Submitted:
17 May 2023
Posted:
18 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
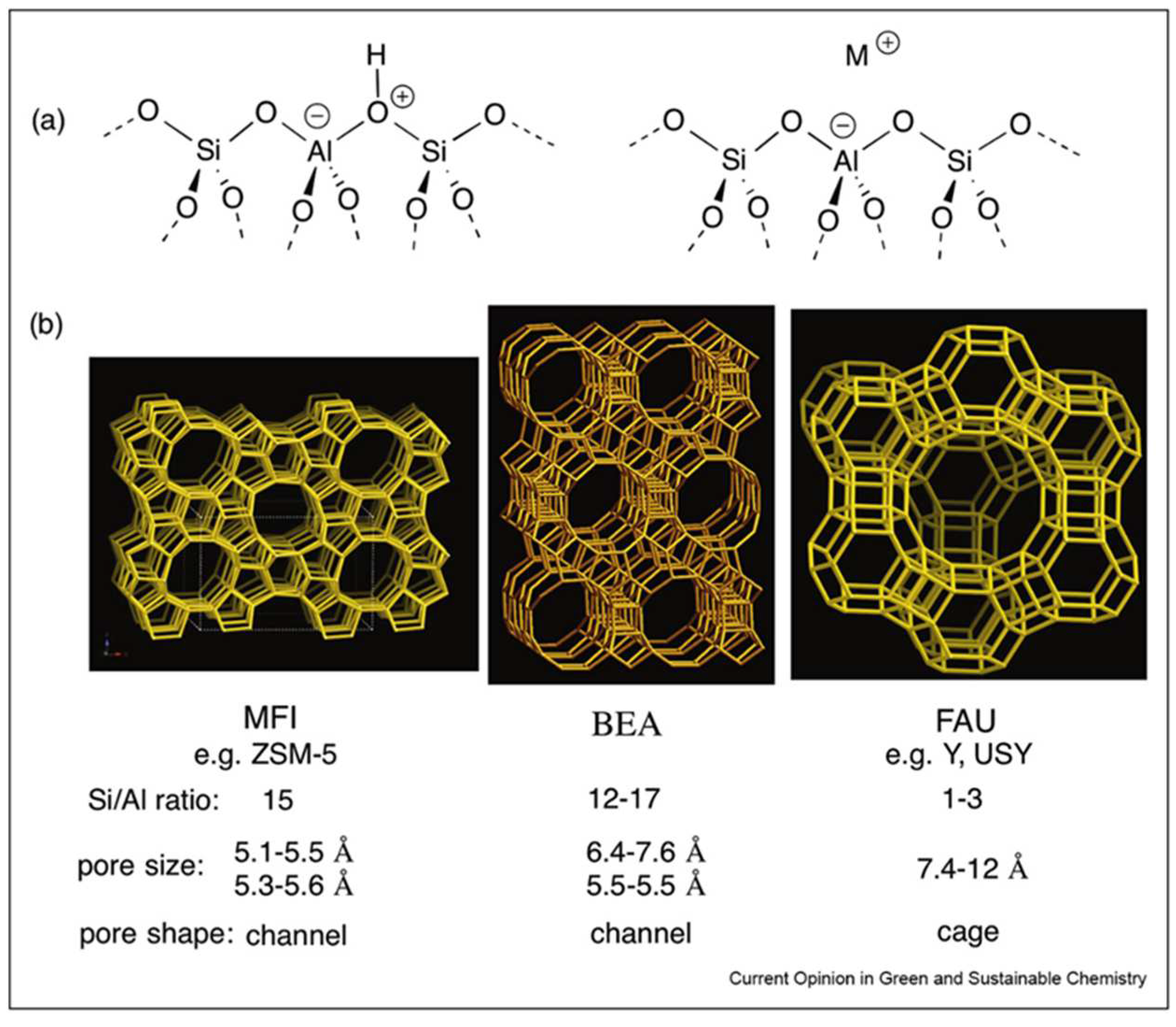
2. Types of Zeolites
3. Methods of the NP Formation in Zeolites
3.1. Impregnation
3.2. Mixing
3.3. Ion Exchange
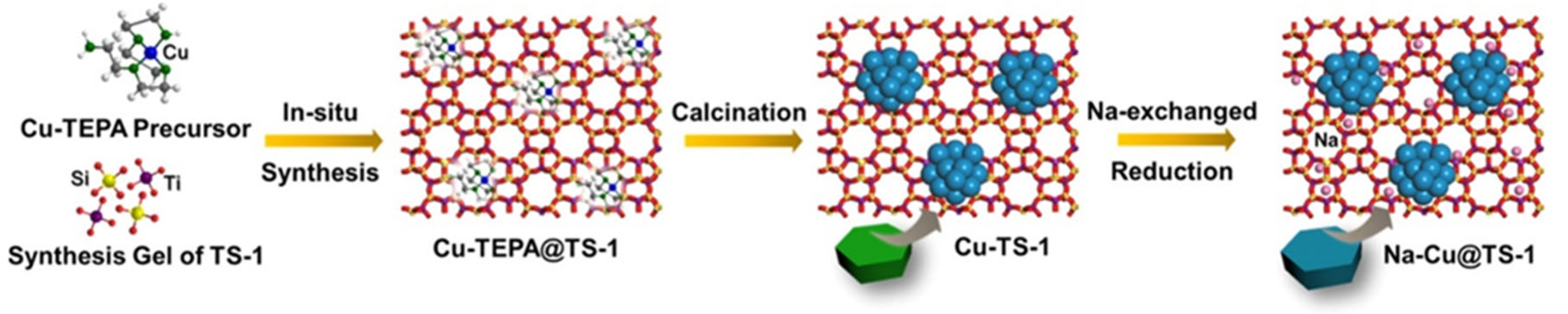
3.4. Encapsulation
4. Zeolite Modification for the Enhancement of Bifunctional Catalysts
4.1. Modification with Ions
4.2. Dealumination, Desilication
4.3. Incorporation of Fluorine
4.4. Post-Fabrication Modification
4.5. Modification with Magnetic NPs
4.6. Morphology Modification
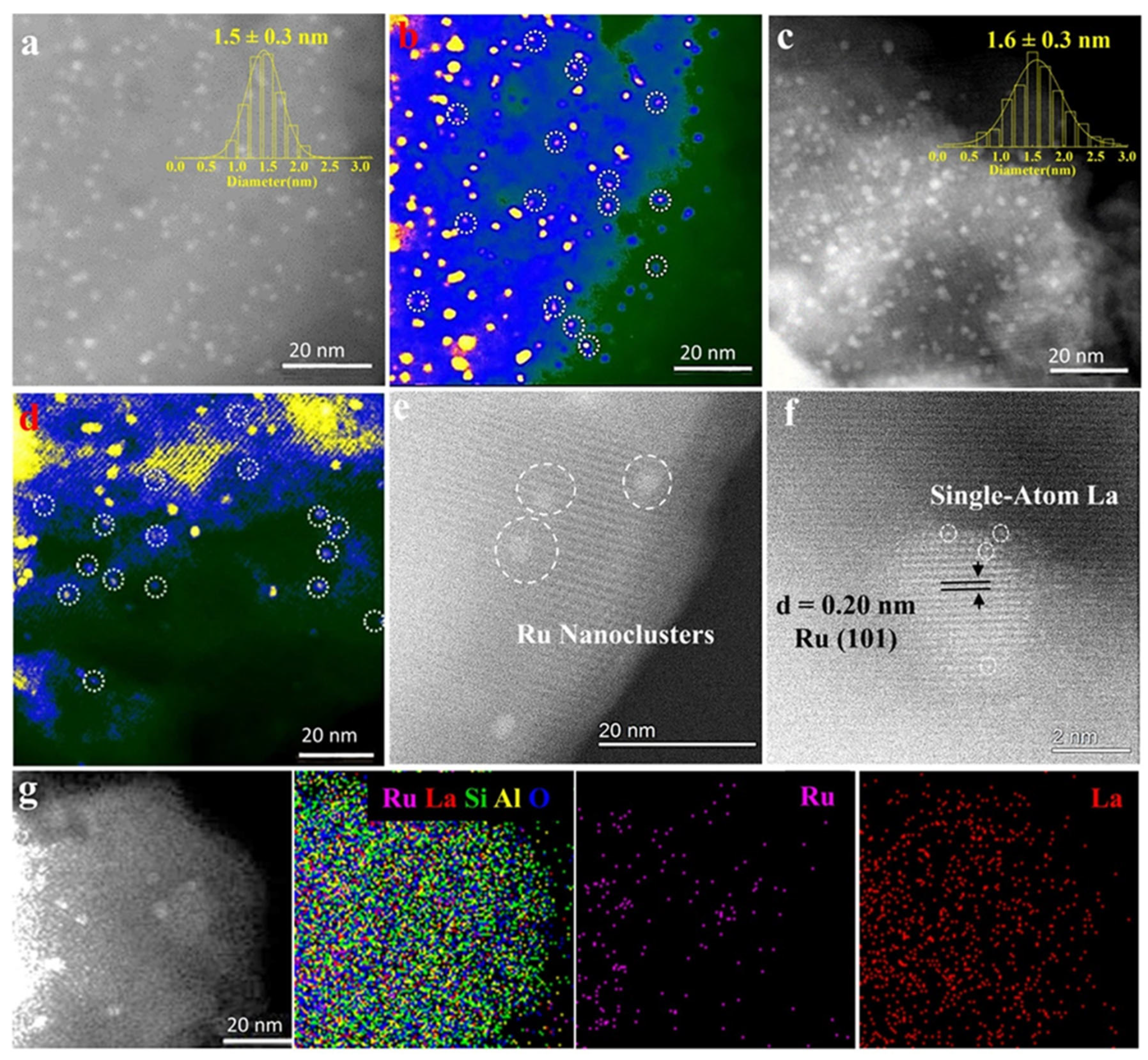
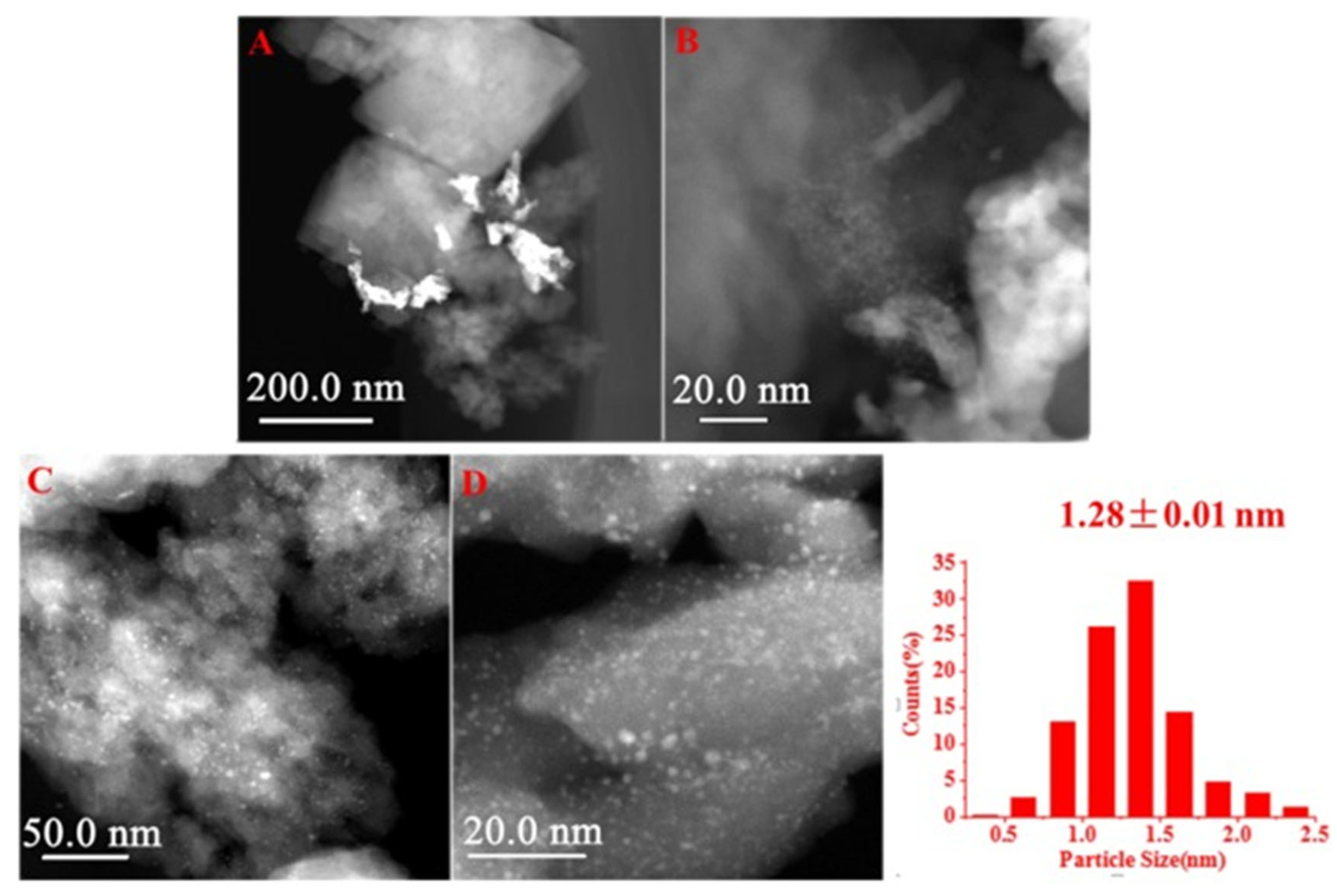
5. Interactions between Zeolite Acid Sites and NPs
6. Biomass Processing Catalytic Reactions with Bifunctional Catalysts
6.1. Direct Processing of Biomass
6.2. Indirect Processing of Biomass
7. Conclusions
Funding
Conflicts of Interest
References
- Tomczyk, A.; Sokolowska, Z.; Boguta, P. Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Khiari, B.; Jeguirim, M.; Limousy, L.; Bennici, S. Biomass derived chars for energy applications. Renewable Sustainable Energy Rev. 2019, 108, 253–273. [Google Scholar] [CrossRef]
- Buaki-Sogo, M.; Zubizarreta, L.; Garcia-Pellicer, M.; Quijano-Lopez, A. Sustainable carbon as efficient support for metal-based nanocatalyst: applications in energy harvesting and storage. Molecules 2020, 25, 3123. [Google Scholar] [CrossRef] [PubMed]
- Fakayode, O.A.; Aboagarib, E.A.A.; Zhou, C.; Ma, H. Co-pyrolysis of lignocellulosic and macroalgae biomasses for the production of biochar - A review. Bioresour. Technol. 2020, 297, 122408. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Raj, M.C.; B, A.K.; H, R.M.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. (Washington, DC, U. S.) 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kharkar, P.S.; Pethe, A.M. Biomass and waste materials as potential sources of nanocrystalline cellulose: Comparative review of preparation methods (2016 - Till date). Carbohydr. Polym. 2019, 207, 418–427. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Agarwal, U.P.; Ciesielski, P.N.; Himmel, M.E.; Gao, R.; Deng, Y.; Morits, M.; Osterberg, M. Towards sustainable production and utilization of plant-biomass-based nanomaterials: a review and analysis of recent developments. Biotechnol. Biofuels 2021, 14, 114. [Google Scholar] [CrossRef]
- Lam, E.; Hemraz, U.D. Preparation and Surface Functionalization of Carboxylated Cellulose Nanocrystals. Nanomaterials 2021, 11, 1641. [Google Scholar] [CrossRef]
- Magagula, L.P.; Masemola, C.M.; Ballim, M.A.a.; Tetana, Z.N.; Moloto, N.; Linganiso, E.C. Lignocellulosic Biomass Waste-Derived Cellulose Nanocrystals and Carbon Nanomaterials: A Review. Int. J. Mol. Sci. 2022, 23, 4310. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging technologies for the production of nanocellulose from lignocellulosic biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, H.; Vignolini, S. Recent Progress in Production Methods for Cellulose Nanocrystals: Leading to More Sustainable Processes. Adv. Sustainable Syst. 2022, 6, 2100100. [Google Scholar] [CrossRef]
- Elisadiki, J.; Kibona, T.E.; Machunda, R.L.; Saleem, M.W.; Kim, W.-S.; Jande, Y.A.C. Biomass-based carbon electrode materials for capacitive deionization: a review. Biomass Convers. Biorefin. 2019. DOI: 10.1007/s13399-13019-00463-13399. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, S.; Li, L.; Dou, S. Bio-Nanotechnology in High-Performance Supercapacitors. Adv. Energy Mater. 2017, 7, 1700592. [Google Scholar] [CrossRef]
- Shrestha, R.G.; Maji, S.; Shrestha, L.K.; Ariga, K.; Ariga, K. Nanoarchitectonics of Nanoporous Carbon Materials in Supercapacitors Applications. Nanomaterials (Basel) 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, H.; Kong, F.; Zhang, Y.; Wang, S.; Liu, S.; Lucia, L.A.; Fatehi, P.; Pang, H. Fabrication, characteristics and applications of carbon materials with different morphologies and porous structures produced from wood liquefaction: A review. Chem. Eng. J. (Amsterdam, Neth.) 2019, 364, 226–243. [Google Scholar] [CrossRef]
- Jo, C.-H.; Voronina, N.; Sun, Y.-K.; Myung, S.-T. Gifts from Nature: Bio-Inspired Materials for Rechargeable Secondary Batteries. Adv. Mater. (Weinheim, Ger.) 2021, 33, 2006019. [Google Scholar] [CrossRef]
- Azwar, E.; Wan Mahari, W.A.; Chuah, J.H.; Vo, D.-V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application - A review. Int. J. Hydrogen Energy 2018, 43, 20811–20821. [Google Scholar] [CrossRef]
- Omoriyekomwan, J.E.; Tahmasebi, A.; Dou, J.; Wang, R.; Yu, J. A review on the recent advances in the production of carbon nanotubes and carbon nanofibers via microwave-assisted pyrolysis of biomass. Fuel Process. Technol. 2021, 214, 106686. [Google Scholar] [CrossRef]
- Dhenadhayalan, N.; Lin, K.-C.; Saleh, T.A. Recent Advances in Functionalized Carbon Dots toward the Design of Efficient Materials for Sensing and Catalysis Applications. Small 2020, 16, 1905767. [Google Scholar] [CrossRef]
- Bhandari, S.; Mondal, D.; Nataraj, S.K.; Balakrishna, R.G. Biomolecule-derived quantum dots for sustainable optoelectronics. Nanoscale Adv. 2019, 1, 913–936. [Google Scholar] [CrossRef]
- Rodriguez-Padron, D.; Luque, R.; Munoz-Batista, M.J. Waste-derived Materials: Opportunities in Photocatalysis. Top. Curr. Chem. 2020, 378, 3. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, B.; Solomon, P.R.; Ranganathan, S. Synthesis of Carbon Quantum Dots with Special Reference to Biomass as a Source - A Review. Curr. Pharm. Des. 2019, 25, 1455–1476. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Mariana, L.T.; Phan, A.N. Biomass-waste derived graphene quantum dots and their applications. Carbon 2018, 140, 77–99. [Google Scholar] [CrossRef]
- Berenice Gonzalez-Gonzalez, R.; Parra-Saldivar, R.; Ramirez-Mendoza, R.A.; Iqbal, H.M.N. Carbon dots as a new fluorescent nanomaterial with switchable sensing potential and its sustainable deployment for metal sensing applications. Mater. Lett. 2022, 309, 131372. [Google Scholar] [CrossRef]
- Danial, W.H.; Md Bahri, N.F.; Abdul Majid, Z. Preparation, marriage chemistry and applications of graphene quantum dots-nanocellulose composite : brief review. Molecules 2021, 26, 6158. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Jin, W. Carbon nanomaterials: Synthesis, properties and applications in electrochemical sensors and energy conversion systems. Mater. Sci. Eng., B 2021, 272, 115341. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Y.; Balasubramanian, R. Synthesis, formation mechanisms and applications of biomass-derived carbonaceous materials: a critical review. J. Mater. Chem. A 2021, 9, 24759–24802. [Google Scholar] [CrossRef]
- Safian, M.T.-U.; Haron, U.S.; Ibrahim, M.N.M. A review on bio-based graphene derived from biomass wastes. BioResources 2020, 15, 1–30. [Google Scholar] [CrossRef]
- Grossman, A.; Wilfred, V. Lignin-based polymers and nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef]
- Addepally, U.; Gandham, V.; Palety, K.K.; Kanakaraju, Y. Lignin-based carbon nanomaterials-the future scope. Mater. Perform. Charact. 2019, 8, 401–420. [Google Scholar] [CrossRef]
- Beisl, S.; Miltner, A.; Friedl, A. Lignin from micro-to nanosize: production methods. Int. J. Mol. Sci. 2017, 18, 1244–1274. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and Specialty Industrial Applications of Lignocellulosic Biomass. Waste Biomass Valorization 2020. Ahead of Print. [Google Scholar] [CrossRef]
- Glasser, W.G. About Making Lignin Great Again-Some Lessons From the Past. Front Chem 2019, 7, 565. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.; Vickram, S.; Thanigaivel, S.; Subbaiya, R.; Kim, W.; Karmegam, N.; Govarthanan, M. Nanomaterials for transforming barrier properties of lignocellulosic biomass towards potential applications - A review. Fuel 2022, 316, 123444. [Google Scholar] [CrossRef]
- Yan, Z.; Song, B.; Fang, G.; Wu, T.; Chen, N.; Zhao, M.; Zou, X.; Liao, G. Bringing Material Concepts into Conventional Biorefineries: Considerations of Sources, Preparations, and Applications of Lignin Nanomaterials. ACS Sustainable Chem. Eng. 2021, 9, 10403–10423. [Google Scholar] [CrossRef]
- Vignesh, N.S.; Soosai, M.R.; Chia, W.Y.; Wahid, S.N.; Varalakshmi, P.; Moorthy, I.M.G.; Ashokkumar, B.; Arumugasamy, S.K.; Selvarajoo, A.; Chew, K.W. Microwave-assisted pyrolysis for carbon catalyst, nanomaterials and biofuel production. Fuel 2022, 313, 123023. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Zheng, X.; Wang, X.; Xu, Y.; Tang, H.; Kang, F.; Yang, Q.-H.; Luo, J. Biomass Organs Control the Porosity of Their Pyrolyzed Carbon. Adv. Funct. Mater. 2017, 27, 1604687. [Google Scholar] [CrossRef]
- Wang, C.; Liang, W.; Yang, Y.; Liu, F.; Sun, H.; Zhu, Z.; Li, A. Biomass carbon aerogels based shape-stable phase change composites with high light-to-thermal efficiency for energy storage. Renewable Energy 2020, 153, 182–192. [Google Scholar] [CrossRef]
- Matveeva, V.G.; Bronstein, L.M. From renewable biomass to nanomaterials: Does biomass origin matter. Prog. Mater. Sci. 2022, 130, 100999. [Google Scholar] [CrossRef]
- Bender, M.T.; Yuan, X.; Goetz, M.K.; Choi, K.-S. Electrochemical Hydrogenation, Hydrogenolysis, and Dehydrogenation for Reductive and Oxidative Biomass Upgrading Using 5-Hydroxymethylfurfural as a Model System. ACS Catal. 2022, 12, 12349–12368. [Google Scholar] [CrossRef]
- Coutanceau, C.; Neha, N.; Rafaideen, T. Electrocatalytic transformation of biosourced organic molecules. Curr. Opin. Electrochem. 2023, 38, 101210. [Google Scholar] [CrossRef]
- Guo, M.; Lu, X.; Xiong, J.; Zhang, R.; Li, X.; Qiao, Y.; Ji, N.; Yu, Z. Alloy-Driven Efficient Electrocatalytic Oxidation of Biomass-Derived 5-Hydroxymethylfurfural towards 2,5-Furandicarboxylic Acid: A Review. ChemSusChem 2022, 15, e202201074. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Reif, P.; Palenicek, P.; Rose, M. Toward Renewable Amines: Recent Advances in the Catalytic Amination of Biomass-Derived Oxygenates. ACS Catal. 2022, 12, 10400–10440. [Google Scholar] [CrossRef]
- Ouyang, D.; Wang, F.; Gao, D.; Han, W.; Hu, X.; Qiao, D.; Zhao, X. Light-driven lignocellulosic biomass conversion for production of energy and chemicals. iScience 2022, 25, 105221. [Google Scholar] [CrossRef] [PubMed]
- Terzan, J.; Sedminek, A.; Lavric, Z.; Grilc, M.; Hus, M.; Likozar, B. Selective oxidation of biomass-derived carbohydrate monomers. Green Chem. 2023, 25, 2220–2240. [Google Scholar] [CrossRef]
- Turkin, A.A.; Makshina, E.V.; Sels, B.F. Catalytic Hydroconversion of 5-HMF to Value-Added Chemicals: Insights into the Role of Catalyst Properties and Feedstock Purity. ChemSusChem 2022, 15, e202200412. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhang, S.; Ke, L.; Wu, Q.; Zhang, Q.; Cui, X.; Dai, A.; Xu, C.; Cobb, K.; Liu, Y.; et al. Research progress on pyrolysis of nitrogen-containing biomass for fuels, materials, and chemicals production. Sci. Total Environ. 2023, 872, 162214. [Google Scholar] [CrossRef]
- Yuan, Z.; Dai, W.; Zhang, S.; Wang, F.; Jian, J.; Zeng, J.; Zhou, H. Heterogeneous strategies for selective conversion of lignocellulosic polysaccharides. Cellulose (Dordrecht, Neth.) 2022, 29, 3059–3077. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, Z.; Watanabe, M. Production of solid fuels by hydrothermal treatment of wastes of biomass, plastic, and biomass/plastic mixtures: A review. J. Bioresour. Bioprod. 2022, 7, 221–244. [Google Scholar] [CrossRef]
- Advani, J.H.; More, G.S.; Srivastava, R. Spinel-based catalysts for the biomass valorisation of platform molecules via oxidative and reductive transformations. Green Chem. 2022, 24, 3574–3604. [Google Scholar] [CrossRef]
- Aljammal, N.; Jabbour, C.; Thybaut, J.W.; Demeestere, K.; Verpoort, F.; Heynderickx, P.M. Metal-organic frameworks as catalysts for sugar conversion into platform chemicals: State-of-the-art and prospects. Coord. Chem. Rev. 2019, 401, 213064. [Google Scholar] [CrossRef]
- Chen, X.; Song, S.; Li, H.; Gozaydln, G.; Yan, N. Expanding the Boundary of Biorefinery: Organonitrogen Chemicals from Biomass. Acc. Chem. Res. 2021, 54, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Gale, M.; Cai, C.M.; Gilliard-Abdul-Aziz, K.L. Heterogeneous Catalyst Design Principles for the Conversion of Lignin into High-Value Commodity Fuels and Chemicals. ChemSusChem 2020, 13, 1947–1966. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Rai, R.K.; Singh, S.K. Metal Catalysts for the Efficient Transformation of Biomass-derived HMF and Furfural to Value Added Chemicals. ChemCatChem 2018, 10, 2326–2349. [Google Scholar] [CrossRef]
- Hu, Y.; He, Q.; Xu, C. Catalytic Conversion of Glycerol into Hydrogen and Value-Added Chemicals: Recent Research Advances. Catalysts 2021, 11, 1455. [Google Scholar] [CrossRef]
- Lazzarini, A.; Colaiezzi, R.; Gabriele, F.; Crucianelli, M. Support-Activity Relationship in Heterogeneous Catalysis for Biomass Valorization and Fine-Chemicals Production. Materials 2021, 14, 6796. [Google Scholar] [CrossRef] [PubMed]
- Redina, E.; Tkachenko, O.; Salmi, T. Recent Advances in C5 and C6 Sugar Alcohol Synthesis by Hydrogenation of Monosaccharides and Cellulose Hydrolytic Hydrogenation over Non-Noble Metal Catalysts. Molecules 2022, 27, 1353. [Google Scholar] [CrossRef]
- Sharma, V.; Getahun, T.; Verma, M.; Villa, A.; Gupta, N. Carbon based catalysts for the hydrodeoxygenation of lignin and related molecules: A powerful tool for the generation of non-petroleum chemical products including hydrocarbons. Renewable Sustainable Energy Rev. 2020, 133, 110280. [Google Scholar] [CrossRef]
- Shivhare, A.; Kumar, A.; Srivastava, R. Metal phosphate catalysts to upgrade lignocellulose biomass into value-added chemicals and biofuels. Green Chem. 2021, 23, 3818–3841. [Google Scholar] [CrossRef]
- Shivhare, A.; Kumar, A.; Srivastava, R. Account of the Catalytic Transfer Hydrogenation and Hydrogenolysis of Carbohydrate-Derived Renewable Platform Chemicals over Non-Precious Heterogeneous Metal Catalysts. ChemCatChem 2021, 13, 59–80. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Hu, W.; Lu, L.; Chen, J.; Zhu, Y.; Zhou, H.; Si, C. Recent advances on solid acid catalyic systems for production of 5-Hydroxymethylfurfural from biomass derivatives. Fuel Process. Technol. 2022, 234, 107338. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Liu, S.; Zhao, N.; Jiang, X.; Su, M.; Li, Z. Enhanced gasoline selectivity through Fischer-Tropsch synthesis on a bifunctional catalyst: Effects of active sites proximity and reaction temperature. Chem. Eng. J. (Amsterdam, Neth.) 2021, 416, 129180. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Liu, S.; Wu, F.; Zhang, Q.; Zhang, S.; Cheng, K.; Wang, Y. Distance for Communication between Metal and Acid Sites for Syngas Conversion. ACS Catal. 2022, 12, 8793–8801. [Google Scholar] [CrossRef]
- Xu, L.; Li, C.-g.; Zhang, K.; Wu, P. Bifunctional Tandem Catalysis on Multilamellar Organic-Inorganic Hybrid Zeolites. ACS Catal. 2014, 4, 2959–2968. [Google Scholar] [CrossRef]
- Yuan, E.-H.; Niu, Y.; Huang, X.; Li, M.; Bao, J.; Song, Y.-H.; Zhang, B.; Liu, Z.-T.; Willinger, M.-G.; Liu, Z.-W. Finned Zn-MFI zeolite encapsulated noble metal nanoparticle catalysts for the oxidative dehydrogenation of propane with carbon dioxide. J. Energy Chem. 2023, 80, 479–491. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, C.; Yin, H.; Shi, J.; Zhang, G.; Zheng, X.; Min, X.; Zhang, Z.; Cheng, K.; Kang, J.; et al. Direct conversion of syngas into aromatics over a bifunctional catalyst: inhibiting net CO2 release. Chem. Commun. (Cambridge, U. K.) 2020, 56, 5239–5242. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Zhan, G.; Huang, J.; Li, Q. Hydrogenation of CO2 to Dimethyl Ether over Tandem Catalysts Based on Biotemplated Hierarchical ZSM-5 and Pd/ZnO. ACS Sustainable Chem. Eng. 2020, 8, 14058–14070. [Google Scholar] [CrossRef]
- Fu, X.-X.; Li, J.-P.; Li, Z.-Q.; Liu, Y.; Feng, C.-X.; Wang, H.-Y.; Zhao, Z.-P.; Liu, Q.-Y.; Liu, Z.-Y.; Peng, Z.-K. Selective conversion of 2-methylfuran to 3-acetyl-1-propanol in water over Pd@HZSM-5 catalyst with balanced metal-acid cooperation. J. Catal. 2022, 413, 648–657. [Google Scholar] [CrossRef]
- He, J.; Lin, L.; Liu, M.; Miao, C.; Wu, Z.; Chen, R.; Chen, S.; Chen, T.; Su, Y.; Zhang, T.; et al. A durable Ni/La-Y catalyst for efficient hydrogenation of γ-valerolactone into pentanoic biofuels. J. Energy Chem. 2022, 70, 347–355. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, K.; Kang, J.; Deng, W.; Wang, Y. Fischer-Tropsch Catalysts for the Production of Hydrocarbon Fuels with High Selectivity. ChemSusChem 2014, 7, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Ao, S.; Rokhum, S.L. Recent advances in the valorization of biodiesel by-product glycerol to solketal. J. Chem. 2022, 4938672. [Google Scholar] [CrossRef]
- Boronat, M.; Climent, M.J.; Concepcion, P.; Diaz, U.; Garcia, H.; Iborra, S.; Leyva-Perez, A.; Liu, L.; Martinez, A.; Martinez, C.; et al. A Career in Catalysis: Avelino Corma. ACS Catal. 2022, 12, 7054–7123. [Google Scholar] [CrossRef]
- De, S.; Dutta, S.; Saha, B. Critical design of heterogeneous catalysts for biomass valorization: current thrust and emerging prospects. Catal. Sci. Technol. 2016, 6, 7364–7385. [Google Scholar] [CrossRef]
- Hara, M.; Nakajima, K.; Kamata, K. Recent progress in the development of solid catalysts for biomass conversion into high value-added chemicals. Sci. Technol. Adv. Mater. 2015, 16, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.; Janiak, C. MOF catalysts in biomass upgrading towards value-added fine chemicals. CrystEngComm 2017, 19, 4092–4117. [Google Scholar] [CrossRef]
- Limlamthong, M.; Yip, A.C.K. Recent advances in zeolite-encapsulated metal catalysts: A suitable catalyst design for catalytic biomass conversion. Bioresour. Technol. 2020, 297, 122488. [Google Scholar] [CrossRef]
- Qi, S.-C.; Wei, X.-Y.; Zong, Z.-M.; Wang, Y.-K. Application of supported metallic catalysts in catalytic hydrogenation of arenes. RSC Adv. 2013, 3, 14219–14232. [Google Scholar] [CrossRef]
- Shamzhy, M.; Opanasenko, M.; Concepcion, P.; Martinez, A. New trends in tailoring active sites in zeolite-based catalysts. Chem. Soc. Rev. 2019, 48, 1095–1149. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Baiker, A.; Huang, J. Pentacoordinated Aluminum Species: New Frontier for Tailoring Acidity-Enhanced Silica-Alumina Catalysts. Acc. Chem. Res. 2020, 53, 2648–2658. [Google Scholar] [CrossRef]
- Krol, M. Natural vs. Synthetic Zeolites. Crystals 2020, 10, 622. [Google Scholar] [CrossRef]
- Sherman, J.D. Synthetic zeolites and other microporous oxide molecular sieves. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 3471–3478. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, S.; Beneteau, V.; Pale, P. Green catalysts based on zeolites for heterocycle synthesis. Current Opinion in Green and Sustainable Chemistry 2018, 10, 35–39. [Google Scholar] [CrossRef]
- Kuz'micheva, G.M.; Domoroshchina, E.N.; Kravchenko, G.V. Design of MFI Type Aluminum- and Titanium-Containing Zeolites. Crystals 2021, 11, 1451. [Google Scholar] [CrossRef]
- Diaz, I.; Kokkoli, E.; Terasaki, O.; Tsapatsis, M. Surface Structure of Zeolite (MFI) Crystals. Chem. Mater. 2004, 16, 5226–5232. [Google Scholar] [CrossRef]
- Shamzhy, M.; Gil, B.; Opanasenko, M.; Roth, W.J.; Cejka, J. MWW and MFI Frameworks as Model Layered Zeolites: Structures, Transformations, Properties, and Activity. ACS Catal. 2021, 11, 2366–2396. [Google Scholar] [CrossRef]
- Li, X.Y.; Han, H.; Xu, W.Q.; Hwang, S.J.; Shi, Z.C.; Lu, P.; Bhan, A.; Tsapatsis, M. Acid Catalysis over Low-Silica Faujasite Zeolites. Journal of the American Chemical Society 2022, 144, 9324–9329. [Google Scholar] [CrossRef] [PubMed]
- Bok, T.O.; Andriako, E.P.; Knyazeva, E.E.; Ivanova, II. Engineering of zeolite BEA crystal size and morphology via seed-directed steam assisted conversion. Rsc Advances 2020, 10, 38505–38514. [Google Scholar] [CrossRef]
- Medeiros-Costa, I.C.; Laroche, C.; Perez-Pellitero, J.; Coasne, B. Characterization of hierarchical zeolites: Combining adsorption/intrusion, electron microscopy, diffraction and spectroscopic techniques. Microporous and Mesoporous Materials 2019, 287, 167–176. [Google Scholar] [CrossRef]
- Reiprich, B.; Weissenberger, T.; Schwieger, W.; Inayat, A. Layer-like FAU-type zeolites: A comparative view on different preparation routes. Frontiers of Chemical Science and Engineering 2020, 14, 127–142. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Makowski, W.; Marszalek, B.; Eliasova, P. Layer like porous materials with hierarchical structure. Chemical Society Reviews 2016, 45, 3400–3438. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.X.; He, C.J.; Sun, Y.Y.; Dong, H.; Ge, M.; Xia, X.P. A layer-like FAU zeolite with palladium particles embedded in situ as catalyst for hydrogenation of 1,4-bis(phenylethynyl) benzene. Surfaces and Interfaces 2022, 34. [Google Scholar] [CrossRef]
- de Aguiar Pedott, V.; Moraes, G.H.; Soares, C.; Padoin, N.; Riella, H.G.; Jose de Andrade, C. Biosurfactants as structure directing agents of porous siliceous materials. Microporous Mesoporous Mater. 2022, 345, 112279. [Google Scholar] [CrossRef]
- Schlumberger, C.; Thommes, M. Characterization of Hierarchically Ordered Porous Materials by Physisorption and Mercury Porosimetry-A Tutorial Review. Adv. Mater. Interfaces 2021, 8, 2002181. [Google Scholar] [CrossRef]
- Singh, B.K.; Kim, Y.; Kwon, S.; Na, K. Synthesis of Mesoporous Zeolites and Their Opportunities in Heterogeneous Catalysis. Catalysts 2021, 11, 1541. [Google Scholar] [CrossRef]
- Weissenberger, T.; Machoke, A.G.F.; Reiprich, B.; Schwieger, W. Preparation and Potential Catalytic Applications of Hierarchically Structured Zeolites with Macropores. Adv. Mater. Interfaces 2021, 8, 2001653. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, D.; Xu, B. Pore Size Engineering Enabled Selectivity Control in Tandem Catalytic Upgrading of Cyclopentanone on Zeolite-Encapsulated Pt Nanoparticles. ACS Catal. 2020, 10, 8850–8859. [Google Scholar] [CrossRef]
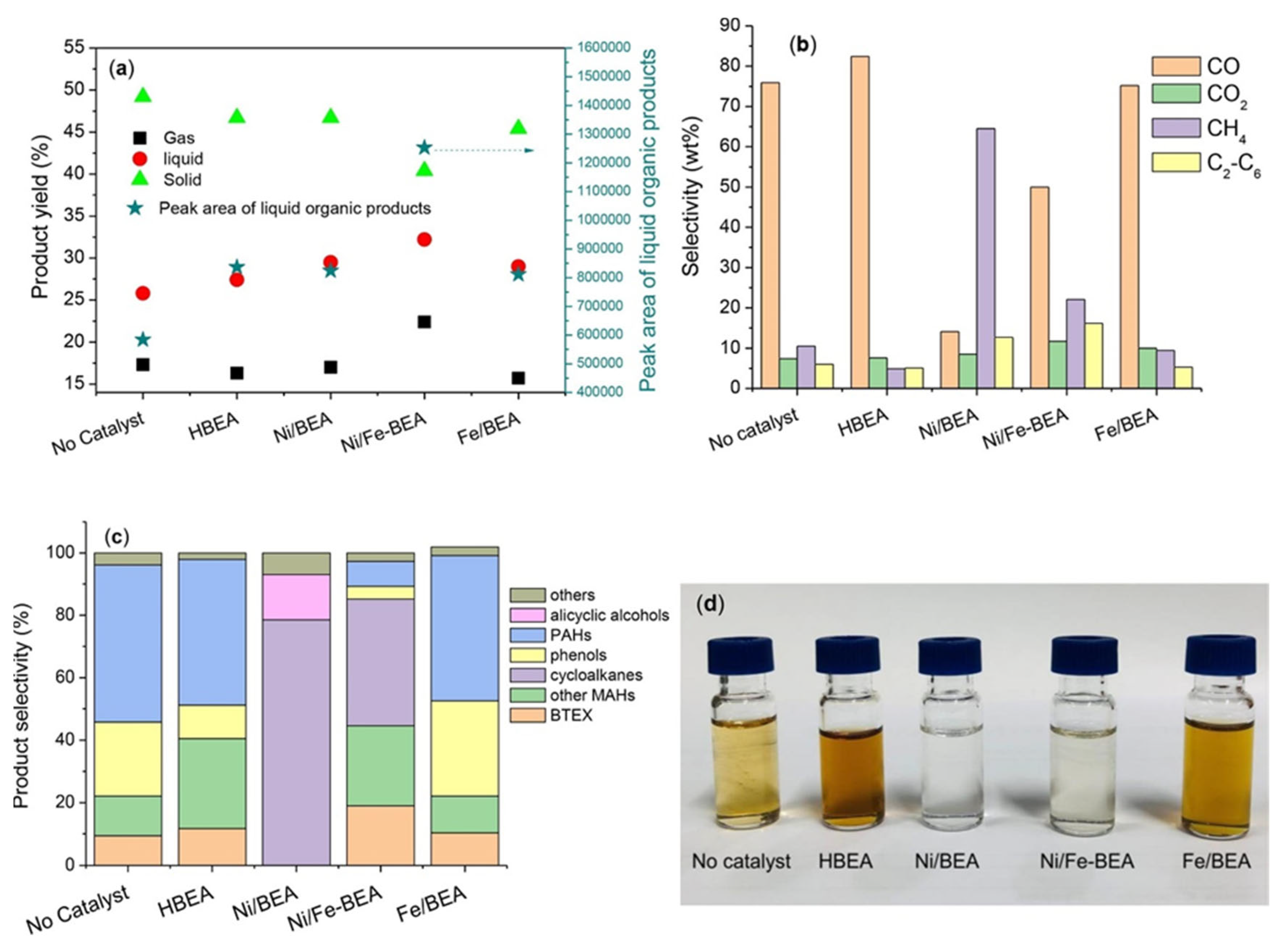
- Yan, P.; Kennedy, E.M.; Zhang, H.; Stockenhuber, M. Catalytic hydropyrolysis of lignocellulosic biomass to BTX and biofuels over zeolite beta based catalysts. Fuel 2023, 332, 125946. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Tong, Y.; Wu, Y.; Peng, J.; Gu, X.-K.; Ding, M. Selective C-O bond activation of biomass-derived γ-valerolactone to biofuels over MFI-mediated Co-based synergetic catalysts. Appl. Catal., B 2022, 318, 121840. [Google Scholar] [CrossRef]
- El-Deeb, Z.M.; Aboutaleb, W.A.; Dhmees, A.S.; El Naggar, A.M.A.; Emara, K.; Elgendy, A.T.; Ahmed, A.I. Bio-fuels production through waste tires pyrolytic oil upgrading over Ni-W/zeolite composites derived from blast furnace slag. Int. J. Energy Res. 2022, 46, 17376–17390. [Google Scholar] [CrossRef]
- Gao, X.; Ding, Y.; Peng, L.; Yang, D.; Wan, X.; Zhou, C.; Liu, W.; Dai, Y.; Yang, Y. On the effect of zeolite acid property and reaction pathway in Pd-catalyzed hydrogenation of furfural to cyclopentanone. Fuel 2022, 314, 123074. [Google Scholar] [CrossRef]
- Meng, Q.; Yan, J.; Wu, R.; Liu, H.; Sun, Y.; Wu, N.; Xiang, J.; Zheng, L.; Zhang, J.; Han, B. Sustainable production of benzene from lignin. Nat. Commun. 2021, 12, 4534. [Google Scholar] [CrossRef] [PubMed]
- Salakhum, S.; Prasertsab, A.; Pornsetmetakul, P.; Saenluang, K.; Iadrat, P.; Chareonpanich, M.; Wattanakit, C. Pt Nanoparticles on ZSM-5 Nanoparticles for Base-Free Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. ACS Appl. Nano Mater. 2021, 4, 14047–14059. [Google Scholar] [CrossRef]
- Karanwal, N.; Sibi, M.G.; Khan, M.K.; Myint, A.A.; Chan Ryu, B.; Kang, J.W.; Kim, J. Trimetallic Cu-Ni-Zn/H-ZSM-5 Catalyst for the One-Pot Conversion of Levulinic Acid to High-Yield 1,4-Pentanediol under Mild Conditions in an Aqueous Medium. ACS Catal. 2021, 11, 2846–2864. [Google Scholar] [CrossRef]
- Joshi, H.; Ochoa-Hernandez, C.; Nuerenberg, E.; Kang, L.; Wang, F.R.; Weidenthaler, C.; Schmidt, W.; Schueth, F. Insights into the mechanochemical synthesis of Sn-β: Solid-state metal incorporation in beta zeolite. Microporous Mesoporous Mater. 2020, 309, 110566. [Google Scholar] [CrossRef]
- Arslan, M.T.; Qureshi, B.A.; Gilani, S.Z.A.; Cai, D.; Ma, Y.; Usman, M.; Chen, X.; Wang, Y.; Wei, F. Single-Step Conversion of H2-Deficient Syngas into High Yield of Tetramethylbenzene. ACS Catal. 2019, 9, 2203–2212. [Google Scholar] [CrossRef]
- Gentzen, M.; Doronkin, D.E.; Sheppard, T.L.; Grunwaldt, J.D.; Sauer, J.; Behrens, S. Bifunctional catalysts based on colloidal Cu/Zn nanoparticles for the direct conversion of synthesis gas to dimethyl ether and hydrocarbons. Appl. Catal., A 2018, 557, 99–107. [Google Scholar] [CrossRef]
- Cabanillas, M.; Franco, A.; Lazaro, N.; Balu, A.M.; Luque, R.; Pineda, A. Continuous flow transfer hydrogenation of biomass derived methyl levulinate over Zr containing zeolites: Insights into the role of the catalyst acidity. Mol. Catal. 2019, 477. [Google Scholar] [CrossRef]
- Yan, P.; Mensah, J.; Adesina, A.; Kennedy, E.; Stockenhuber, M. Highly-dispersed Ni on BEA catalyst prepared by ion-exchange-deposition-precipitation for improved hydrodeoxygenation activity. Appl. Catal., B 2020, 267, 118690. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, F.; Zhang, Y.; Su, Y.; Wang, A.; Zhang, T. Ru/H-beta as an efficient catalyst for the conversion of furfural into 3-acetyl-1-propanol (3-AP) toward one-pot transformation of xylan to 3-AP. Mol. Catal. 2019, 476. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Wu, Y.; Peng, J.; Gu, X.-K.; Ding, M. Controlling reaction pathways via selective C-O activation for highly efficient biomass oriented-upgrading. Chem. Eng. J. (Amsterdam, Neth.) 2022, 446, 137404. [Google Scholar] [CrossRef]
- Lin, L.; Cao, P.; Pang, J.; Wang, Z.; Jiang, Q.; Su, Y.; Chen, R.; Wu, Z.; Zheng, M.; Luo, W. Zeolite-encapsulated Cu nanoparticles with enhanced performance for ethanol dehydrogenation. J. Catal. 2022, 413, 565–574. [Google Scholar] [CrossRef]
- Cao, P.; Lin, L.; Qi, H.; Chen, R.; Wu, Z.; Li, N.; Zhang, T.; Luo, W. Zeolite-Encapsulated Cu Nanoparticles for the Selective Hydrogenation of Furfural to Furfuryl Alcohol. ACS Catal. 2021, 11, 10246–10256. [Google Scholar] [CrossRef]
- Ojeda, M.; Osterman, N.; Drazic, G.; Fele Zilnik, L.; Meden, A.; Kwapinski, W.; Balu, A.M.; Likozar, B.; Novak Tusar, N. Conversion of Palmitic Acid Over Bi-functional Ni/ZSM-5 Catalyst: Effect of Stoichiometric Ni/Al Molar Ratio. Top. Catal. 2018, 61, 1757–1768. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, J. Combination of microwave discharge electrodeless lamp and a TiO2/HZSM-5 composite for the photocatalytic degradation of dimethyl sulphide. Environ. Res. 2021, 197, 111082. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wu, Z.; Gu, Q.; Liu, Y.; Chu, S.; Chen, S.; Zhang, Y.; Yang, B.; Chen, T.; Wang, A.; et al. Zeolite-Tailored Active Site Proximity for the Efficient Production of Pentanoic Biofuels. Angew. Chem., Int. Ed. 2021, 60, 23713–23721. [Google Scholar] [CrossRef]
- Zong, R.; Li, H.; Ding, W.; Huang, H. Highly Dispersed Pd on Zeolite/Carbon Nanocomposites for Selective Hydrodeoxygenation of Biomass-Derived Molecules under Mild Conditions. ACS Sustainable Chem. Eng. 2021, 9, 9891–9902. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Tan, L.; Zhang, P.; Peng, X.; Oruganti, A.; Yang, G.; Abe, H.; Wang, Y.; Tsubaki, N. Integrated tuneable synthesis of liquid fuels via Fischer-Tropsch technology. Nat. Catal. 2018, 1, 787–793. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, J.; Xing, E.; Xie, Y.; Zhao, H.; Ning, P.; Shi, Y. Upgrading of palmitic acid to diesel-like fuels over Ni@HZSM-5 bi-functional catalysts through the in situ encapsulation method. Mol. Catal. 2021, 511, 111715. [Google Scholar] [CrossRef]
- Duan, Y.; Luo, Q.; Nie, R.; Wang, J.; Zhang, Y.; Lu, T.; Xu, C. Catalytic Conversion of Glycerol to Methyl Lactate over Au-CuO/Sn-Beta: The Roles of Sn-Beta. Catalysts 2022, 12, 104. [Google Scholar] [CrossRef]
- Jin, Z.; Yi, X.; Wang, L.; Xu, S.; Wang, C.; Wu, Q.; Wang, L.; Zheng, A.; Xiao, F.-S. Metal-acid interfaces enveloped in zeolite crystals for cascade biomass hydrodeoxygenation. Appl. Catal., B 2019, 254, 560–568. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, X.-Y.; Zhang, M.; Zong, Z.-M. Catalytic hydroconversion of aryl ethers over a nickel catalyst supported on acid-modified zeolite 5A. Fuel Process. Technol. 2018, 177, 345–352. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, J.; Qiu, Y.; Zhang, X.; Zhou, J.; Cen, K. Competitive conversion pathways of methyl palmitate to produce jet biofuel over Ni/desilicated meso-Y zeolite catalyst. Fuel 2019, 244, 472–478. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Wang, L.; Dong, X.; Zhang, J.; Wang, G.; Han, S.; Meng, X.; Zheng, A.; Xiao, F.-S. Importance of Zeolite Wettability for Selective Hydrogenation of Furfural over Pd@Zeolite Catalysts. ACS Catal. 2018, 8, 474–481. [Google Scholar] [CrossRef]
- Jiang, J.; Ding, W.; Li, H. Promotional effect of F for Pd/HZSM-5 catalyst on selective HDO of biobased ketones. Renewable Energy 2021, 179, 1262–1270. [Google Scholar] [CrossRef]
- Zhang, B.; Douthwaite, M.; Liu, Q.; Zhang, C.; Wu, Q.; Shi, R.; Wu, P.; Liu, K.; Wang, Z.; Lin, W.; et al. Seed- and solvent-free synthesis of ZSM-5 with tuneable Si/Al ratios for biomass hydrogenation. Green Chem. 2020, 22, 1630–1638. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, J.; Gu, M.; Gao, F.; Shen, Z. Catalytic production of 1,2-propanediol from sucrose over a functionalized Pt/deAl-beta zeolite catalyst. RSC Adv. 2023, 13, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Angamuthu, V.; Tai, D.-F. Asymmetric reactions of chiral organo-magnetic nanoparticles. Appl. Catal., A 2015, 506, 254–260. [Google Scholar] [CrossRef]
- Aqeel Ashraf, M.; Liu, Z.; Li, Y.-Y.; Li, C.; Zhang, D. Zinc nanomagnetic catalysts in organic synthesis. Synth. Commun. 2021, 51, 37–56. [Google Scholar] [CrossRef]
- Bodaghifard, M.A.; Hamidinasab, M.; Ahadi, N. Recent Advances in the Preparation and Application of Organic- inorganic Hybrid Magnetic Nanocatalysts on Multicomponent Reactions. Curr. Org. Chem. 2018, 22, 234–267. [Google Scholar] [CrossRef]
- Dalpozzo, R. Magnetic nanoparticle supports for asymmetric catalysts. Green Chem. 2015, 17, 3671–3686. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Varma, R.S. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 2013, 42, 3371–3393. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, M.; Pourmoghaddam Qhazvini, P.; Eslami, M.; Kazemi, M. Magnetic nanoparticles supported bromine sources: Catalysis in organic synthesis. Synth. Commun. 2021, 51, 325–350. [Google Scholar] [CrossRef]
- Kainz, Q.M.; Reiser, O. Polymer- and Dendrimer-Coated Magnetic Nanoparticles as Versatile Supports for Catalysts, Scavengers, and Reagents. Acc. Chem. Res. 2014, 47, 667–677. [Google Scholar] [CrossRef]
- Kazemi, M. Based on magnetic nanoparticles: Gold reusable nanomagnetic catalysts in organic synthesis. Synth. Commun. 2020, 50, 2079–2094. [Google Scholar] [CrossRef]
- Kothandapani, J.; Ganesan, S.S. Concise Review on the Applications of Magnetically Separable Bronsted Acidic Catalysts. Curr. Org. Chem. 2019, 23, 313–334. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z. Catalytic conversion of biomass into chemicals and fuels over magnetic catalysts. ACS Catal. 2016, 6, 326–338. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Mrowczynski, R.; Nan, A.; Liebscher, J. Magnetic nanoparticle-supported organocatalysts - an efficient way of recycling and reuse. RSC Adv. 2014, 4, 5927–5952. [Google Scholar] [CrossRef]
- Nasir Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Magnetically retrievable catalysts for asymmetric synthesis. Coord. Chem. Rev. 2015, 287, 137–156. [Google Scholar] [CrossRef]
- Wang, A.; Sudarsanam, P.; Xu, Y.; Zhang, H.; Li, H.; Yang, S. Functionalized magnetic nanosized materials for efficient biodiesel synthesis via acid-base/enzyme catalysis. Green Chem. 2020, 22, 2977–3012. [Google Scholar] [CrossRef]
- Wang, D.; Deraedt, C.; Ruiz, J.; Astruc, D. Magnetic and Dendritic Catalysts. Acc. Chem. Res. 2015, 48, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, L.M. Magnetically Recoverable Catalysts with Dendritic Ligands for Enhanced Catalysis and Easy Separation. ChemCatChem 2015, 7, 1058–1060. [Google Scholar] [CrossRef]
- Matveeva, V.G.; Bronstein, L.M. Magnetic Nanoparticle-Containing Supports as Carriers of Immobilized Enzymes: Key Factors Influencing the Biocatalyst Performance. Nanomaterials 2021, 11, 2257. [Google Scholar] [CrossRef] [PubMed]
- Shifrina, Z.B.; Matveeva, V.G.; Bronstein, L.M. Role of Polymer Structures in Catalysis by Transition Metal and Metal Oxide Nanoparticle Composites. Chem. Rev. (Washington, DC, U. S.) 2020, 120, 1350–1396. [Google Scholar] [CrossRef]
- Alamgholiloo, H.; Hashemzadeh, B.; Noroozi Pesyan, N.; Sheikhmohammadi, A.; Asgari, E.; Yeganeh, J.; Hashemzadeh, H. A facile strategy for designing core-shell nanocomposite of ZIF-67/Fe3O4: A novel insight into ciprofloxacin removal from wastewater. Process Saf. Environ. Prot. 2021, 147, 392–404. [Google Scholar] [CrossRef]
- Chen, J.; Lian, J.; Fang, Z. A comparative study on adsorption and catalytic degradation of tetracycline by five magnetic mineral functional materials prepared from steel pickling waste liquor. Environ. Sci. Pollut. Res. 2022, 29, 78926–78941. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Khoris, I.M.; Chowdhury, A.D.; Li, T.-C.; Park, E.Y. Ultrasensitive Detection of the Hepatitis E Virus by Electrocatalytic Water Oxidation Using Pt-Co3O4 Hollow Cages. ACS Appl. Mater. Interfaces 2020, 12, 50212–50221. [Google Scholar] [CrossRef]
- Hu, T.; Li, J.; Wang, L.; Wang, H.; Zhang, Z.; Jiang, W.; Xue, C. ZnO/ZnFe2O4/zeolite composite catalyst for peroxymonosulfate oxidation and photocatalysis. Mater. Lett. 2023, 330, 133310. [Google Scholar] [CrossRef]
- Kalhor, M.; Zarnegar, Z.; Janghorban, F.; Mirshokraei, S.A. Fe3O4@zeolite-SO3H as a magnetically bifunctional and retrievable nanocatalyst for green synthesis of perimidines. Res. Chem. Intermed. 2020, 46, 821–836. [Google Scholar] [CrossRef]
- Li, W.; Wan, D.; Wang, G.; Chen, K.; Hu, Q.; Lu, L. Heterogeneous Fenton degradation of Orange II by immobilization of Fe3O4 nanoparticles onto Al-Fe pillared bentonite. Korean J. Chem. Eng. 2016, 33, 1557–1564. [Google Scholar] [CrossRef]
- Liu, M.; Hou, L.-a.; Xi, B.-d.; Li, Q.; Hu, X.; Yu, S. Magnetically separable Ag/AgCl-zero valent iron particles modified zeolite X heterogeneous photocatalysts for tetracycline degradation under visible light. Chem. Eng. J. (Amsterdam, Neth.) 2016, 302, 475–484. [Google Scholar] [CrossRef]
- Prech, J.; Ioannou, E.; Roussis, V.; Kuncser, V.; Podolean, I.; Coman, S.M.; Valtchev, V.; Parvulescu, V.I. Magnetic Fe@Y Composites as Efficient Recoverable Catalysts for the Valorization of the Recalcitrant Marine Sulfated Polysaccharide Ulvan. ACS Sustainable Chem. Eng. 2020, 8, 319–328. [Google Scholar] [CrossRef]
- Sadeghi, M.; Farhadi, S.; Zabardasti, A. Fabrication of a novel magnetic CdS nanorod/NiFe2O4/NaX zeolite nanocomposite with enhanced sonocatalytic performance in the degradation of organic dyes. New J. Chem. 2020, 44, 8386–8401. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, W.D.; Wang, L.; Schwieger, W.; Wang, W.; Huang, J. Tuning Hierarchical ZSM-5 Zeolite for Both Gas- and Liquid-Phase Biorefining. ACS Catal. 2020, 10, 1185–1194. [Google Scholar] [CrossRef]
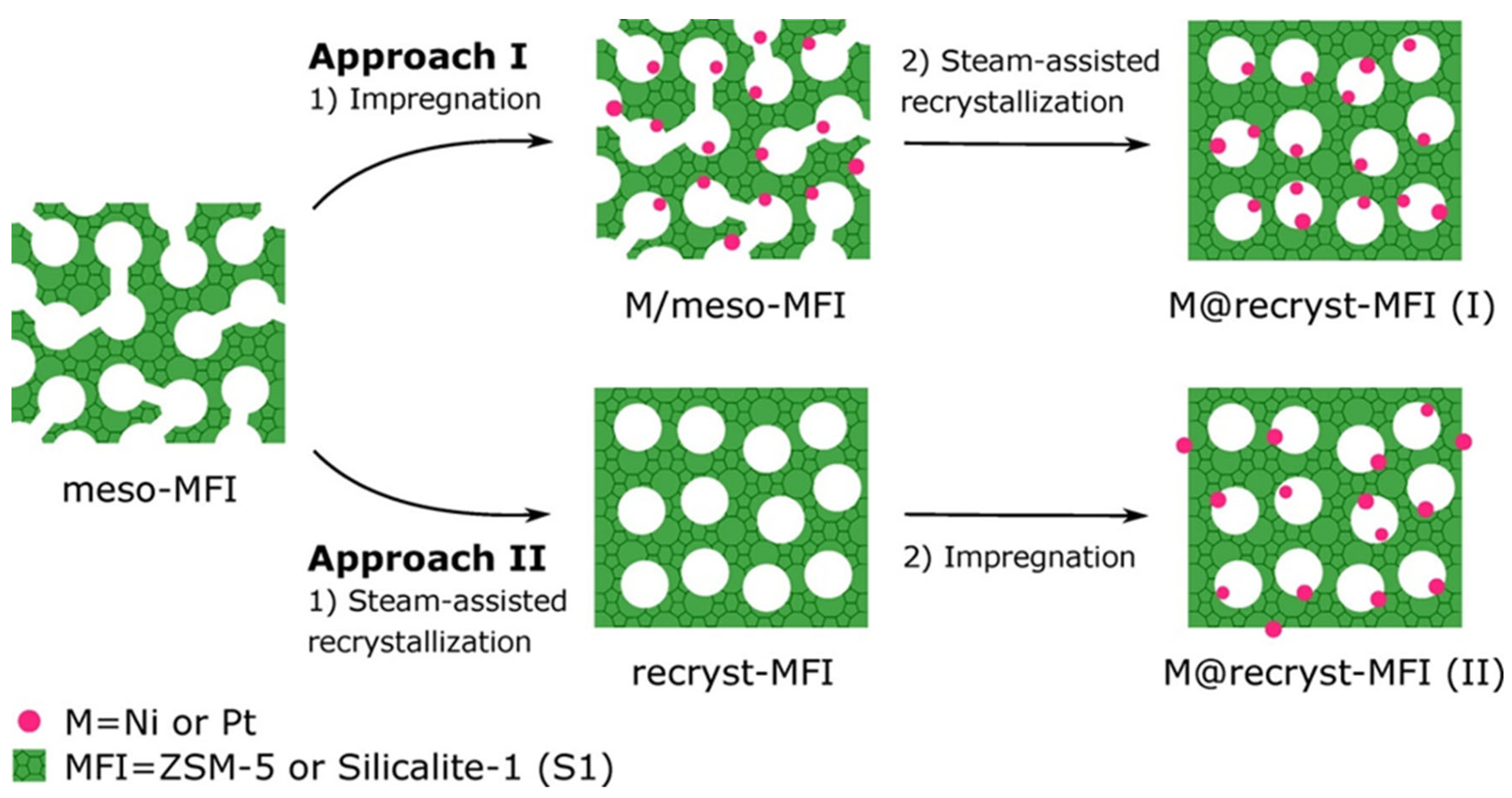
- Rasmussen, K.H.; Goodarzi, F.; Christensen, D.B.; Mielby, J.; Kegnaes, S. Stabilization of Metal Nanoparticle Catalysts via Encapsulation in Mesoporous Zeolites by Steam-Assisted Recrystallization. ACS Appl. Nano Mater. 2019, 2, 8083–8091. [Google Scholar] [CrossRef]
- Xu, S.; Du, J.; Li, H.; Tang, J. Zeolite@Pd/Al2O3 Core-Shell Catalyst for Efficient Hydrodeoxygenation of Phenolic Biomolecules. Ind. Eng. Chem. Res. 2018, 57, 14088–14095. [Google Scholar] [CrossRef]
- Zheng, Z.; Luo, Z.; Zhao, C. Morphologically Cross-Shaped Ru/HZSM-5 Catalyzes Tandem Hydrogenolysis of Guaiacol to Benzene in Water. ChemCatChem 2018, 10, 1376–1384. [Google Scholar] [CrossRef]
- Fan, H.; Zheng, T.; Liao, X.; Sun, M.; Fu, J.; Zheng, J.; Zhang, N.; Chen, B. Stereoselective hydrogenation of platform molecule alpha-pinene under solvent-free conditions. ChemCatChem 2022, 14, e202101690. [Google Scholar] [CrossRef]
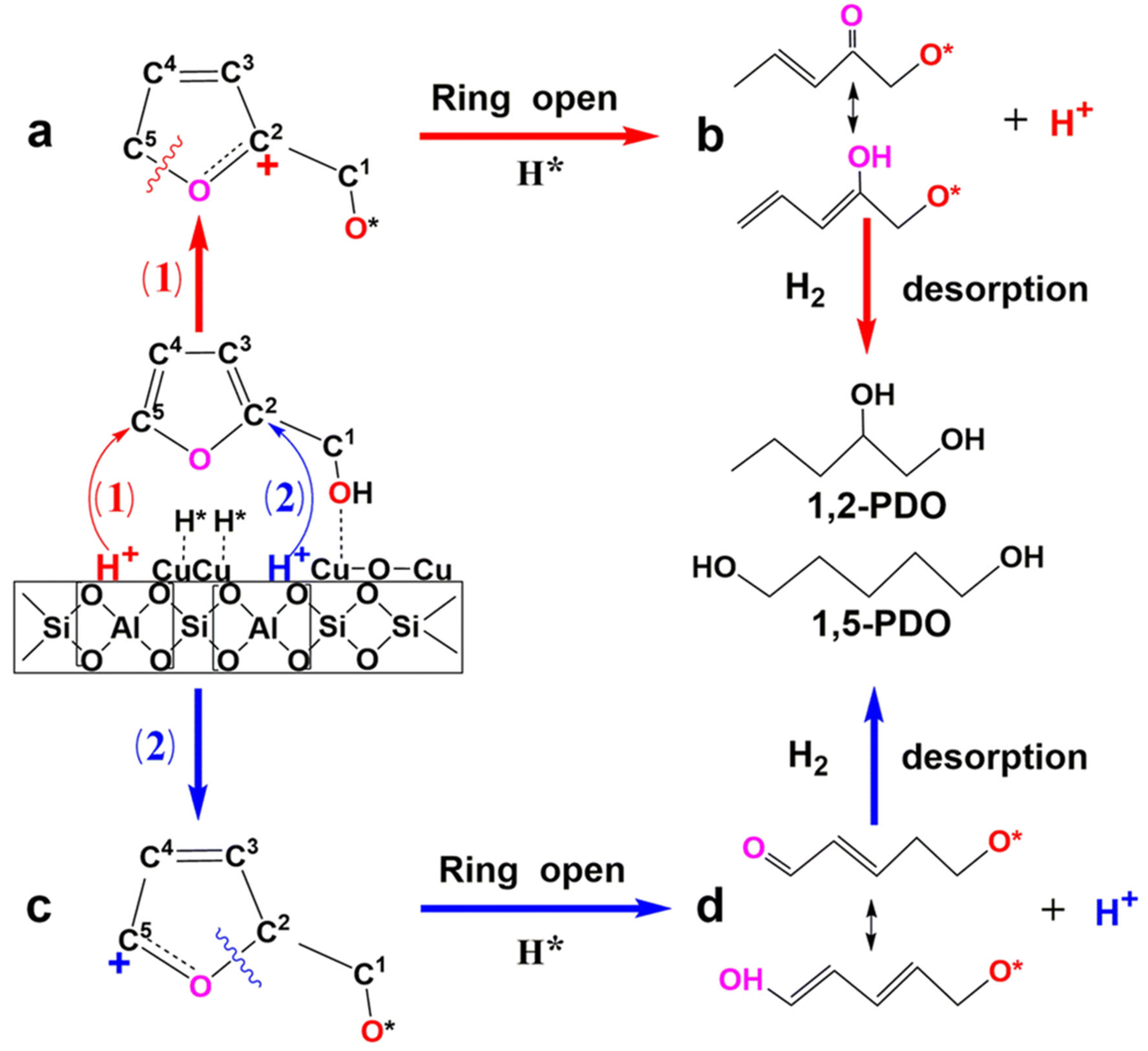
- Dai, D.; Feng, C.; Wang, M.; Du, Q.; Liu, D.; Pan, Y.; Liu, Y. Ring-opening of furfuryl alcohol to pentanediol with extremely high selectivity over Cu/MFI catalysts with balanced Cu0-Cu+ and Bronsted acid sites. Catal. Sci. Technol. 2022, 12, 5879–5890. [Google Scholar] [CrossRef]
- Wetchasat, P.; Salakhum, S.; Imyen, T.; Suttipat, D.; Wannapakdee, W.; Ketkaew, M.; Prasertsab, A.; Kidkhunthod, P.; Witoon, T.; Wattanakit, C. One-pot synthesis of ultra-small Pt dispersed on hierarchical zeolite nanosheet surfaces for mild hydrodeoxygenation of 4-propylphenol. Catalysts 2021, 11, 333. [Google Scholar] [CrossRef]
- Kostyniuk, A.; Bajec, D.; Prasnikar, A.; Likozar, B. Catalytic hydrocracking, hydrogenation, and isomerization reactions of model biomass tar over (W/Ni)-zeolites. J. Ind. Eng. Chem. (Amsterdam, Neth.) 2021, 101, 293–306. [Google Scholar] [CrossRef]
- Li, A.; Zhang, Y.; Heard, C.J.; Golabek, K.; Ju, X.; Cejka, J.; Mazur, M. Encapsulating Metal Nanoparticles into a Layered Zeolite Precursor with Surface Silanol Nests Enhances Sintering Resistance. Angew. Chem., Int. Ed. 2023, 62, e202213361. [Google Scholar] [CrossRef] [PubMed]
- Mandal, K.; Gu, Y.; Westendorff, K.S.; Li, S.; Pihl, J.A.; Grabow, L.C.; Epling, W.S.; Paolucci, C. Condition-Dependent Pd Speciation and NO Adsorption in Pd/Zeolites. ACS Catal. 2020, 10, 12801–12818. [Google Scholar] [CrossRef]
- Sharma, M.; Das, B.; Hazarika, A.; Guha, A.K.; Bhargava, S.K.; Bania, K.K. PdO/CuO Nanoparticles on Zeolite-Y for Nitroarene Reduction and Methanol Oxidation. ACS Appl. Nano Mater. 2019, 2, 3769–3779. [Google Scholar] [CrossRef]
- Wang, L.; Dai, J.; Xu, Y.; Hong, Y.; Huang, J.; Sun, D.; Li, Q. Titanium silicalite-1 zeolite encapsulating Au particles as a catalyst for vapor phase propylene epoxidation with H2/O2: a matter of Au-Ti synergic interaction. J. Mater. Chem. A 2020, 8, 4428–4436. [Google Scholar] [CrossRef]
- Wannapakdee, W.; Yutthalekha, T.; Dugkhuntod, P.; Rodponthukwaji, K.; Thivasasith, A.; Nokbin, S.; Witoon, T.; Pengpanich, S.; Wattanakit, C. Dehydrogenation of propane to propylene using promoter-free hierarchical Pt/silicalite-1 nanosheets. Catalysts 2019, 9, 174–171. [Google Scholar] [CrossRef]
- Xu, D.; Wang, S.; Wu, B.; Zhang, B.; Qin, Y.; Huo, C.; Huang, L.; Wen, X.; Yang, Y.; Li, Y. Highly Dispersed Single-Atom Pt and Pt Clusters in the Fe-Modified KL Zeolite with Enhanced Selectivity for n-Heptane Aromatization. ACS Appl. Mater. Interfaces 2019, 11, 29858–29867. [Google Scholar] [CrossRef]
- Yang, Y.; Lyu, Y.; Zhao, L.; Zhan, W.; Fan, L.; Li, F.; Liu, X. Ligand assistance via solid-state coordination for promoting nickel dispersion over the Ni/Beta hydroisomerization catalyst. Fuel 2022, 318, 123568. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Ding, C.; Niu, Y.; Zhang, Y.; Yang, B.; Li, G.; Wang, J.; Ma, Z.; Yu, L.-J. Atomic Dispersion of Pt Clusters Encapsulated Within ZSM-5 Depending on Aluminum Sites and Calcination Temperature. Small Struct. 2023, 4, 2200115. [Google Scholar] [CrossRef]
- Hernando, H.; Hernandez-Gimenez, A.M.; Ochoa-Hernandez, C.; Bruijnincx, P.C.A.; Houben, K.; Baldus, M.; Pizarro, P.; Coronado, J.M.; Fermoso, J.; Cejka, J.; et al. Engineering the acidity and accessibility of the zeolite ZSM-5 for efficient bio-oil upgrading in catalytic pyrolysis of lignocellulose. Green Chem. 2018, 20, 3499–3511. [Google Scholar] [CrossRef]
- Jaya, G.T.; Insyani, R.; Park, J.; Barus, A.F.; Sibi, M.G.; Ranaware, V.; Verma, D.; Kim, J. One-pot conversion of lignocellulosic biomass to ketones and aromatics over a multifunctional Cu-Ru/ZSM-5 catalyst. Appl. Catal., B 2022, 312, 121368. [Google Scholar] [CrossRef]
- Chen, W.; Xu, X.; Zhang, C.; Feng, Y.; Wang, Y.; Wang, J.; Zhang, R. A novel production of monocyclic aromatic hydrocarbons via one-step catalytic conversion of pine sawdust and waste plastics over Pd/trap-HZSM-5. Fuel 2021, 293, 120503. [Google Scholar] [CrossRef]
- Wang, G.X.; Xu, S.D.; Wang, L.; Liu, Z.Q.; Dong, X.; Wang, L.X.; Zheng, A.M.; Meng, X.J.; Xiao, F.S. Fish-in-hole: rationally positioning palladium into traps of zeolite crystals for sinter-resistant catalysts. Chemical Communications 2018, 54, 3274–3277. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, M.; Fang, Y.; Tan, T. Resolving challenges in biomass catalytic pyrolysis by co-optimization of process and catalyst: Removal of heavy fraction in pyrolysis vapours and application of novel zeolite catalyst with high thermal conductivity. Renewable Energy 2020, 156, 951–963. [Google Scholar] [CrossRef]
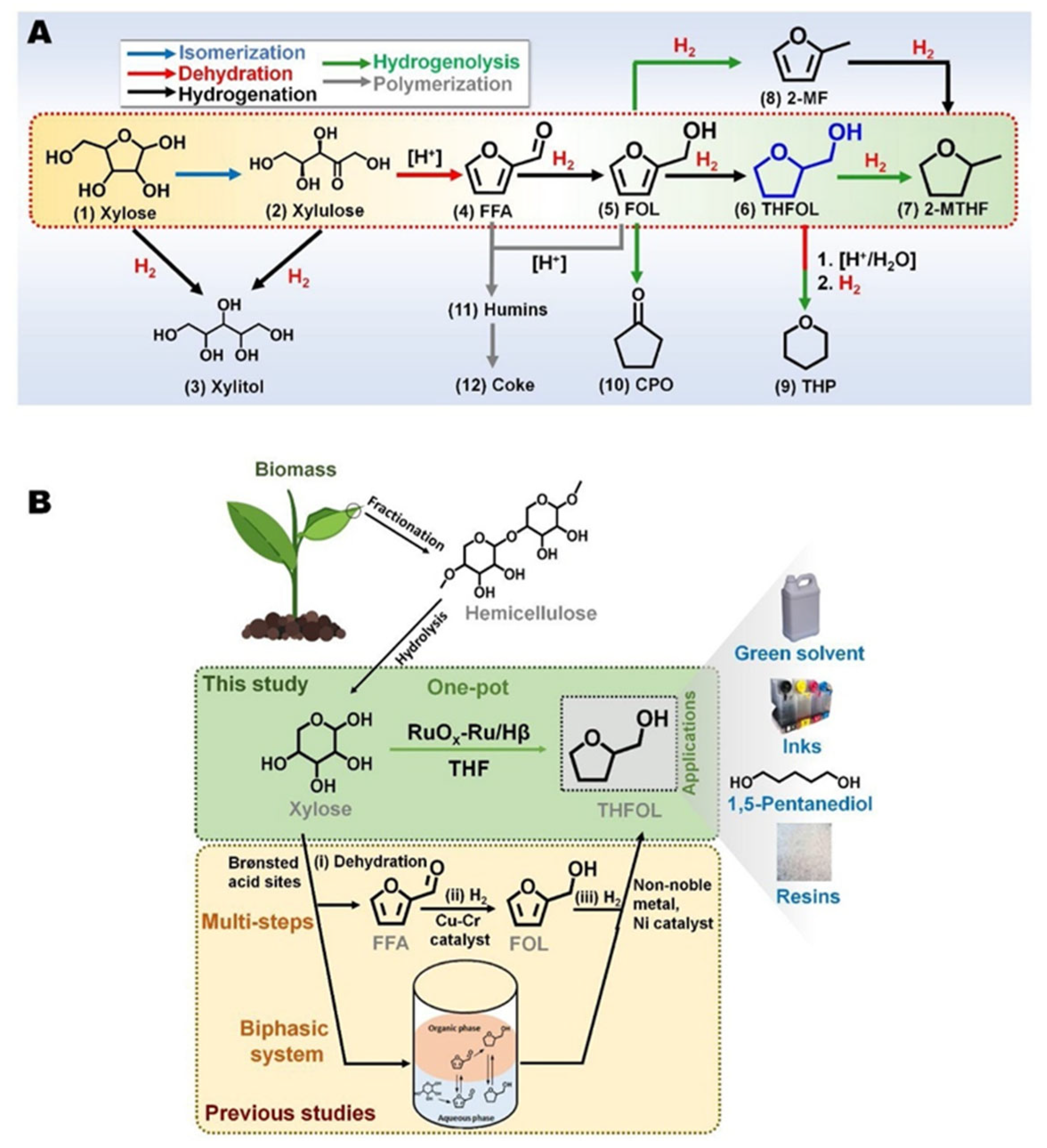
- Insyani, R.; Barus, A.F.; Gunawan, R.; Park, J.; Jaya, G.T.; Cahyadi, H.S.; Sibi, M.G.; Kwak, S.K.; Verma, D.; Kim, J. RuO2-Ru/Hβ zeolite catalyst for high-yield direct conversion of xylose to tetrahydrofurfuryl alcohol. Appl. Catal., B 2021, 291, 120120. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Guo, L.; Chen, Z.; Li, C. Photothermally promoted cleavage of β-1,4-glycosidic bonds of cellulosic biomass on Ir/HY catalyst under mild conditions. Appl. Catal., B 2018, 237, 660–664. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Zhang, J.; Guo, Q.; Unocic, K.A.; Tao, L.; Li, Z. A hybrid pathway to biojet fuel via 2,3-butanediol. Sustainable Energy Fuels 2020, 4, 3904–3914. [Google Scholar] [CrossRef]
- Gogoi, P.; Nagpure, A.S.; Kandasamy, P.; Satyanarayana, C.V.V.; Raja, T. Insights into the catalytic activity of Ru/NaY catalysts for efficient H2 production through aqueous phase reforming. Sustainable Energy Fuels 2020, 4, 678–690. [Google Scholar] [CrossRef]
- Gutierrez-Rubio, S.; Moreno, I.; Serrano, D.P.; Coronado, J.M. Hydrotreating of Guaiacol and Acetic Acid Blends over Ni2P/ZSM-5 Catalysts: Elucidating Molecular Interactions during Bio-Oil Upgrading. ACS Omega 2019, 4, 21516–21528. [Google Scholar] [CrossRef]
- Chai, Y.; Liu, S.; Zhao, Z.-J.; Gong, J.; Dai, W.; Wu, G.; Guan, N.; Li, L. Selectivity Modulation of Encapsulated Palladium Nanoparticles by Zeolite Microenvironment for Biomass Catalytic Upgrading. ACS Catal. 2018, 8, 8578–8589. [Google Scholar] [CrossRef]
- Du, F.; You, Z.; Meng, K.; Qu, X.; Zhang, D.; Zhang, W.; Liu, M.; Shen, Y.; Deng, W.; Jin, X. Dealuminization for a Modified (Si-OH)n-Pt Interface: Self-Activation of Pt/NaY Catalysts for Oxidation of Ethylene Glycol in a Base-Free Medium. ACS Sustainable Chem. Eng. 2021, 9, 14416–14429. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




