Submitted:
18 May 2023
Posted:
18 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Section
2.1. Materials and Methods
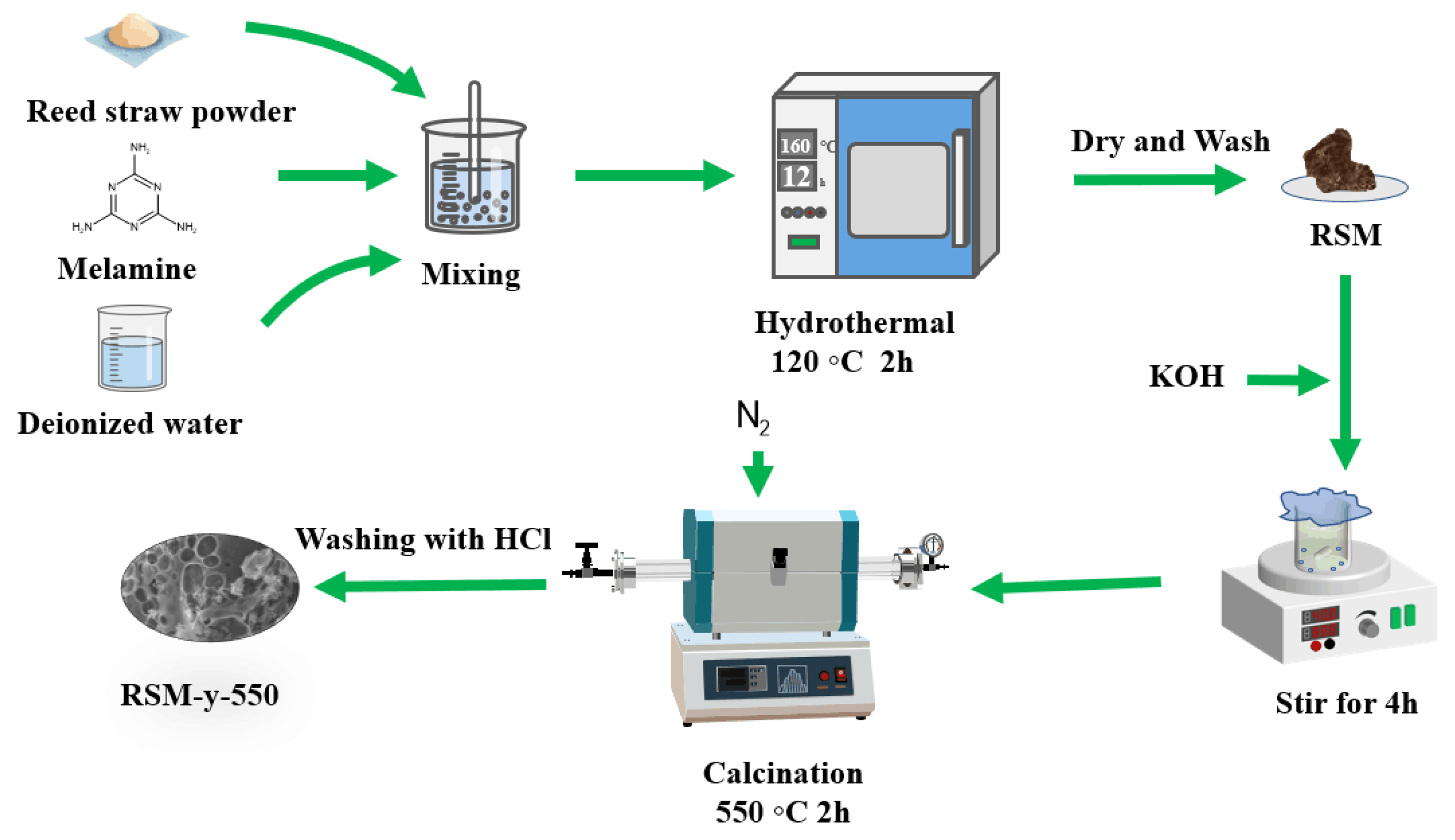
2.2. Preparation of RSM-y-550
2.3. Characterization
2.4. Electrochemical Measurement
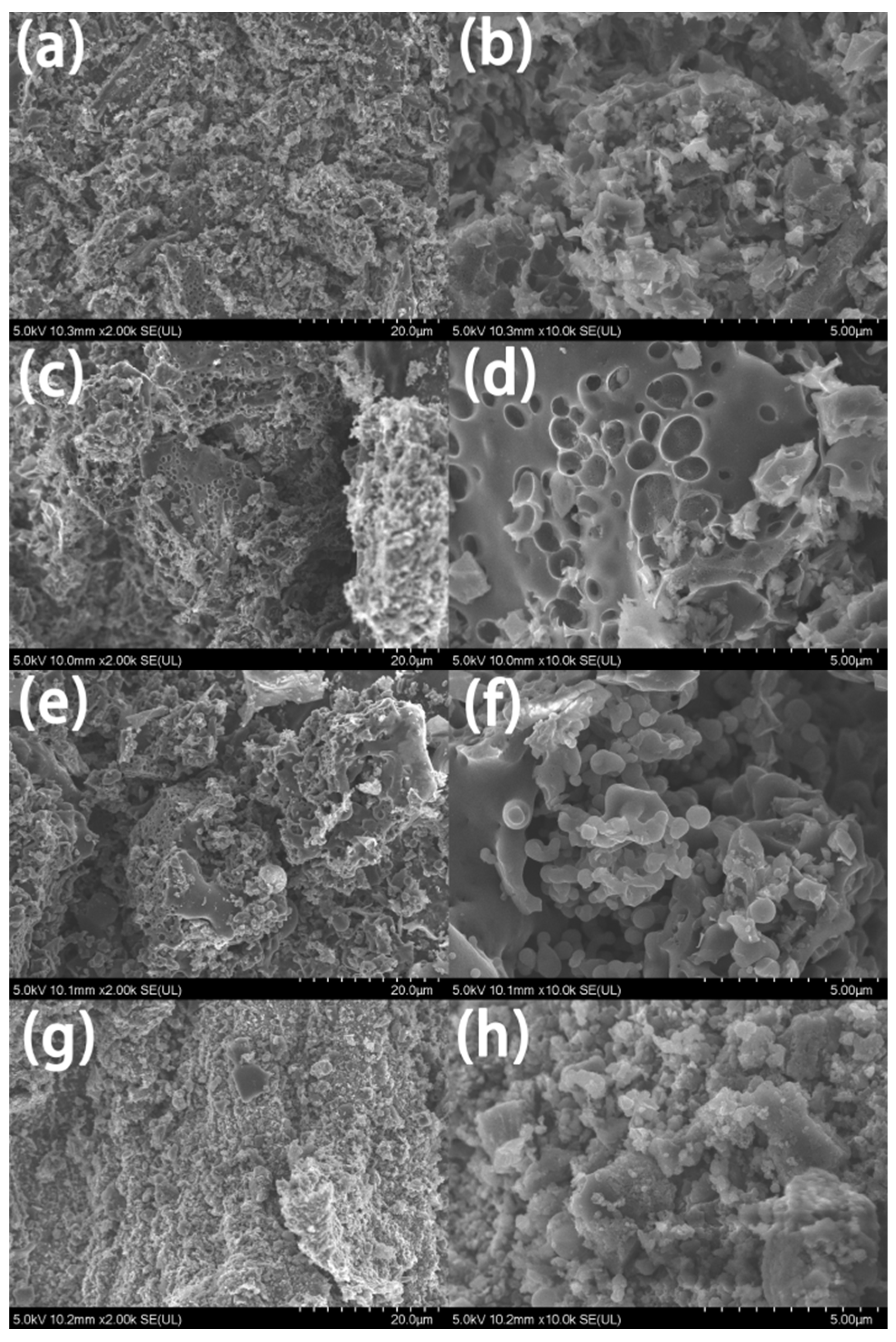
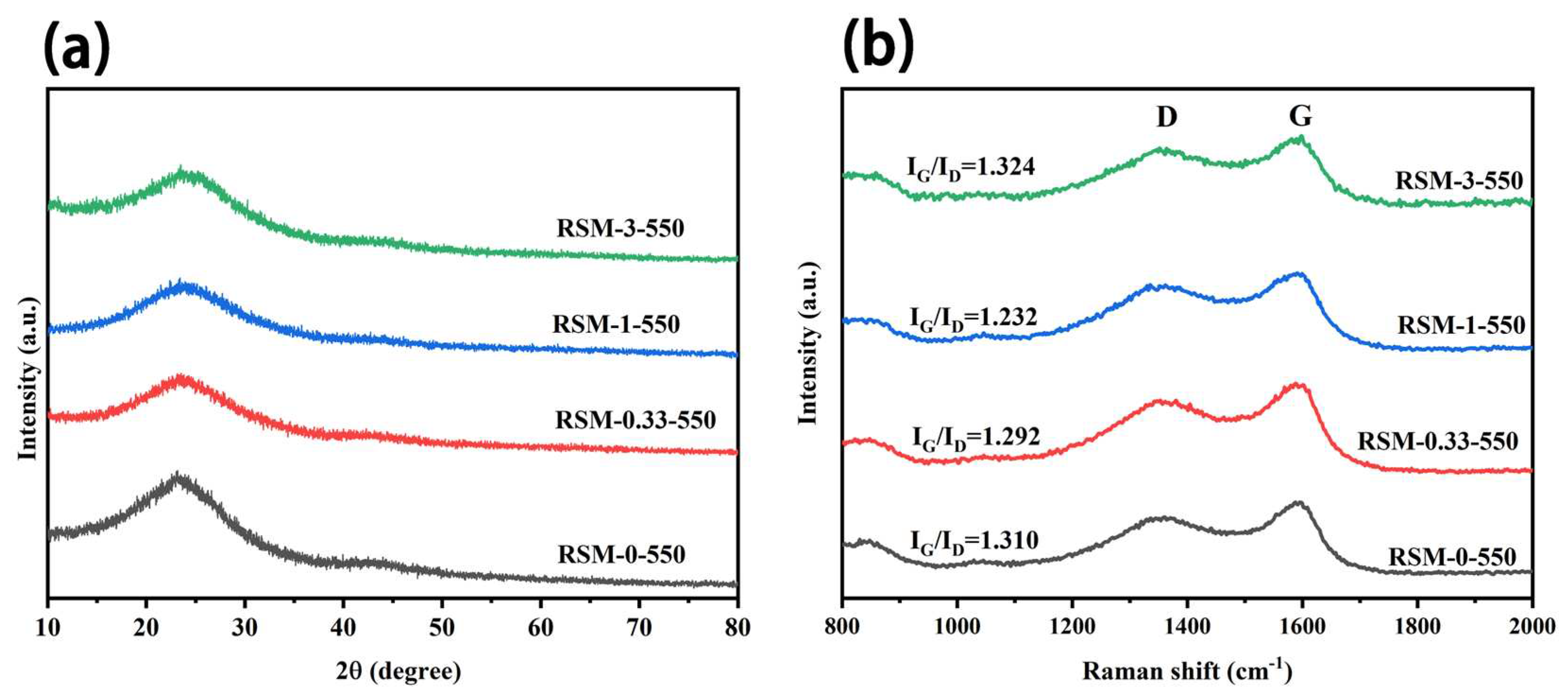
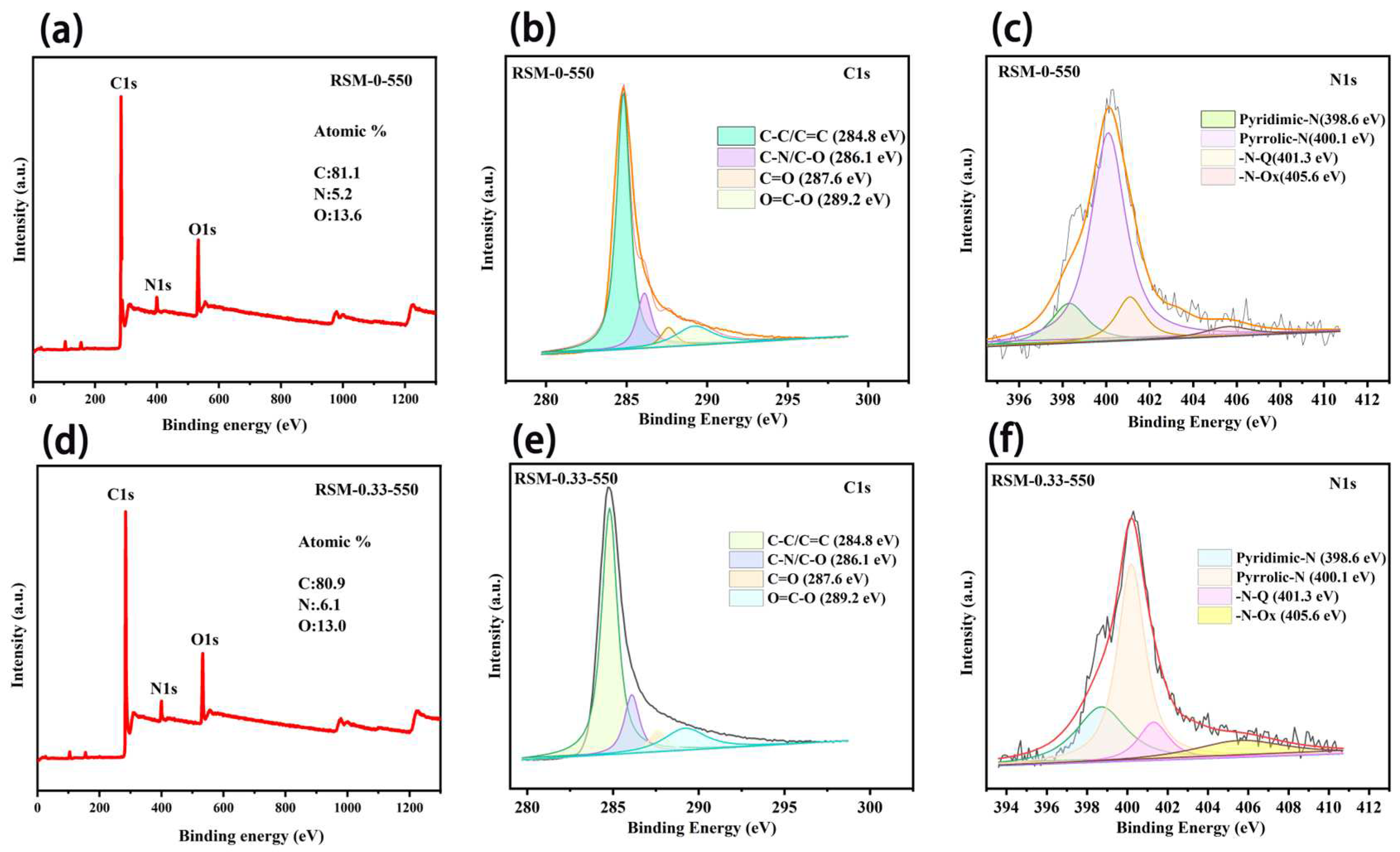
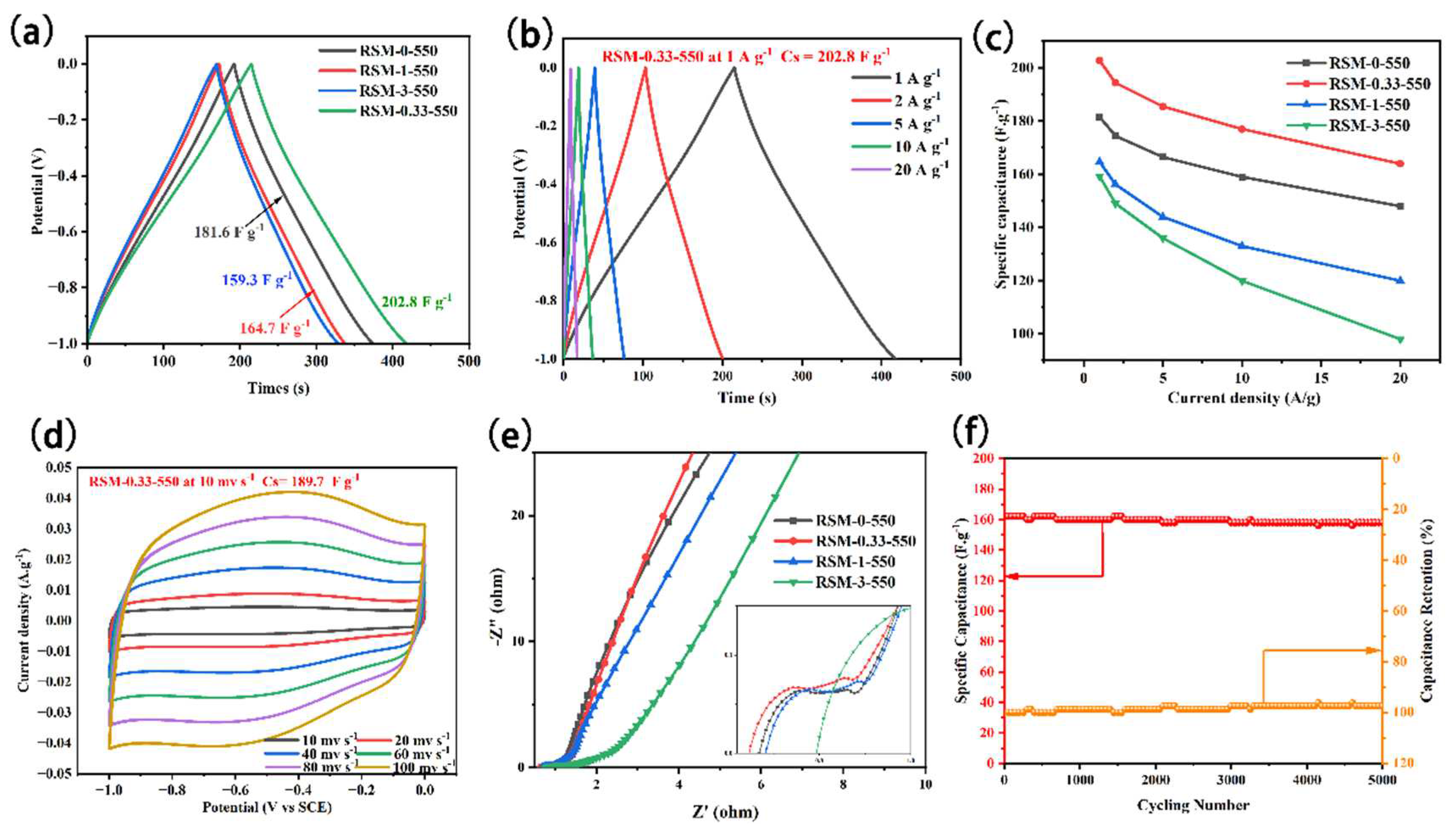
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, C.; Wu, Z.; Cui, G.; Xie, F.; Guo, X.; Sun, X. FeP nanorod arrays on carbon cloth: a high-performance anode for sodium-ion batteries. Chemical Communications 2018, 54, 9341–9344. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renewable and Sustainable Energy Reviews 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nature Materials 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Pohlmann, S. Metrics and methods for moving from research to innovation in energy storage. Nature Communications 2022, 13, 1538. [Google Scholar] [CrossRef]
- Tang, J.; Yuan, H.; Duan, Q.; Liu, Y.; Wang, Y.; Yuan, S. Phosphorus-functionalized low-crystallinity transition-metal oxide nanorod arrays grown on carbon cloth for high-performance asymmetric supercapacitors. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2022, 654, 130189. [Google Scholar] [CrossRef]
- Wu, C.; Pei, Z.; Lv, M.; Huang, D.; Wang, Y.; Yuan, S. Polypyrrole-coated low-crystallinity iron oxide grown on carbon cloth enabling enhanced electrochemical supercapacitor performance. Molecules, 2023, 28, 434. [Google Scholar] [CrossRef]
- Tang, H.; Yao, J.; Zhu, Y. Recent developments and future prospects for zinc-ion hybrid capacitors: a review. Advanced Energy Materials 2021, 11, 2003994. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, L.; Yılmaz, M.; Zhang, T.C.; Wang, Y.; Yuan, S. MgFe2O4-loaded N-doped biochar derived from waste cooked rice for efficient low-temperature desulfurization of H2S. Fuel 2023, 339, 127385. [Google Scholar] [CrossRef]
- Huang, L.; Liu, H.; Wang, Y.; Zhang, T.C.; Yuan, S. Construction of ternary Bi2O3/biochar/g-C3N4 heterojunction to accelerate photoinduced carrier separation for enhanced tetracycline photodegradation. Applied Surface Science 2023, 616, 156509. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Xiao, J.; Tian, X.; Yuan, S. Enhancing electrochemical performance of ultrasmall Fe2O3-embedded carbon nanotubes via combusting-induced high-valence dopants. Journal of Materials Science & Technology 2023, 134, 142–150. [Google Scholar]
- Xiao, J.; Zhang, Y.; Zhang, T.C.; Yuan, S. Prussian blue-impregnated waste pomelo peels-derived biochar for enhanced adsorption of NH3. Journal of Cleaner Production 2023, 382, 135393. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, L.; Zhang, T.C.; Ouyang, L.; Yuan, S. One-step synthesis of ZnFe2O4-loaded biochar derived from leftover rice for high-performance H2S removal. Separation and Purification Technology 2021, 279, 119686. [Google Scholar] [CrossRef]
- Momodu, D.; Madito, M.; Barzegar, F.; Bello, A.; Khaleed, A.; Olaniyan, O.; Dangbegnon, J.; Manyala, N. Activated carbon derived from tree bark biomass with promising material properties for supercapacitors. Journal of Solid State Electrochemistry 2017, 21, 859–872. [Google Scholar] [CrossRef]
- Kesavan, T.; Raaju Sundhar, A.S.; Dharaneshwar, S.; Prabu, N.; Manickam, S. N-Doped carbon nanosheets from biomass for ultra long-cycling and high energy density symmetric supercapacitors. ECS Journal of Solid State Science and Technology 2021, 10, 051004. [Google Scholar] [CrossRef]
- Taer, E.; Apriwandi, A.; Febriani, W.; Taslim, R. Suitable micro/mesoporous carbon derived from galangal leaves (Alpinia galanga L. ) biomass for enhancing symmetric electrochemical double-layer capacitor performances. ChemistrySelect 2022, 7, e202201810. [Google Scholar]
- Kang, W.; Lin, B.; Huang, G.; Zhang, C.; Yao, Y.; Hou, W.; Xu, B.; Xing, B. Peanut bran derived hierarchical porous carbon for supercapacitor. Journal of Materials Science: Materials in Electronics 2018, 29, 6361–6368. [Google Scholar] [CrossRef]
- Dai, C.; Wan, J.; Shao, J.; Ma, F. Hollow activated carbon with unique through-pore structure derived from reed straw for high-performance supercapacitors. Materials Letters 2017, 193, 279–282. [Google Scholar] [CrossRef]
- Xie, Q.; Zheng, A.; Zhai, S.; Wu, S.; Xie, C.; Zhang, Y.; Guan, Y. Reed straw derived active carbon/graphene hybrids as sustainable high-performance electrodes for advanced supercapacitors. Journal of Solid State Electrochemistry 2016, 20, 449–457. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Ji, S.; Han, Y.; Kim, D. Dendritic nanostructured waste copper wires for high-energy alkaline battery. Nano-Micro Letters 2019, 12, 1. [Google Scholar] [CrossRef]
- Wei, Q.; Chen, Z.; Cheng, Y.; Wang, X.; Yang, X.; Wang, Z. Preparation and electrochemical performance of orange peel based-activated carbons activated by different activators. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2019, 574, 221–227. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, X.; Xu, X.; Zhang, M.; Wang, L.; Zhao, X.; An, Z.; Yao, H.; Gao, J. A novel porous carbon material made from wild rice stem and its application in supercapacitors. Materials Chemistry and Physics 2018, 213, 267–276. [Google Scholar] [CrossRef]
- Deng, J.; Xiong, T.; Xu, F.; Li, M.; Han, C.; Gong, Y.; Wang, H.; Wang, Y. Inspired by bread leavening: one-pot synthesis of hierarchically porous carbon for supercapacitors. Green Chemistry 2015, 17, 4053–4060. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Li, A.; Zhang, L. Facile synthesis of high-surface area mesoporous biochar for energy storage via in-situ template strategy. Materials Letters 2018, 230, 183–186. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Zhang, T.; Ouyang, L.; Yuan, S. Single-step preparation of ultrasmall iron oxide-embedded carbon nanotubes on carbon cloth with excellent superhydrophilicity and enhanced supercapacitor performance. ACS Applied Materials & Interfaces 2021, 13, 45670–45678. [Google Scholar]
- Xiao, J.; Wang, Y.; Zhang, T.C.; Ouyang, L.; Yuan, S. Phytic acid-induced self-assembled chitosan gel-derived N, P–co-doped porous carbon for high-performance CO2 capture and supercapacitor. Journal of Power Sources 2022, 517, 230727. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Y.; Zhang, T.C.; Yuan, S. N,S-containing polycondensate-derived porous carbon materials for superior CO2 adsorption and supercapacitor. Applied Surface Science 2021, 562, 150128. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, J.; Zhang, T.C.; Ouyang, L.; Yuan, S. Synthesis of CuSiO3-loaded P-doped porous biochar derived from phytic acid-activated lemon peel for enhanced adsorption of NH3. Separation and Purification Technology 2022, 283, 120179. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy & Environmental Science 2021, 14, 2036–2089. [Google Scholar]
- Xiao, J.; Wang, Y.; Zhang, T.C.; Yuan, S. rGO/N-porous carbon composites for enhanced CO2 capture and energy storage performances. Journal of Alloys and Compounds 2021, 857, 157534. [Google Scholar] [CrossRef]
- Yuan, X.; Xiao, J.; Yılmaz, M.; Zhang, T.C.; Yuan, S. N, P Co-doped porous biochar derived from cornstalk for high performance CO2 adsorption and electrochemical energy storage. Separation and Purification Technology 2022, 299, 121719. [Google Scholar] [CrossRef]
- Liu, H.; Huo, W.; Zhang, T.C.; Ouyang, L.; Yuan, S. Photocatalytic removal of tetracycline by a Z-scheme heterojunction of bismuth oxyiodide/exfoliated g-C3N4: performance, mechanism, and degradation pathway. Materials Today Chemistry 2022, 23, 100729. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, M.; Kim, S.K.; Bae, G.; Song, W.; Myung, S.; Lim, J.; Lee, S.S.; Zyung, T.; An, K.-S. A strategy for synthesis of carbon nitride induced chemically doped 2D MXene for high-performance Supercapacitor Electrodes. Advanced Energy Materials 2018, 8, 1703173. [Google Scholar] [CrossRef]
- Szubzda, B.; Szmaja, A.; Halama, A. Influence of structure and wettability of supercapacitor electrodes carbon materials on their electrochemical properties in water and organic solutions. Electrochimica Acta 2012, 86, 255–259. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, T.C.; Yuan, S.; Liang, B. N-doped porous carbon derived from rGO-Incorporated polyphenylenediamine composites for CO2 adsorption and supercapacitors. Journal of Power Sources 2020, 472, 228610. [Google Scholar] [CrossRef]
- Wang, Q.; Juan, J.; Xiao, T.; Zhang, J.; Chen, H.; Song, X.; Chen, M.; Huang, J. The physical structure of compost and C and N utilization during composting and mushroom growth in Agaricus bisporus cultivation with rice, wheat, and reed straw-based composts. Applied Microbiology and Biotechnology 2021, 105, 3811–3823. [Google Scholar] [CrossRef]
- Fan, X.; Tan, F.; Meng, F.; Liu, J. Hierarchical porous N-doped carbon nanosheets obtained by organic–inorganic bipolymeric engineering for improved lithium–sulfur batteries. Chemistry – A European Journal 2019, 25, 4040–4046. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Zhang, T.C.; Xiang, G.; Wang, X.; Pehkonen, S.; Yuan, S. A magnetic γ-Fe2O3@PANI@TiO2 core–shell nanocomposite for arsenic removal via a coupled visible-light-induced photocatalytic oxidation–adsorption process. Nanoscale Advances 2020, 2, 2018–2024. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhang, T.C.; Xiang, G.; Wang, X.; Yuan, S. Removal of trace arsenite through simultaneous photocatalytic oxidation and adsorption by magnetic Fe3O4@PpPDA@TiO2 core–shell nanoparticles. ACS Applied Nano Materials 2020, 3, 8495–8504. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Z.; Xiao, J.; Cen, W.; Yuan, S. Polypyrrole-encapsulated Fe2O3 nanotube arrays on a carbon cloth support: Achieving synergistic effect for enhanced supercapacitor performance. Electrochimica Acta 2021, 386, 138486. [Google Scholar] [CrossRef]
- He, H.; Huang, D.; Tang, Y.; Wang, Q.; Ji, X.; Wang, H.; Guo, Z. Tuning nitrogen species in three-dimensional porous carbon via phosphorus doping for ultra-fast potassium storage. Nano Energy 2019, 57, 728–736. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Wang, H.; Zhang, T.C.; Yuan, S. Binary doping of nitrogen and phosphorus into porous carbon: A novel di-functional material for enhancing CO2 capture and super-capacitance. Journal of Materials Science & Technology 2022, 99, 73–81. [Google Scholar]
- He, H.; Gan, Q.; Wang, H.; Xu, G.-L.; Zhang, X.; Huang, D.; Fu, F.; Tang, Y.; Amine, K.; Shao, M. Structure-dependent performance of TiO2/C as anode material for Na-ion batteries. Nano Energy 2018, 44, 217–227. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Y.; Shi, J.; Gu, L.; Yu, Y. Multichannel porous TiO2 hollow nanofibers with rich oxygen vacancies and high grain boundary density enabling superior sodium storage performance. Small 2017, 13, 1700129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, J.; Wang, H.; Zhang, T.C.; Yuan, S. N-doped porous carbon derived from solvent-free synthesis of cross-linked triazine polymers for simultaneously achieving CO2 capture and supercapacitors. Chemistry–A European Journal 2021, 27, 7908–7914. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, K.; Pan, Q.; Xu, Y.; Liu, Q.; Cui, G.; Guo, X.; Sun, X. Boron-doped TiO2 for efficient electrocatalytic N2 fixation to NH3 at ambient conditions. ACS Sustainable Chemistry & Engineering 2019, 7, 117–122. [Google Scholar]
- Zhang, J.; Pan, L.; Zhang, X.; Shi, C.; Zou, J. Donor-acceptor carbon nitride with electron-withdrawing chlorine group to promote exciton dissociation. Chinese Journal of Catalysis 2021, 42, 1168–1175. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




