1. Introduction
Soybean (
Glycine max (L.) Merr) is an important food source for people in Asia including Indonesia. Soybean is also said to be Gold from the Soil or World's Miracle because of their balanced nutritional composition. Soybean has a chemical composition that is almost like meat, with high protein content [
1], low saturated fat content, and as a source of fiber. Soybean seeds with good nutritional content can be used as a functional food source [
1,
2].
Soybean seeds as a source of vegetable protein with good nutritional content contain about 40% protein, 20% unsaturated fat, and 17% soluble and insoluble fiber. In addition, soybean is also a source of calcium, iron, zinc, phosphor, magnesium, thiamine, riboflavin, niacin, and folic acid. Soybean contains large amounts of essential amino acids for humans and is a good source of protein and vegetable oil[
3]
.Soybean seeds contain minerals in the form of calcium around 276 mg/100g, magnesium 280 mg/100g, potassium 1,797 mg/100g, iron 16 mg/100g, and zinc 4.8 mg/100g [
4]
.
Soybean are the biggest and best sources of isoflavones. Isoflavones are known as bioactive compounds that can reduce the risk of chronic disease, osteoporosis, and colon cancer [
5,
6]Besides, soybean also contains alpha-linolenic acid, omega-6 fatty acids and isoflavones, genistein, daidzein, and several other components including vitamins.
Soybean seeds from Rwanda have a protein content ranging from 34.7% - 36.7% with a fat content of around 11.1% - 16.6% [
7]. The Indonesian soybean seeds characteristics have a fairly high water content of 9.95%, ash content of 5.15% - 5.36%, and protein content of 30.33% -36.49%. Soybean seeds originating from abroad have lower water content, such as soybean seeds originating from Argentina at 9.71% and soybean seeds from the United States at 8.86%, with a lower protein content of 30.33% and 34.07 % respectively[
8].
The Dega-1, Grobogan, and Gepak Kuning are soybean varieties released by the Ministry of Agriculture of the Republic of Indonesia. Grobogan and Gepak Kuning were released in 2008 which are early maturing superior varieties with productivity of almost 2.5 t ha
-1. As additional information, the size of soybean seeds is grouped into 3 groups, namely small, medium, and large, with small sizes< 10g/100 seeds, medium sizes 10-14 g/100 seeds, and large sizes> 14 g/100 seeds. The size and weight of soybean arefactorsin determining soybean quality [
9]
.The Gepak Kuning has a small seed size and it is suitable for tofu raw material because it has a high yield and is higher than imported soybean [
10]. This soybean has a relatively low weight compared to other varieties (<10 g/100 seeds), whereas the Grobogan has wide adaptive properties and large seed size. Likewise, Dega 1 is an early maturing soybean (77 DAP) and has large seeds (23 g/100 seeds)[
11].
Indonesian soybean varieties have been widely cultivated with various types of varieties. The selection of these varieties is based on farmers' preferences in terms of seed size, seed weight, and productivity result. Public understanding and knowledge regarding the chemical composition and nutritional content of soybean is still low and has not been used as a basis for selecting varieties for cultivation. This study aims to determine the chemical composition of three early maturing soybean varieties (Dega-1, Grobogan, and Gepak Kuning) in Indonesia to support the development of food and non-food products.
2. Materials and Methods
The soybean samples were resulting from the soybean varieties adaptation test conducted by South Sulawesi Assessment Institute for Agricultural Technology (South Sulawesi-AIAT) Experimental Garden in Maros, Indonesia in 2022. These varieties consist of Dega-1, Grobogan, and Gepak Kuning which are early-maturing superior varieties released by the Ministry of Agriculture the Republic of Indonesia. The study was conducted by Completely Randomized Design (CRD) and six replication.Sample analysis was carried out in the laboratory according to the parameters required. Each soybean varietywas collected 1 kg sample with 3 replicates. The soybean used for each variety was 1 kg, n-Hexane, Chloroform, and KBr.
The tools used were digital scales on a kg scale (Sonic, Model ACS, Capacity 30 kg) and a gr scale (ION Scale EPSO5, Max 200g, d=0.01g), blender, filter, 1000 ml measuring cup, slide rule, GC- MS (QP.1000), microscope (USB digital microscope 1000 x 8 LED 2 MP digital), FTIR test kit – 8400S – Shimadzu.
2.1. Observation Parameters and Data Processing
Observation parameters include physical testing and soybean seeds’ chemical composition. Parameters for soybean seeds physical testing include weight per 100 seeds, bulk density[
12] and the number of damaged seeds per 100 seeds, and the shape of soybean seed granules. Testing the soybean seed’s chemical composition includes water content, protein content with the Kjeldhal micro method, salt content, and fat content with the Soxhlet method [
13], ash content [
14], calcium content [
15], phosphor content [
16]free fatty acids (FFA), peroxide number [
15]. Analysis of soybean compound components using Shimadzu's GCMS-QP2010, testing the soybean seeds structure with FTIR (Fourier Transform Infra-Red).
2.2. Sample Preparation
Soybean seeds to be used, before testing, grind them first using a blender. Soybean seeds that have been in the form of flour were then filtered to obtain uniform particle sizes. From the sample in the form of soybean flour, it was then analyzed for its proximate content, granule shape, FFA, peroxide number of soybean fat, and testing the components of soybean compounds with their functional groups.
2.3. Observation of Soybean Granule Shape
The shape of the starch granules was observed using a microscope (USB Digital Microscope 1000 x 8 LED 2MP Digital) equipped with a camera. Soybeans that have been mashed and filtered were then placed in an object glass, then drops of water were then observed under a microscope with 1000X magnification[
17]
2.4. Water Content Testing (AOAC 2005)
The porcelain cup was dried in the oven, and the sample was weighed as much as ± 2.0 grams, and put in the porcelain cup. The samples were dried for 2 hours (135°C), cooled in a desiccator, and weighed. The results of the difference in weight were recorded as water content.
2.5. Ash Content Testing (SNI 01-3709-1995)
The empty and dry porcelain cups were weighed, and ± 2.0-gram sample was put into the porcelain cup. The cup containing the sample was put into the furnace at 550oC for 4 hours. Removed and cooled in the desiccator, and weighed.
2.6. Protein Content Testing (AOAC 2005)
The sample was weighed ± 0.4-0.6 grams, and put into the Kjehdal tube. Catalyst Na2SO4+CuSO4.H2O was added as much as 6.4 g and catalyst (1 kg Na2SO4 + 68 grams CuSO4.H2O then mixed thoroughly), added 12 ml of 98% H2SO4. The sample is heated at 450ºC for about 2 hours (the color turns green), then cooled in an open place if it's not too hot, slowly add ±20 ml of distilled water and let it cool down. Hereafter the sample was distilled for 6 minutes with the addition of 40% NaOH until the sample looks cloudy. A receiving solution was prepared to consist of 30 ml of 4% H3BO3 + mix indicator. At the distillation stage, dip the alkaline hose in 40% NaOH, then it was titrated with 0.2 M HCl until the color changed from green to red.
2.7. Crude Fat Content Testing (AOAC, 1996)
The sample was weighed ± 2-3 gr (m), then the sample was put into a filter paper package, then put into a petri dish + lid whose weight is known. Samples were heated at 105°C for 3 hours in an open petri dish or 130°C for 40 minutes. Cooled in a desiccator for 30 minutes. The petri dish was weighed containing the sample and its lid (W1). The sample pack was put into the fat flask. Approximately 200 ml of petroleum ether or petroleum benzene was added. Fattenization was carried out at 60-63°C for 5 hours. The fattenization indicator was perfect if the fat flask was filled with a yellowish liquid. If the fattenization process has been perfect, drying was carried out by taking the distillate petroleum ether, placing the sample packs in a petri dish, heating at 105°C for 3 hours or 130°C for 40 minutes in the open position of the petri dish. The further stage was cooling in a desiccator for 30 minutes, and the petri dish was weighed containing the sample pack and its lid (W2).
2.8. Free Fatty Acids (FFA) Determination (AOAC, 1990)
Weigh 14 grams of oil that have been extracted from soybean seeds of each variety at each purification stage and put it into a 250 ml Erlenmeyer. Later, add 25 ml of 95% ethanol and heat at 40°C, after that add 2 ml of pp indicator, and titrate with 0.05 M NaOH solution until a pink color appears and does not disappear for 30 seconds.
2.9. Soybean Chemical Compound Components Testing
Testing the components of soybean seed compounds was carried out using the Shimadzu QP2010 Gas Chromatography Mass Spectroscopy (GC-MS) instrument. The column used was DB-5MS (non-polar column) with a length of 30 mm, diameter of 0.25 mm, injector temperature of 250
°C with detector temperature of 280
°C. The sample testing method was carried out by extracting floured soybean seeds using the Soxhlet extraction method. Samples in the form of flour were weighed as much as 20 grams and put into the chamber. The chamber containing the sample was put into the soxhlet and added 30 ml of n-hexane, which was then extracted for 7 hours at 300
°C. The extraction results were evaporated using a vacuum evaporator with a pressure of 20 Ag for 30 minutes at a speed of 5 rpm, with a water bath temperature of 40
°C. The resulting fat was then evaporated using a vacuum oven at 80
°C for 2 hours. The evaporated soybean fat was then diluted using chloroform in a ratio of 1: 9. The results of the dilution were then analyzed for the compound components by injecting the sample into the Shimadzu GC-MS QP2010 instrument[
18]
.
2.10. Data Analyses
The resulting data were tabulated and processed using Excel and SPSS software. Data were analyzed by ANOVA to determine the significance of the observed parameter. If it has a significant effect, it will be tested further with the Tukey test.
3. Results and Discussion
3.1. Morphological and Physical Characteristics of the Seeds of Three Soybean Varieties
3.1.1. Morphological Characteristics of Three Soybean Varieties
Dega-1, Grobogan and Gepak Kuning have different characteristics. According to the Soybean Varieties Description [
19], the the Dega-1 has an oval leaf shape, plant height is 53 cm, weight of 100 seeds is 22.80 g per 100 seeds, quite vulnerable to armyworms, average seed yield of 2.78 t ha
-1, protein content of 37.78%, and fat content 17.29%. While the Grobogan has a pointed leaf shape, plant height is 50-60 cm, the weight of 100 seeds is 18 g per 100 seeds, flowering age of30-32 days, average seed yield of 2.77 t Ha
-1, 43.9% protein content, and 18.4% fat content. Furthermore, the Gepak Kuning has an oval leaf shape, plant height is 55 cm, weight of 100 seeds is 8.25 g per 100 seeds, flowering age at 28 days, resistance to
Spodoptera litura attack, an average yield of 2.22 t Ha
-1, protein content of 35.38%, and fat content 15.10%. According to[
20]the Grobogan has a weight of 100 seeds of about 18.57 g and Gepak Kuning 10.18 g and has a pest attack rate of
Spodoptera litura of 10.10% on GepakKuning and 13.14% on Grobogan.While the seed yields for the Groboganisare 2.08 ha-1 and Gepak Kuning is 2.00 t ha
-1. In line with[
21], the Grobogan has a plant height of 41.24 cm and a number of leaves is 4.50, while the Gepak Kuning has a plant height of 31.82 cm and a number of leaves is 4.83. The study report from[
22], the Dega-1 has a plant height of 50 cm, a flowering age of 28.67 days, and a growing percentage of 98.33%, while the Grobogan has a plant height of 53.04 cm, a flowering age of 29.00 days and a growing percentage of 100.0%. The same report from[
23], Dega-1 has a plant height of about 45.7-64.00 cm, a flowering age of 29.6-36.6 days, a number of pods per plant of around 38.5-60.00, and a plant age of 77.9-83.9 days.
Figure 1.
Seed morphology characteristics and seed size in three soybean varieties under normal conditions.
Figure 1.
Seed morphology characteristics and seed size in three soybean varieties under normal conditions.
3.1.2. Soybean Seeds Physical Characteristics
Soybean seeds have different characteristics for each variety. These characteristics include the weight of seeds for every 100 seeds, as well as the condition of the seeds produced. The weight of the soybean seeds produced for each varietywas quite different as shown in
Table 1. The Dega-1was heavier than the Grobogan and Gepak Kuning varieties.
The weight of the seeds produced is different from the weight in the description of the soybean varieties[
19]showing the soybean weight with a difference for Dega-1, Grobogan, and Gepak Kuning each 1.5 g; 4.67gr; and 0.75gr per 100 soybeans respectively. Soybean seed weight can be caused by soil nutrient availability, climatic or environmental conditions during cultivation, and the ability of each genotype to carry out its physiological functions [
24].
The soybean seeds'weight did not have a large effect on the bulk density of the soybean seeds produced. As can be seen in
Table 1, the Dega-1 and Grobogan with different seed weights have the same bulk density. The Gepak Kuning with a lighter seed weight compared to Dega-1 and Grobogan has almost the same bulkdensity as the two varieties. In other words, the bulk density of grain was affected by the size of soybean seeds for each variety.
The seed quality for 100 seeds also differs for each variety. The quality was seen from the level of damage to the soybean seeds produced. The highest level of damage was found in the Grobogan and the lowest level of damage was in the Dega-1. The damage was caused by pests and diseases during the cultivation period, resulting in small, flat, and black seeds, also found black soybean seeds with a very hard seed texture.
The soybean seeds damage can be caused by pest attacks on crops such as grasshoppers, pod borers, bean flies, leaf rollers, and several other types of pests. Damage caused by these pests can reduce the quantity and quality of crop yields because they can interfere photosynthesis process in plants. The previous study reported that disruption to the photosynthesis process can cause a decrease in yield of up to 70% to 80%[
25].
The damage during cultivation can be caused by
Nezara viridula [
26], as well as
Riptortus sp. causing changes in the nutrition and soybean seeds quality[
27]. Another pest that damages soybean seeds was the soybean pod borer
Etiella zinckenella. Similarly, the abiatic factor for instance heat determines physical and chemical damage so that the soybean seeds experience discoloration, protein, starch, oil, moisture, and other components[
28].
Figure 2.
Seeds damaged by purple seed spot disease Cercospora kikuchii (a), leaf spot Cercospora sojina (b), pod borer Etiella zinckenella (c), and pod sucker Nezara viridula.
Figure 2.
Seeds damaged by purple seed spot disease Cercospora kikuchii (a), leaf spot Cercospora sojina (b), pod borer Etiella zinckenella (c), and pod sucker Nezara viridula.
3.2. Form of Soybean Starch Granules
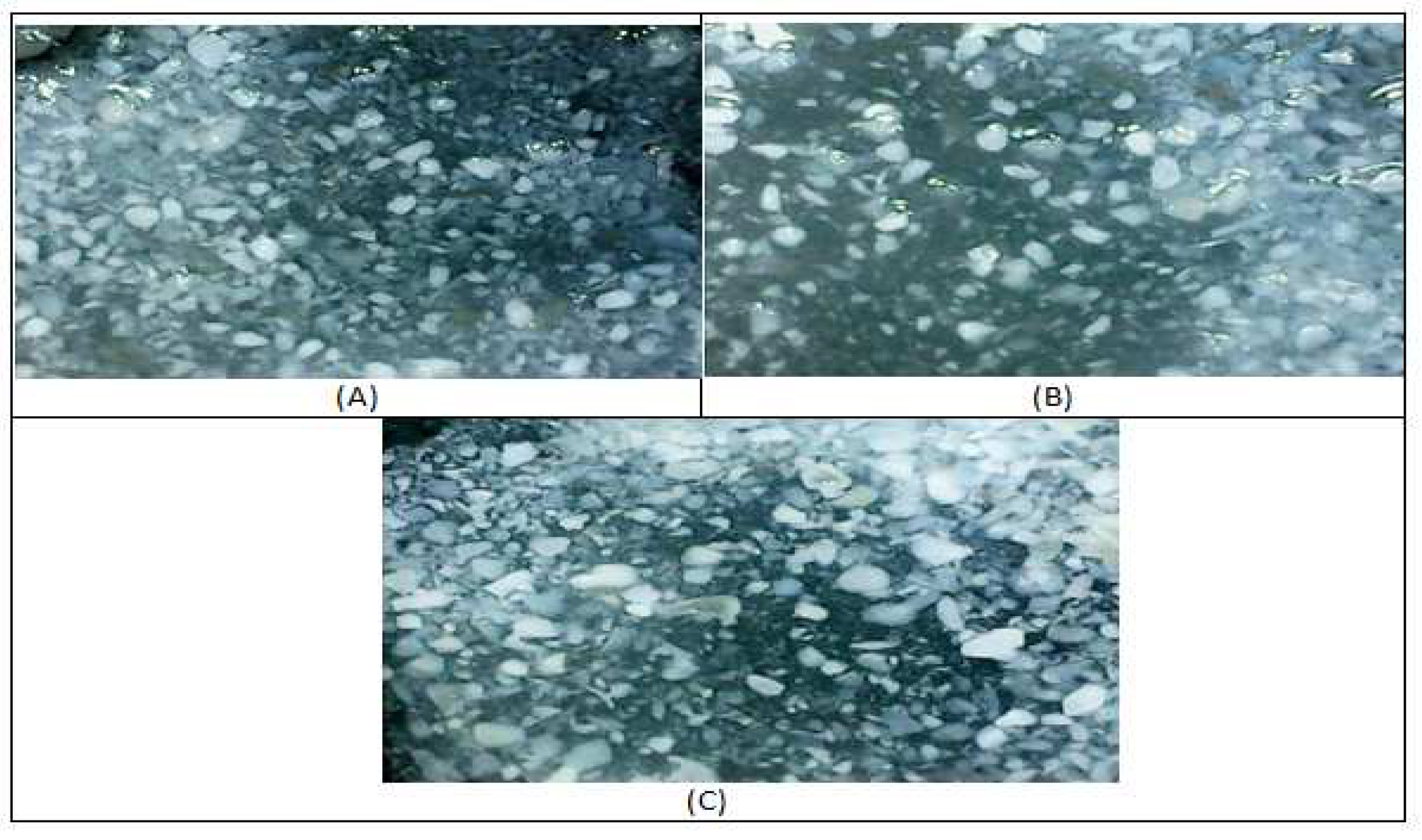
Starch granules observation on all soybean varieties with a microscope showed images that were still intact with irregular shapes. The appearance of the granules indicates that the starch has undergone a gelatinization process with a smooth and intact surface.
Figure 3.
The form of soybean starch granules in Dega 1 (A), Grobogan (B), Gepak Kuning (C).
Figure 3.
The form of soybean starch granules in Dega 1 (A), Grobogan (B), Gepak Kuning (C).
The soybean starch granules in all samples collected have almost the same shape but with different sizes.The Gepak Kuning has a larger starch granule size compared to the Grobogan and Dega-1 varieties with Dega-1 having the smallest size compared to the others. The larger starch granule size can also be affected by the protein content found in the Gepak Kuning as shown in
Table 2. Research conducted by [
29] states that the high protein content affects the amount of crystallization in starch induced by protein molecules and the encouragement of hydrogen bonds so it requires greater energy to release these bonds.
The shape of starch granules is influenced by the chemical composition of each different soybean variety. This is in accordance with the results of research conducted by [
30]that the fat and minerals content is an important part that affects the physicochemical properties of wheat starch grains. Soybean starch grains tend to gather and were in groups as shown in
Figure 1. The tendency to cluster was influenced by the fat content in the soybean seeds, which affects the shape of the soybean starch granules. The soybean starch granules shape is like irregular or polygonal shape, and some have a polygonal granule shape on one side and ovoid on the other [
31].Granule size plays an important role in the material processing, related to the gelatinization temperature or the energy requirements. Starch with a small granule size has a high gelatinization temperature because it tends to have stronger intermolecular bonds, resulting in a higher energy requirement for the gelatinization process.
3.3. Soybean Seeds Chemical Characteristics
Our data showed that soybean seeds have different chemical characteristics for each variety. Based on chemical compositionconsisting;of ofprotein, fat, moisture, ash content, phosphor, and FFA, these valuesare weresignificantly different between soybean varieties. The Gepak Kuning has a higher average chemical composition compared to the Dega-1 and Grobogan as shown in
Table 2.
The fat content of the soybean seeds of the three varieties ranged from 12.627 – 14.488 %. The highest soybean fat content was obtained in the GepakKuning. This data is quite low when compared to the fat content of the Wilis and Anjasmoro varieties [
32]. The research result of[
33], reported thatsoybean fat contains quite large essential fatty acids ranging from 7 – 54%, namely linoleic acid (omega 6) and linolenic acid (omega 3). Furthermore, a study by [
34]showed that in general soybean contains about 18-20% fat and 25% of this amount consists of cholesterol-free unsaturated fatty acids.
The higher the soy protein content, the better the quality of the soybean. Soybean contains an average of 35% protein, even in superior varieties the protein content can reach 40-44%[
34]. The protein content of the samples in this study ranged from 36,623 – 39,723 %. Our experiment figure out that the highest protein content was found in the Gepak Kuning.The previous study conducted by [
8] states that each type of soybean has various components depending on the varieties developed and the location where it grows.In this experiment, the Grobogan has a protein contentof 37.610 % lower than the results of studies of 42.32% and 43.90%[
35] even though this value is better than the Dega-1 varieties.
The amount of ash content is related to the minerals of the material[
36]. The soybean seeds ash content ranges from 4.487 – 5.187 % and the highest was obtained in the Gepak Kuning. Ash content in soybeans is around 5% and affects mineral levels such as potassium, calcium, and magnesium[
8]. Previously, research explained minerals that predominate in soybean were phosphorus (P), calcium (Ca), and iron (Fe) [
32]. The ash content and composition depend on the type of material and the method of ashing used[
37].
The water content of the three soybean varieties meets the maximum SNI requirements of 13% [
38]namely 11.513 - 11.860%. This showed that the post-harvest handling process specifically in the drying stage has been carried out properly. The highest water content was obtained in the Grobongan (11.860%).This figure corresponds[
39], the differences in seed size affect the value of moisture content and the Grobogan has larger seed sizes than other varieties. The high water content in a material can be caused by the low fiber content of the material [
40].
3.4. The Chemical Content of Soybean’s FFA
The level of free fatty acids (FFA) in the soybean oil content is one of the determining factors for the soybean quality. Since seed storage, the fat content will slowly undergo hydrolysis by water in high-temperature conditions or due to natural lipolytic enzymes or those produced by bacteria or fungi which will contribute to product rancidity [
41]. For all samples observed, the Grobogan has the highest FFA content (1.033 %) and was significantly different from the other varieties. The increase in FFA indicates that the oil has increased damage due to hydrolysis[
42,
43,
44] FFAs can alter the taste and give a dreadful flavor and noxious oxygenated compounds, on the other hand, physical refining is the alternative way for FFAs deacidification such as distillation, membrane, solvent extraction, enzymatic, and adsorption [
45]
The surface composition and structure of the oil bodies depend on the plant oil content and these factors influence the behavior of gastrointestinal digestion in vitro. Free fatty acids in soybean oil emulsion were significantly higher than in rapeseed oil after 20 minutes of digestion time under simulated intestinal aqueous conditions. The results obtained indicate that plant oils can be useful as natural emulsifiers in the development of functional foods and achieve controlled inhibition of bioactive compounds from emulsions during gastrointestinal digestion [
46].
3.4.1. The Peroxide Number
Peroxide number is an index of the amount of fat or oil that has undergone oxidation. Peroxide can accelerate the process of rancidity and unwanted odors in food. If the amount of peroxide is more than 100 meq peroxide/kg the oil will be very toxic and have an unpleasant odor. An increase in peroxide value is an indicator that the oil will smell rancid.
Table 2 displays that the highest peroxide number was in the Grobogan, followed by the Gepak Kuning, and the lowest in the Dega-1. This means that the oil reacts with oxygen in the double bond and a chain reaction occurs which continuously provides free radicals which produce further peroxides. Pure cooking oil that has not been used for frying has the lowest peroxide content, namely 0.3986 mg 0/100 g. Until the third process, the maximum limit of oil peroxide content is 1 mg 0/100 g of oil[
14]. Thereafter, exceeding this maximum limit is feared to poison the body, especially in foods that contain fat with a peroxide value of more than 100. The quality requirements for cooking oil used by the public must be based on the Ministry of Industry as presented in
Table 2 because the cooking oil used can have an impact negative for health [
47].
The high peroxide value of the three tested soybean varieties was thought to be due to the handling of the samples with the heat factor. The longer the heating time, the higher the peroxide value of the three varieties of soybean oil. That means that the three oils are easily oxidized during cooking. According to [
48], oxidation during cooking occurs easily in soybean oil and is most difficult in Bohai algae. Bohai algae oil is better cooked in a microwave oven with P-20 power and cooked on an electric stove with 1000W power, especially in 2~3 minutes. Olive oil is suitable for cooking in a microwave oven with P-20 power or cooking on induction hobs and electric hobs. Most of the cooking methods are suitable for soybean oil except the induction cooker heating method because the peroxide value does not change.
In general, our experiment showed that the Gepak Kuning has a better chemical composition compared to two other soybean varieties (
Table 2). The high chemical composition has no effect on the weight and bulk density as well as on the physical quality of the soybean seeds produced (
Table 1). The quality of the oil produced from the three varieties, the soybean oil produced from the Grobogan has lower quality in terms of free fatty acid content compared to the Dega-1 and Gepak Kuning varieties. The high content of free fatty acids in soybean fat is caused by the water content in an ingredientas the Grobogan has the highest water content compared to other varieties. The water content in the material can function as a catalyst that causes the hydrolysis of triglycerides with the help of lipase enzymes. The high water content in a material can be caused by the fiber content of the material[
40]. In addition to water content, fat oxidation is also affected by the unsaturation of fat, transition metal content in fat, oxygen content, and temperature[
49,
50,
51,
52]
.
3.4.2. Soybean Seeds Compound Components
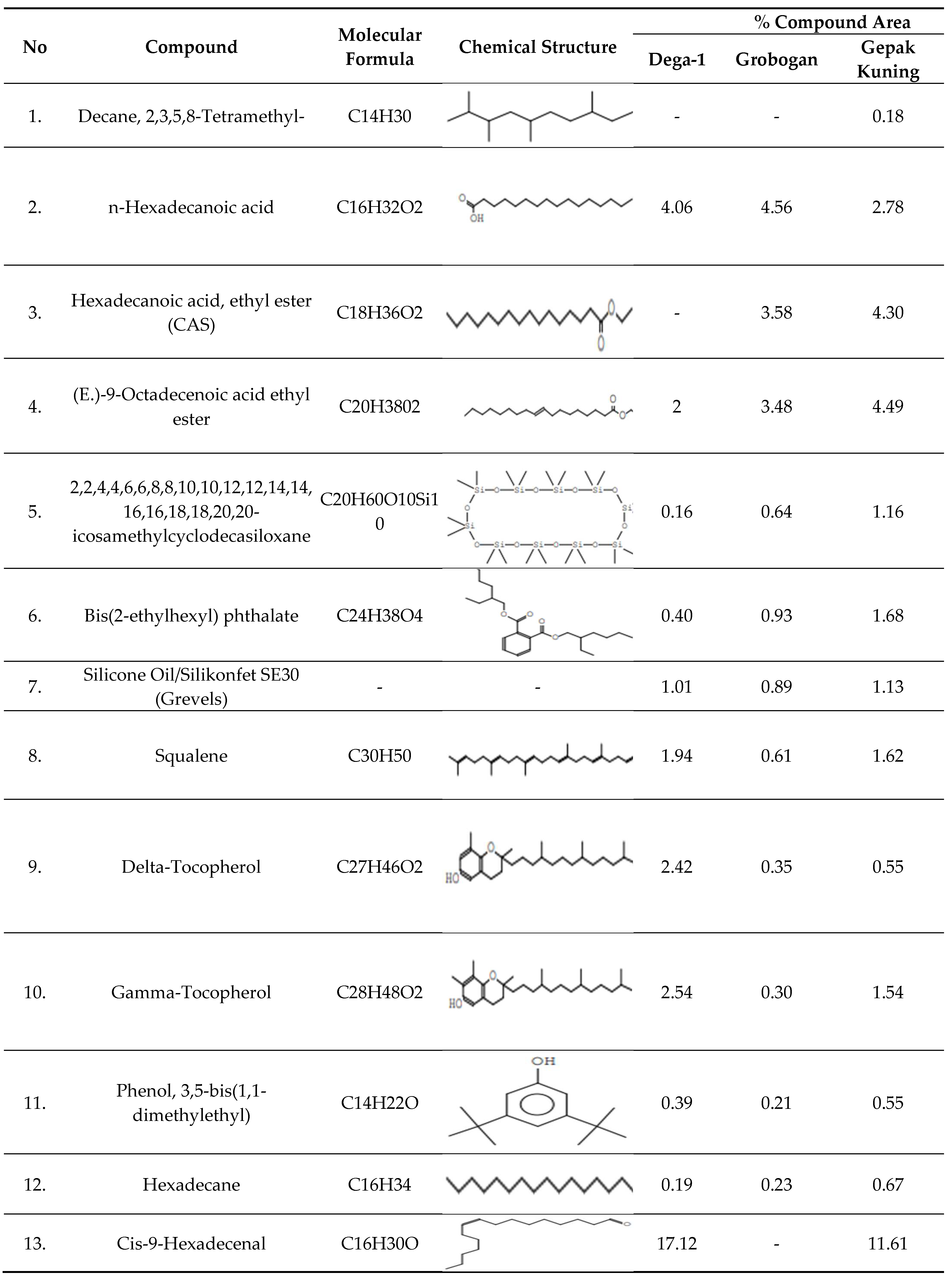
The GCMS analysis obtained 18 compounds that predominated in the three soybean varieties including saturated and unsaturated fatty acids, most of which contain ingredients such as n-Hexadecanoic acid, Hexadecanoic acid, ethyl ester (CAS), (E.)-9-Octadecenoic acid ethyl ester, Hexadecane, Cis-9-Hexadecenal, Octadecena, 9-Octadecenoic acid (Z)-(CAS)/olead acid [
53,
54]. For Cis-9-Hexadecenal fatty acids, the Dega-1 has an area of 17.12% and 11.61% for Gepak Kuning while the Grobongan does not detect these fatty acid compounds, but almost all fats must have Cis-9-Hexadecenal fatty acid compounds[
54,
55,
56]. Cis-9-Hexadecenal is classified as an antifungal activity that has the potential for anti-melanogenic antifungal properties [
57]. Decane 2, 3, 5, and 8-Tetramethyl compounds belong to a class of organic compounds known as acyclic alkanes, and these compounds belong to the pheromone class [
58].
Figure 4.
Chemical compound components of Dega-1, Grobogan, andGepakKuning.
Figure 4.
Chemical compound components of Dega-1, Grobogan, andGepakKuning.
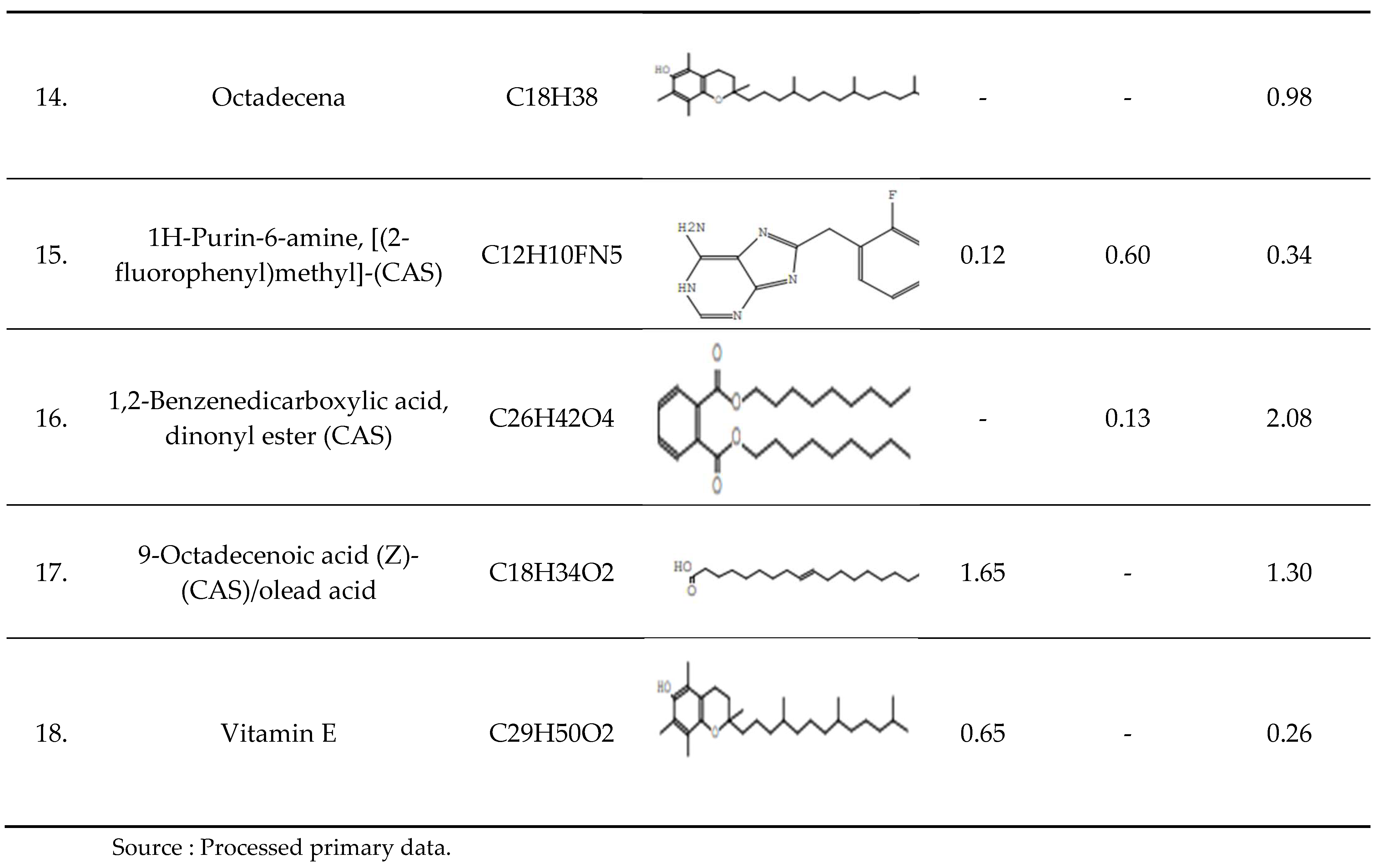
The compound components in the table above apart from those classified as fatty acids, there are other compounds identified from the three soybean varieties, namely Decane, 2,3,5,8-Tetramethyl-, icosamethylcyclodecasilloxane, Bis(2-ethylhexyl) phthalate, Silicone Oil/Siliconfet SE30, Squalene, Delta-Tocopherol, Gamma-Tocopherol, Phenol, 3,5-bis(1,1-dimethylethyl), 1H-Purin-6-amine, [(2-fluorophenyl) methyl]-(CAS) 1,2-Benzenedicarboxylic acid, dinonyl ester (CAS), and Vitamin E. The presence of these bioactive compounds in soybean gives credence to their use by the human community. Soybean is also applicable for the production of new drugs by the isolation of certain compounds. It can be concluded that soybeans contain various bioactive compounds and are recommended as an important phytopharmaca plant.
3.4.3. Organic Compound Components
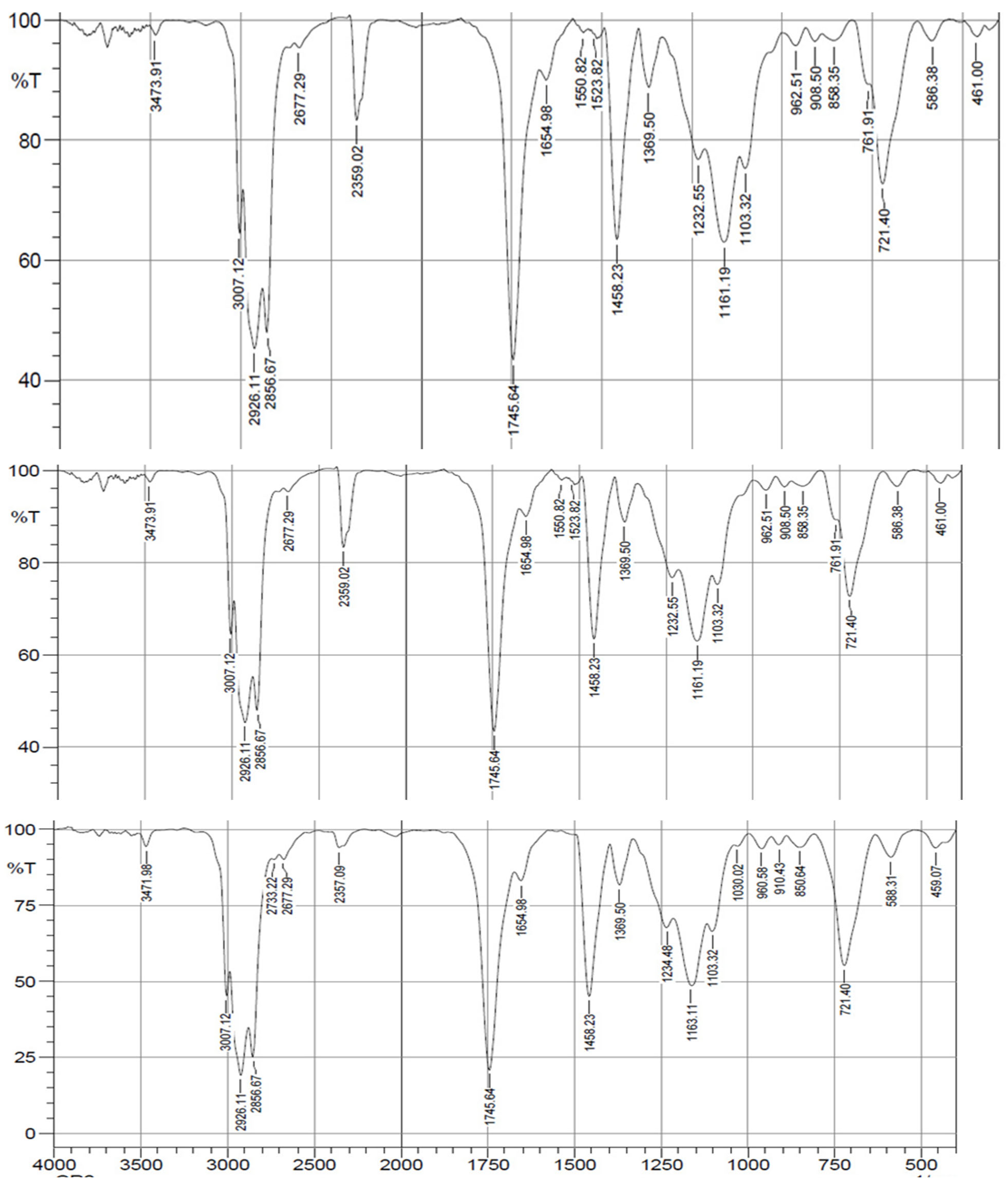
The results of the FTIR analysis for all soybean varieties showed the same value at each peak. The infrared spectrum of the soybean oil sample showed various organic components. The small peak above 3007 cm−1 corresponds to the aromatic group of the C=CH symmetric stretching vibration. The peak between 2800 and 3000 cm−1 corresponds to the C–H stretching vibration of the aliphatic group [
59].In this region, the peaks at 2926 and 2856 cm-1 (which are asymmetric and symmetrical absorptions of the methylene group stretching vibrations)[
59,
60]. There are two peaks at 1745 and 1161 which correlate with the stretching vibration of the carbonyl (C = O) of the ester bond, and with the asymmetric stretching vibration of the C-O ester bond,[
61]and the peak above 961 cm−1 corresponds to the bending vibration of the CH functional group which is associated with the aromatic structure. The peak above 723 cm−1 corresponds to deformation vibrations by unbranched aliphatic chains with more than four CH2 groups [
59].
Figure 5.
Components of organic compounds from the results of FTIR analysis on Dega-1, Grobogan, and Gepak Kuning.
Figure 5.
Components of organic compounds from the results of FTIR analysis on Dega-1, Grobogan, and Gepak Kuning.
4. Conclusions
Our experiment revealed the physical and chemical characteristics of three early maturing soybean varieties in Indonesia. Based on the physical characteristics of weight per 100 seeds, bulk density, and the number of damaged seeds, the Dega-1 showed the best performance compared to the other two soybean varieties. In contrast, Gepak Kuning gave the best results forstarch granule size parameters. For chemical characteristics, in general,Gepak Kuning has the best quality compared to Grobogan and Dega-1. The parameters for determining quality were protein content, moisture, ash, calcium, phosphor, and FFA content.On the other hand, Grobogan has the lowest oil quality because this variety had the highest FFA and peroxide value than the Dega-1 and Gepak Kuning varieties.It can be concluded that Gepak Kuning has the most potential to be developed in supporting food and non-food products, especially in Indonesia.
Funding
Please add: This research received no external funding or This research was funded by [name of funder] grant number [xxx] And The APC was funded by [XXX]. Information regarding the funder and the funding number should be provided. Please check the accuracy of funding data and any other information carefully.
Author Contribution
Conceptualization, A.F., I.D.R, H.W., Y.S.N., A.S., W.D., N.N., N.H.,A.Y.F., E.W.,M.F.H, A.E. Methodology, A.F., I.D.R, S.L., H.W., Y.S.N., N.H.,W.D. W.D.,S.W.M., A.S., N.N., A.E. Software, S.L., A.Y.F., A.S., H.S.W., S.N., M.F.H. Literature, A.F., I.D.R., S.L., N.N., W.D., A.Y.F., A.E., E.W., H.S.W., W.D.Y., S.W.M., S.R., N.H.., S.L.,W.D.,I.D.R. Validation, A.F., Y.S.N, H.W.,E.W., A.S., W.D., S.W.M., M.F.H., A.E.,N.H. Formal Analysis, A.F., S.L., A.Y.F., E.W., A.P., M.F.H., S.W.M. N.H., I.D.R., M.R.Investigation, A.F., I.D.R., H.W., S.L., W.D., S.N., A.S., N.N., A.E., W.D., E.W., H.S.W., S.W.M., S.R., N.H.S., E.T.Y. Resources, Y.S.N., S.L., S.M., A.S.,W.D., N.N., A.G.T., A.Y.F., A.E., W.D.Y., S.W.M., M.R. Writing-Preparation of the Original Draft, A.F., I.D.R., W.D., H.W., S.L., N.N., A.S., A.E., A.Y.F., E.W., Y.S.N., M.F.H.,W.D., E.W., S.N., N.H., W.D., H.S.W., A.Y.F.,, S.W.M. , S.W.M., H.W. Review-Writing and Editing, A.F., I.D.R., H.W., Y.S.N., S.L., W.D., E.E., S.N., S.L.,M.F.H., N.N.,A.E., A.Y.F., A.S., W.D., N.N., H.S.W. Visualization, S.L., A.E., E.W., M.F.H., N.H.., A.F. Supervision, A.F., H.W., Y.S.N., A.S., A.E. All authors have read and approved the published version of the manuscript.
Acknowledgments
We thank Dr. Ir. Syamsuddin, M.Sc. The Head of the South Sulawesi Assessment Institute for Agricultural Technology (South Sulawesi-AIAT) for his assistance has lent a research location at the Maros Soil Laboratory and other equipment. This research activity is independently funded by a group of researchers/authors sharing with South Sulawesi-AIAT.
Conflicts of Interest
Declare conflicts of interest or state “The authors declare no conflict of interest.”
References
- Ningrum, I.H.; Irianto, H.; Riptanti, E.W. Analysis of soybean production and import trends and its import factors in Indonesia. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: 2018; Volume 142.
- Ayda, K. Kedelaisebagai Sumber Pangan Fungsional. Iptek Tanaman Pangan 2017, 12, 57–65. [Google Scholar]
- Kanchana, P.; Santha, M.L.; Raja, K.D. A review on Glycine max (L.) Merr.(soybean). World J. Pharm. Pharm. Sci.
- Mateos-Aparicio, I.; Cuenca, A.R.; Villanueva-Suárez, M.J.; Zapata-Revilla, M.A. Soybean, a promising health source. Nutr Hosp 2008, 23, 305–312. [Google Scholar] [PubMed]
- Astawan, M.; Mardhiyyah, Y.S.; Wijaya, C.H. Potential of Bioactive Components in Tempe for the Treatment of Obesity. Jurnal Gizi Pangan 2018, 13, 79–86. [Google Scholar] [CrossRef]
- Sulistyowati, E.; Martono, S.; Riyanto, S.; Lukitaningsih, E. Development and validation for free aglycones daidzein and genistein in soybeans (Glycine max (l.) Merr.) using RP HPLC method. Int. J. Appl. Pharm. 2019, 11, 138–142. [Google Scholar] [CrossRef]
- Niyibituronsa, M.; Onyango, A.N.; Gaidashova, S.; Imathiu, S.; Uwizerwa, M.; Ochieng, E.P.; et al. The effect of different processing methods on nutrient and isoflavone content of soymilk obtained from six varieties of soybean grown in Rwanda. Food Sci. Nutr. 2019, 7, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Andarwulan, N.; Nuraida, L.; Adawiyah, D.R.; Triana, N.R.; Agustin, D.; Gitapratiwi, D. PengaruhPerbedaanJenisKedelaiterhadapKualitasMutuTahu. J. Mutu Pangan 2018, 5, 66–72. [Google Scholar]
- Adie, M.M.; Krisnawati, A. Identification of soybean genotypes adaptive to tropical area and suitable for industry. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: 2018; Voluem 102.
- Rahajeng, W.; Adie, M.M. VARIETAS KEDELAI UMUR GENJAH. Bul. Palawija 2013, 26, 91–100. [Google Scholar]
- Patriyawaty, N.R.; Anggara, G.W. Pertumbuhandanhasilgenotipekedelai (Glycinemax (L.) Merril) padatigatingkatcekamankekeringan. AGROMIX 2020, 11, 151–165. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, L.; Singh Sodhi, N.; Singh Sekhon, K. Physicochemical, cooking and textural properties of milled rice from different Indian rice cultivars. Food Chem. 2005, 89, 253–259. [Google Scholar] [CrossRef]
- AOAC, Chemist. Official Method of Analysis of AOAC International. 18th Edition. Official Method of Analysis of AOAC International. 18th Edition. AOAC International. 2005.
- Nasional, B.S. Standar Nasional Indonesia SNI 01-2891-1992. CaraUjiMakananMinuman. BadanStandardisasiNasional. 1992. [Google Scholar]
- AOAC. Official Method 927.02. 16th Edition. Official Method 927.02. 16th Edition. AOAC International. AOAC International 1997.
- Nasional, B.S. SNI 6989-31:2021. Air dan Air Limbah -Bagian 31 : Cara Uji Kadar Ortofosfat dan Total FosforMenggunakanSpektrofotometerdenganReduksiAsamAskorbat. 2021.
- Aulana, L.N.; Sugiyono, S.; Syamsir, E. Characterization of Physicochemical and Functional Properties of Heat Treated Wheat Flours. J. Mutu Pangan 2015, 2, 98–104. [Google Scholar]
- Kumar, P.S.; CR, A.; Vinaya, B.; Dinesh Babu, J. Variation of the antioxidant activity with the extraction method and solvent selection. Int. Res. J. Pharm. Appl. Sci. 2020, 10, 39–42. [Google Scholar]
- 19. Balitkabi. DeskripsiVarietasKedelai, 2016.
- Fattah, A.; Syam, S.; Daud, I.D.; Sartika Dewi, V.; Rahman, A. The Intensity of Leaf Damage Caused by Attack of Spodoptera litura F and Seed Yield on Some Soybean Varieties in South Sulawesi Indonesia. Sci. Res. J. (SCIRJ) 2018, 55. [Google Scholar]
- Sembiring, M.J.; Damanik, R.I.M.; Siregar, L.A.M. ResponPertumbuhanBeberapaVarietasKedelai (Glycine Max L. Merrill) Pada KeadaanTergenangTerhadapPemberian GA3 Growth Response on Various Varieties of Soybean (Glycine Max L. Merrill) at Flooded Condition to Application of GA3; 2016; Vol. 4.
- Haitami, A.; Indrawanis, E.; Ezward, C. Agronomic displays of some superior varieties soybean (Glycine max l.) In ultisol soil Singingi Kuantan District. MENARA Ilmu 2021, 15, 1–8. [Google Scholar]
- Rasyad, A.; Fiarahman, B. The Performance of Soybean (Glycine max.) Generation M1 Mutation Result of Dega 1 Variety with Several Concentrations of Ethyl Methane Sulphonate. J. Din. Pertan. Ed. 2022, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mawardi; Ichsan Cut Nur; Syamsuddin. Pertumbuhan dan Hasil BeberapaVarietasTanamanPadi (Oryza sativa L.) pada Tingkat KondisiKekeringan (Growth and yield of some varieties of rice plant (Oryza sativa L.) at the level of drought conditions ). J. Ilm. Mhs. Pertan. Unsyiah 2016, 1, 176–187. [Google Scholar]
- Utami, N.R.E. OPT utamakedelai dan pengendaliannyasecararamahlingkungan. Direktorat Jenderal Tanaman Pangan. https://tanamanpangan.pertanian.go.id/detil-konten/iptek/73.
- Giacometti, R.; Barneto, J.; Barriga, L.G.; Sardoy, P.M.; Balestrasse, K.; Andrade, A.M.; et al. Early perception of stink bug damage in developing seeds of field-grown soybean induces chemical defences and reduces bug attack. Pest Manag. Sci. 2016, 72, 1585–1594. [Google Scholar] [CrossRef]
- Herlina, L.; Istiaji, B.; Koswanudin, D. RESISTANT LEVEL OF SOYBEAN GERMPLASM AGAINST POD SUCKING BUGS (Riptortus spp.) Ketahanan Plasma NutfahKedelaiTerhadapKepikPengisapPolong (Riptortus spp.). Indones. J. Agric. Sci. 2021, 22, 39–57. [Google Scholar] [CrossRef]
- Liu, X.; Hao, Q.; Yue, J.; Hou, L.; Xia, X.; Zhao, W.; et al. Sarcopenia, Obesity and Sarcopenia Obesity in Comparison: Prevalence, Metabolic Profile, and Key Differences: Results from WCHAT Study. J.Nutr. Health Aging 2020, 24, 429–437. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, F.; Douglas Goff, H.; Li, Y. Study on starch-protein interactions and their effects on physicochemical and digestible properties of the blends. Food Chem. 2019, 280, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Sandhu, K.S.; Dhull, S.B.; Siroha, A.K.; Purewal, S.S.; Kaur, M.; et al. Oat starch: Physico-chemical, morphological, rheological characteristics and its applications - A review. Int. J. Biol. Macromol. 2020, 154, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Masoodi, F.A.; Gani, A.; Ashwar, B. Dual enzyme modified oat starch: Structural characterisation, rheological properties, and digestibility in simulated GI tract. Int. J. Biol. Macromol. 2018, 106, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Ginting, E.; Muchlish Adie, M.; DidikHarnowo, dan. Sifat Fisikokimia dan KandunganSeratPanganGalur-Galur Harapan Kedelai. J. Penelit. Pascapanen Pertan. 2017, 14, 35–45. [Google Scholar]
- Isa, I. PENETAPAN ASAM LEMAK LINOLEAT DAN LINOLENAT PADA MINYAK KEDELAI SECARA KROMATOGRAFI GAS. J. Sainstek Dan Ter. 2011, 6, 76–81. [Google Scholar]
- Astawan, M.; Adiningsih, N.R.; Palupi, N.S. Evaluation on Tempeh Nugget Quality Madefrom Different Soybean Varieties. J. Pangan 2014, 23, 244–255. [Google Scholar]
- Ginting, E.; Antarlina, SS.; Widowati, S. Varietaskedelaiuntukbahanbakuindustripangan. J. Litbang Pertan. 2009, 28, 79–87. [Google Scholar]
- Sudarmadji, S.; Suhardi, S.; Haryono, B. Analisa Bahan Makanan Dan Pertanian; Yogyakarta: Liberty Yogyakarta bekerjasamadengan Pusat Antar Universitas Pangan dan Gizi Universitas Gadjah Mada, 1989. [Google Scholar]
- Jayanti, E.T. Kandungan Protein Biji Dan Tempe Berbahan Dasar Kacang-KacanganLokal (Fabaceae) Non Kedelai (Seeds and Tempeh Protein Content From Non Soybean Fabaceae). Biosci. J. Ilm. Biol. 2019, 7. [Google Scholar]
- Ginting, E.; Tastra, I.K. StandarMutuBijiKedelai. Kedelai: Teknik Produksi Dan Pengembangan. 2016; pp 444–463.
- ArdianPurnama, F. Kadar Air, Abu, Protein, danKarbohidratpadaTahapanPembuatanTempe.DoktoralDisertation, Program Studi Kimia FSM-UKSW, 2012.
- Giri, S.K.; Tripathi, M.K.; Kotwaliwale, N. Effect of composition and storage time on some physico-chemical and rheological properties of probiotic soy-cheese spread. J. Food Sci. Technol. 2018, 55, 1667–1674. [Google Scholar] [CrossRef]
- Araújo, J.M.A. Química de Alimentos: Teoria e Prática [Editorial] UFV. In Química de alimentos: Teoria e prática.
- Baena, A.; Orjuela, A.; Rakshit, S.K.; Clark, J.H. Enzymatic hydrolysis of waste fats, oils and greases (FOGs): Status, prospective, and process intensification alternatives. Chem. Eng. Process. Process Intensif. 2022, 175, 108930. [Google Scholar] [CrossRef]
- dos Santos, J.J.A.; Pascoal, L.A.F.; Grisi, C.V.B.; da Costa Santos, V.; de Santana Neto, D.C.; Jordão Filho, J.; et al. Soybean oil and selenium yeast levels in the diet of rabbits on performance, fatty acid profile, enzyme activity and oxidative stability of meat. Livest. Sci. 2022, 263, 105021. [Google Scholar] [CrossRef]
- dos Santos, L.K.; Hatanaka, R.R.; de Oliveira, J.E.; Flumignan, D.L. Production of biodiesel from crude palm oil by a sequential hydrolysis/esterification process using subcritical water. Renew. Energy 2019, 130, 633–640. [Google Scholar] [CrossRef]
- Nor Shafizah, I.; Irmawati, R.; Omar, H.; Yahaya, M.; Alia Aina, A. Removal of free fatty acid (FFA) in crude palm oil (CPO) using potassium oxide/dolomite as an adsorbent: Optimization by Taguchi method. Food Chem. 2022, 373, 131668. [Google Scholar] [CrossRef] [PubMed]
- He, S.H.; Liu, C.H.; Wang, R.C.; Zhou, S.J.; Guo, W.Y.; Wang, Y.H. Comparison of two different natural oil body emulsions: In vitro gastrointestinal digestion. J. Oleo Sci. 2020, 69, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Cheng, C.C.; Chou, S.S. Determination of arsenic in edible oils by direct graphite furnace atomic absorption spectrometry. J. Food Drug Anal. 2003, 11, 214–219. [Google Scholar] [CrossRef]
- Geng, L.; Zhou, W.; Qu, X.; Sa, R.; Liang, J.; Wang, X.; et al. Iodine values, peroxide values and acid values of Bohai algae oil compared with other oils during the 1 cooking 2. Heliyon 2022, 9, e15088. [Google Scholar] [CrossRef]
- Chaiyasit, W.; Elias, R.J.; McClements, D.J.; Decker, E.A. Role of physical structures in bulk oils on lipid oxidation. Crit. Rev. Food Sci. Nutr. 2007, 47, 299–317. [Google Scholar] [CrossRef]
- Chaiyasit, W.; Stanley, C.B.; Strey, H.H.; McClements, D.J.; Decker, E.A. Impact of surface active compounds on iron catalyzed oxidation of methyl linolenate in AOT-water-hexadecane systems. Food Biophys. 2007, 2, 57–66. [Google Scholar] [CrossRef]
- Choe, E.; David, B. Min. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Mcclements, D.J.; Decker, E.A. Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems; 2000; Vol. 65.
- dosSantos, J.J.A.; Pascoal, L.A.F.; Grisi, C.V.B.; Santos, V. da C.; de Santana Neto, D.C.; JordãoFilho, J.; et al. Soybean oil and selenium yeast levels in the diet of rabbits on performance, fatty acid profile, enzyme activity and oxidative stability of meat. Livest. Sci. 2022, 263, 105021. [Google Scholar] [CrossRef]
- Gobikrishnan, S.; Park, J.H.; Park, S.H.; Indrawan, N.; Rahman, S.F.; Park, D.H. Sonication-assisted production of biodiesel using soybean oil and supercritical methanol. Bioprocess Biosyst. Eng. 2013, 36, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Reisig, D.D.; Dean, L.L.; Reay-Jones, F.P.F.; Greene, J.K.; Carter, T.E.; et al. Mechanisms of Soybean Host-Plant Resistance against Megacoptacribraria (Hemiptera: Plataspidae). Environ. Entomol. 2020, 49, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Abdulmumin, T.M.; Yunusa, A.; Alhassan, A.J.; Muhammad, I.A.; Dalhatu, M. Amino Acid Evaluation and GC-MS Analysis of Bambaranut (Vigna subterranea (L) Verdc.) † Plant Toxicology View project Hepato protective effects of ethanolic root extracts of c. Procera on ccl4 induced hepatotoxicity in rats View project. Malays. J. Chem. 2020, 22, 78–86. [Google Scholar]
- Anjali Patil, S.; Biranje, S. Biochemical characterization of Ophiocordyceps nutans (pat.) G. UGC Care Group 1 J. 2022, 52, 70–83. [Google Scholar]
- Widihastuty; Susanti, R.; Fadhillah, W. SEMIOCHEMICAL INTERACTION BETWEEN Myopoponecastanea SMITH WITH ITS PREY Oryctes rhinoceros LINN. LARVAE Serangga, 2021; 26, 99–109. [Google Scholar]
- Kraiem, T.; Hassen, A. Ben; Belayouni, H.; Jeguirim, M. Production and characterization of bio-oil from the pyrolysis of waste frying oil. Environ. Sci. Pollut. Res. 2017, 24, 9951–9961. [Google Scholar] [CrossRef]
- Yang, R.; Wang, B.; Li, M.; Zhang, X.; Li, J. Preparation, characterization and thermal degradation behavior of rigid polyurethane foam using a malic acid based polyols. Ind. Crops Prod. 2019, 136, 121–128. [Google Scholar] [CrossRef]
- Valasi, L.; Kokotou, M.G.; Pappas, C.S. GC-MS, FTIR and Raman spectroscopic analysis of fatty acids of Pistacia vera (Greek variety “Aegina”) oils from two consecutive harvest periods and chemometric differentiation of oils quality. Food Res. Int. 2021, 148, 110590. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).