Submitted:
18 May 2023
Posted:
18 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. RNA sequencing data collection
2.2. Network Pathway Analysis
2.3. Cell culture of gastric cancer cells
2.4. Characterization of EMT markers in scirrhous gastric cancer cells
2.5. Statistic Analysis
3. Results
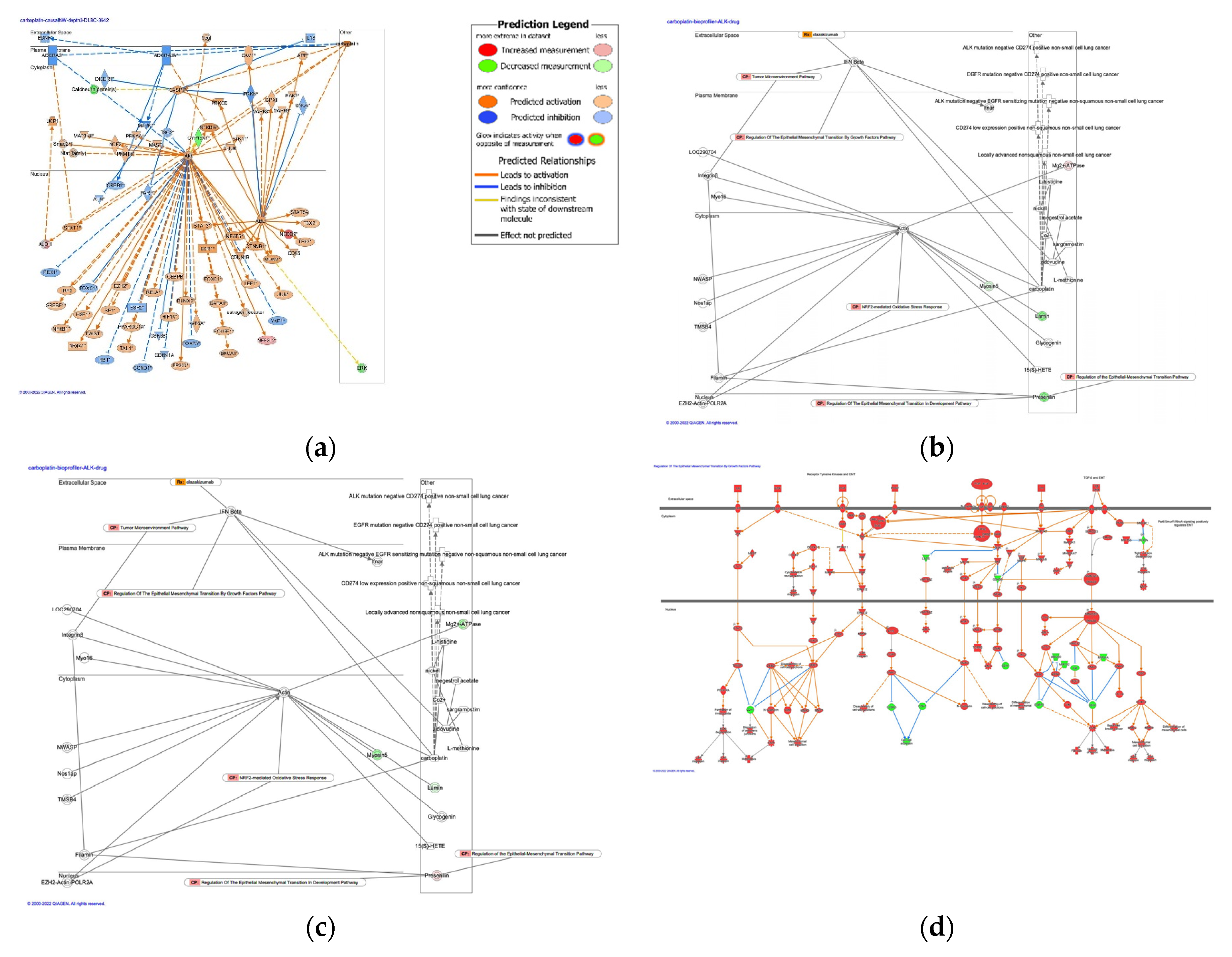
3.1. Causal networks of carboplatin activity plot in cancer treatment
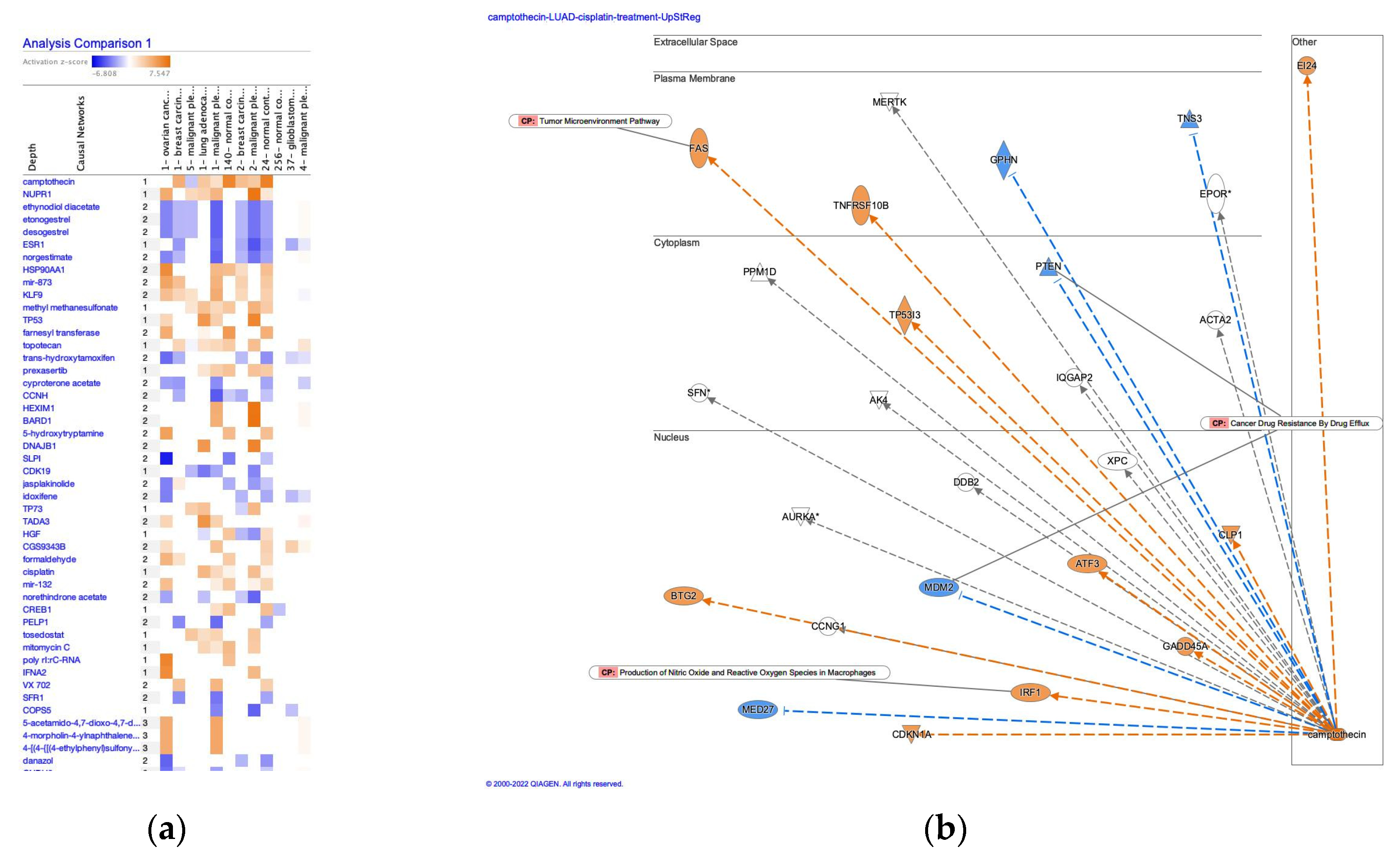
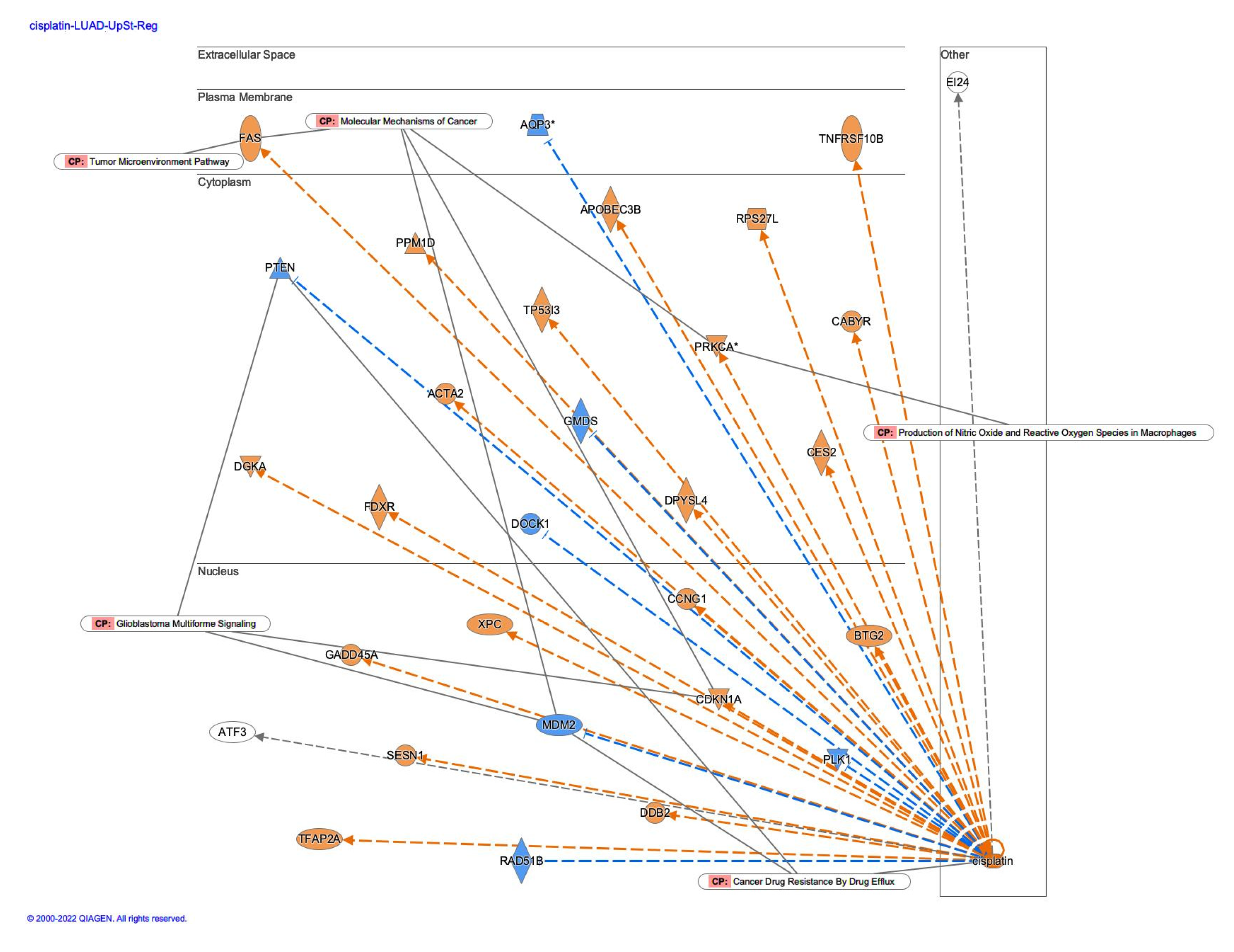
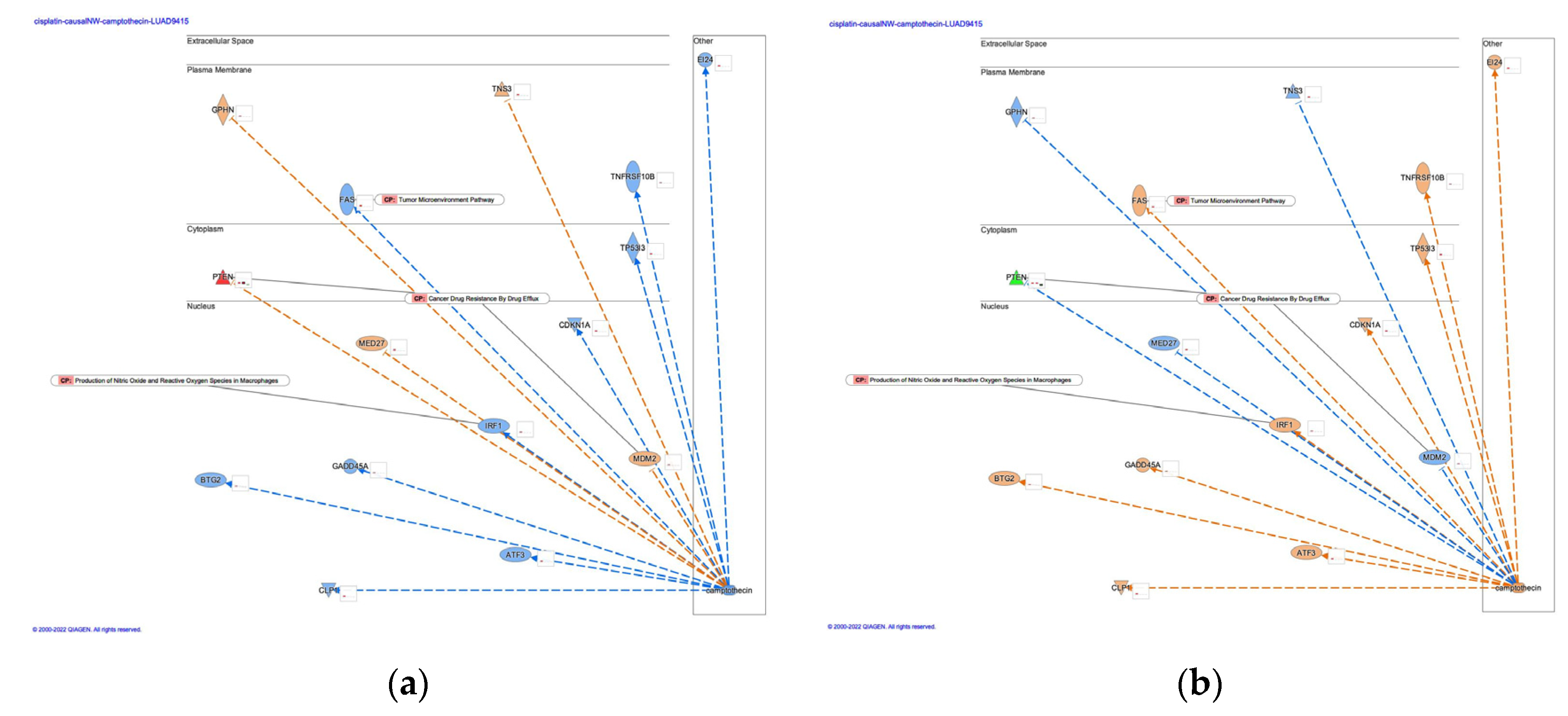
3.2. Causal networks of cisplatin-treated lung adenocarcinoma
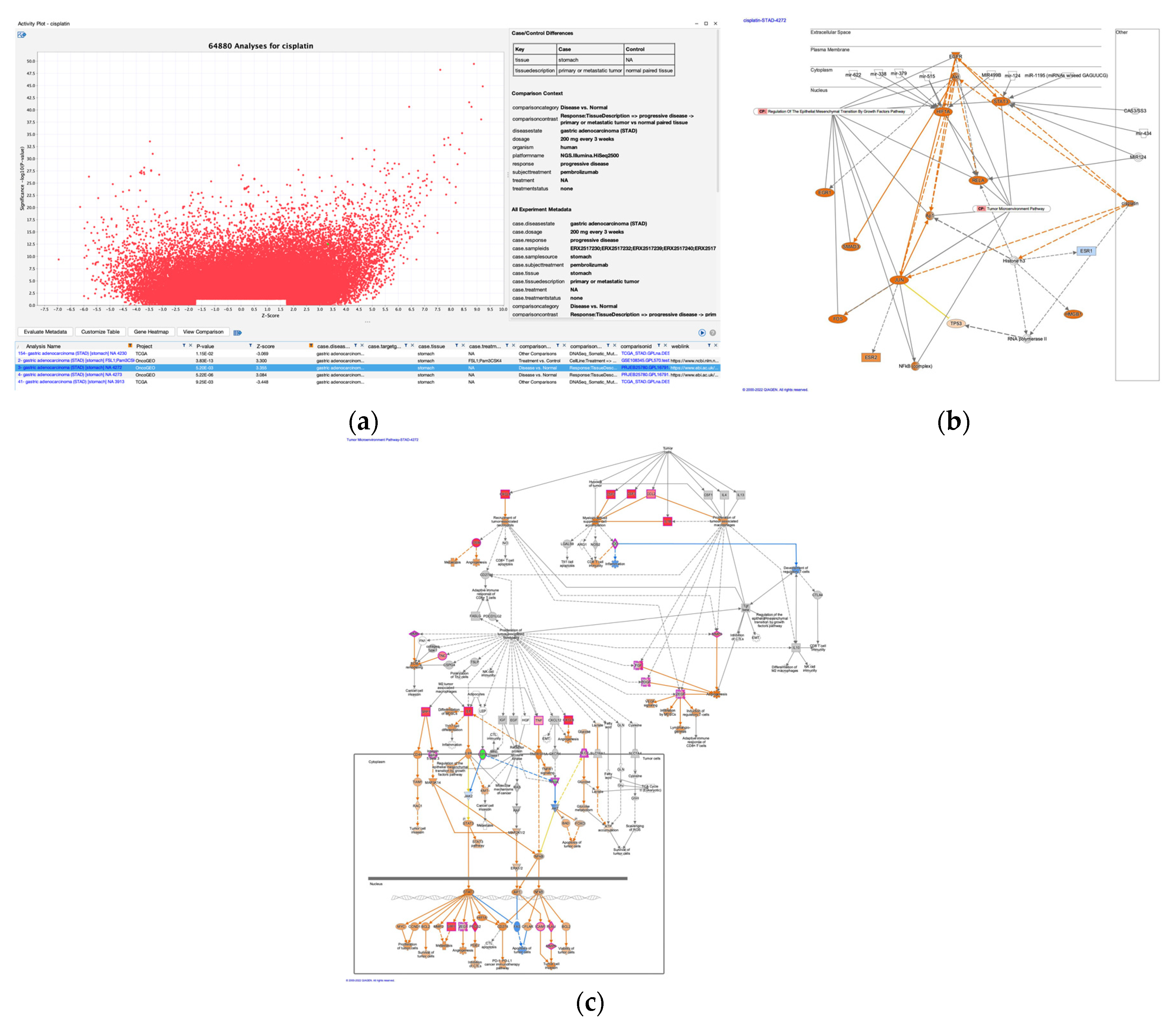
3.3. Cisplatin as an upstream regulator of gastric adenocarcinoma
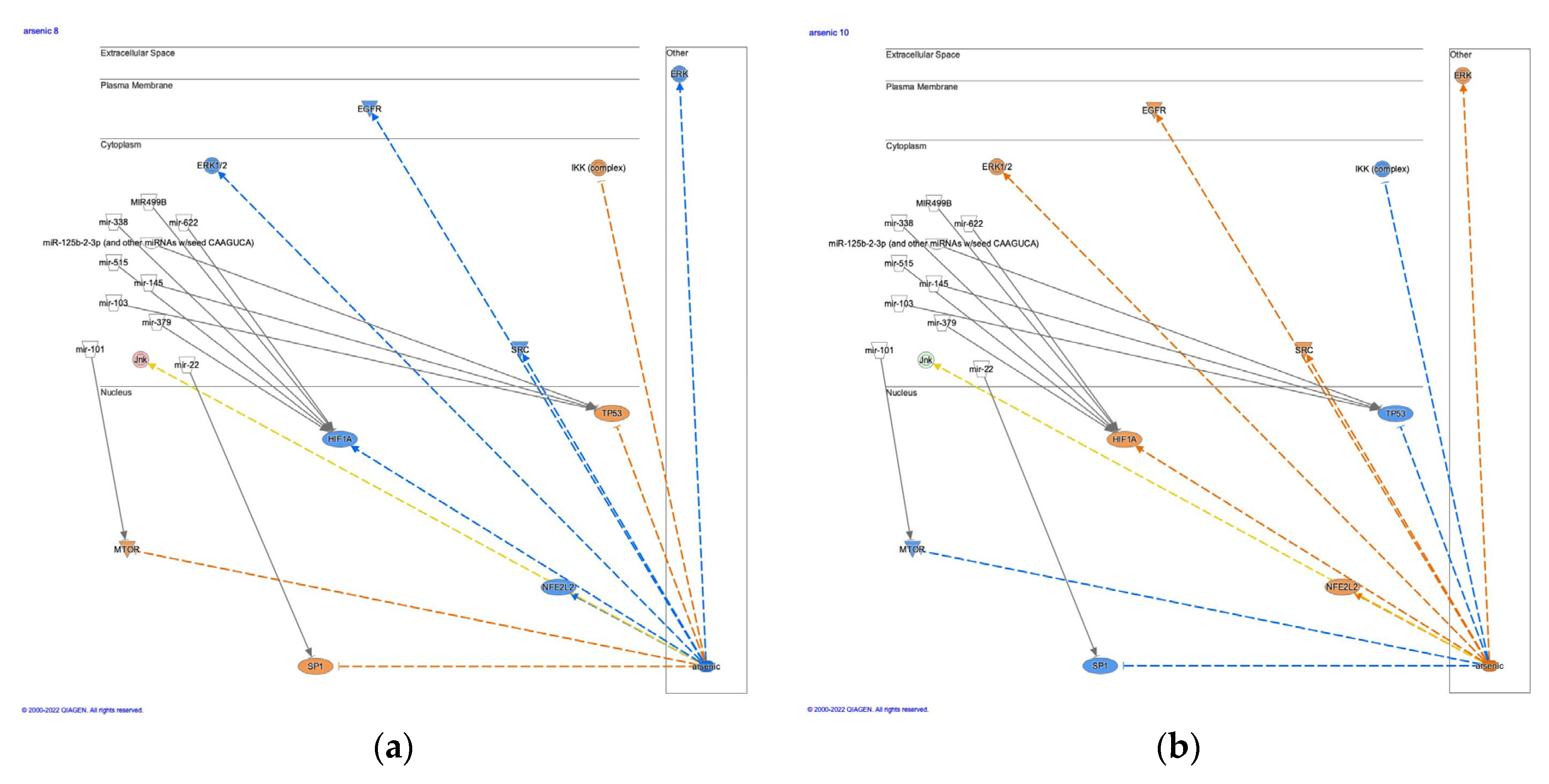
3.4. Arsenic treatment in the EMT by growth factors pathway
3.4.1. Regulatory networks in arsenic-treated liver carcinoma
3.4.2. Causal networks of arsenic and direct interaction with microRNAs
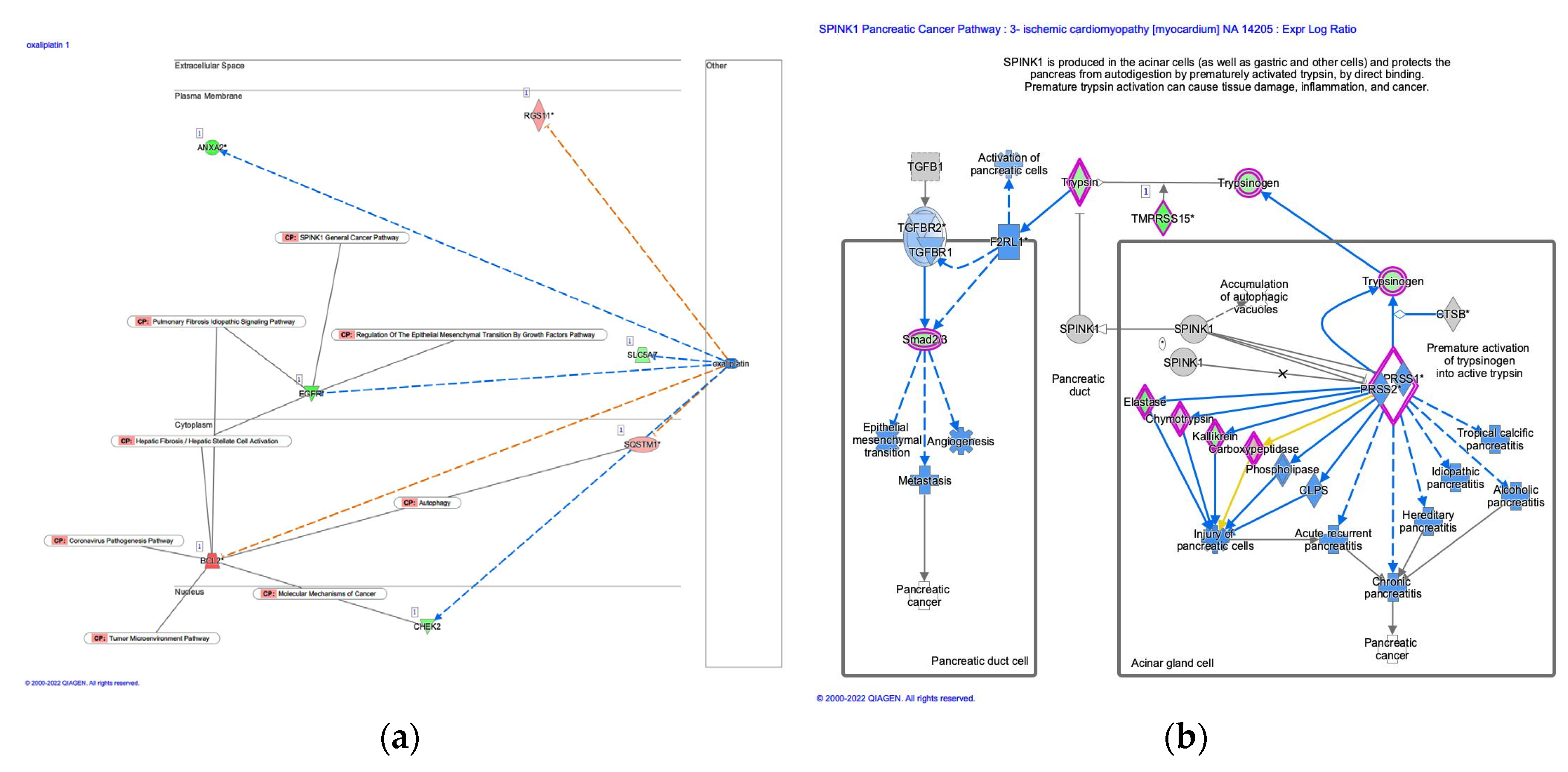
3.5. Analysis of oxaliplatin and SPINK1 pancreatic cancer pathway
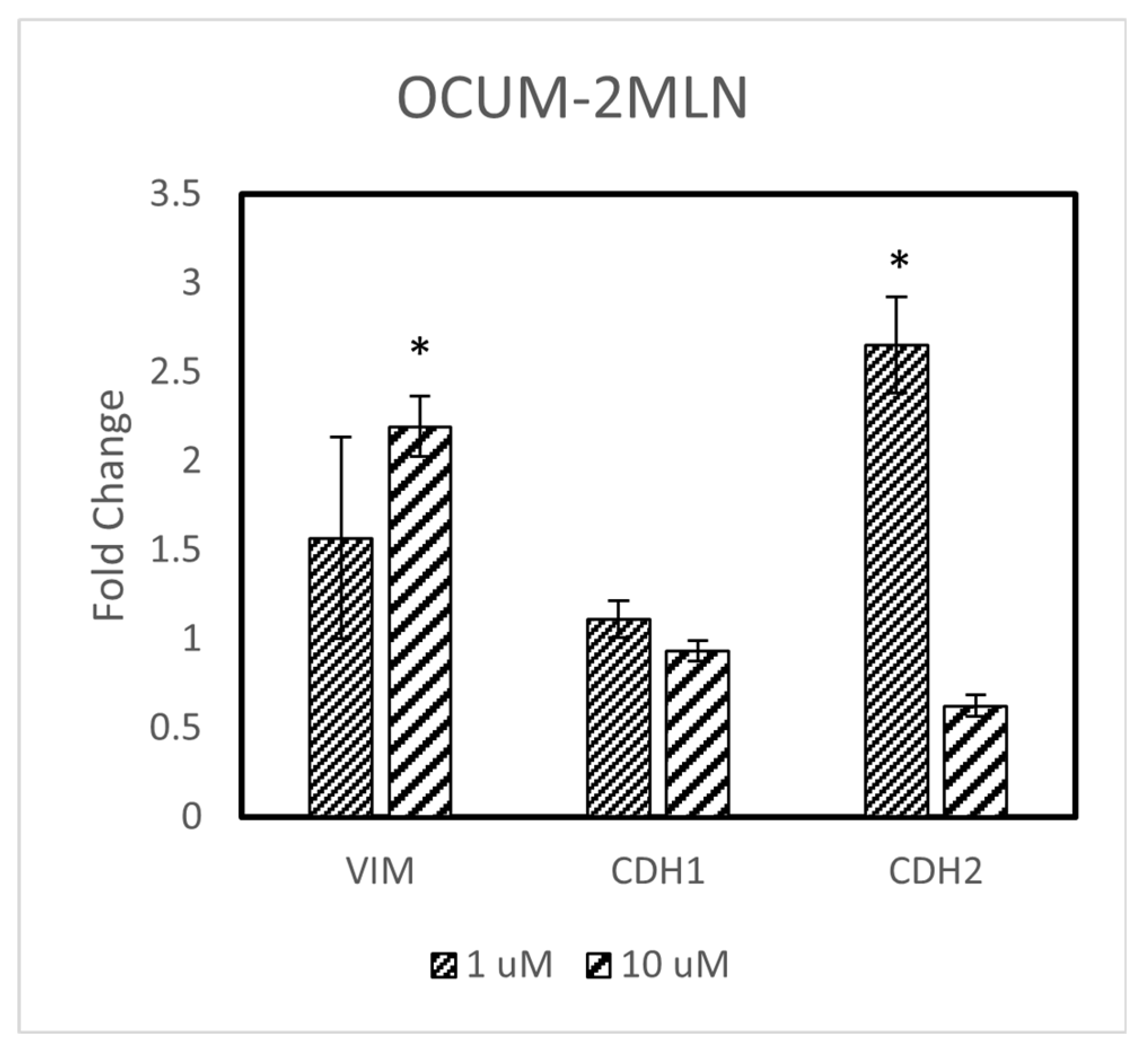
3.6. Cisplatin treatment increased vimentin expression in scirrhous gastric cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roman, D.A.; Pizarro, I.; Rivera, L.; Avila, J.; Cortes, P. Magnesium, zinc, arsenic, selenium and platinum urinary excretion from cancer patients of Antofagasta region, Chile: Multi-metal approach. JRSM Open 2016, 7, 2054270416660932. [CrossRef]
- Zhang, Y.; Pan, D.; Yang, H.; Huang, J.; He, Z.; Li, H.; Li, D. Effects of arsenic trioxide combined with platinum drugs in treatment of cervical cancer: A protocol for systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2020, 99, e22950. [CrossRef]
- Chen, H.; Pazicni, S.; Krett, N.L.; Ahn, R.W.; Penner-Hahn, J.E.; Rosen, S.T.; O'Halloran, T.V. Coencapsulation of arsenic- and platinum-based drugs for targeted cancer treatment. Angew Chem Int Ed Engl 2009, 48, 9295-9299. [CrossRef]
- He, Z.Y.; Li, H.Y.; Yan, J.; Li, S.J.; Li, D.C.; Liang, Z.Z. A prospective trial to evaluate the clinical efficacy and safety of neoadjuvant chemotherapy with arsenic trioxide and carboplatin in locally advanced cervical cancer: A study protocol for randomized controlled clinical. Trials 2022, 23, 556. [CrossRef]
- Desoize, B. Metals and metal compounds in cancer treatment. Anticancer Res 2004, 24, 1529-1544.
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol 2014, 740, 364-378. [CrossRef]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol 2019, 53, 148-158. [CrossRef]
- Hoang, D.H.; Buettner, R.; Valerio, M.; Ghoda, L.; Zhang, B.; Kuo, Y.H.; Rosen, S.T.; Burnett, J.; Marcucci, G.; Pullarkat, V.; et al. Arsenic Trioxide and Venetoclax Synergize against AML Progenitors by ROS Induction and Inhibition of Nrf2 Activation. Int J Mol Sci 2022, 23. [CrossRef]
- Cingam, S.R.; Koshy, N.V. Acute Promyelocytic Leukemia. In StatPearls, StatPearls Publishing, Copyright © 2022, StatPearls Publishing LLC.: Treasure Island (FL), 2022.
- Jang, T.H.; Huang, W.C.; Tung, S.L.; Lin, S.C.; Chen, P.M.; Cho, C.Y.; Yang, Y.Y.; Yen, T.C.; Lo, G.H.; Chuang, S.E.; et al. MicroRNA-485-5p targets keratin 17 to regulate oral cancer stemness and chemoresistance via the integrin/FAK/Src/ERK/beta-catenin pathway. J Biomed Sci 2022, 29, 42. [CrossRef]
- Lin, X.; Wang, F.; Chen, J.; Liu, J.; Lin, Y.B.; Li, L.; Chen, C.B.; Xu, Q. N(6)-methyladenosine modification of CENPK mRNA by ZC3H13 promotes cervical cancer stemness and chemoresistance. Mil Med Res 2022, 9, 19. [CrossRef]
- Zang, H.; Peng, J.; Wang, W.; Fan, S. Roles of microRNAs in the resistance to platinum based chemotherapy in the non-small cell lung cancer. J Cancer 2017, 8, 3856-3861. [CrossRef]
- Zhang, S.; Deen, S.; Storr, S.J.; Chondrou, P.S.; Nicholls, H.; Yao, A.; Rungsakaolert, P.; Martin, S.G. Calpain system protein expression and activity in ovarian cancer. J Cancer Res Clin Oncol 2019, 145, 345-361. [CrossRef]
- Du, B.; Shim, J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21. [CrossRef]
- Nilsson, M.B.; Sun, H.; Robichaux, J.; Pfeifer, M.; McDermott, U.; Travers, J.; Diao, L.; Xi, Y.; Tong, P.; Shen, L.; et al. A YAP/FOXM1 axis mediates EMT-associated EGFR inhibitor resistance and increased expression of spindle assembly checkpoint components. Sci Transl Med 2020, 12. [CrossRef]
- Jin, H.; He, Y.; Zhao, P.; Hu, Y.; Tao, J.; Chen, J.; Huang, Y. Targeting lipid metabolism to overcome EMT-associated drug resistance via integrin beta3/FAK pathway and tumor-associated macrophage repolarization using legumain-activatable delivery. Theranostics 2019, 9, 265-278. [CrossRef]
- Gaianigo, N.; Melisi, D.; Carbone, C. EMT and Treatment Resistance in Pancreatic Cancer. Cancers (Basel) 2017, 9. [CrossRef]
- Liu, X.; He, M.; Li, L.; Wang, X.; Han, S.; Zhao, J.; Dong, Y.; Ahmad, M.; Li, L.; Zhang, X.; et al. EMT and Cancer Cell Stemness Associated With Chemotherapeutic Resistance in Esophageal Cancer. Front Oncol 2021, 11, 672222. [CrossRef]
- Han, M.L.; Zhao, Y.F.; Tan, C.H.; Xiong, Y.J.; Wang, W.J.; Wu, F.; Fei, Y.; Wang, L.; Liang, Z.Q. Cathepsin L upregulation-induced EMT phenotype is associated with the acquisition of cisplatin or paclitaxel resistance in A549 cells. Acta Pharmacol Sin 2016, 37, 1606-1622. [CrossRef]
- Liang, F.; Ren, C.; Wang, J.; Wang, S.; Yang, L.; Han, X.; Chen, Y.; Tong, G.; Yang, G. The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis 2019, 8, 59. [CrossRef]
- Luo, J.; Yao, J.F.; Deng, X.F.; Zheng, X.D.; Jia, M.; Wang, Y.Q.; Huang, Y.; Zhu, J.H. 14, 15-EET induces breast cancer cell EMT and cisplatin resistance by up-regulating integrin alphavbeta3 and activating FAK/PI3K/AKT signaling. J Exp Clin Cancer Res 2018, 37, 23. [CrossRef]
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T.; Laird, P.W.; Curtis, C.; Shen, H.; et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202-209. [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discovery 2012, 2, 401-404. [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Science Signaling 2013, 6, pl1-pl1. [CrossRef]
- Grossman, R.L.; Heath, A.P.; Ferretti, V.; Varmus, H.E.; Lowy, D.R.; Kibbe, W.A.; Staudt, L.M. Toward a Shared Vision for Cancer Genomic Data. New England Journal of Medicine 2016, 375, 1109-1112. [CrossRef]
- Tanabe, S.; Quader, S.; Ono, R.; Cabral, H.; Aoyagi, K.; Hirose, A.; Yokozaki, H.; Sasaki, H. Molecular Network Profiling in Intestinal- and Diffuse-Type Gastric Cancer. Cancers 2020, 12, 3833. [CrossRef]
- Tanabe, S.; Quader, S.; Ono, R.; Cabral, H.; Aoyagi, K.; Hirose, A.; Yokozaki, H.; Sasaki, H. Cell Cycle Regulation and DNA Damage Response Networks in Diffuse- and Intestinal-Type Gastric Cancer. Cancers 2021, 13, 5786. [CrossRef]
- Krämer, A.; Green, J.; Pollard, J., Jr; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2013, 30, 523-530. [CrossRef]
- Qi, W.; Zhao, K.; Gu, J.; Huang, Y.; Wang, Y.; Zhang, H.; Zhang, M.; Zhang, J.; Yu, Z.; Li, L.; et al. An allosteric PRC2 inhibitor targeting the H3K27me3 binding pocket of EED. Nat Chem Biol 2017, 13, 381-388. [CrossRef]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018, 24, 1449-1458. [CrossRef]
- Kawata, K.; Yokoo, H.; Shimazaki, R.; Okabe, S. Classification of heavy-metal toxicity by human DNA microarray analysis. Environ Sci Technol 2007, 41, 3769-3774. [CrossRef]
- Høye, E.; Fromm, B.; Böttger, P.H.M.; Domanska, D.; Torgunrud, A.; Lund-Andersen, C.; Abrahamsen, T.W.; Fretland Å, A.; Dagenborg, V.J.; Lorenz, S.; et al. A comprehensive framework for analysis of microRNA sequencing data in metastatic colorectal cancer. NAR Cancer 2022, 4, zcab051. [CrossRef]
- Biersack, B. Relations between approved platinum drugs and non-coding RNAs in mesothelioma. Noncoding RNA Res 2018, 3, 161-173. [CrossRef]
- Pan, G.; Liu, Y.; Shang, L.; Zhou, F.; Yang, S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun (Lond) 2021, 41, 199-217. [CrossRef]
- Petri, B.J.; Klinge, C.M. Regulation of breast cancer metastasis signaling by miRNAs. Cancer Metastasis Rev 2020, 39, 837-886. [CrossRef]
- Khan, A.Q.; Ahmed, E.I.; Elareer, N.R.; Junejo, K.; Steinhoff, M.; Uddin, S. Role of miRNA-Regulated Cancer Stem Cells in the Pathogenesis of Human Malignancies. Cells 2019, 8. [CrossRef]
- Wong, C.H.; Lou, U.K.; Fung, F.K.; Tong, J.H.M.; Zhang, C.H.; To, K.F.; Chan, S.L.; Chen, Y. CircRTN4 promotes pancreatic cancer progression through a novel CircRNA-miRNA-lncRNA pathway and stabilizing epithelial-mesenchymal transition protein. Mol Cancer 2022, 21, 10. [CrossRef]
- Wang, R.; Sun, Y.; Yu, W.; Yan, Y.; Qiao, M.; Jiang, R.; Guan, W.; Wang, L. Downregulation of miRNA-214 in cancer-associated fibroblasts contributes to migration and invasion of gastric cancer cells through targeting FGF9 and inducing EMT. J Exp Clin Cancer Res 2019, 38, 20. [CrossRef]
- Takei, S.; Kawazoe, A.; Shitara, K. The New Era of Immunotherapy in Gastric Cancer. Cancers (Basel) 2022, 14. [CrossRef]
- Chen, K.H.; Yuan, C.T.; Tseng, L.H.; Shun, C.T.; Yeh, K.H. Case report: Mismatch repair proficiency and microsatellite stability in gastric cancer may not predict programmed death-1 blockade resistance. J Hematol Oncol 2016, 9, 29. [CrossRef]
- Zhang, P.; Gu, Y.; Fang, H.; Cao, Y.; Wang, J.; Liu, H.; Zhang, H.; Li, H.; He, H.; Li, R.; et al. Intratumoral IL-1R1 expression delineates a distinctive molecular subset with therapeutic resistance in patients with gastric cancer. J Immunother Cancer 2022, 10. [CrossRef]
- Sundar, R.; Huang, K.K.; Qamra, A.; Kim, K.M.; Kim, S.T.; Kang, W.K.; Tan, A.L.K.; Lee, J.; Tan, P. Epigenomic promoter alterations predict for benefit from immune checkpoint inhibition in metastatic gastric cancer. Ann Oncol 2019, 30, 424-430. [CrossRef]
- 4Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017, 127, 2930-2940. [CrossRef]
- Santangelo, A.; Rossato, M.; Lombardi, G.; Benfatto, S.; Lavezzari, D.; De Salvo, G.L.; Indraccolo, S.; Dechecchi, M.C.; Prandini, P.; Gambari, R.; et al. A molecular signature associated with prolonged survival in glioblastoma patients treated with regorafenib. Neuro Oncol 2021, 23, 264-276. [CrossRef]
- Liu, N.; Xia, W.Y.; Liu, S.S.; Chen, H.Y.; Sun, L.; Liu, M.Y.; Li, L.F.; Lu, H.M.; Fu, Y.J.; Wang, P.; et al. MicroRNA-101 targets von Hippel-Lindau tumor suppressor (VHL) to induce HIF1alpha mediated apoptosis and cell cycle arrest in normoxia condition. Sci Rep 2016, 6, 20489. [CrossRef]
| Primer name | Primer sequence |
|---|---|
| CDH1 FWD (forward) | GGGGTAGTGAGGATCTTGAT |
| CDH1 REV (reverse) | TCCTTTTCCACCCCCAAAGA |
| CDH2 FWD (forward) | GGCATAGTCTATGGAGAAGT |
| CDH2 REV (reverse) | GCTGTTGTCAGAAGTCTCTC |
| VIM FWD (forward) | GCTTTCAAGTGCCTTTCTGC |
| VIM REV (reverse) | GTTGGTTGGATACTTGCTGG |
| ACTB FWD (forward) | CCCAAAGTTCACAATGTGG |
| ACTB REV (reverse) | AAGGGACTTCCTGTAACAAC |
| # | Symbol |
|---|---|
| 1 | platinum |
| 2 | Pt2+ |
| 3 | carboplatin |
| 4 | dicycloplatin |
| 5 | enloplatin |
| 6 | eptaplatin |
| 7 | iproplatin |
| 8 | nedaplatin |
| 9 | oxaliplatin |
| 10 | picoplatin |
| 11 | satraplatin |
| 12 | sebriplatin |
| 13 | zeniplatin |
| 14 | platinum agent |
| 15 | platinum chemotherapy regimen |
| 16 | platinum-based doublet chemotherapy |
| 17 | E platinum |
| 18 | platinum(II) chloride |
| 19 | platinum agent/trastuzumab |
| 20 | cisplatin |
| 21 | platinum chemotherapy regimen/radiotherapy |
| 22 | platinum-based doublet chemotherapy/taxane |
| 23 | platinum chemotherapy regimen/vinorelbine |
| 24 | platinum chemotherapy/taxoid derivative |
| 25 | platinum-based doublet chemotherapy/vinorelbine |
| 26 | platinum-based triplet chemotherapy |
| 27 | adjuvant chemotherapy/platinum agent |
| 28 | cetuximab/platinum chemotherapy regimen |
| 29 | gemcitabine/platinum chemotherapy |
| 30 | paclitaxel/platinum chemotherapy regimen |
| 31 | platinum-based neoadjuvant chemotherapy |
| 32 | bevacizumab/platinum chemotherapy/taxoid derivative |
| 33 | platinum-norsperimidine complex Pt3NSpd2 |
| 34 | capecitabine/platinum chemotherapy regimen |
| 35 | bevacizumab/gemcitabine/platinum chemotherapy regimen |
| 36 | platinum chemotherapy regimen/thoracic radiotherapy |
| 37 | nonplatinum-based doublet chemotherapy |
| 38 | platinum acetylacetonate-titanium dioxide nanoparticles |
| 39 | BP-Cx1-platinum complex BP-C1 |
| 40 | cetuximab/5-fluorouracil/platinum chemotherapy |
| 41 | etoposide/platinum chemotherapy regimen |
| 42 | ormaplatin |
| 43 | Bamet-UD2 |
| 44 | NC-4016 |
| 45 | fluoropyrimidine/platinum-based triplet chemotherapy |
| 46 | (diaminocyclohexane)(diacetato)(dichloro)platinum |
| 47 | lobaplatin |
| 48 | non-pemetrexed containing platinum chemotherapy regimen |
| 49 | triplatin tetranitrate |
| 50 | dacplatinum |
| 51 | cisplatin/etoposide |
| 52 | cisplatin/docetaxel |
| 53 | cisplatin/paclitaxel |
| 54 | carboplatin/paclitaxel |
| 55 | cisplatin/epirubicin/5-fluorouracil |
| Location | Symbol |
|---|---|
| Cytoplasm | AKT |
| CDC42 | |
| DOCK10 | |
| ERK1/2 | |
| FRS2-GRB2-SHP2 | |
| GAB1 | |
| GRB2 | |
| Grb2-Shc1-Sos | |
| Ikk | |
| JAK | |
| Jnk | |
| MAP2K4/7 | |
| MAP3K1 | |
| MAP3K7 | |
| MAPK1 | |
| MEK | |
| MEST | |
| MIR155 | |
| MIR192 | |
| MIR34A | |
| MKK3/6 | |
| p38MAPK | |
| PI3K | |
| PTPN11 | |
| RAF | |
| Ras | |
| RHOA | |
| SHC1 | |
| SMAD2/3 | |
| SMURF1 | |
| SOS | |
| TAB1 | |
| VIM | |
| Extracellular Space | EGF |
| FGF | |
| FGF dimer | |
| HGF | |
| IL6 | |
| MMP1 | |
| MMP2 | |
| MMP9 | |
| PDGF | |
| Tgfbeta | |
| TGFB2 | |
| Nucleus | AP1 |
| EGR1 | |
| ESRP2 | |
| ETS1 | |
| FOS | |
| FOXC2 | |
| FOXO1 | |
| GSC | |
| GSK3B | |
| HMGA2 | |
| HSF1 | |
| ID2 | |
| MTOR | |
| NFkB | |
| SMAD2/3/4 | |
| SMAD4 | |
| SNAI1 | |
| SNAI2 | |
| STAT3 | |
| TCF3 | |
| TWIST1 | |
| ZEB1 | |
| ZEB2 | |
| Plasma Membrane | CDH1 |
| CDH2 | |
| CLDN3 | |
| EGFR | |
| ERBB2 | |
| FGFR | |
| FRS2 | |
| IL6R | |
| MET | |
| OCLN | |
| Pdgfr | |
| PDGFRA | |
| RAC1 | |
| TGFBR1 | |
| TGFBR2 | |
| Tnfreceptor | |
| Other | LATS |
| MIR200 | |
| N-Cadherin | |
| PAR6 | |
| Tnf | |
| YAP/TAZ |
| Analysis Name | Comparison contrast | Upregulated log2 cutoff | Project name | Organism |
|---|---|---|---|---|
| 1- ovarian cancer [ovary] cisplatin 1667 | ExperimentGroup => cisplatin 1 day recovery 2 weeks vs monolayer culture | 0.6374 | GSE144232 | human |
| 1- breast carcinoma [breast] cisplatin 7438 | SamplingTime => 10 to 11 hours after treatment vs NA | 0.344 | GSE28274 | human |
| 5- malignant pleural mesothelioma [mesothelium] cisplatin;piroxicam 6825 | Treatment:TreatTime[hours] => 8 -> cisplatin;piroxicam vs none | 0.1488 | GSE22445 | human |
| 1- lung adenocarcinoma (LUAD) [lung] cisplatin 9800 | Treatment => cisplatin vs none | 0.1224 | GSE6410 | human |
| 1- malignant pleural mesothelioma [mesothelium] cisplatin 6821 | Treatment:TreatTime[hours] => 24 -> cisplatin vs none | 0.1633 | GSE22445 | human |
| 140- normal control [liver] cisplatin 2334 | TreatTime[days]:Treatment => 1 -> cisplatin vs DMSO | 1.1422 | GSE57805 | rat |
| 2- breast carcinoma [breast] cisplatin 7439 | SamplingTime => 8 to 9 hours after treatment vs NA | 0.0815 | GSE28274 | human |
| 2- malignant pleural mesothelioma [mesothelium] cisplatin;piroxicam 6822 | Treatment:TreatTime[hours] => 24 -> cisplatin;piroxicam vs none | 0.539 | GSE22445 | human |
| 24- normal control [liver] cisplatin 2200 | TreatTime[days]:Treatment => 0.67 -> cisplatin vs DMSO | 1.0049 | GSE57805 | rat |
| 256- normal control [liver] cisplatin 2324 | Treatment:TreatTime[days] => cisplatin -> 1 vs 0.67 | 0.2943 | GSE57805 | rat |
| 37- glioblastoma (GBM) [brain] cisplatin 4128 | Treatment => cisplatin vs DMSO | 0.4672 | GSE97460 | human |
| 4- malignant pleural mesothelioma [mesothelium] cisplatin 6824 | Treatment:TreatTime[hours] => 8 -> cisplatin vs none | 0.1641 | GSE22445 | human |
| Symbol | Family |
|---|---|
| CAS3/SS3 | biologic drug |
| miR-1195 (miRNAs w/seed GAGUUCG) | mature microRNA |
| mir-124 | microRNA |
| mir-338 | microRNA |
| mir-379 | microRNA |
| mir-434 | microRNA |
| mir-515 | microRNA |
| mir-622 | microRNA |
| MIR124 | group |
| MIR499B | microRNA |
| ID | Diseases & Functions | Known Regulator-Disease/Function Relationship |
|---|---|---|
| 1 | Cell death of carcinoma cell lines,Cell proliferation of adenocarcinoma cell lines | 11% (2/18) |
| 2 | Death of embryo,Homologous recombination of DNA | 0% (0/18) |
| 3 | Cell cycle progression,Cell proliferation of tumor cell lines | 65% (13/20) |
| 4 | Cell death of breast cancer cell lines,Cell survival | 0% (0/6) |
| 5 | DNA recombination,Formation of gamma H2AX nuclear focus,Incidence of lymphoma | 0% (0/15) |
| 6 | Chromosomal aberration,DNA damage,Homologous recombination of DNA,T-cell non-Hodgkin lymphoma | 13% (1/8) |
| 7 | Formation of gamma H2AX nuclear focus | 13% (1/8) |
| 8 | Chromosomal aberration,DNA damage,T-cell non-Hodgkin lymphoma | 11% (1/9) |
| 9 | Death of embryo | 0% (0/5) |
| 10 | Chromosomal aberration,DNA damage | 13% (1/8) |
| microRNAs |
|---|
| mir-101 |
| mir-103 |
| miR-125b-2-3p (and other miRNAs w/seed CAAGUCA) |
| mir-145 |
| mir-22 |
| mir-338 |
| mir-379 |
| mir-515 |
| mir-622 |
| MIR499B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





