Submitted:
18 May 2023
Posted:
19 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
Results
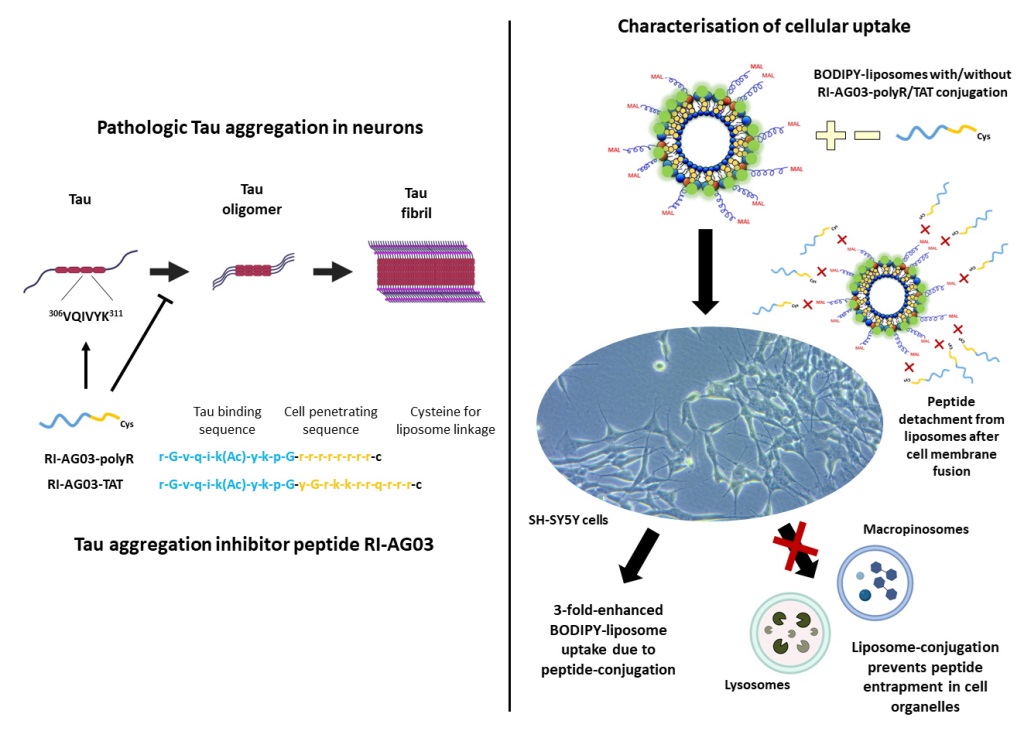
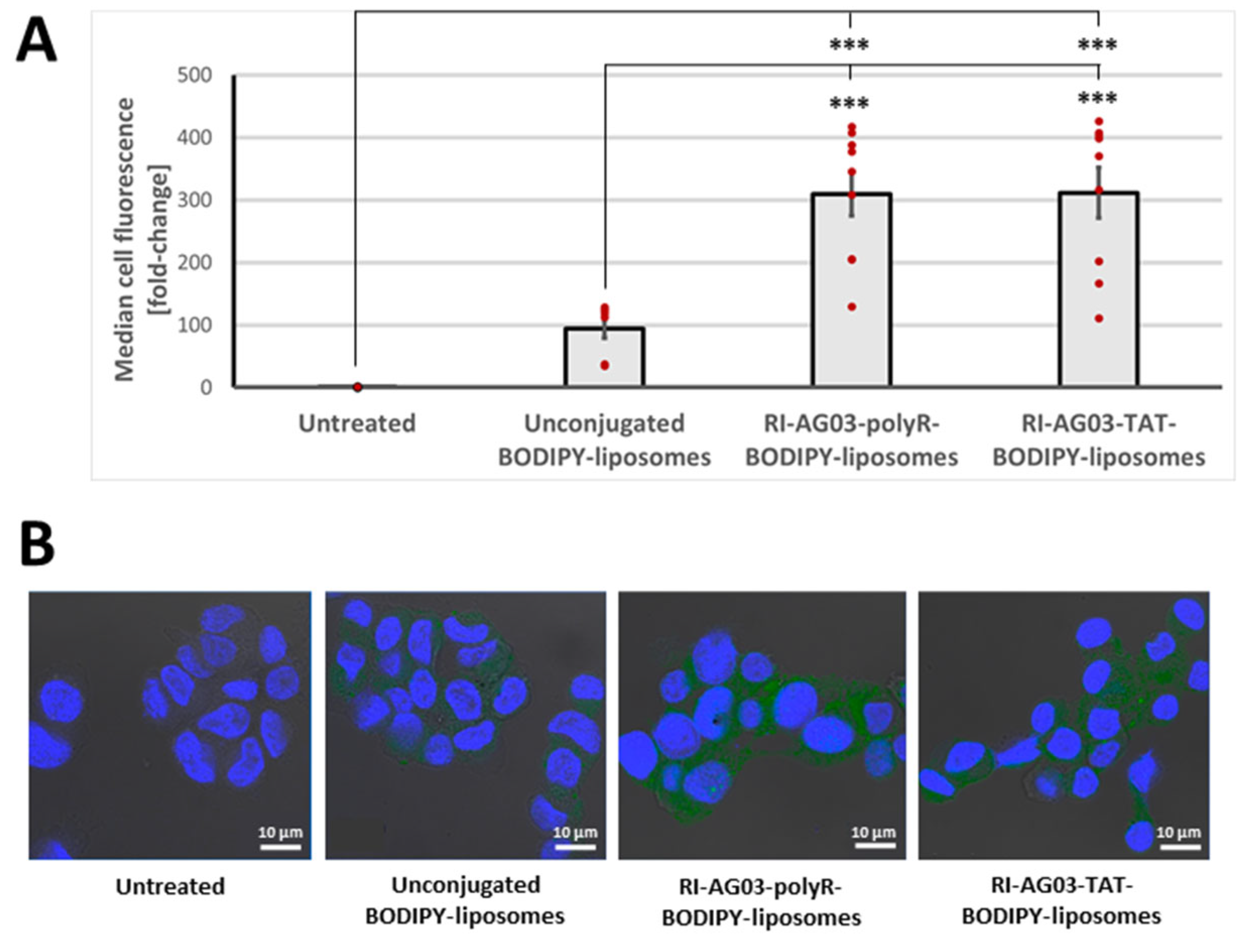
Conjugating RI-AG03-polyR and RI-AG03-TAT to Liposomes Enhances Cellular Liposome Uptake
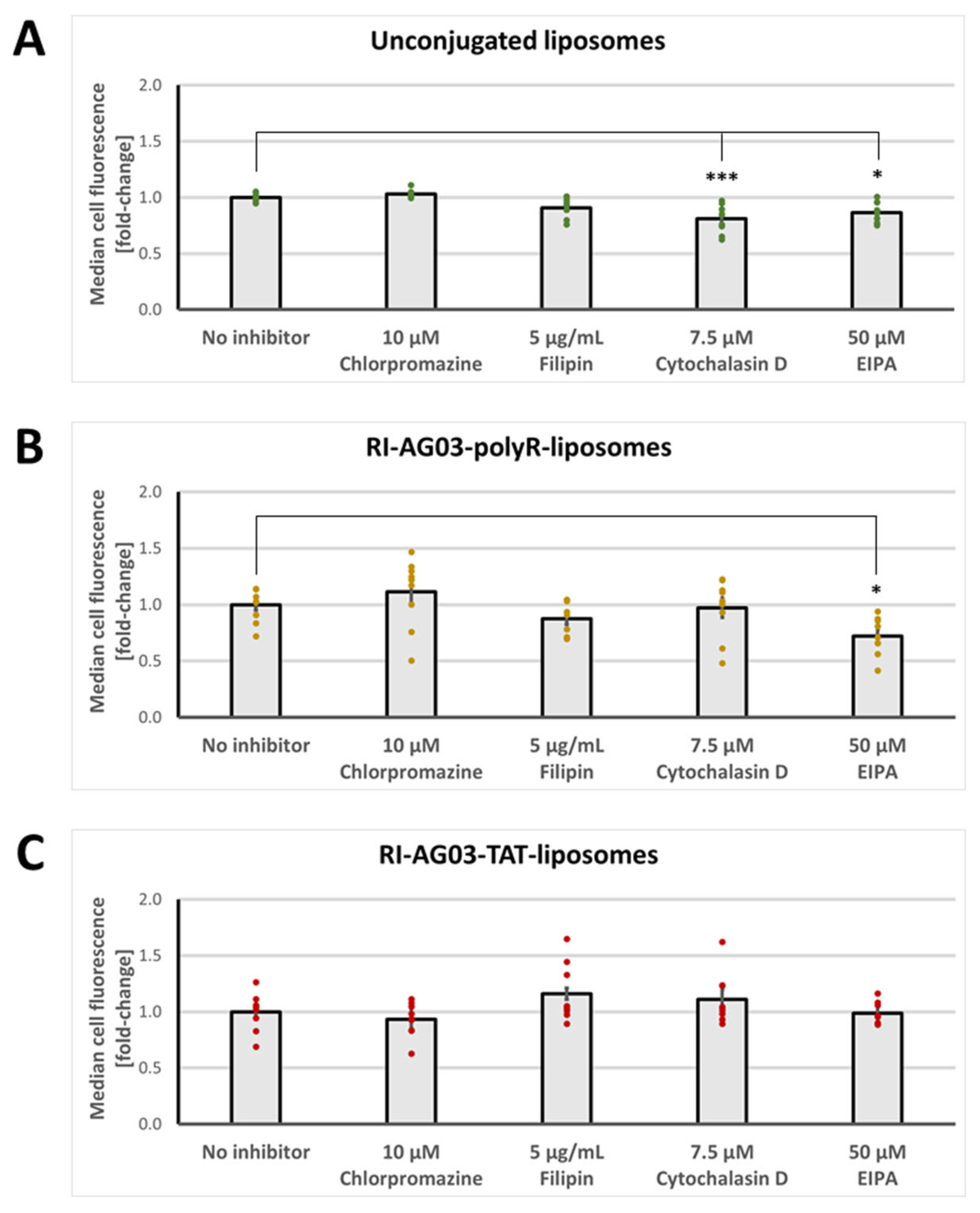
Unconjugated and RI-AG03-polyR Conjugated Liposomes, but not RI-AG03-TAT-Conjugated Liposomes, Are Partially Internalised by Macropinocytosis
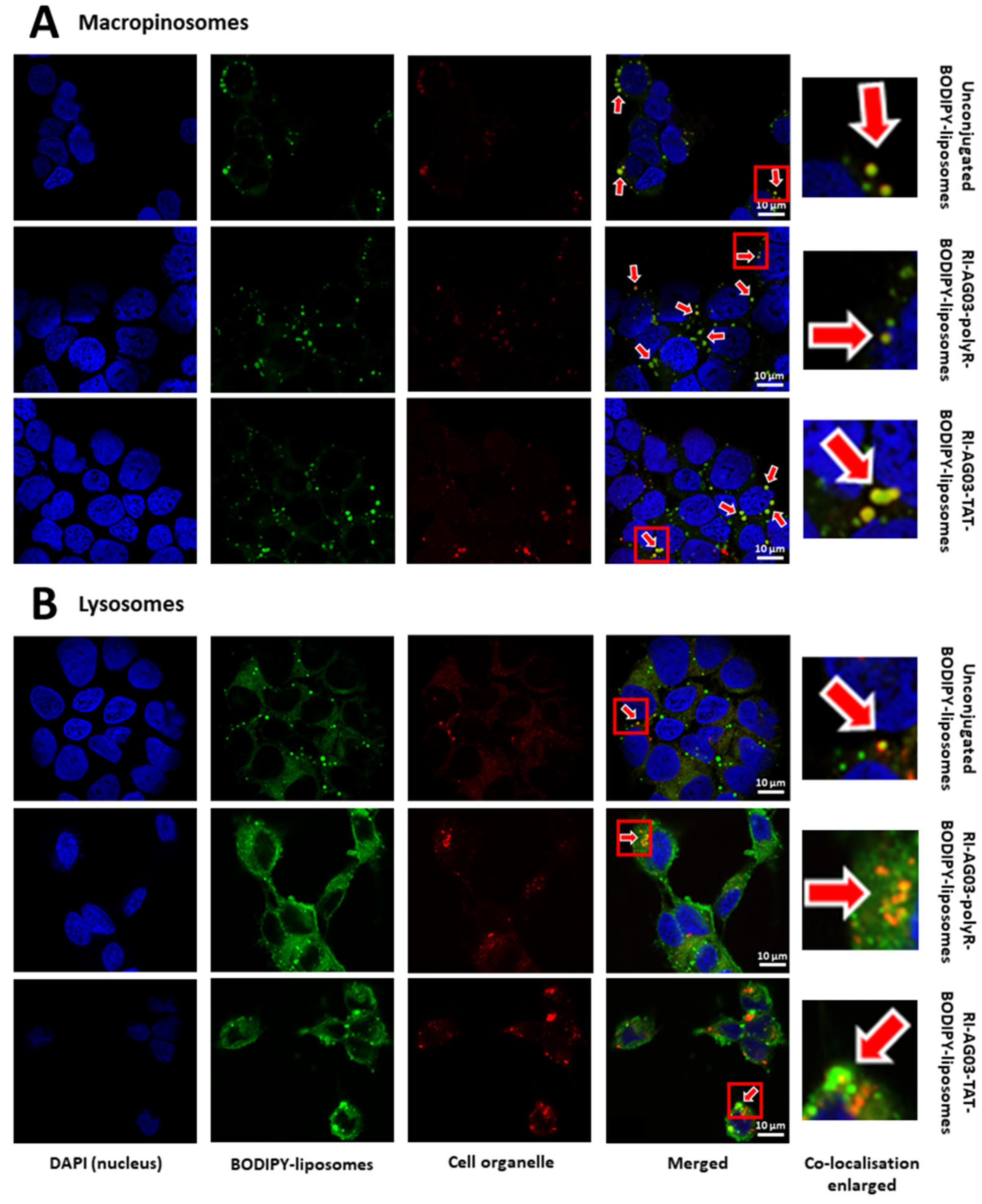
BODIPY Fluorescence from Unconjugated and Peptide-Conjugated BODIPY-Liposomes Localises to Macropinocytosis-Associated Cell Organelles Following Uptake
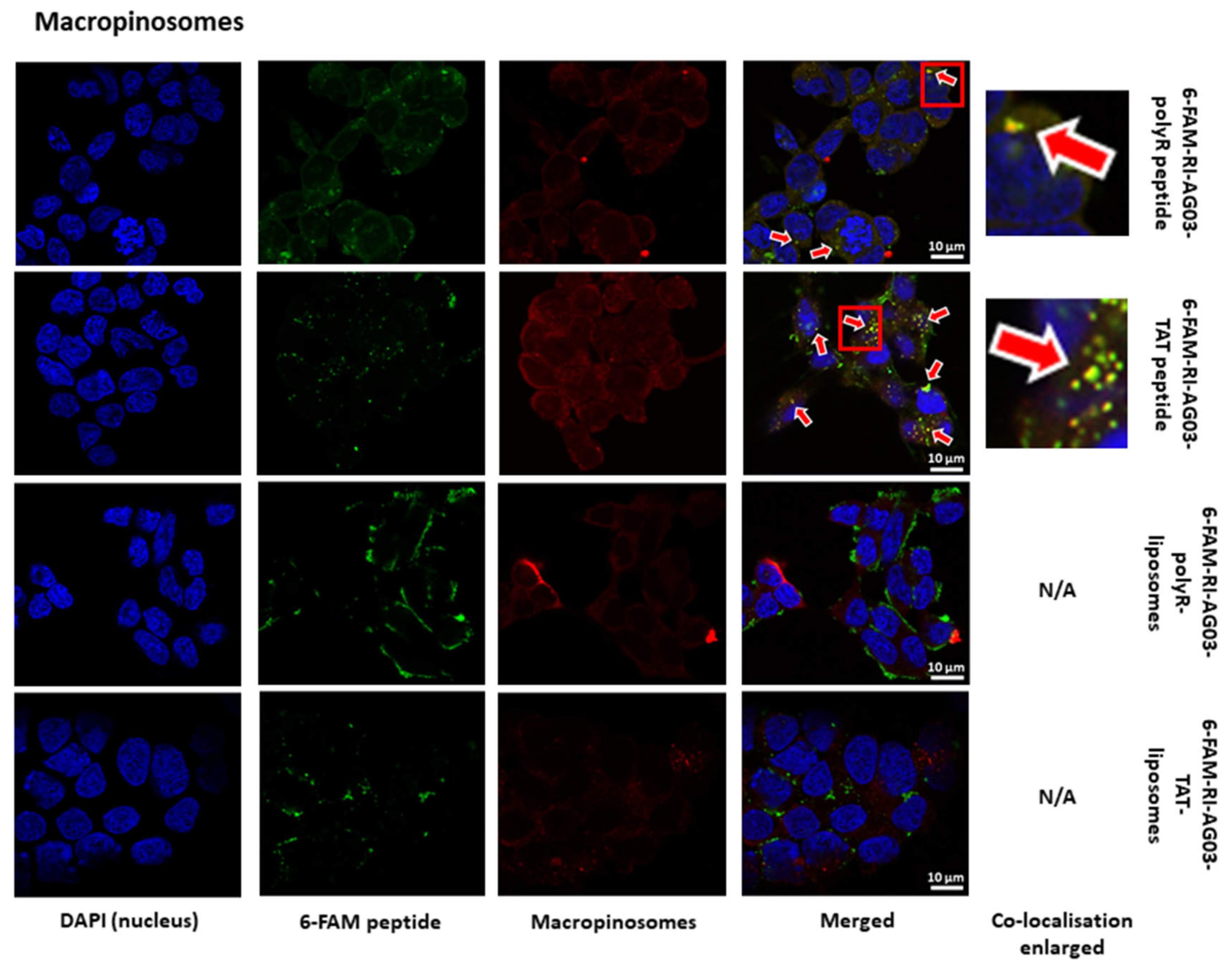
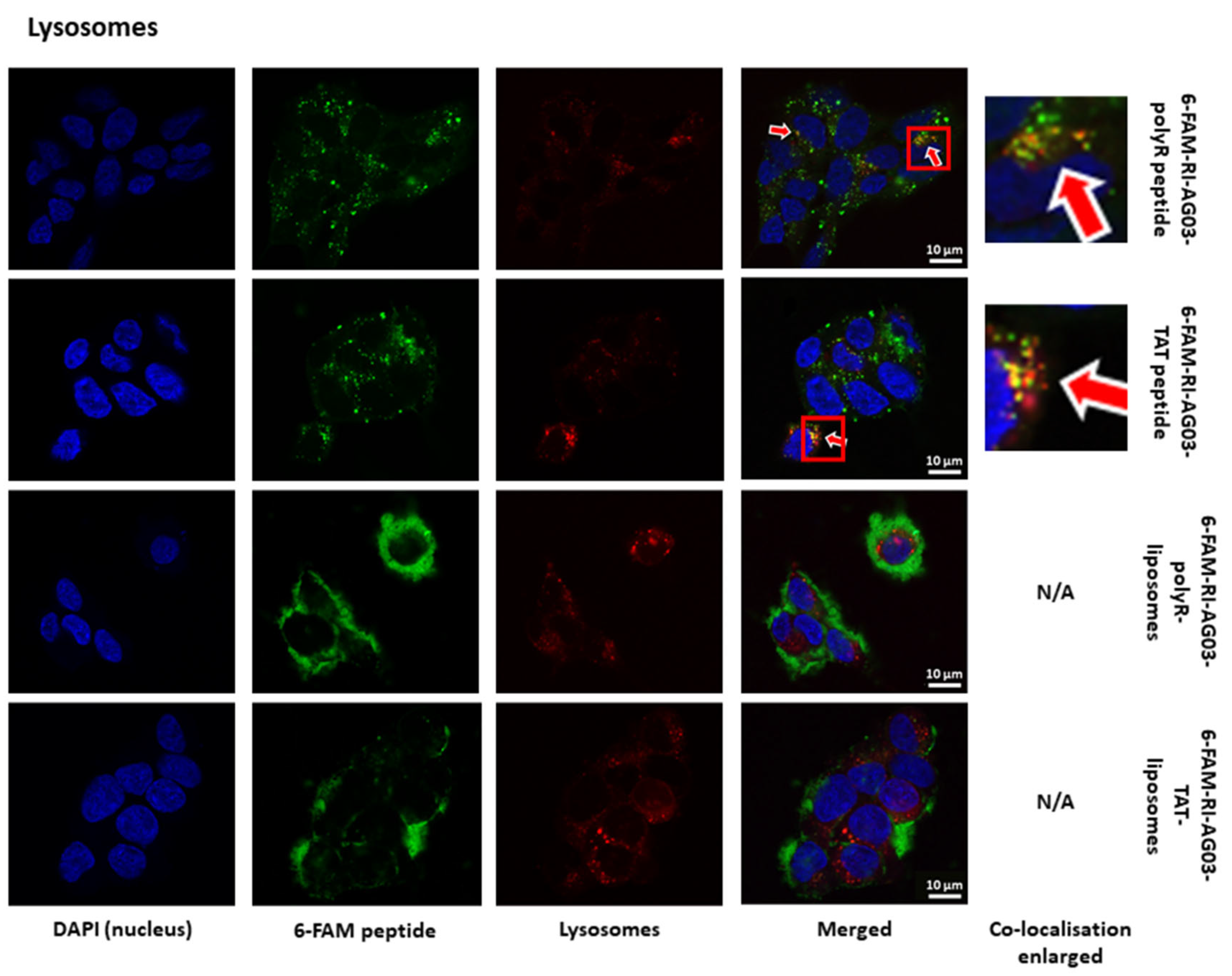
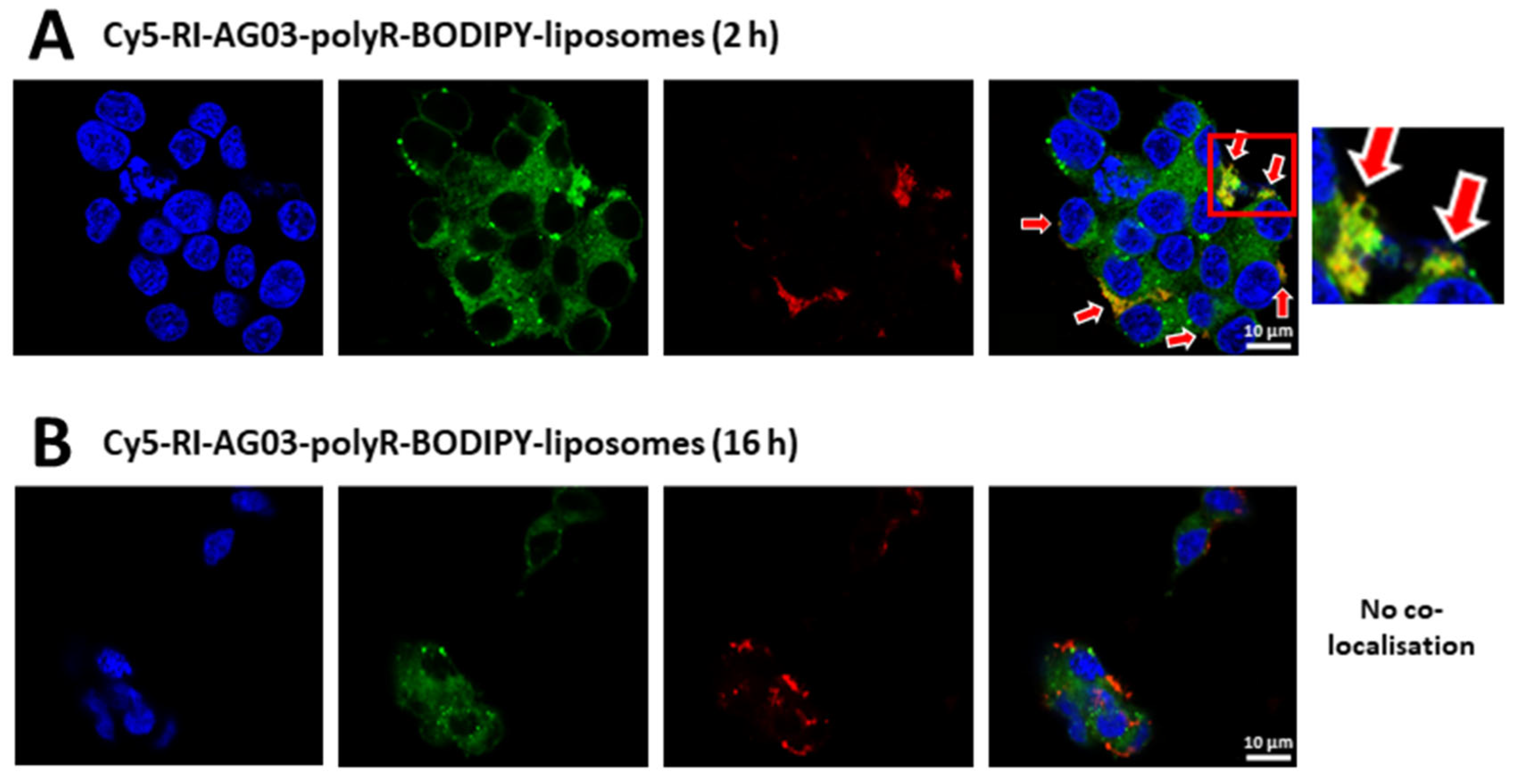
Following Internalisation of RI-AG03 Peptide-Liposomes, the Intracellular Distribution of the Peptide Differs from that of the Liposome Vehicle
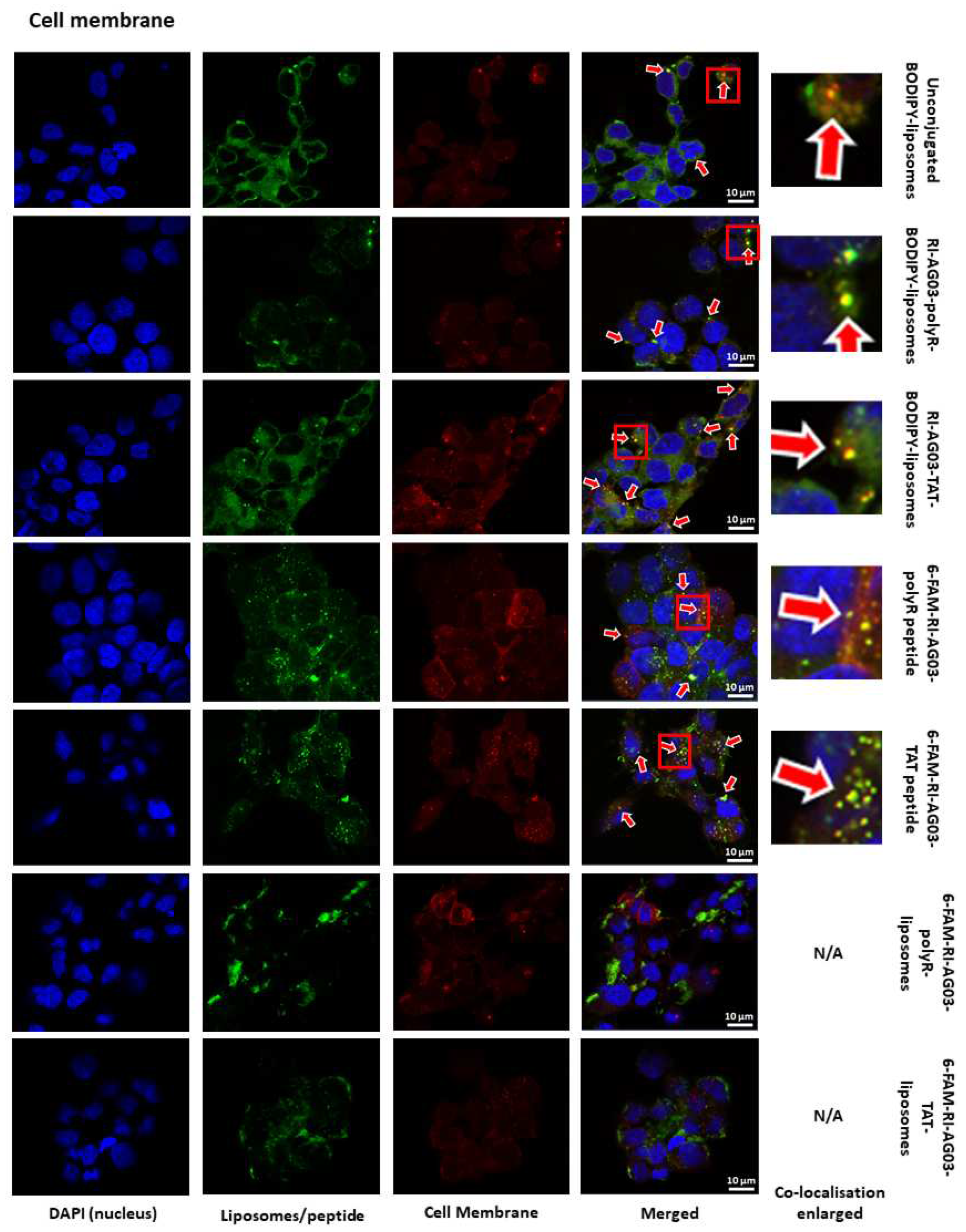
RI-AG03 Dissociates from Its Liposome Vehicle Following Cellular Membrane Fusion
Discussion
Material & Methods
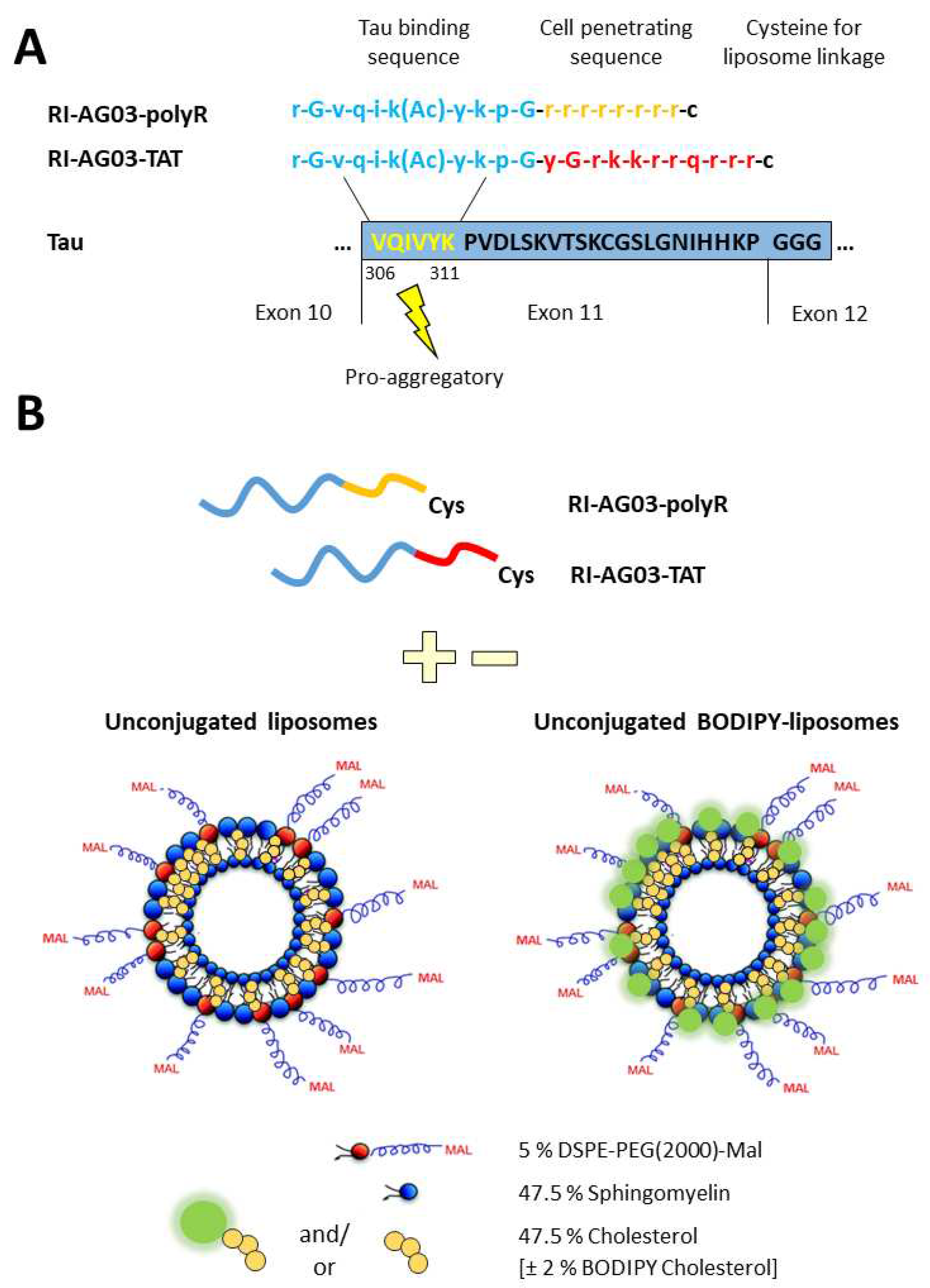
Peptide Synthesis
Preparation of Liposomes and Click Chemistry Attachment of RI-AGO3
Cell Culture
Flow Cytometry
Immunocytochemistry
Viability Assays
Statistical Analysis
Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of interest
References
- Breijyeh, Z., and R. Karaman. "Comprehensive Review on Alzheimer's Disease: Causes and Treatment." Molecules 25, no. 24 (2020). [CrossRef]
- Pinheiro, L., and C. Faustino. "Therapeutic Strategies Targeting Amyloid-Beta in Alzheimer's Disease." Current Alzheimer Research 16, no. 5 (2019): 418-52.
- Panza, F., M. Lozupone, G. Logroscino, and B. P. Imbimbo. "A Critical Appraisal of Amyloid-Beta-Targeting Therapies for Alzheimer Disease." Nat Rev Neurol 15, no. 2 (2019): 73-88.
- Tampi, R. R., B. P. Forester, and M. Agronin. "Aducanumab: Evidence from Clinical Trial Data and Controversies." Drugs Context 10 (2021). [CrossRef]
- Vaz, M., V. Silva, C. Monteiro, and S. Silvestre. "Role of Aducanumab in the Treatment of Alzheimer's Disease: Challenges and Opportunities." Clinical Interventions in Aging 17 (2022): 797-810. [CrossRef]
- Congdon, E. E., and E. M. Sigurdsson. "Tau-Targeting Therapies for Alzheimer Disease." Nat Rev Neurol 14, no. 7 (2018): 399-415. [CrossRef]
- Sandusky-Beltran, L. A., and E. M. Sigurdsson. "Tau Immunotherapies: Lessons Learned, Current Status and Future Considerations." Neuropharmacology 175 (2020): 108104. [CrossRef]
- Guo, T., W. Noble, and D. P. Hanger. "Roles of Tau Protein in Health and Disease." Acta Neuropathologica 133, no. 5 (2017): 665-704. [CrossRef]
- Kametani, F., and M. Hasegawa. "Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer's Disease." Front Neurosci 12 (2018): 25.
- Gulisano, W., D. Maugeri, M. A. Baltrons, M. Fa, A. Amato, A. Palmeri, L. D'Adamio, C. Grassi, D. P. Devanand, L. S. Honig, D. Puzzo, and O. Arancio. "Role of Amyloid-Beta and Tau Proteins in Alzheimer's Disease: Confuting the Amyloid Cascade." Journal of Alzheimers Disease 64, no. s1 (2018): S611-S31.
- Simic, G., M. Babic Leko, S. Wray, C. Harrington, I. Delalle, N. Jovanov-Milosevic, D. Bazadona, L. Buee, R. de Silva, G. Di Giovanni, C. Wischik, and P. R. Hof. "Tau Protein Hyperphosphorylation and Aggregation in Alzheimer's Disease and Other Tauopathies, and Possible Neuroprotective Strategies." Biomolecules 6, no. 1 (2016): 6.
- Wang, Y., V. Balaji, S. Kaniyappan, L. Kruger, S. Irsen, K. Tepper, R. Chandupatla, W. Maetzler, A. Schneider, E. Mandelkow, and E. M. Mandelkow. "The Release and Trans-Synaptic Transmission of Tau Via Exosomes." Molecular Neurodegeneration 12, no. 1 (2017): 5. [CrossRef]
- Saman, S., W. Kim, M. Raya, Y. Visnick, S. Miro, S. Saman, B. Jackson, A. C. McKee, V. E. Alvarez, N. C. Lee, and G. F. Hall. "Exosome-Associated Tau Is Secreted in Tauopathy Models and Is Selectively Phosphorylated in Cerebrospinal Fluid in Early Alzheimer Disease." Journal of Biological Chemistry 287, no. 6 (2012): 3842-9. [CrossRef]
- Mirbaha, H., B. B. Holmes, D. W. Sanders, J. Bieschke, and M. I. Diamond. "Tau Trimers Are the Minimal Propagation Unit Spontaneously Internalized to Seed Intracellular Aggregation." Journal of Biological Chemistry 290, no. 24 (2015): 14893-903. [CrossRef]
- Braak, H., and E. Braak. "Neuropathological Stageing of Alzheimer-Related Changes." Acta Neuropathologica 82, no. 4 (1991): 239-59. [CrossRef]
- Wischik, C. M., C. R. Harrington, and J. M. Storey. "Tau-Aggregation Inhibitor Therapy for Alzheimer's Disease." Biochemical Pharmacology 88, no. 4 (2014): 529-39.
- Austen, B. M., K. E. Paleologou, S. A. Ali, M. M. Qureshi, D. Allsop, and O. M. El-Agnaf. "Designing Peptide Inhibitors for Oligomerization and Toxicity of Alzheimer's Beta-Amyloid Peptide." Biochemistry 47, no. 7 (2008): 1984-92.
- Taylor, M., S. Moore, J. Mayes, E. Parkin, M. Beeg, M. Canovi, M. Gobbi, D. M. Mann, and D. Allsop. "Development of a Proteolytically Stable Retro-Inverso Peptide Inhibitor of Beta-Amyloid Oligomerization as a Potential Novel Treatment for Alzheimer's Disease." Biochemistry 49, no. 15 (2010): 3261-72.
- Tian, X. H., F. Wei, T. X. Wang, D. Wang, J. Wang, X. N. Lin, P. Wang, and L. Ren. "Blood-Brain Barrier Transport of Tat Peptide and Polyethylene Glycol Decorated Gelatin-Siloxane Nanoparticle." Materials Letters 68 (2012): 94-96. [CrossRef]
- Al Humaidan, E.L., S.L. Pedersen, A. Burkhart, C.L.M. Rasmussen, T. Moos, P. Fuchs, E.F.A. Fernandes, B. Ozgür, K. Strømgaard, A. Bach, B. Brodin, and M. Kristensen. "The Cell-Penetrating Peptide Tat Facilitates Effective Internalization of Psd-95 Inhibitors into Blood–Brain Barrier Endothelial Cells but Less Efficient Permeation across the Blood–Brain Barrier in Vitro and in Vivo." Front. Drug Deliv. 2, no. 854703 (2022). [CrossRef]
- Parthsarathy, V., P. L. McClean, C. Holscher, M. Taylor, C. Tinker, G. Jones, O. Kolosov, E. Salvati, M. Gregori, M. Masserini, and D. Allsop. "A Novel Retro-Inverso Peptide Inhibitor Reduces Amyloid Deposition, Oxidation and Inflammation and Stimulates Neurogenesis in the Appswe/Ps1deltae9 Mouse Model of Alzheimer's Disease." Plos One 8, no. 1 (2013): e54769.
- Gregori, M., M. Taylor, E. Salvati, F. Re, S. Mancini, C. Balducci, G. Forloni, V. Zambelli, S. Sesana, M. Michael, C. Michail, C. Tinker-Mill, O. Kolosov, M. Sherer, S. Harris, N. J. Fullwood, M. Masserini, and D. Allsop. "Retro-Inverso Peptide Inhibitor Nanoparticles as Potent Inhibitors of Aggregation of the Alzheimer's Abeta Peptide." Nanomedicine 13, no. 2 (2017): 723-32.
- von Bergen, M., S. Barghorn, L. Li, A. Marx, J. Biernat, E. M. Mandelkow, and E. Mandelkow. "Mutations of Tau Protein in Frontotemporal Dementia Promote Aggregation of Paired Helical Filaments by Enhancing Local Beta-Structure." Journal of Biological Chemistry 276, no. 51 (2001): 48165-74.
- von Bergen, M., P. Friedhoff, J. Biernat, J. Heberle, E. M. Mandelkow, and E. Mandelkow. "Assembly of Tau Protein into Alzheimer Paired Helical Filaments Depends on a Local Sequence Motif ((306)Vqivyk(311)) Forming Beta Structure." Proceedings of the National Academy of Sciences of the United States of America 97, no. 10 (2000): 5129-34.
- Aggidis A., Chatterjee S., Townsend D., Fullwood N.J., Ortega E. R., Tarutani A., Hasegawa M., Lucas H., Mudher A., and Allsop D. "Peptide-Based Inhibitors of Tau Aggregation as a Potential Therapeutic for Alzheimer’s Disease and Other Tauopathies." bioRxiv, (2021). [CrossRef]
- Chandrasekaran, S., M. J. McGuire, and M. R. King. "Sweeping Lymph Node Micrometastases Off Their Feet: An Engineered Model to Evaluate Natural Killer Cell Mediated Therapeutic Intervention of Circulating Tumor Cells That Disseminate to the Lymph Nodes." Lab Chip 14, no. 1 (2014): 118-27. [CrossRef]
- Futaki, S., W. Ohashi, T. Suzuki, M. Niwa, S. Tanaka, K. Ueda, H. Harashima, and Y. Sugiura. "Stearylated Arginine-Rich Peptides: A New Class of Transfection Systems." Bioconjug Chem 12, no. 6 (2001): 1005-11. [CrossRef]
- Mitchell, D. J., D. T. Kim, L. Steinman, C. G. Fathman, and J. B. Rothbard. "Polyarginine Enters Cells More Efficiently Than Other Polycationic Homopolymers." J Pept Res 56, no. 5 (2000): 318-25. [CrossRef]
- Gotanda, Y., F. Y. Wei, H. Harada, K. Ohta, K. I. Nakamura, K. Tomizawa, and K. Ushijima. "Efficient Transduction of 11 Poly-Arginine Peptide in an Ischemic Lesion of Mouse Brain." J Stroke Cerebrovasc Dis 23, no. 8 (2014): 2023-30. [CrossRef]
- Pham, W., B. Q. Zhao, E. H. Lo, Z. Medarova, B. Rosen, and A. Moore. "Crossing the Blood-Brain Barrier: A Potential Application of Myristoylated Polyarginine for in Vivo Neuroimaging." Neuroimage 28, no. 1 (2005): 287-92. [CrossRef]
- Mamsa, S. S. A., and B. P. Meloni. "Arginine and Arginine-Rich Peptides as Modulators of Protein Aggregation and Cytotoxicity Associated with Alzheimer's Disease." Frontiers in Molecular Neuroscience 14 (2021): 759729. [CrossRef]
- Doherty, G. J., and H. T. McMahon. "Mechanisms of Endocytosis." Annu Rev Biochem 78 (2009): 857-902.
- Foroozandeh, P., and A. A. Aziz. "Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles." Nanoscale Res Lett 13, no. 1 (2018): 339. [CrossRef]
- Vercauteren, D., R. E. Vandenbroucke, A. T. Jones, J. Rejman, J. Demeester, S. C. De Smedt, N. N. Sanders, and K. Braeckmans. "The Use of Inhibitors to Study Endocytic Pathways of Gene Carriers: Optimization and Pitfalls." Molecular Therapy 18, no. 3 (2010): 561-9. [CrossRef]
- El-Sayed, A., S. Futaki, and H. Harashima. "Delivery of Macromolecules Using Arginine-Rich Cell-Penetrating Peptides: Ways to Overcome Endosomal Entrapment." AAPS J 11, no. 1 (2009): 13-22. [CrossRef]
- Pei, D., and M. Buyanova. "Overcoming Endosomal Entrapment in Drug Delivery." Bioconjug Chem 30, no. 2 (2019): 273-83. [CrossRef]
- Ross, C., M. Taylor, N. Fullwood, and D. Allsop. "Liposome Delivery Systems for the Treatment of Alzheimer's Disease." Int J Nanomedicine 13 (2018): 8507-22. [CrossRef]
- Shi, N. Q., X. R. Qi, B. Xiang, and Y. Zhang. "A Survey on "Trojan Horse" Peptides: Opportunities, Issues and Controlled Entry to "Troy"." J Control Release 194 (2014): 53-70.
- Mason, A. F., and P. Thordarson. "Synthesis of Protein Bioconjugates Via Cysteine-Maleimide Chemistry." J Vis Exp, no. 113 (2016).
- Dutta, D., and J. G. Donaldson. "Search for Inhibitors of Endocytosis: Intended Specificity and Unintended Consequences." Cell Logist 2, no. 4 (2012): 203-08.
- Ivanov, A. I. "Pharmacological Inhibition of Endocytic Pathways: Is It Specific Enough to Be Useful?" Methods Mol Biol 440 (2008): 15-33.
- Curtis, E. M., A. H. Bahrami, T. R. Weikl, and C. K. Hall. "Modeling Nanoparticle Wrapping or Translocation in Bilayer Membranes." Nanoscale 7, no. 34 (2015): 14505-14. [CrossRef]
- Lee, S., A. T. Ashizawa, K. S. Kim, D. J. Falk, and L. Notterpek. "Liposomes to Target Peripheral Neurons and Schwann Cells." Plos One 8, no. 11 (2013): e78724. [CrossRef]
- Vogel, J. W., Y. Iturria-Medina, O. T. Strandberg, R. Smith, E. Levitis, A. C. Evans, O. Hansson, Initiative Alzheimer's Disease Neuroimaging, and Study Swedish BioFinder. "Author Correction: Spread of Pathological Tau Proteins through Communicating Neurons in Human Alzheimer's Disease." Nature Communications 12, no. 1 (2021): 4862. [CrossRef]
- Narasimhan, S., L. Changolkar, D. M. Riddle, A. Kats, A. Stieber, S. A. Weitzman, B. Zhang, Z. Li, E. D. Roberson, J. Q. Trojanowski, and V. M. Y. Lee. "Human Tau Pathology Transmits Glial Tau Aggregates in the Absence of Neuronal Tau." J Exp Med 217, no. 2 (2020). [CrossRef]
- Zareba-Paslawska, J., K. Patra, L. Kluzer, T. Revesz, and P. Svenningsson. "Tau Isoform-Driven Cbd Pathology Transmission in Oligodendrocytes in Humanized Tau Mice." Frontiers in Neurology 11 (2020): 589471. [CrossRef]
- Higuchi, M., B. Zhang, M. S. Forman, Y. Yoshiyama, J. Q. Trojanowski, and V. M. Lee. "Axonal Degeneration Induced by Targeted Expression of Mutant Human Tau in Oligodendrocytes of Transgenic Mice That Model Glial Tauopathies." Journal of Neuroscience 25, no. 41 (2005): 9434-43. [CrossRef]
- Li, S., and N. Malmstadt. "Deformation and Poration of Lipid Bilayer Membranes by Cationic Nanoparticles." Soft Matter 9, no. 20 (2013): 4969-76. [CrossRef]
- Magarkar, A., V. Dhawan, P. Kallinteri, T. Viitala, M. Elmowafy, T. Rog, and A. Bunker. "Cholesterol Level Affects Surface Charge of Lipid Membranes in Saline Solution." Sci Rep 4 (2014): 5005. [CrossRef]
- Rye, K. A., N. J. Hime, and P. J. Barter. "The Influence of Sphingomyelin on the Structure and Function of Reconstituted High Density Lipoproteins." Journal of Biological Chemistry 271, no. 8 (1996): 4243-50. [CrossRef]
- Oswald, M., S. Geissler, and A. Goepferich. "Determination of the Activity of Maleimide-Functionalized Phospholipids During Preparation of Liposomes." Int J Pharm 514, no. 1 (2016): 93-102. [CrossRef]
- Taylor, M., S. Moore, S. Mourtas, A. Niarakis, F. Re, C. Zona, B. La Ferla, F. Nicotra, M. Masserini, S. G. Antimisiaris, M. Gregori, and D. Allsop. "Effect of Curcumin-Associated and Lipid Ligand-Functionalized Nanoliposomes on Aggregation of the Alzheimer's Abeta Peptide." Nanomedicine 7, no. 5 (2011): 541-50.
- Juliano, R., M. R. Alam, V. Dixit, and H. Kang. "Mechanisms and Strategies for Effective Delivery of Antisense and Sirna Oligonucleotides." Nucleic Acids Res 36, no. 12 (2008): 4158-71. [CrossRef]
- Opanasopit, P., J. Tragulpakseerojn, A. Apirakaramwong, T. Ngawhirunpat, T. Rojanarata, and U. Ruktanonchai. "The Development of Poly-L-Arginine-Coated Liposomes for Gene Delivery." Int J Nanomedicine 6 (2011): 2245-52. [CrossRef]
- Cho, E. C., J. Xie, P. A. Wurm, and Y. Xia. "Understanding the Role of Surface Charges in Cellular Adsorption Versus Internalization by Selectively Removing Gold Nanoparticles on the Cell Surface with a I2/Ki Etchant." Nano Lett 9, no. 3 (2009): 1080-4.
- Kim, H. K., E. Davaa, C. S. Myung, and J. S. Park. "Enhanced Sirna Delivery Using Cationic Liposomes with New Polyarginine-Conjugated Peg-Lipid." Int J Pharm 392, no. 1-2 (2010): 141-7. [CrossRef]
- Suk, J. S., J. Suh, K. Choy, S. K. Lai, J. Fu, and J. Hanes. "Gene Delivery to Differentiated Neurotypic Cells with Rgd and Hiv Tat Peptide Functionalized Polymeric Nanoparticles." Biomaterials 27, no. 29 (2006): 5143-50. doi:10.1016/j.biomaterials.2006.05.013.
- Lim, J. P., and P. A. Gleeson. "Macropinocytosis: An Endocytic Pathway for Internalising Large Gulps." Immunol Cell Biol 89, no. 8 (2011): 836-43. [CrossRef]
- Mosconi, L., A. Pupi, and M. J. De Leon. "Brain Glucose Hypometabolism and Oxidative Stress in Preclinical Alzheimer's Disease." Ann N Y Acad Sci 1147 (2008): 180-95.
- Neth, B. J., and S. Craft. "Insulin Resistance and Alzheimer's Disease: Bioenergetic Linkages." Frontiers in Aging Neuroscience 9 (2017): 345. doi:10.3389/fnagi.2017.00345.
- Grimmer, S., B. van Deurs, and K. Sandvig. "Membrane Ruffling and Macropinocytosis in A431 Cells Require Cholesterol." Journal of Cell Science 115, no. Pt 14 (2002): 2953-62. [CrossRef]
- Allolio, C., A. Magarkar, P. Jurkiewicz, K. Baxova, M. Javanainen, P. E. Mason, R. Sachl, M. Cebecauer, M. Hof, D. Horinek, V. Heinz, R. Rachel, C. M. Ziegler, A. Schrofel, and P. Jungwirth. "Arginine-Rich Cell-Penetrating Peptides Induce Membrane Multilamellarity and Subsequently Enter Via Formation of a Fusion Pore." Proc Natl Acad Sci U S A 115, no. 47 (2018): 11923-28. [CrossRef]
- Duchardt, F., M. Fotin-Mleczek, H. Schwarz, R. Fischer, and R. Brock. "A Comprehensive Model for the Cellular Uptake of Cationic Cell-Penetrating Peptides." Traffic 8, no. 7 (2007): 848-66. [CrossRef]
- Daniel, J. A., N. Chau, M. K. Abdel-Hamid, L. Hu, L. von Kleist, A. Whiting, S. Krishnan, P. Maamary, S. R. Joseph, F. Simpson, V. Haucke, A. McCluskey, and P. J. Robinson. "Phenothiazine-Derived Antipsychotic Drugs Inhibit Dynamin and Clathrin-Mediated Endocytosis." Traffic 16, no. 6 (2015): 635-54. [CrossRef]
- Hakeem, I.J., N.J. Hodges, and F. Michelangeli. "Cytotoxicity of Sh-Sy5y Neuroblastoma Cells to the Antipsychotic Drugs, Chlorpromazine and Trifluoperazine, Is Via a Ca2+-Mediated Apoptosis Process and Differentiation of These Cells with Retinoic Acid Makes Them More Resistant to Cell Death." Clin Oncol Res (2020).
- McMahon, H. T., and E. Boucrot. "Molecular Mechanism and Physiological Functions of Clathrin-Mediated Endocytosis." Nat Rev Mol Cell Biol 12, no. 8 (2011): 517-33. [CrossRef]
- Ruan, G., A. Agrawal, A. I. Marcus, and S. Nie. "Imaging and Tracking of Tat Peptide-Conjugated Quantum Dots in Living Cells: New Insights into Nanoparticle Uptake, Intracellular Transport, and Vesicle Shedding." J Am Chem Soc 129, no. 47 (2007): 14759-66.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




