1. Introduction
Burgeoning popularity and acceptance of plant-based protein consumption have brought us a step closer to a more sustainable global food system. Among various sources of plant-based products, wild edible seaweeds (or macroalgae) have traditionally been harvested in many cultures [
1,
2] but pressures on natural supplies [
3] have instigated industrial-scale marine macroalgae farming [
4]; algae farming now supplies most of the market demand. China is the largest producer of edible macroalgae farmed on suspended ropes in the ocean; similar technology is used in Indonesia and other countries. Macroalgae is an important source of hydrocolloids such as alginate, agar, and carrageenan which have been used as thickening and gelling agents in food, pharmaceuticals, and textile printing. Macroalgae have also long been used as a fertiliser, the high fibre content acts as a soil conditioner and the mineral content as a fertiliser. Increasing concerns about the environment as well as global warming and peak oil have driven interest in microalgae for biofuel. The potential for algae production particularly in developing countries has been recognised including by the Food and Agriculture Organisation of the United Nations [
5]. Microalgae farming technology has enabled a shift in agricultural operations from the sea onto land over recent decades, offering better control, economies of scale, and greater ease of harvesting than macroalgae farming. Microalgae are a highly-productive and low-greenhouse-gas emission cash crop requiring only modest investment in equipment: a microalgae farm does not require arable land and can use waste water [
6,
7]; microalgae grow fast, are continually harvestable [
7], and can sequester carbon dioxide [
8,
9]. Moreover, microalgae are highly nutritious with up to 60% protein content [
8], rich in vitamins and minerals [
10], and are associated with health benefits [
10], which adds to their appeal and value. As a plant-based source of omega-3, microalgae are an alternative to unsustainable fish wild-catch which contain omega-3 only because of consumption of microalgae in the food-chain.
Microalgae farming has commonalities with other crops in that it needs water, nutrients as well as light to grow. As with any other crop grown in monoculture, microalgae crops can attract pests and diseases. Problems can include attack by predators, invasion by competitive species, toxic byproducts, bacteria, and viruses in the crop as well as in wastewater and harvested products. The harvested microalgae need to be dried as soon as possible before rot sets in. Management of the growing medium is one of the challenges of land-based microalgae farming. Water stagnation, toxic blooms, and slime proliferation are risks associated with poor quality control, which can cause concern among communities and jurisdictions located near microalgae production facilities. Local government approval of a microalgae farm in Goondiwindi, Queensland, Australia was contingent upon on the owners, AlgaePharm, properly managing water run-off, storm water, wastewater, security, landscaping, as well as fauna that might be attracted to the farm [
11]. Meticulous monitoring of water and control of the media culture is critical to optimising the growth of the preferred microalgae species, and identifying and controlling competitor species and predators [
12]. Manual media monitoring methods are laborious, require a high level of expertise and are prone to human error [
13]. At the aforementioned AlgaePharm microalgae farm, which uses an open phototropic system to cultivate the Nannochloropsis oculate microalgae strain, manual microalgae crop monitoring at each pond currently requires one hour per day of skilled personnel trained in the microscopic analysis of microalgae species (AlgaePharm management, personal communication).
Considering the growth of digital automated technologies in applications of agriculture and the specific challenges faced by the microalgae farmers in managing their ponds, there is a need for efficient automated models for microalgae species identification. Deep learning-enabled image recognition, which has been widely applied to agricultural pest identification [
14,
15], can be harnessed to automate the process of microalgae culture species classification, which is a key to building a sustainable healthy microalgae ecosystem and for detecting potential invaders and other threats. Wang et al., 2022 [
16], reviewed IoT technologies that could be adopted for microalgae biorefinery and presented a model for microalgae environmental management that encompassed automation, sensors, lab-on-chip, machine learning, and the Internet of Things. Xu et al., 2022 [
17], combined three-dimensional fluorescence with machine learning and deep learning to successfully distinguish microalgae that cause paralytic shellfish poisoning from those that do not. However, a fully automated microalgae species identification and pond management require more accurately trained models that are yet to be implemented.
The purpose of this study was to develop a model for the systematic detection of microalgae species based on microscopic images, which is computationally lightweight and easy to implement for monitoring the growth media in microalgae farms using automatic detection models.
Specifically, this study: (i) acquired 595 images of the Nannochloropsis sp microalgae and competitor Spirulina and developed a transfer learning model, (ii) applied cross-validation to demonstrate excellent classification accuracy for both species on a tenfold cross-validation approach, and (iii) demonstrated the usefulness of the method in microalgae species identification and potential pond management applications for algae farming. Digital automation methods are essential to meet next-generation agriculture and food technologies needs [
18]. Based on our studies, the proposed automation models can help streamline processes to reduce time and costs, increase accuracy, and allow for more data-driven decisions. The proposed automation approaches can also help increase efficiency of existing monitoring systems that are largely manual and reduce time spent, leading to a more sustainable future for agriculture and food production. The methods developed can give farmers a better approach for managing the algae ponds by automating monitoring and early detection of invader species and the other environmental perturbations that might require adjustments to environmental variables.
2. Materials and Methods
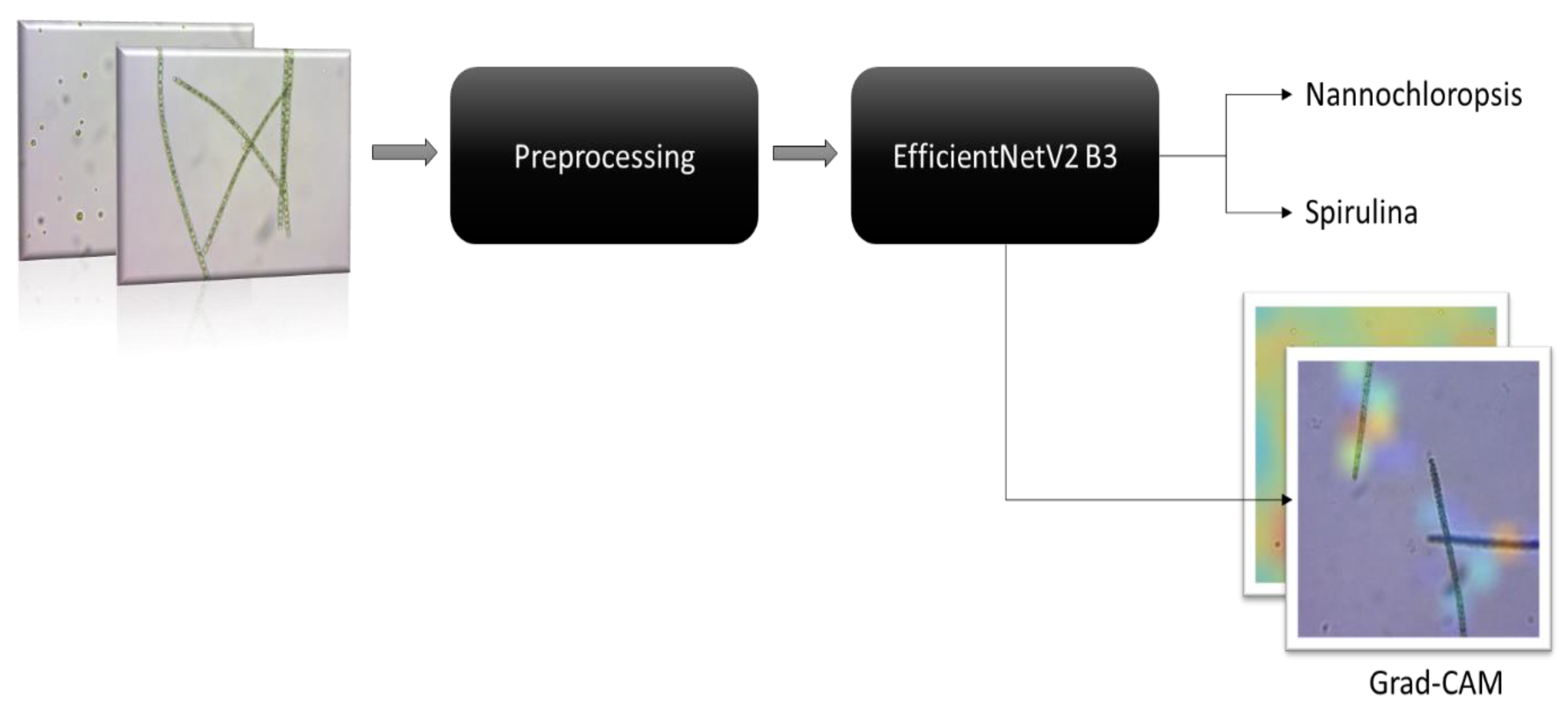
Our study is based on the premise that one of the challenges faced by researchers in this area is the limited availability of high-quality labelled image datasets to help identify the microalgae species, which constitute a barrier to research into and uptake of microalgae farming. For this study, we have prospectively acquired 434 images of healthy Nannochloropsis sp. microalgae, which was the preferred crop species, and 161 images of Spirulina from the Goondiwindi AlgaePharm growing ponds. The images were pre-processed by cropping with a 1:1 aspect ratio, resizing to 300 x 300, and then the step of converting the images to a red-green-blue (RGB) scale and normalising the pixels to -1 and 1.
Using an important technique of ten-fold cross-validation, the pre-processed images were fed to a pre-trained neural network called EfficientNetV2 B3 [
19] which then classified them into Nannochloropsis oculate and Spirulina classes based on the pre-processed images. To visualise the most informative parts of the pre-processed input images, we also applied gradient-weighted class activation mapping (Grad-CAM) to the modified EfficientNetV2 B3 transfer learning model (
Figure 1). It should be noted that EfficientNetV2 B3, like many deep learning models, is a "black box" model in which the inner workings of how the model predicts the results are indecipherable by design [
20], which may prevent its acceptance by application developers. An explainable artificial intelligence technique, Grad-CAM is typically applied to the final layer of CNN models to generate a heatmap of the relative contributory importance of different regions within the input images to the prediction [
21], which allows for some degree of interpretation of model predictions.
Table 2 B3 [
19], which is a type of convolutional neural network (CNN). While convolutional neural network models are widely used for image recognition tasks, EfficientNetV2 B3 takes it up to another level with training-aware neural architecture search, which accelerates the learning rate and improves parameter effectiveness. In our study, we have modified EfficientNetV2 B3 by freezing the base model except for the fully-connected layers, which were removed and replaced by batch normalisation, the dropout layer (0.2), and three other trainable fully-connected layers (
Table 1). The model was trained with the Adam optimiser [
22] with a 0.001 learning rate, batch size of 5, and run with 10 epochs. A weighted loss function was also incorporated into the model to mitigate dataset class imbalance during training.
Classification performance was evaluated using standard metrics: accuracy, sensitivity, specificity, and precision.
3. Results
Based on the results of the classification experiment, the modified EfficientNetV2 B3 model was able to correctly identify 98.32% of the dataset (
Table 2). As shown in the table below, we have calculated performance metrics separately for Nannochloropsis and Spirulina samples. Our model is reliable since it can independently determine whether a sample is Nannochloropsis or Spirulina, it does not classify all samples in a binary fashion as one of the two. After training the model with multiple data augmentation techniques, we observed an increase in the accuracy of the model compared to the baseline model. This demonstrates that our model is able to identify the differences between Nannochloropsis and Spirulina samples more accurately. Furthermore, the model was able to correctly identify 98.32% of the dataset, which further confirms the robustness of our model.
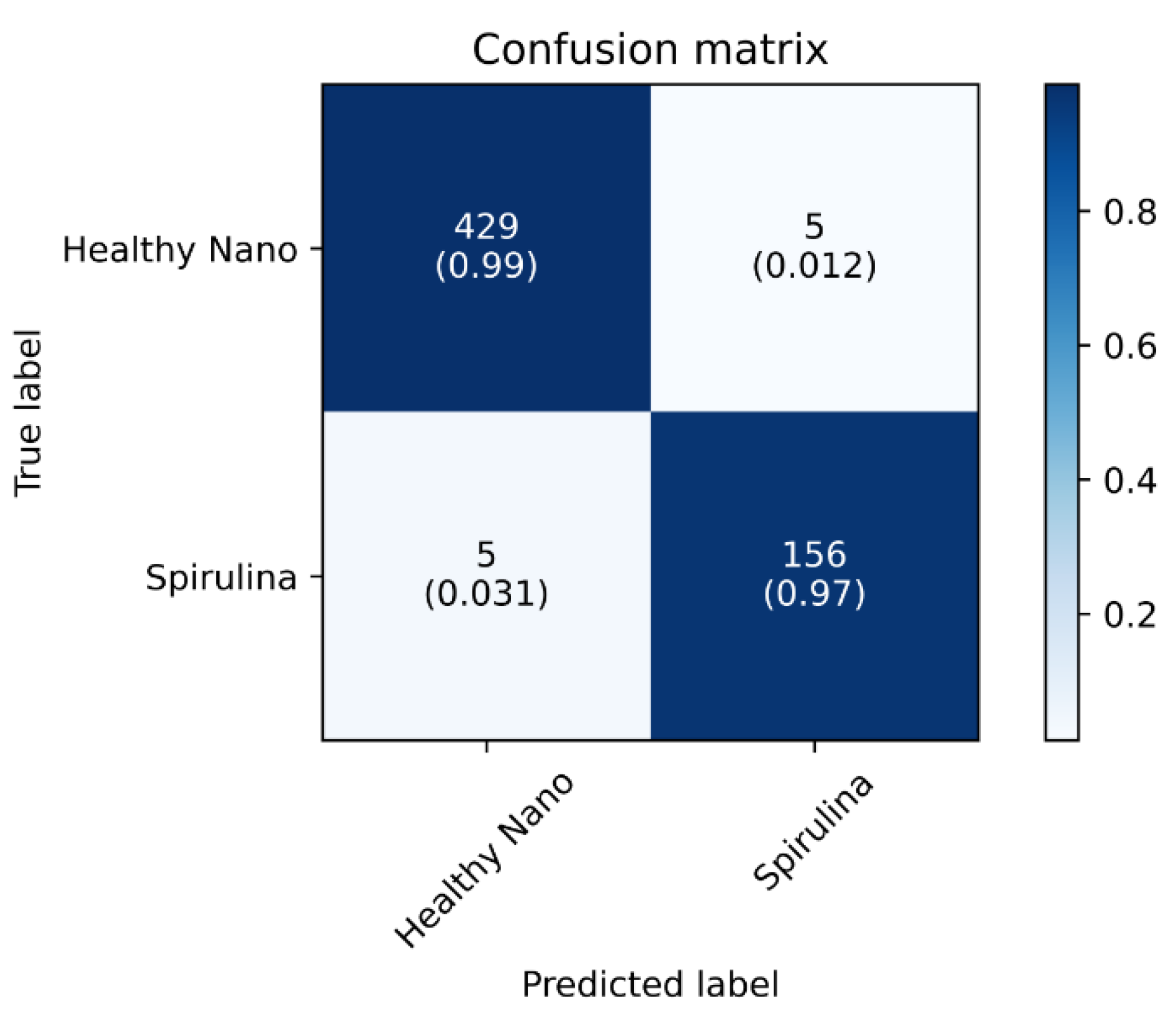
As can be seen from the confusion matrix, there is a very low rate of misclassification within each class, resulting in only five samples being classified incorrectly from each (
Figure 2). Based on the confusion matrix, the classification can either be binary or non-binary. For example, Nano 429/434 is correctly classified as Spirulina, while Spirulina 5/434 is incorrectly classified. As illustrated in
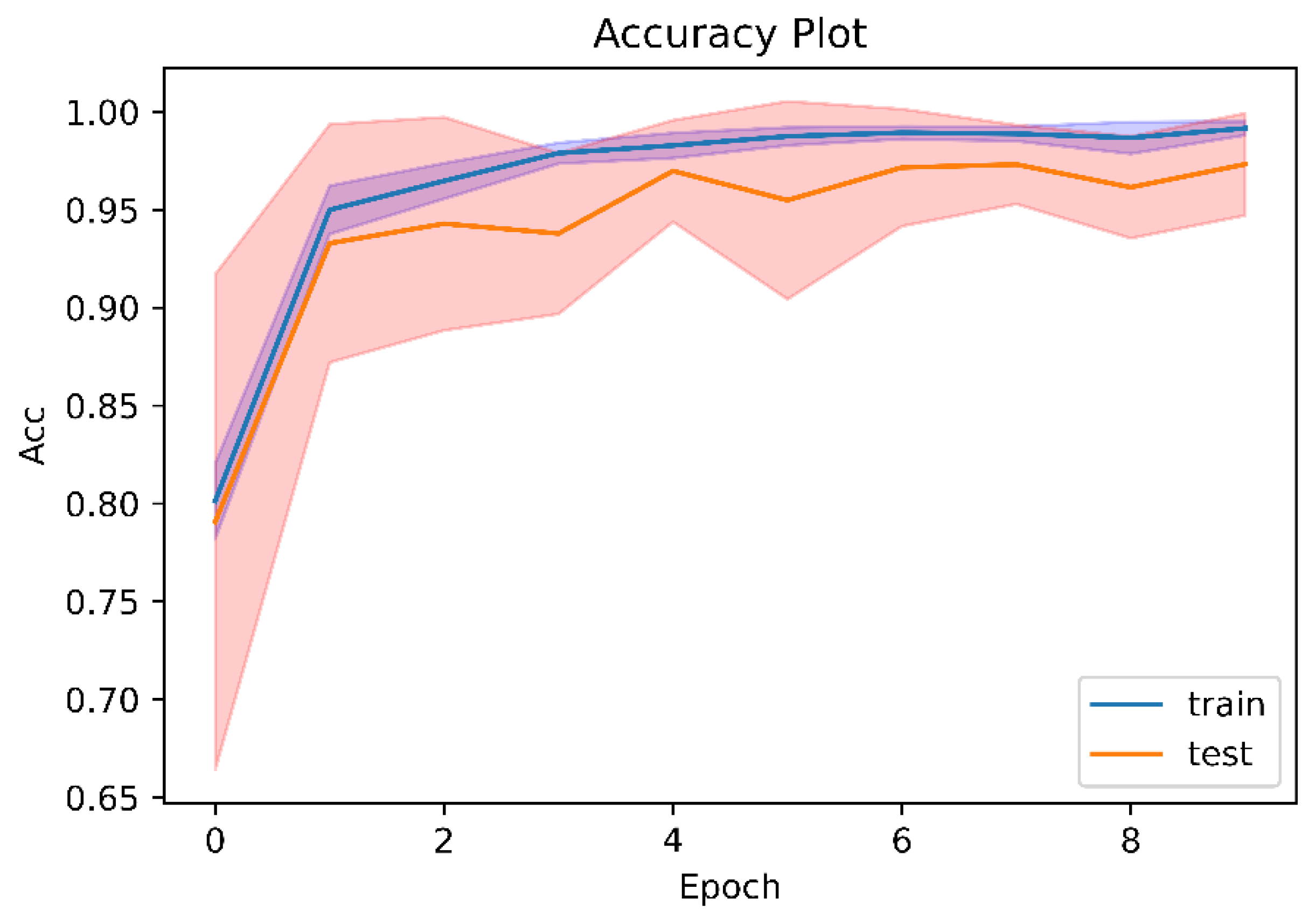
Figure 3, the performance graph generated during EfficientNetV2 B3 training also indicates that the model did not overfit, which corresponds to the number in brackets. The confusion matrix provides a visual representation of the model’s performance. It shows the number of true positives, false positives, false negatives and true negatives, which can then be used to calculate the accuracy, precision, recall and other metrics that measure the model’s performance. The performance graph also shows the model’s performance over the course of training, which can be used to detect overfitting.
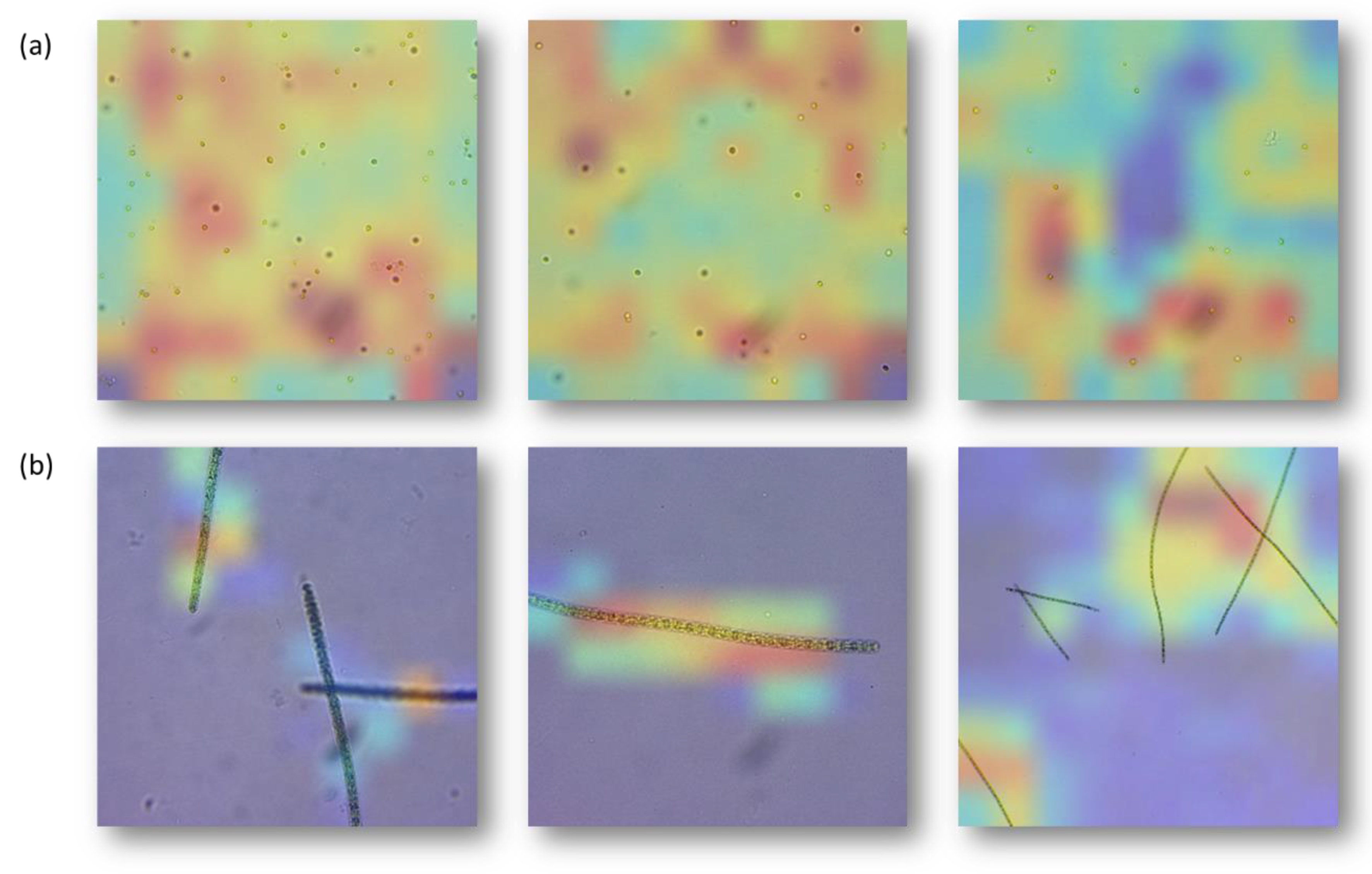
As illustrated in
Figure 4, heatmaps created by Grad-CAM were superimposed over pre-processed images of Nannochloropsis sp. and Spirulina samples as inputs. On the basis of a visual inspection, the regions that were flagged as highly relevant for model classification by Grad-CAM also overlap significantly with the microorganisms. It supports the contention that the EfficientNetV2 B3 model is capable of automatically focusing attention more specifically on relevant areas of an image to perform the classification process. This suggests that EfficientNetV2 B3 model has the potential to be a reliable classifier for microorganism identification in microscopic images.
Figure 4 shows Grad-CAM generated heatmaps for Nannochloropsis sp. (a) and Spirulina (b). The red regions denote regions that were highly relevant for classification; and the blue regions are irrelevant. These heatmaps enable us to quantitatively measure the relative importance of each region for the classification, with red regions indicating the most important features and blue regions indicating the least important features. By using the heatmaps, it became clear which regions of the images were most important for the classification and which were not, allowing us to gain a better understanding of the models used.
4. Discussion
We modified an existing deep transfer learning classifier, EfficientNetV2 B3, to perform automated image-based classification of microalgae species. The application is novel and the proposed system is simpler, requires minimal pre-processing, is less computationally demanding and yields commensurate accuracy compared with previous works (
Table 3). Luo et al., 2021 [
23], proposed a support vector machine or deep learning model to distinguish one species of individual microalgae from another with high accuracy. They also demonstrated the feasibility of the use of a confocal hyperspectral microscopic imager in the analysis of the concentration, species, and distribution differences of microalgae. Specifically, they used the model to identify the species of microalgae in a range of different concentrations, including those found in natural environments. The model was able to accurately classify the species with a high degree of accuracy, demonstrating the potential of the model for use in microalgae research.
Baladehi et al., 2021 [
24], established an approach for rapidly identifying and metabolically profiling single cells, either cultured or uncultured, using pigment spectrum and whole spectrum, which could accelerate the mining of microalgae and their products. Mirasbekov et al., 2021 [
25], employed machine learning to detect and quantify Microcystis colonial morphospecies using the FlowCAM-based imaging flow device. They demonstrated the proof-of-concept of how machine learning approaches could be applied to analyse microalgae. The authors used an imaging flow device to capture images of the microalgae and then used machine learning algorithms to analyse the images. They were able to accurately classify the different morphospecies of Microcystis, showing how machine learning can be used as a powerful tool for analysis.
Wang et al., 2020 [
26], used a label-free method to identify living and dead algae cells based on digital holographic microscopy and machine learning. Memmolo et al 2020 [
27], used convolutional neural network models to classify diatoms on dry slide images to avoid the time-consuming steps of water sampling and labelling by skilled marine biologists. The network model was then validated by using holographic recordings of live diatoms imaged in water samples. Xu et al., 2020 [
28], performed hyperspectral imaging of three species of microalgae to verify their absorption characteristics. They demonstrated the feasibility of the technology for evaluating the growth state of microalgae through their transmission spectra.
Building on all these studies, we have developed a model that could automatically identify microalgae ―both the preferred and invader species―with high accuracy, thereby realising the potential of transfer deep learning for real-world applications. With appropriate and larger training datasets, the work could be extended for the classification of other microalgae classes. For microalgae farming, species identification is but the first step. By linking the classification results to a real-time intelligent database, deep learning could provide timely decision-support: upon detecting populations it could advise the farmer of required interventions to produce the desired response in crop growth or time to harvest, e.g., water rotation, nutrient supply, water treatment, etc.
Our results are of practical value considering that in order to gain the most benefit from microalgae farming, farmers must have a thorough understanding of the species being grown, and the ability to implement the necessary adjustments to produce desired results. Deep learning technology can provide real-time decision-support, helping the farmer optimise crop growth and harvest times through interventions such as water rotation, nutrient supply, and water treatment.
The advantages of the proposed method in this study are as follows:
To the best of our knowledge, we are the first group to classify healthy Nannochloropsis sp. microalgae and Spirulina classes with an accuracy of >98%.
The developed model is robust as we have employed a ten-fold cross-validation strategy.
We have provided explainable AI to visualise the heatmaps in the Nannochloropsis sp. and Spirulina classes.
In spite of good performance of the proposed model for microalgae identification, one of the limitations of our work is that we have developed the model using a small dataset of 434 images of healthy Nannochloropsis sp. microalgae, which was the preferred crop species, and 161 images of Spirulina. In the future, we plan to validate our model using more images taken from other ponds. Also, we intend to consider more classes of algae images. This means that the model is unable to accurately identify microalgae of other species, as it has only been trained on images of Nannochloropsis and Spirulina. Additionally, since the dataset is relatively small, the model may not be able to generalise well to other ponds and environments, and more images from different ponds would be needed to improve accuracy.
In an era of increasing concern about the environment and global warnings as well as peak oil, there is potential for greater investment in microalgae for biofuel. , for its high nutritional value and its high productivity compared to other agricultural crops. Grown on non-arable land and utilising almost any source of water, microalgae farming has the potential internationally as an environmentally friendly source of biofuels, animal/human food, nutraceuticals, cosmetics, and plastics. A key threat to microalgae farming is pest attack, damaging a crop overnight (a ‘crash’), and taking a long time to recover. Current pest or invader identification methods are largely manual, time-consuming, and prone to human error, impacting productivity. Our innovation will make microalgae farming accessible to more farmers around the world. Our innovation uses machine learning and image recognition technology to identify pests and invaders quickly and accurately. By automating the pest identification process, microalgae farmers will be able to act more quickly to protect their crops and reduce the risk of crop failure.
In the past the technology for algae farming was simple, such as harvesting from the sea or growing macroalgae on structures that can be pulled out to harvest it. Technology has opened up large-scale microalgae monoculture production in controlled environments primarily on land which provides economies of scale. It is a potential additional or main crop for many farmers; it could be an attractive option for Australia and many countries with abundant sunlight. Whilst building the required shallow ponds and installing equipment may not be challenging in terms of cost or expertise, few farmers are likely to have the expertise to identify and monitor the populations. An AI-based system could help farmers with monitoring their microalgae crop to identify the health of the preferred species and the presence of invaders and respond appropriately in adjusting variables. With an AI-based automation system, farmers will be able to monitor the health of the microalgae crop in real-time, while also being able to adjust variables in order to create an optimal environment for the desired species. Additionally, AI-based systems can also be used to detect any unwanted invaders before they become a problem, allowing farmers to take preventative measures to protect their crop.
The preliminary development of the modified EfficientNetV2 B3 model presented in this paper offers potential public benefit by making microalgae farming expertise more available to the farming community, improving drought resistance and farm productivity, reducing labour needs, and reducing pesticide use through early detection of unwanted species. Improving farm resilience benefits regions, the economy, and human health through the high nutritional value of microalgae and the environment to develop and improve productivity and environmental resilience.
5. Conclusions
In this study, we have demonstrated that a simple deep transfer learning model, denoted as modified EfficientNetV2 B3, developed for the classification of microalgae species can be feasible and accurate in order to classify microalgae. There is a practical need for robust automated monitoring of preferred and invader microalgae populations in industrial microalgae farms to ensure the sustainability of the farms. Therefore this detection model has been developed in order to satisfy that practical need. This model also has the potential to help improve the efficiency and sustainability of industrial microalgae farms, thereby helping to ensure their future success.
For effective management of the culture, it is necessary to identify the culture in a way that produces a consistent and predictable output. It is anticipated that if the model is successfully implemented, there is the potential to increase the productivity of microalgae farming, as well as to promote the use of microalgae as a viable alternative source of plant-based protein and other products once the model has been successfully implemented.
By creating a model that is based on the identification of the culture, managers can better understand the unique characteristics of the microalgae, allowing them to make informed decisions about how to best utilise the resources available to them. This in turn can lead to more efficient management of the microalgae farm, as well as improved outcomes for the products produced.
Author Contributions
Conceptualisation, J.S., R.A., A.W.; methodology, R.A.; software, O.D.L, L.H.W.; validation, J.S., R.A., E.S, R.C.D.; formal analysis, R.A., E.S., R.C.D., O.S.L, L.H.W; investigation, J.S., R.A, O.S.L, E.S, R.C.D., A.W., L.H.W.; resources, E.R., L.H.W.; data curation, E.R., E.S, R.C.D.; writing—original draft preparation, J.S., R.A., E.S., R.C.D.; writing—review and editing, J.S., R.A., E.S, R.C.D., A.W.; visualisation, J.S., R.A.; supervision and project administration, J.S., R.A.; data acquisition, E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable
Data Availability Statement
Data are available on request to the Corresponding Author.
Acknowledgments
The authors acknowledge AlgaePharm, Goondiwindi, Australia for the supply of all images that were used in the research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buchholz, C.M.; Krause, G.; Buck, B.H. Seaweed and Man. In: Seaweed Biology. Ecological Studies (Analysis and Synthesis), Wiencke, C.; Bischof, K.; Springer, Berlin, Heidelberg. 2012, Volume 219. [CrossRef]
- Merz, C.; Main, K. Microalgae (diatom) production — The aquaculture and biofuel nexus. Oceans’14 MTS/IEEE Conference Proceedings – IEEE Xplore, St. John’s, NL, Canada, 2014. [CrossRef]
- Mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable harvesting of wild seaweed resources. European Journal of Phycology 2017, 52, 371–390. [Google Scholar] [CrossRef]
- Marsham, S.; Scott, G.W.; Tobin, M.L. Comparison of nutritive chemistry of a range of temperate seaweeds. Food Chemistry 2007, 100, 1331–1336. [Google Scholar] [CrossRef]
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A.; Lucente, D.; Mair, G.; Miao, W.; Potin, P.; Przybyla, C.; Reantaso, M.; Roubach, R.; Tauati, M.; Yuan, X. Seaweeds and microalgae: an overview for unlocking their potential in global aquaculture development. FAO Fisheries and Aquaculture Circular, (1229). 2021. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Andrade, D.S.; Amaral, H.F.; Gavilanes, F.Z.; Morioka, L.R.; Nassar, J.M.; Muniz de Melo, J.; Rodrigues, S.H.; Santos Telles, T. Microalgae: Cultivation, Biotechnological, Environmental, and Agricultural Applications. In Advances in the Domain of Environmental Biotechnology; Gavilanes, F, Morioka, L, Nassar, J, Muniz de Melo, J., Rodrigues, S, Santos Telles, T., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: the potential of domestication towards sustainable biofactories. Microb Cell Fact 2018, 17. [Google Scholar] [CrossRef]
- McGinn, P.J.; Dickinson, K.E.; Bhatti, S.; Frigon, J.-C.; Guiot, S.R.; O’Leary, S.J.B. Integration of microalgae cultivation with industrial waste remediation for biofuel and bioenergy production: opportunities and limitations. Photosynth Res 2011, 109, 231–247. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation Into Innovative Food Products With Potential Health Benefits. Front. Nutr. 2018, 5. [Google Scholar] [CrossRef]
- Goondiwindi Regional Council. Communication of decision. mail@grc.qld.gov.au - Goondiwindi Regional Council. 27 November 2018. Available online: https://www.grc.qld.gov.au/downloads/file/1028/17-48g-decision-notice.
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: current trends, prospects and challenges. Biotechnology Advances 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- Deore, P.; Beardall, J.; Noronha, S. A perspective on the current status of approaches for early detection of microalgal grazing. J Appl Phycol 2020, 32, 3723–3733. [Google Scholar] [CrossRef]
- He, Y.; Zeng, H.; Fan, Y.; Ji, S.; Wu, J. Application of Deep Learning in Integrated Pest Management: A Real-Time System for Detection and Diagnosis of Oilseed Rape Pests. Mobile Information Systems 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Liu, L.; Wang, R.; Xie, C.; Yang, P.; Wang, F.; Sudirman, S.; Liu, W. PestNet: An End-to-End Deep Learning Approach for Large-Scale Multi-Class Pest Detection and Classification. IEEE Access 2019, 7, 45301–45312. [Google Scholar] [CrossRef]
- Wang, K.; Khoo, K.S.; Leong, H.Y.; Nagarajan, D.; Chew, K.W.; Ting, H.Y.; Selvarajoo, A.; Chang, J.-S.; Show, P.L. How does the Internet of Things (IoT) help in microalgae biorefinery? Biotechnology Advances 2022, 54, 107819. [Google Scholar] [CrossRef]
- Xu, W.; Niu, J.; Gan, W.; Gou, S.; Zhang, S.; Qiu, H.; Jiang, T. Identification of paralytic shellfish toxin-producing microalgae using machine learning and deep learning methods. J. Ocean. Limnol. 2022, 40, 2202–2217. [Google Scholar] [CrossRef]
- Austrade, Australian Government. Australia: shaping the future of food and agriculture. Available online: https://www.austrade.gov.au/agriculture40 (accessed on 11 February 2022).
- Tan, M.; Le, Q. EfficientNetV2: Smaller Models and Faster Training. In Proceedings of the 38th International Conference on Machine Learning/Proceedings of Machine Learning Research. PMLR 139, 14 December 2021. Available online: https://proceedings.mlr.press/v139/tan21a.html.
- Selvaraju, R.R.; Das, A.; Vedantam, R.; Cogswell, M.; Parikh, D.; Batra, D. Grad-CAM: Why did you say that? 2016, arXiv:1611.07450. [Google Scholar]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-CAM: Visual Explanations from Deep Networks via Gradient-Based Localization. Int J Comput Vis 2020, 128, 336–359. [Google Scholar] [CrossRef]
- Zhang, Z. Improved Adam Optimizer for Deep Neural Networks. IEEE/ACM 26th International Symposium on Quality of Service (IWQoS). 2018. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, H.; Forsberg, E.; Hou, S.; Li, S.; Xu, Z.; Chen, X.; Sun, X.; He, S. Confocal hyperspectral microscopic imager for the detection and classification of individual microalgae. Opt. Express 2021, 29, 37281. [Google Scholar] [CrossRef]
- Heidari Baladehi, M.; Hekmatara, M.; He, Y.; Bhaskar, Y.; Wang, Z.; Liu, L.; Ji, Y.; Xu, J. Culture-Free Identification and Metabolic Profiling of Microalgal Single Cells via Ensemble Learning of Ramanomes. Anal. Chem. 2021, 93, 8872–8880. [Google Scholar] [CrossRef]
- Mirasbekov, Y.; Zhumakhanova, A.; Zhantuyakova, A.; Sarkytbayev, K.; Malashenkov, D.V.; Baishulakova, A.; Dashkova, V.; Davidson, T.A.; Vorobjev, I.A.; Jeppesen, E. Semi-automated classification of colonial Microcystis by FlowCAM imaging flow cytometry in mesocosm experiment reveals high heterogeneity during seasonal bloom. Sci Rep 2021, 11. [Google Scholar] [CrossRef]
- Wang, Y.; Ju, P.; Wang, S.; Su, J.; Zhai, W.; Wu, C. Identification of living and dead microalgae cells with digital holography and verified in the East China Sea. Marine Pollution Bulletin 2021, 163, 111927. [Google Scholar] [CrossRef]
- Memmolo, P.; Carcagnì, P.; Bianco, V.; Merola, F.; Goncalves da Silva Junior, A.; Garcia Goncalves, L.M.; Ferraro, P.; Distante, C. Learning Diatoms Classification from a Dry Test Slide by Holographic Microscopy. Sensors 2020, 20, 6353. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Ji, J.; Forsberg, E.; Li, Y.; He, S. Classification, identification, and growth stage estimation of microalgae based on transmission hyperspectral microscopic imaging and machine learning. Opt. Express 2020, 28, 30686. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).