Submitted:
18 May 2023
Posted:
19 May 2023
You are already at the latest version
Abstract
Keywords:
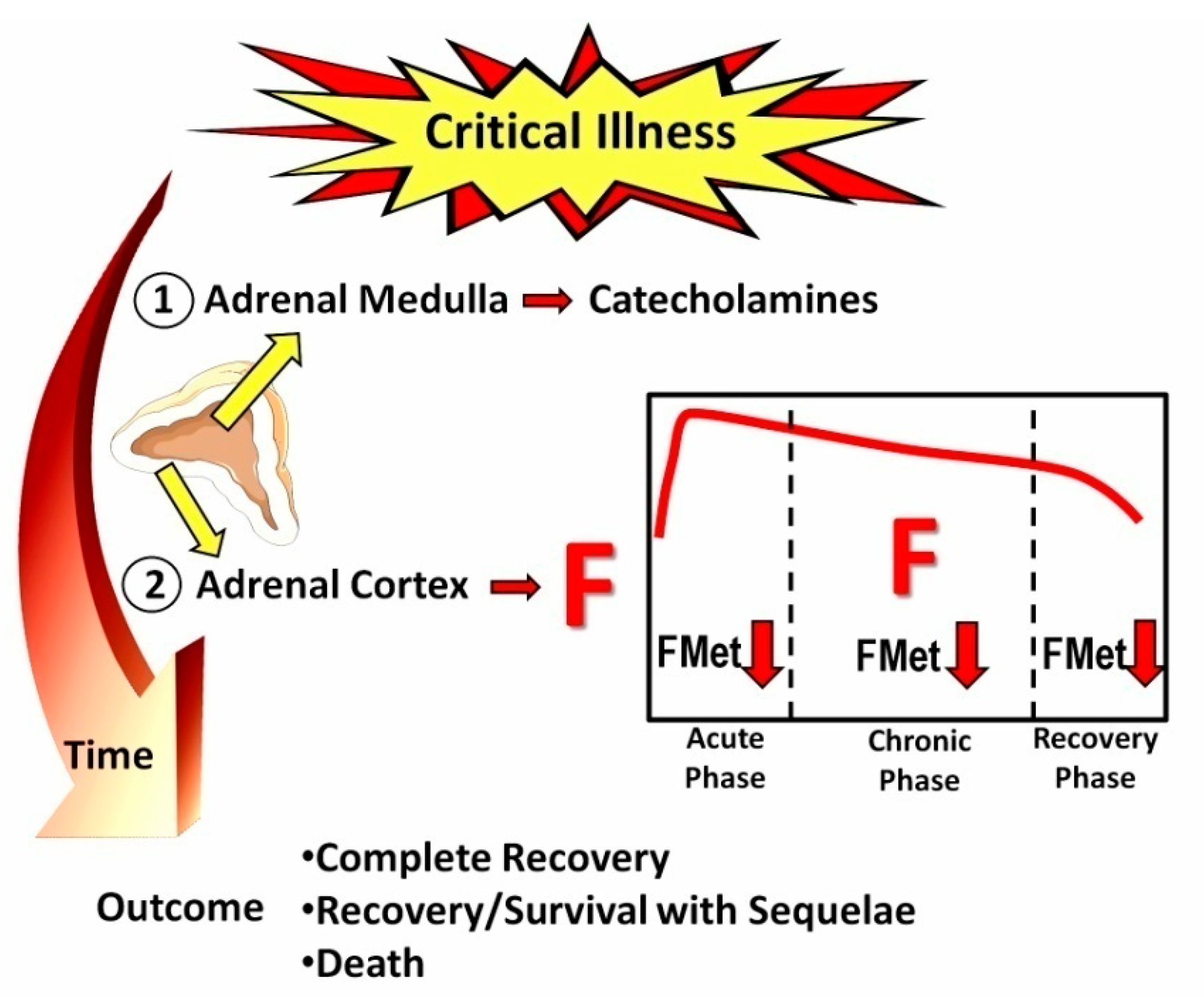
1. Introduction—Critical illness
1.1. Sepsis and septic shock
1.2. HPA axis and critical illness and/or sepsis
1.3. Critical-illness-related corticosteroid insufficiency
1.4. COVID-19 and Adrenal function
1.5. COVID-19 and Sepsis/Septic Shock
2. GCR Expression in Critical Illness and Sepsis
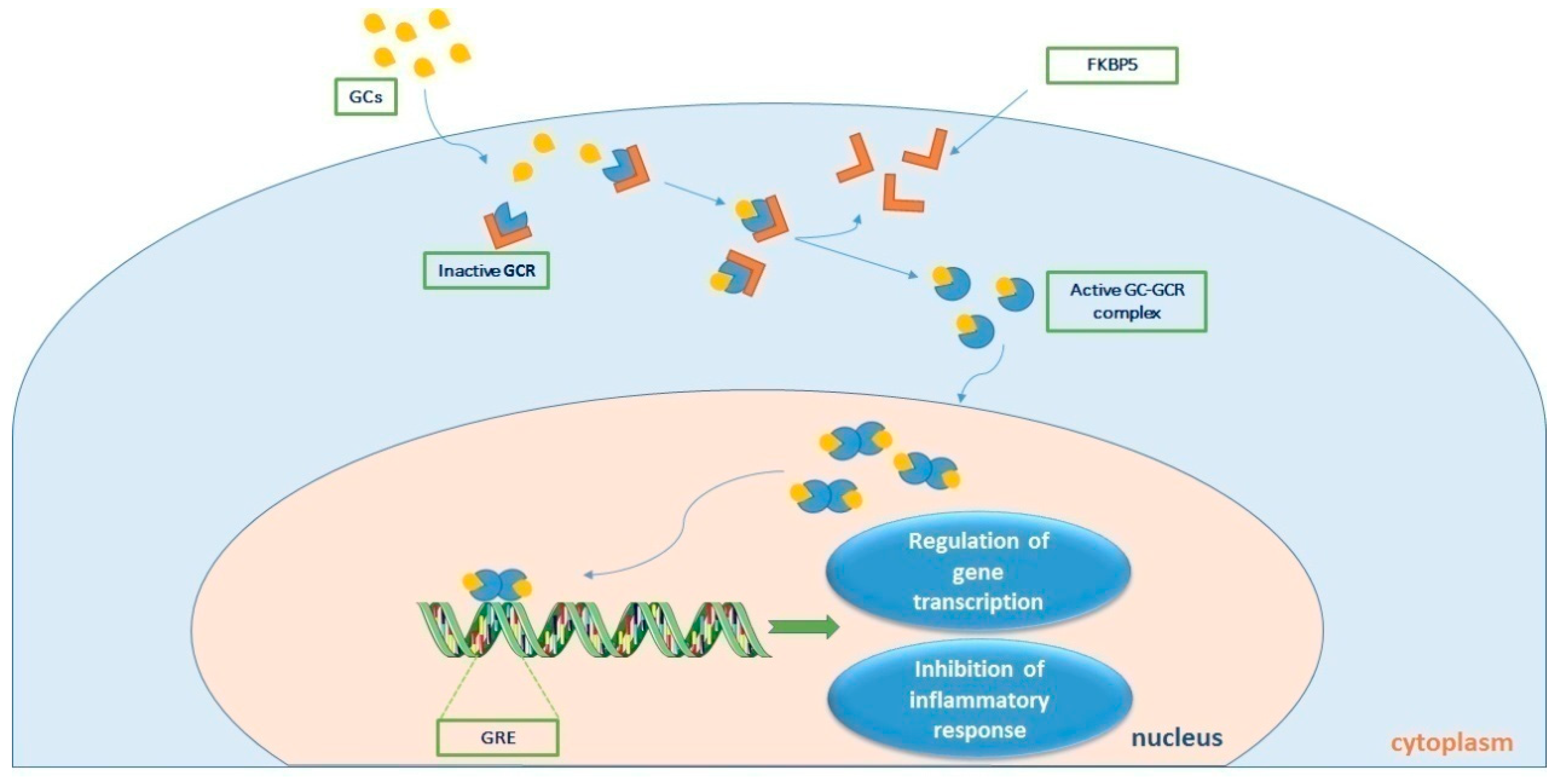
2.1. The GCR
2.2. GCR Expression and Glucocorticoid Resistance in Critical Illness and Sepsis
2.3. GCR Εxpression in COVID-19
3. Glucocorticoids in Septic Shock: Therapeutics, Clinical Trials and Future Research
4. Current Guidelines for Glucocorticoids in Septic Shock
5. COVID-19 and treatment with Steroids
6. Conclusion
Author Contributions
Conflicts of Interest
References
- Maslove, D.M.; Tang, B.; Shankar-Hari, M.; Lawler, P.R.; Angus, D.C.; Baillie, J.K.; Baron, R.M.; Bauer, M.; Buchman, T.G.; Calfee, C.S.; et al. Redefining critical illness. Nature Medicine 2022, 28, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, G.; Téblick, A.; Langouche, L.; Gunst, J. The hypothalamus-pituitary-adrenal axis in sepsis- and hyperinflammation-induced critical illness: Gaps in current knowledge and future translational research directions. EBioMedicine 2022, 84, 104284. [Google Scholar] [CrossRef]
- Vincent, J.-L. Sepsis and infection: Two words that should not be confused. Frontiers in Medicine 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Téblick, A.; Gunst, J.; Van den Berghe, G. Critical Illness–induced Corticosteroid Insufficiency: What It Is Not and What It Could Be. The Journal of Clinical Endocrinology & Metabolism 2022, 107, 2057–2064. [Google Scholar] [CrossRef]
- Marik, P.E.; Zaloga, G.P. Adrenal insufficiency in the critically ill: A new look at an old problem. Chest 2002, 122, 1784–1796. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M.; Bion, J.; Parker, M.M.; Jaeschke, R.; Reinhart, K.; Angus, D.C.; Brun-Buisson, C.; Beale, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008, 36, 296–327. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Pastores, S.M.; Annane, D.; Meduri, G.U.; Sprung, C.L.; Arlt, W.; Keh, D.; Briegel, J.; Beishuizen, A.; Dimopoulou, I.; et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: Consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 2008, 36, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Pastores, S.M.; Rochwerg, B.; Arlt, W.; Balk, R.A.; Beishuizen, A.; Briegel, J.; Carcillo, J.; Christ-Crain, M.; Cooper, M.S.; et al. Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med 2017, 45, 2078–2088. [Google Scholar] [CrossRef]
- Lazartigues, E.; Qadir, M.M.F.; Mauvais-Jarvis, F. Endocrine Significance of SARS-CoV-2's Reliance on ACE2. Endocrinology 2020, 161. [Google Scholar] [CrossRef]
- Tan, T.; Khoo, B.; Mills, E.G.; Phylactou, M.; Patel, B.; Eng, P.C.; Thurston, L.; Muzi, B.; Meeran, K.; Prevost, A.T.; et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol 2020, 8, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Tomo, S.; Banerjee, M.; Karli, S.; Purohit, P.; Mitra, P.; Sharma, P.; Garg, M.K.; Kumar, B. Assessment of DHEAS, cortisol, and DHEAS/cortisol ratio in patients with COVID-19: A pilot study. Hormones 2022, 21, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, I.; Estabraghnia Babaki, H.; Maleki, M.; Jarineshin, H.; Kaffashian, M.R.; Hassaniazad, M.; Kenarkoohi, A.; Ghanbarnejad, A.; Falahi, S.; Kazemi Jahromi, M.; et al. Changes in Physiological Levels of Cortisol and Adrenocorticotropic Hormone upon Hospitalization Can Predict SARS-CoV-2 Mortality: A Cohort Study. Int J Endocrinol 2022, 2022, 4280691. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Dashatan, N.; Koushki, M.; Parsamanesh, N.; Chiti, H. Serum cortisol concentration and COVID-19 severity: A systematic review and meta-analysis. J Investig Med 2022, 70, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Vakhshoori, M.; Heidarpour, M.; Bondariyan, N.; Sadeghpour, N.; Mousavi, Z. Adrenal Insufficiency in Coronavirus Disease 2019 (COVID-19)-Infected Patients without Preexisting Adrenal Diseases: A Systematic Literature Review. Int J Endocrinol 2021, 2021, 2271514. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Xu, B.; Guan, W.; Xu, D.; Li, F.; Ren, R.; Zhu, X.; Gao, Y.; Jiang, L. The Adrenal Cortex, an Underestimated Site of SARS-CoV-2 Infection. Front Endocrinol 2020, 11, 593179. [Google Scholar] [CrossRef] [PubMed]
- Kanczkowski, W.; Gaba, W.H.; Krone, N.; Varga, Z.; Beuschlein, F.; Hantel, C.; Andoniadou, C.; Bornstein, S.R. Adrenal Gland Function and Dysfunction During COVID-19. Horm Metab Res 2022, 54, 532–539. [Google Scholar] [CrossRef]
- Bryce, C.; Grimes, Z.; Pujadas, E.; Ahuja, S.; Beasley, M.B.; Albrecht, R.; Hernandez, T.; Stock, A.; Zhao, Z.; AlRasheed, M.R.; et al. Pathophysiology of SARS-CoV-2: The Mount Sinai COVID-19 autopsy experience. Mod Pathol 2021, 34, 1456–1467. [Google Scholar] [CrossRef]
- Wheatland, R. Molecular mimicry of ACTH in SARS - implications for corticosteroid treatment and prophylaxis. Med Hypotheses 2004, 63, 855–862. [Google Scholar] [CrossRef]
- Shappell, C.N.; Klompas, M.; Kanjilal, S.; Chan, C.; Rhee, C. Prevalence, Clinical Characteristics, and Outcomes of Sepsis Caused by Severe Acute Respiratory Syndrome Coronavirus 2 Versus Other Pathogens in Hospitalized Patients With COVID-19. Crit Care Explor 2022, 4, e0703. [Google Scholar] [CrossRef]
- Karakike, E.; Giamarellos-Bourboulis, E.J.; Kyprianou, M.; Fleischmann-Struzek, C.; Pletz, M.W.; Netea, M.G.; Reinhart, K.; Kyriazopoulou, E. Coronavirus Disease 2019 as Cause of Viral Sepsis: A Systematic Review and Meta-Analysis. Crit Care Med 2021, 49, 2042–2057. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, C.; Mostert, C.; Hentschker, C.; Voshaar, T.; Malzahn, J.; Schillinger, G.; Klauber, J.; Janssens, U.; Marx, G.; Weber-Carstens, S.; et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: An observational study. The Lancet Respiratory Medicine 2020, 8, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, S.M.; Weinberger, C.; Ong, E.S.; Cerelli, G.; Oro, A.; Lebo, R.; Thompson, E.B.; Rosenfeld, M.G.; Evans, R.M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature 1985, 318, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S186–S195. [Google Scholar] [CrossRef] [PubMed]
- Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci 2013, 34, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Smoak, K.A.; Cidlowski, J.A. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev 2004, 125, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Gottlicher, M.; Heck, S.; Herrlich, P. Transcriptional cross-talk, the second mode of steroid hormone receptor action. J Mol Med 1998, 76, 480–489. [Google Scholar] [CrossRef]
- Bamberger, C.M.; Bamberger, A.M.; de Castro, M.; Chrousos, G.P. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest 1995, 95, 2435–2441. [Google Scholar] [CrossRef]
- Kino, T.; Su, Y.A.; Chrousos, G.P. Human glucocorticoid receptor isoform beta: Recent understanding of its potential implications in physiology and pathophysiology. Cell Mol Life Sci 2009, 66, 3435–3448. [Google Scholar] [CrossRef]
- Oakley, R.H.; Sar, M.; Cidlowski, J.A. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J Biol Chem 1996, 271, 9550–9559. [Google Scholar] [CrossRef]
- Bamberger, C.M.; Schulte, H.M.; Chrousos, G.P. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev 1996, 17, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.U.; Muthiah, M.P.; Carratu, P.; Eltorky, M.; Chrousos, G.P. Nuclear factor-kappaB- and glucocorticoid receptor alpha- mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. Neuroimmunomodulation 2005, 12, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Pretorius, C.J.; Ungerer, J.P.; Cardinal, J.; Blumenthal, A.; Presneill, J.; Gatica-Andrades, M.; Jarrett, P.; Lassig-Smith, M.; Stuart, J.; et al. Glucocorticoid Sensitivity Is Highly Variable in Critically Ill Patients With Septic Shock and Is Associated With Disease Severity. Crit Care Med 2016, 44, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Da, J.; Chen, L.; Hedenstierna, G. Nitric oxide up-regulates the glucocorticoid receptor and blunts the inflammatory reaction in porcine endotoxin sepsis. Crit Care Med 2007, 35, 26–32. [Google Scholar] [CrossRef]
- Koulouras, V.P.; Li, R.; Chen, L.; Hedenstierna, G.G. Effects of inhaled carbon monoxide and glucocorticoids in porcine endotoxin sepsis. Int J Clin Exp Med 2011, 4, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, R.B. Changes in canine leukocyte glucocorticoid receptors during endotoxin shock. Circ Shock 1988, 26, 99–105. [Google Scholar]
- Reichardt, H.M.; Umland, T.; Bauer, A.; Kretz, O.; Schutz, G. Mice with an increased glucocorticoid receptor gene dosage show enhanced resistance to stress and endotoxic shock. Mol Cell Biol 2000, 20, 9009–9017. [Google Scholar] [CrossRef]
- Stith, R.D.; McCallum, R.E. Down regulation of hepatic glucocorticoid receptors after endotoxin treatment. Infect Immun 1983, 40, 613–621. [Google Scholar] [CrossRef]
- Bergquist, M.; Nurkkala, M.; Rylander, C.; Kristiansson, E.; Hedenstierna, G.; Lindholm, C. Expression of the glucocorticoid receptor is decreased in experimental Staphylococcus aureus sepsis. J Infect 2013, 67, 574–583. [Google Scholar] [CrossRef]
- Vettorazzi, S.; Bode, C.; Dejager, L.; Frappart, L.; Shelest, E.; Klaßen, C.; Tasdogan, A.; Reichardt, H.M.; Libert, C.; Schneider, M.; et al. Glucocorticoids limit acute lung inflammation in concert with inflammatory stimuli by induction of SphK1. Nat Commun 2015, 6, 7796. [Google Scholar] [CrossRef]
- Wepler, M.; Preuss, J.M.; Merz, T.; Hartmann, C.; Wachter, U.; McCook, O.; Vogt, J.; Kress, S.; Gröger, M.; Fink, M.; et al. Impaired Glucocorticoid Receptor Dimerization Aggravates LPS-Induced Circulatory and Pulmonary Dysfunction. Front Immunol 2019, 10, 3152. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.N.; Jimenez, D.M.; Fernandes, T.D.; Deutschman, C.S. Cecal Ligation and Puncture Alters Glucocorticoid Receptor Expression. Crit Care Med 2018, 46, e797–e804. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.E.; Feng, Y.; Velazquez, H.; Sessa, W.C. Endothelial glucocorticoid receptor is required for protection against sepsis. Proc Natl Acad Sci U S A 2013, 110, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, K.; Matsuda, N.; Yamamoto, S.; Takano, K.; Takano, Y.; Yamazaki, H.; Kageyama, S.; Yokoo, H.; Nagata, T.; Hatakeyama, N.; et al. Modulation of glucocorticoid receptor expression, inflammation, and cell apoptosis in septic guinea pig lungs using methylprednisolone. Am J Physiol Lung Cell Mol Physiol 2008, 295, L998–L1006. [Google Scholar] [CrossRef] [PubMed]
- Dekelbab, B.H.; Witchel, S.F.; DeFranco, D.B. TNF-alpha and glucocorticoid receptor interaction in L6 muscle cells: A cooperative downregulation of myosin heavy chain. Steroids 2007, 72, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, J.; Timmermans, S.; Paakinaho, V.; Vancraeynest, L.; Dewyse, L.; Vanderhaeghen, T.; Wallaeys, C.; Van Wyngene, L.; Van Looveren, K.; Nuyttens, L.; et al. Combined glucocorticoid resistance and hyperlactatemia contributes to lethal shock in sepsis. Cell Metab 2021, 33, 1763–1776. [Google Scholar] [CrossRef] [PubMed]
- Ledderose, C.; Mohnle, P.; Limbeck, E.; Schutz, S.; Weis, F.; Rink, J.; Briegel, J.; Kreth, S. Corticosteroid resistance in sepsis is influenced by microRNA-124--induced downregulation of glucocorticoid receptor-alpha. Crit Care Med 2012, 40, 2745–2753. [Google Scholar] [CrossRef]
- Guerrero, J.; Gatica, H.A.; Rodriguez, M.; Estay, R.; Goecke, I.A. Septic serum induces glucocorticoid resistance and modifies the expression of glucocorticoid isoforms receptors: A prospective cohort study and in vitro experimental assay. Crit Care 2013, 17, R107. [Google Scholar] [CrossRef]
- Molijn, G.J.; Koper, J.W.; van Uffelen, C.J.; de Jong, F.H.; Brinkmann, A.O.; Bruining, H.A.; Lamberts, S.W. Temperature-induced down-regulation of the glucocorticoid receptor in peripheral blood mononuclear leucocyte in patients with sepsis or septic shock. Clin Endocrinol 1995, 43, 197–203. [Google Scholar] [CrossRef]
- Bergquist, M.; Lindholm, C.; Strinnholm, M.; Hedenstierna, G.; Rylander, C. Impairment of neutrophilic glucocorticoid receptor function in patients treated with steroids for septic shock. Intensive Care Med Exp 2015, 3, 59. [Google Scholar] [CrossRef]
- Li, J.; Xie, M.; Yu, Y.; Tang, Z.; Hang, C.; Li, C. Leukocyte glucocorticoid receptor expression and related transcriptomic gene signatures during early sepsis. Clin Immunol 2021, 223, 108660. [Google Scholar] [CrossRef] [PubMed]
- Sigal, G.A.; Maria, D.A.; Katayama, M.L.; Wajchenberg, B.L.; Brentani, M.M. Glucocorticoid receptors in mononuclear cells of patients with sepsis. Scand J Infect Dis 1993, 25, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Vardas, K.; Ilia, S.; Sertedaki, A.; Charmandari, E.; Briassouli, E.; Goukos, D.; Apostolou, K.; Psarra, K.; Botoula, E.; Tsagarakis, S.; et al. Increased glucocorticoid receptor expression in sepsis is related to heat shock proteins, cytokines, and cortisol and is associated with increased mortality. Intensive Care Med Exp 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Siebig, S.; Meinel, A.; Rogler, G.; Klebl, E.; Wrede, C.E.; Gelbmann, C.; Froh, S.; Rockmann, F.; Bruennler, T.; Schoelmerich, J.; et al. Decreased cytosolic glucocorticoid receptor levels in critically ill patients. Anaesth Intensive Care 2010, 38, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Floros, G.; Jahaj, E.; Stamogiannos, G.; Gennimata, S.; Vassiliadi, D.A.; Tsagarakis, S.; Tzanela, M.; Ilias, I.; Orfanos, S.E.; et al. Decreased glucocorticoid receptor expression during critical illness. Eur J Clin Invest 2019, 49, e13073. [Google Scholar] [CrossRef]
- Vassiliou, A.G.; Stamogiannos, G.; Jahaj, E.; Botoula, E.; Floros, G.; Vassiliadi, D.A.; Ilias, I.; Tsagarakis, S.; Tzanela, M.; Orfanos, S.E.; et al. Longitudinal evaluation of glucocorticoid receptor alpha/beta expression and signalling, adrenocortical function and cytokines in critically ill steroid-free patients. Mol Cell Endocrinol 2020, 501, 110656. [Google Scholar] [CrossRef]
- Téblick, A.; Van Dyck, L.; Van Aerde, N.; Van der Perre, S.; Pauwels, L.; Derese, I.; Debaveye, Y.; Wouters, P.J.; Vanhorebeek, I.; Langouche, L.; et al. Impact of duration of critical illness and level of systemic glucocorticoid availability on tissue-specific glucocorticoid receptor expression and actions: A prospective, observational, cross-sectional human and two translational mouse studies. EBioMedicine 2022, 80, 104057. [Google Scholar] [CrossRef]
- Grigsby, M.J.; Green, T.L.; Lim, D.; Cho, K.; Greenhalgh, D.G. A Novel Human Glucocorticoid Receptor Variant, G459V, is Hyperactive in Response to Steroids. Shock 2021, 56, 318–324. [Google Scholar] [CrossRef]
- Diaz, P.V.; Pinto, R.A.; Mamani, R.; Uasapud, P.A.; Bono, M.R.; Gaggero, A.A.; Guerrero, J.; Goecke, A. Increased expression of the glucocorticoid receptor beta in infants with RSV bronchiolitis. Pediatrics 2012, 130, e804–e811. [Google Scholar] [CrossRef]
- Indyk, J.A.; Candido-Vitto, C.; Wolf, I.M.; Venkataraman, S.; Munoz, R.; Saladino, R.A.; Witchel, S.F.; Defranco, D.B. Reduced glucocorticoid receptor protein expression in children with critical illness. Horm Res Paediatr 2013, 79, 169–178. [Google Scholar] [CrossRef]
- Shibata, A.R.; Troster, E.J.; Wong, H.R. Glucocorticoid Receptor Expression in Peripheral WBCs of Critically Ill Children. Pediatr Crit Care Med 2015, 16, e132–e140. [Google Scholar] [CrossRef] [PubMed]
- Alder, M.N.; Opoka, A.M.; Wong, H.R. The glucocorticoid receptor and cortisol levels in pediatric septic shock. Crit Care 2018, 22, 244. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Athanasiou, N.; Keskinidou, C.; Jahaj, E.; Tsipilis, S.; Zacharis, A.; Botoula, E.; Diamantopoulos, A.; Ilias, I.; Vassiliadi, D.A.; et al. Increased Glucocorticoid Receptor Alpha Expression and Signaling in Critically Ill Coronavirus Disease 2019 Patients. Crit Care Med 2021, 49, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Godot, V. Glucocorticoid-Glucocorticoid Receptor Response to Severe Acute Respiratory Syndrome Coronavirus 2. Crit Care Med 2021, 49, 2157–2160. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Wagner, T.; Venkatakrishnan, A.J.; Puranik, A.; Hurchik, M.; Agarwal, V.; Conrad, I.; Kirkup, C.; Arunachalam, R.; O'Horo, J.; et al. Plasma IL-6 levels following corticosteroid therapy as an indicator of ICU length of stay in critically ill COVID-19 patients. Cell Death Discov 2021, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Aliska, G.; Nafrialdi, N.; Lie, K.C.; Setiabudy, R.; Putra, A.E.; Widyahening, I.S.; Harahap, A.R. The role of the glucocorticoid receptor and its impact on steroid response in moderate-severe COVID-19 patients. Eur J Pharmacol 2023, 943, 175555. [Google Scholar] [CrossRef]
- Ammar, M.A.; Ammar, A.A.; Wieruszewski, P.M.; Bissell, B.D.; M, T.L.; Albert, L.; Khanna, A.K.; Sacha, G.L. Timing of vasoactive agents and corticosteroid initiation in septic shock. Ann Intensive Care 2022, 12, 47. [Google Scholar] [CrossRef]
- Lansing, A.M. Septic Shock. Can Med Assoc J 1963, 89, 583–588. [Google Scholar]
- Schumer, W. Steroids in the treatment of clinical septic shock. Ann Surg 1976, 184, 333–341. [Google Scholar] [CrossRef]
- Sprung, C.L.; Caralis, P.V.; Marcial, E.H.; Pierce, M.; Gelbard, M.A.; Long, W.M.; Duncan, R.C.; Tendler, M.D.; Karpf, M. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med 1984, 311, 1137–1143. [Google Scholar] [CrossRef]
- Sprung, C.L.; Goodman, S.; Weiss, Y.G. Corticosteroid Treatment of Patients in Septic Shock. In Yearbook of Intensive Care and Emegency Medicine 2009, Vincent, J.L., Ed. Springer: Berlin-Heidelberg, 2009; pp. 753–762.
- Bauer, W.; Ball, J.; Grounds, M. Unanswered questions from Corticus and pragmatic suggestions. Crit Care 2008, 12, 426. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; Sprung, C.L.; Annane, D.; Chevret, S.; Briegel, J.; Keh, D.; Singer, M.; Weiss, Y.G.; Payen, D.; Cuthbertson, B.H.; et al. Time course of organ failure in patients with septic shock treated with hydrocortisone: Results of the Corticus study. Intensive Care Med 2011, 37, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Sprung, C.L.; Annane, D.; Keh, D.; Moreno, R.; Singer, M.; Freivogel, K.; Weiss, Y.G.; Benbenishty, J.; Kalenka, A.; Forst, H.; et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008, 358, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.; Stone, A.; Green, R.S.; Gray, S. Steroids for patients in septic shock: The results of the CORTICUS trial. CJEM 2011, 13, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, B.; Myburgh, J.; Finfer, S.; Webb, S.A.; Cohen, J.; Bellomo, R.; McArthur, C.; Joyce, C.J.; Rajbhandari, D.; Glass, P.; et al. The ADRENAL study protocol: Adjunctive corticosteroid treatment in critically ill patients with septic shock. Crit Care Resusc 2013, 15, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Glass, P.; Joyce, C.; Li, Q.; et al. Hydrocortisone Compared with Placebo in Patients with Septic Shock Satisfying the Sepsis-3 Diagnostic Criteria and APROCCHSS Study Inclusion Criteria: A Post Hoc Analysis of the ADRENAL Trial. Anesthesiology 2019, 131, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Timsit, J.F.; Megarbane, B.; Martin, C.; Misset, B.; Mourvillier, B.; Siami, S.; Chagnon, J.L.; Constantin, J.M.; Petitpas, F.; et al. Recombinant human activated protein C for adults with septic shock: A randomized controlled trial. Am J Respir Crit Care Med 2013, 187, 1091–1097. [Google Scholar] [CrossRef]
- Annane, D.; Renault, A.; Brun-Buisson, C.; Megarbane, B.; Quenot, J.P.; Siami, S.; Cariou, A.; Forceville, X.; Schwebel, C.; Martin, C.; et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N Engl J Med 2018, 378, 809–818. [Google Scholar] [CrossRef]
- Russell, J.A. When and how to use predictive biomarkers for corticosteroid treatment of septic shock. Crit Care 2018, 22, 318. [Google Scholar] [CrossRef]
- Bentzer, P.; Fjell, C.; Walley, K.R.; Boyd, J.; Russell, J.A. Plasma cytokine levels predict response to corticosteroids in septic shock. Intensive Care Med 2016, 42, 1970–1979. [Google Scholar] [CrossRef]
- Bosch, N.A.; Teja, B.; Law, A.C.; Pang, B.; Jafarzadeh, S.R.; Walkey, A.J. Comparative Effectiveness of Fludrocortisone and Hydrocortisone vs Hydrocortisone Alone Among Patients With Septic Shock. JAMA Intern Med 2023. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.J.; Nenke, M.A.; Lewis, J.G.; Torpy, D.J. Corticosteroid-binding globulin: Acute and chronic inflammation. Expert Rev Endocrinol Metab 2017, 12, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Venkatesh, B.; Hammond, N.; Taylor, C.; Finfer, S. Sex differences in response to adjunctive corticosteroid treatment for patients with septic shock. Intensive Care Med 2021, 47, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Anonymous. Corticosteroids. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/. National Institutes of Health: Bethesda, MD, 2023.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




