |
| Sex | 1.934 (0.176) | 0.000 (1.000) |
| Scent origin * Population | 0.048 (0.827) | 4.837(0.037) |
| Scent origin * Sex | 1.867 (0.184) | 1.814 (0.190) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





