Submitted:
19 May 2023
Posted:
22 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experiments
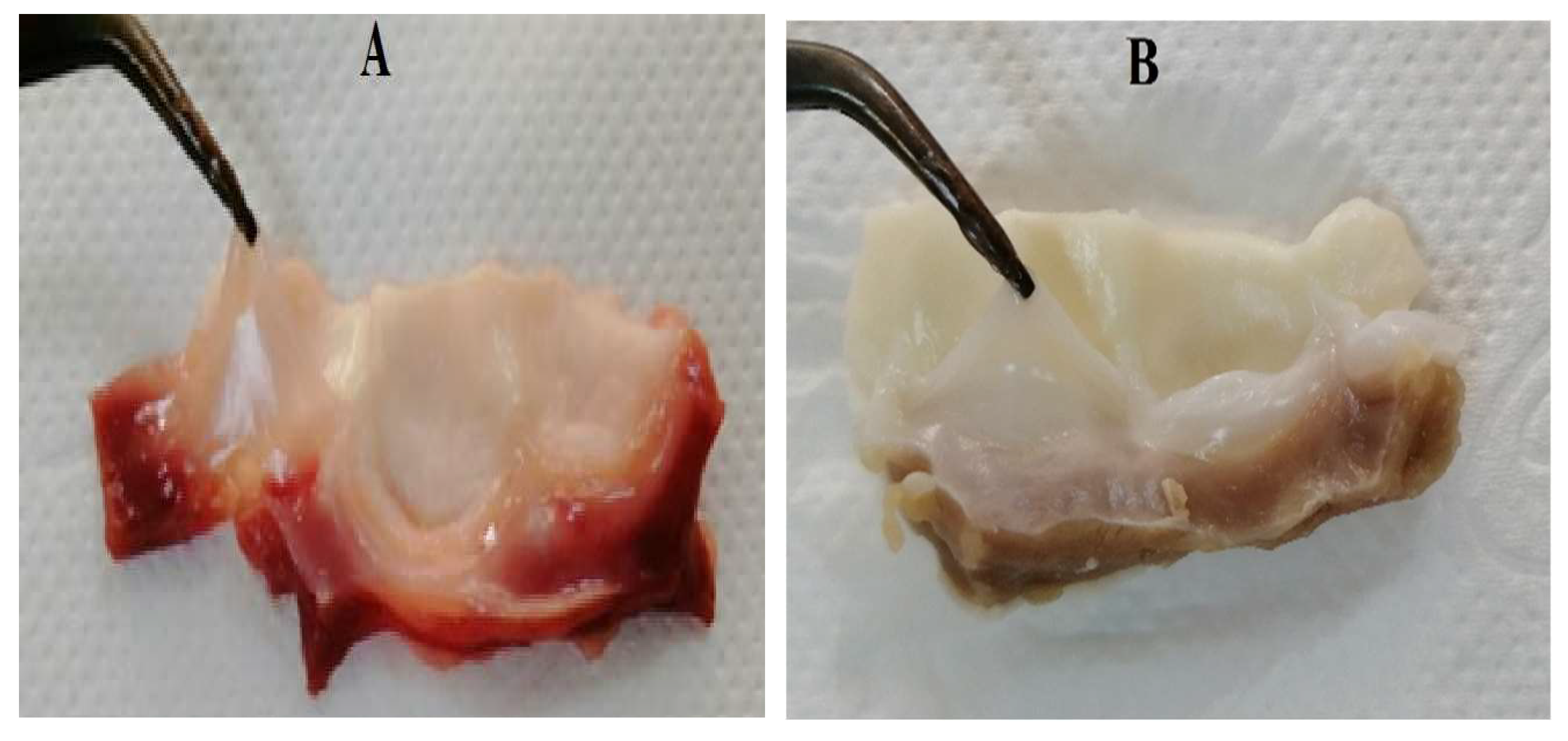
2.1. Decellularization
2.2. Residual DNA Measurement
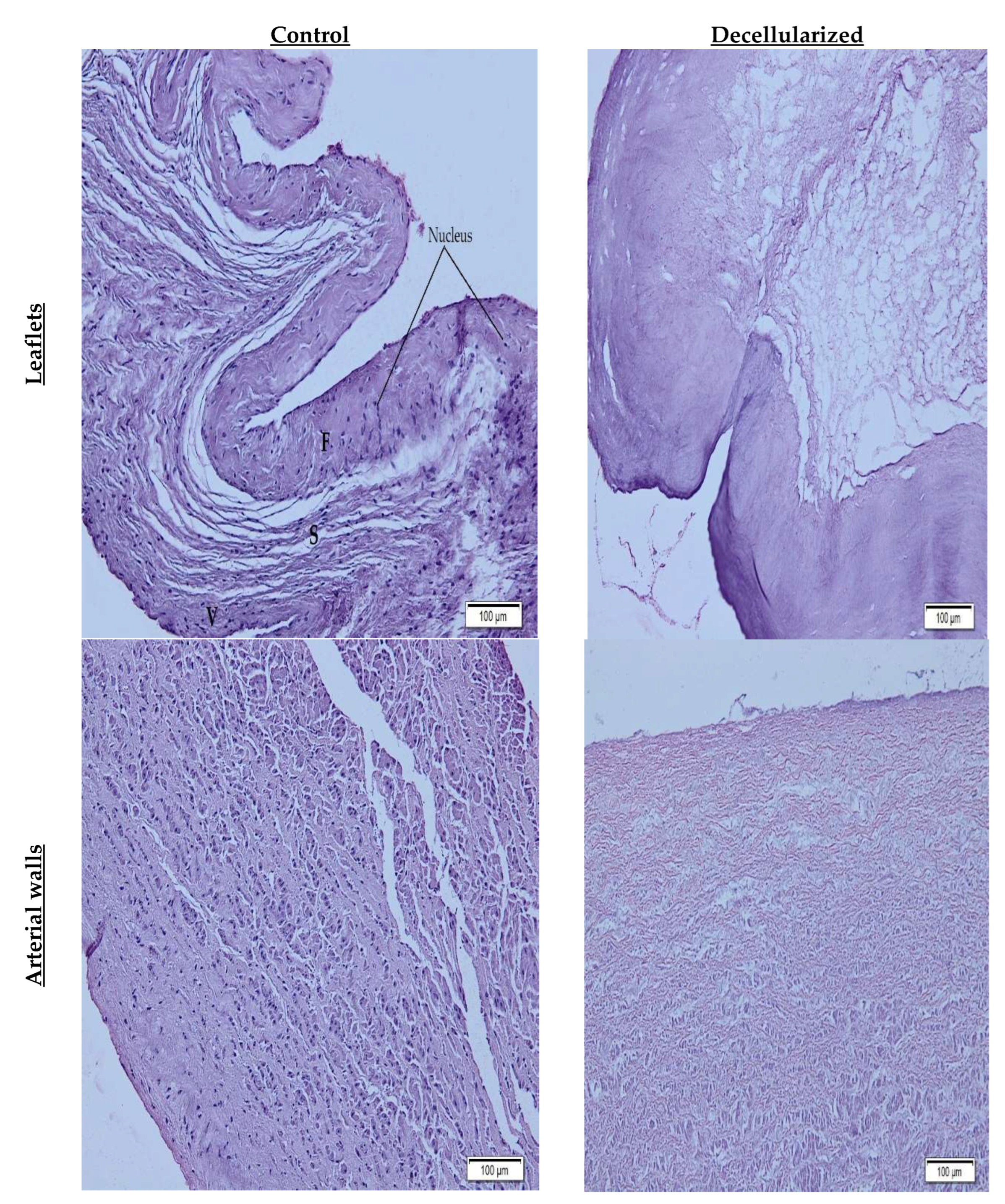
2.3. Histological Characterization
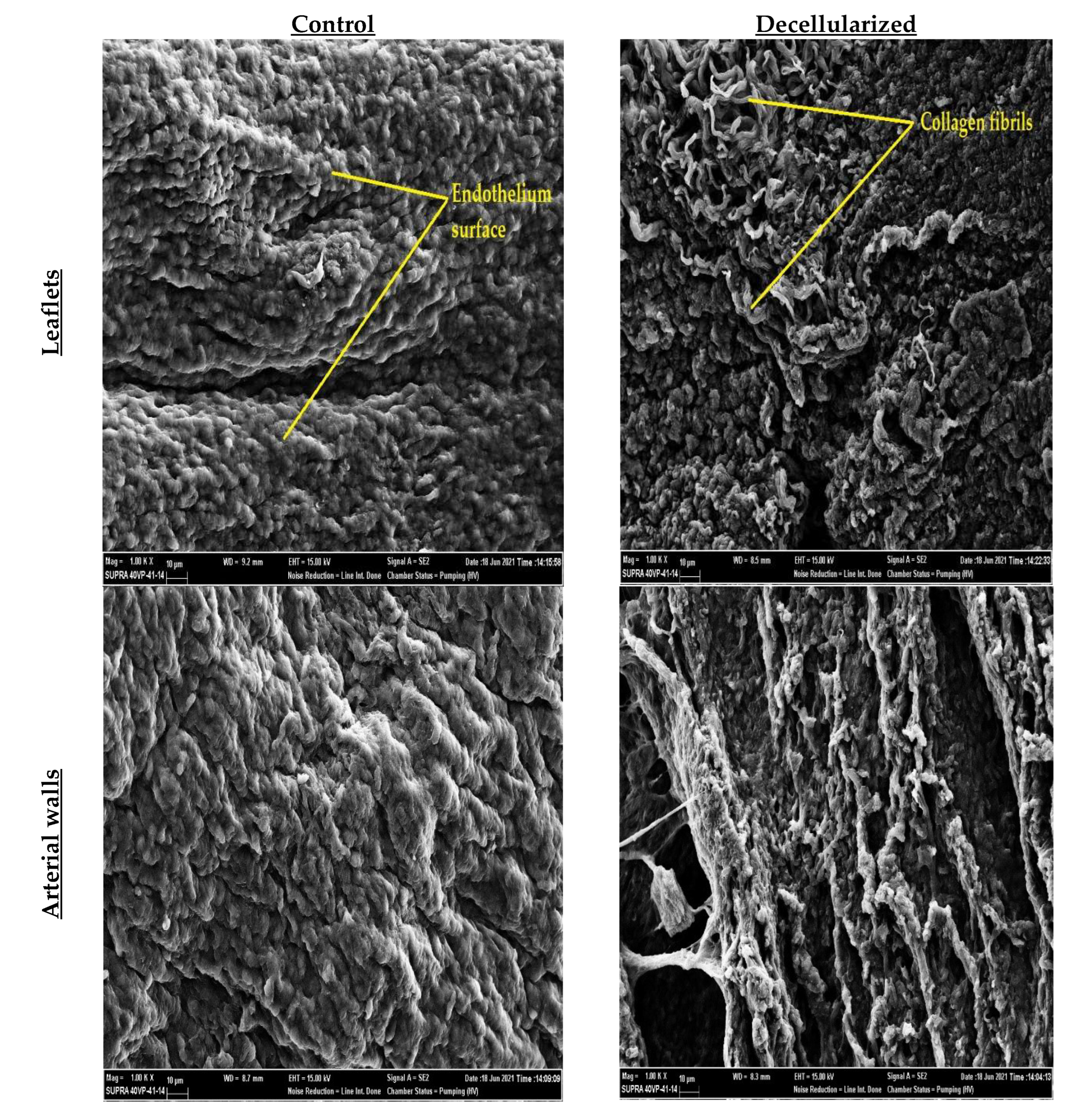
2.4. SEM Imaging
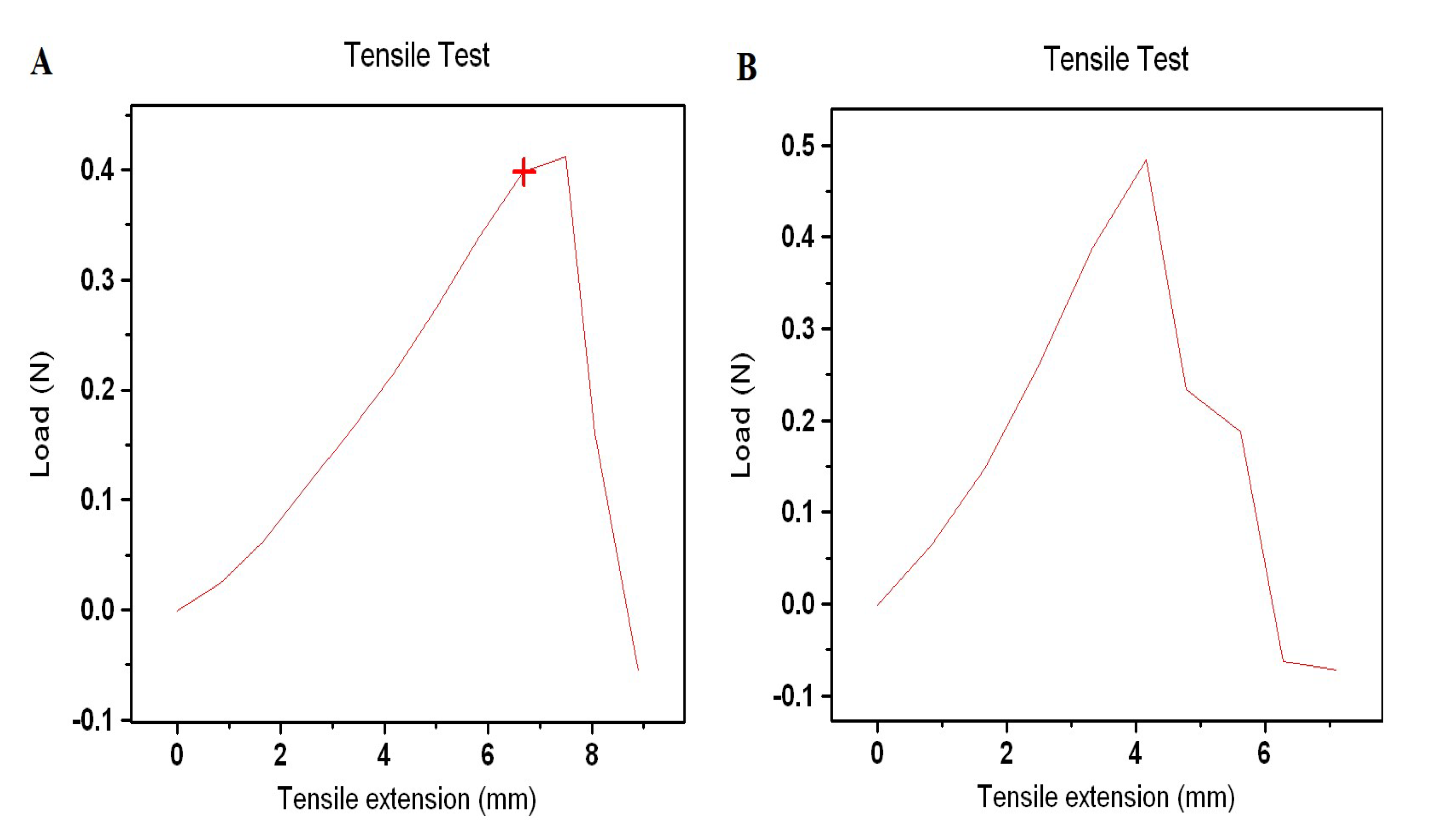
2.5. Tensile Test
2.6. Swelling Ratio
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahnavi, S.; Kumary, T.; Bhuvaneshwar, G.; Natarajan, T.; Verma, R. Engineering of a polymer layered bio-hybrid heart valve scaffold. Mater. Sci. Eng. C 2015, 51, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Tillquist, M.N.; Maddox, T. Cardiac crossroads: deciding between mechanical or bioprosthetic heart valve replacement. Patient Preference Adherence 2011, 5, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Fioretta, E.S.; Motta, S.E.; Lintas, V.; Loerakker, S.; Parker, K.K.; Baaijens, F.P.T.; Falk, V.; Hoerstrup, S.P.; Emmert, M.Y. Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat. Rev. Cardiol. 2020, 18, 92–116. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges. Biomedicines 2022, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Akpek, A. Analysis of biocompatibility characteristics of stereolithography applied three dimensional (3D) bioprinted artifical heart valves. Journal of the Faculty of Engineering and Architecture of Gazi University 2018, 33, 929–938. [Google Scholar]
- Boroumand, S.; Asadpour, S.; Akbarzadeh, A.; Faridi-Majidi, R.; Ghanbari, H. Heart valve tissue engineering: an overview of heart valve decellularization processes. Regen. Med. 2018, 13, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Mela, P.; Hinderer, S.; Kandail, H. S.; Bouten, C. V. C.; Smits, A. I. P. M. Tissue-Engineered Heart Valves. In Principles of Heart Valve Engineering; Elsevier, 2019; pp 123–176.
- Simionescu, D.; Harpa, M.M.; Simionescu, A.; Oprita, C.; Movileanu, I. Tissue Engineering Heart Valves – a Review of More than Two Decades into Preclinical and Clinical Testing for Obtaining the Next Generation of Heart Valve Substitutes. Romanian J. Cardiol. 2021, 31, 501–510. [Google Scholar] [CrossRef]
- Ramm, R.; Niemann, H.; Petersen, B.; Haverich, A.; Hilfiker, A. Decellularized GGTA1-KO pig heart valves do not bind preformed human xenoantibodies. Basic Res. Cardiol. 2016, 111, 1–13. [Google Scholar] [CrossRef]
- Kawecki, M.; Łabuś, W.; Klama-Baryla, A.; Kitala, D.; Kraut, M.; Glik, J.; Misiuga, M.; Nowak, M.; Bielecki, T.; Kasperczyk, A. A review of decellurization methods caused by an urgent need for quality control of cell-free extracellular matrix' scaffolds and their role in regenerative medicine. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2017, 106, 909–923. [Google Scholar] [CrossRef]
- VeDepo, M.C.; Detamore, M.S.; A Hopkins, R.; Converse, G.L. Recellularization of decellularized heart valves: Progress toward the tissue-engineered heart valve. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef]
- Copeland, K. M.; Wang, B.; Shi, X.; Simionescu, D. T.; Hong, Y.; Bajona, P.; Sacks, M. S.; Liao, J. Decellularization in Heart Valve Tissue Engineering. In Advances in Heart Valve Biomechanics; Springer International Publishing: Cham, 2018. [Google Scholar]
- Hussein, K.H.; Park, K.-M.; Kang, K.-S.; Woo, H.-M. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater. Sci. Eng. C 2016, 67, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2015, 11, 942–965. [Google Scholar] [CrossRef] [PubMed]
- Kasimir, M.-T.; Rieder, E.; Seebacher, G.; Silberhumer, G.; Wolner, E.; Weigel, G.; Simon, P. Comparison of Different Decellularization Procedures of Porcine Heart Valves. Int. J. Artif. Organs 2003, 26, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Cebotari, S.; Mertsching, H.; Kallenbach, K.; Kostin, S.; Repin, O.; Batrinac, A.; Kleczka, C.; Ciubotaru, A.; Haverich, A. Construction of Autologous Human Heart Valves Based on an Acellular Allograft Matrix. Circ. 2002, 106, I63–I68. [Google Scholar] [CrossRef]
- Dainese, L.; Guarino, A.; Burba, I.; Esposito, G.; Pompilio, G.; Polvani, G.; Rossini, A. Heart valve engineering: decellularized aortic homograft seeded with human cardiac stromal cells. . 2012, 21, 125–34. [Google Scholar] [PubMed]
- Cigliano, A.; Gandaglia, A.; Lepedda, A.J.; Zinellu, E.; Naso, F.; Gastaldello, A.; Aguiari, P.; De Muro, P.; Gerosa, G.; Spina, M.; et al. Fine Structure of Glycosaminoglycans from Fresh and Decellularized Porcine Cardiac Valves and Pericardium. Biochem. Res. Int. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Spina, M.; Naso, F.; Zancan, I.; Iop, L.; Dettin, M.; Gerosa, G. Biocompatibility Issues of Next Generation Decellularized Bioprosthetic Devices.CONFERENCE NAME, LOCATION OF CONFERENCE, COUNTRYDATE OF CONFERENCE; pp. 1–6.
- Kim, H.; Choi, K.H.; Sung, S.C.; Kim, Y.S. Effect of ethanol washing on porcine pulmonary artery wall decellularization using sodium dodecyl sulfate. Artif. Organs 2022, 46, 1281–1293. [Google Scholar] [CrossRef]
- Amadeo, F.; Boschetti, F.; Polvani, G.; Banfi, C.; Pesce, M.; Santoro, R. Aortic valve cell seeding into decellularized animal pericardium by perfusion-assisted bioreactor. J. Tissue Eng. Regen. Med. 2018, 12, 1481–1493. [Google Scholar] [CrossRef]
- Seyrek, A.; Günal, G.; Aydin, H. M. Development of Antithrombogenic ECM-Based Nanocomposite Heart Valve Leaflets. ACS Appl Bio Mater 2022, 5, 3883–3895. [Google Scholar] [CrossRef]
- Hopkins, R.A.; Bert, A.A.; Hilbert, S.L.; Quinn, R.W.; Brasky, K.M.; Drake, W.B.; Lofland, G.K. Bioengineered human and allogeneic pulmonary valve conduits chronically implanted orthotopically in baboons: Hemodynamic performance and immunologic consequences. J. Thorac. Cardiovasc. Surg. 2012, 145, 1098–1107. [Google Scholar] [CrossRef]
- Godehardt, A.W.; Ramm, R.; Gulich, B.; Tönjes, R.R.; Hilfiker, A. Decellularized pig pulmonary heart valves—Depletion of nucleic acids measured by proviral PERV pol. Xenotransplantation 2019, 27, e12565. [Google Scholar] [CrossRef] [PubMed]
- Schoen, F.J.; Levy, R.J. Calcification of Tissue Heart Valve Substitutes: Progress Toward Understanding and Prevention. Ann. Thorac. Surg. 2005, 79, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Christ, T.; Paun, A.C.; Grubitzsch, H.; Holinski, S.; Falk, V.; Dushe, S. Long-term results after the Ross procedure with the decellularized AutoTissue Matrix P® bioprosthesis used for pulmonary valve replacement. Eur. J. Cardio-Thoracic Surg. 2018, 55, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Rüffer, A.; Cesnjevar, R.; Glöckler, M.; Agaimy, A.; Daniel, W.G.; Garlichs, C.D.; Dittrich, S. Early obstruction of decellularized xenogenic valves in pediatric patients: involvement of inflammatory and fibroproliferative processes. Cardiovasc. Pathol. 2011, 20, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Rüffer, A.; Purbojo, A.; Cicha, I.; Glöckler, M.; Potapov, S.; Dittrich, S.; Cesnjevar, R. A. Early Failure of Xenogenous De-Cellularised Pulmonary Valve Conduits — a Word of Caution! ☆. European Journal of Cardio-Thoracic Surgery 2010, 38, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Leyh, R.G.; Wilhelmi, M.; Rebe, P.; Fischer, S.; Kofidis, T.; Haverich, A.; Mertsching, H. In vivo repopulation of xenogeneic and allogeneic acellular valve matrix conduits in the pulmonary circulation. Ann. Thorac. Surg. 2003, 75, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, I.; Theodoridis, K.; Baraki, H.; Sarikouch, S.; Bara, C.; Meyer, T.; Höffler, K.; Hartung, D.; Hilfiker, A.; Haverich, A.; et al. Decellularized aortic allografts versus pulmonary autografts for aortic valve replacement in the growing sheep model: haemodynamic and morphological results at 20 months after implantation. Eur. J. Cardio-Thoracic Surg. 2015, 49, 1228–1238. [Google Scholar] [CrossRef]
- Abdolghafoorian, H.; Farnia, P.; Sajadi Nia, R. S.; Bahrami, A.; Dorudinia, A.; Ghanavi, J. Effect of Heart Valve Decellularization on Xenograft Rejection. Exp Clin Transplant 2017, 15, 329–336. [Google Scholar]
- Ramm, R.; Goecke, T.; Köhler, P.; Tudorache, I.; Cebotari, S.; Ciubotaru, A.; Sarikouch, S.; Höffler, K.; Bothe, F.; Petersen, B.; et al. Immunological and functional features of decellularized xenogeneic heart valves after transplantation into GGTA1-KO pigs. Regen. Biomater. 2021, 8, rbab036. [Google Scholar] [CrossRef]
- Baraki, H.; Tudorache, I.; Braun, M.; Höffler, K.; Görler, A.; Lichtenberg, A.; Bara, C.; Calistru, A.; Brandes, G.; Hewicker-Trautwein, M.; et al. Orthotopic replacement of the aortic valve with decellularized allograft in a sheep model. Biomaterials 2009, 30, 6240–6246. [Google Scholar] [CrossRef]
- Quinn, R.W.; Hilbert, S.L.; Converse, G.L.; Bert, A.A.; Buse, E.; Drake, W.B.; Armstrong, M.; Moriarty, S.J.; Lofland, G.K.; Hopkins, R.A. Enhanced Autologous Re-endothelialization of Decellularized and Extracellular Matrix Conditioned Allografts Implanted Into the Right Ventricular Outflow Tracts of Juvenile Sheep. Cardiovasc. Eng. Technol. 2012, 3, 217–227. [Google Scholar] [CrossRef]
- Quinn, R.W.; Hilbert, S.L.; Bert, A.A.; Drake, B.W.; Bustamante, J.A.; Fenton, J.E.; Moriarty, S.J.; Neighbors, S.L.; Lofland, G.K.; Hopkins, R.A. Performance and Morphology of Decellularized Pulmonary Valves Implanted in Juvenile Sheep. Ann. Thorac. Surg. 2011, 92, 131–137. [Google Scholar] [CrossRef]
- Qiao, W.-H.; Liu, P.; Hu, D.; Al Shirbini, M.; Zhou, X.-M.; Dong, N.-G. Sequential hydrophile and lipophile solubilization as an efficient method for decellularization of porcine aortic valve leaflets: Structure, mechanical property and biocompatibility study. . 2017, 12, e828–e840. [Google Scholar] [CrossRef]
- van Steenberghe, M.; Schubert, T.; Gerelli, S.; Bouzin, C.; Guiot, Y.; Xhema, D.; Bollen, X.; Abdelhamid, K.; Gianello, P. Porcine Pulmonary Valve Decellularization with NaOH-Based vs Detergent Process: Preliminary in Vitro and in Vivo Assessments. J Cardiothorac Surg 2018, 13, 34. [Google Scholar] [CrossRef]
- Chauvette, V.; Bouhout, I.; Tarabzoni, M.; Pham, M.; Wong, D.; Whitlock, R.; Chu, M.W.; El-Hamamsy, I.; Lefebvre, L.; Poirier, N.; et al. Pulmonary homograft dysfunction after the Ross procedure using decellularized homografts—a multicenter study. J. Thorac. Cardiovasc. Surg. 2020, 163, 1296–1305. [Google Scholar] [CrossRef]
- Tudorache, I.; Calistru, A.; Baraki, H.; Meyer, T.; Höffler, K.; Sarikouch, S.; Bara, C.; Görler, A.; Hartung, D.; Hilfiker, A.; et al. Orthotopic Replacement of Aortic Heart Valves with Tissue-Engineered Grafts. Tissue Eng. Part A 2013, 19, 1686–1694. [Google Scholar] [CrossRef]
- Theodoridis, K.; Tudorache, I.; Calistru, A.; Cebotari, S.; Meyer, T.; Sarikouch, S.; Bara, C.; Brehm, R.; Haverich, A.; Hilfiker, A. Successful matrix guided tissue regeneration of decellularized pulmonary heart valve allografts in elderly sheep. Biomaterials 2015, 52, 221–228. [Google Scholar] [CrossRef]
- Quinn, R.W.; Bert, A.A.; Converse, G.L.; Buse, E.E.; Hilbert, S.L.; Drake, W.B.; Hopkins, R.A. Performance of allogeneic bioengineered replacement pulmonary valves in rapidly growing young lambs. J. Thorac. Cardiovasc. Surg. 2016, 152, 1156–1165. [Google Scholar] [CrossRef]
- Converse, G.L.; Buse, E.E.; Neill, K.R.; McFall, C.R.; Lewis, H.N.; VeDepo, M.C.; Quinn, R.W.; Hopkins, R.A. Design and efficacy of a single-use bioreactor for heart valve tissue engineering. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2015, 105, 249–259. [Google Scholar] [CrossRef]
- Hinderer, S.; Seifert, J.; Votteler, M.; Shen, N.; Rheinlaender, J.; Schäffer, T.E.; Schenke-Layland, K. Engineering of a bio-functionalized hybrid off-the-shelf heart valve. Biomaterials 2014, 35, 2130–2139. [Google Scholar] [CrossRef]
- Lichtenberg, A.; Tudorache, I.; Cebotari, S.; Ringes-Lichtenberg, S.; Sturz, G.; Hoeffler, K.; Hurscheler, C.; Brandes, G.; Hilfiker, A.; Haverich, A. In vitro re-endothelialization of detergent decellularized heart valves under simulated physiological dynamic conditions. Biomaterials 2006, 27, 4221–4229. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Flameng, W.; De Visscher, G.; Mesure, L.; Hermans, H.; Jashari, R.; Meuris, B. Coating with fibronectin and stromal cell–derived factor-1α of decellularized homografts used for right ventricular outflow tract reconstruction eliminates immune response–related degeneration. J. Thorac. Cardiovasc. Surg. 2014, 147, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Haupt, J.; Lutter, G.; Gorb, S.N.; Simionescu, D.T.; Frank, D.; Seiler, J.; Paur, A.; Haben, I. Detergent-based decellularization strategy preserves macro- and microstructure of heart valves. Interact. Cardiovasc. Thorac. Surg. 2017, 26, 230–236. [Google Scholar] [CrossRef]
- VeDepo, M.C.; Buse, E.E.; Quinn, R.W.; Williams, T.D.; Detamore, M.S.; Hopkins, R.A.; Converse, G.L. Species-specific effects of aortic valve decellularization. Acta Biomater. 2017, 50, 249–258. [Google Scholar] [CrossRef]
- Syedain, Z.H.; Bradee, A.R.; Kren, S.; Taylor, D.A.; Tranquillo, R.T. Decellularized Tissue-Engineered Heart Valve Leaflets with Recellularization Potential. Tissue Eng. Part A 2013, 19, 759–769. [Google Scholar] [CrossRef]
- Findeisen, K.; Morticelli, L.; Goecke, T.; Kolbeck, L.; Ramm, R.; Höffler, H.; Brandes, G.; Korossis, S.; Haverich, A.; Hilfiker, A. Toward acellular xenogeneic heart valve prostheses: Histological and biomechanical characterization of decellularized and enzymatically deglycosylated porcine pulmonary heart valve matrices. Xenotransplantation 2020, 27, e12617. [Google Scholar] [CrossRef]
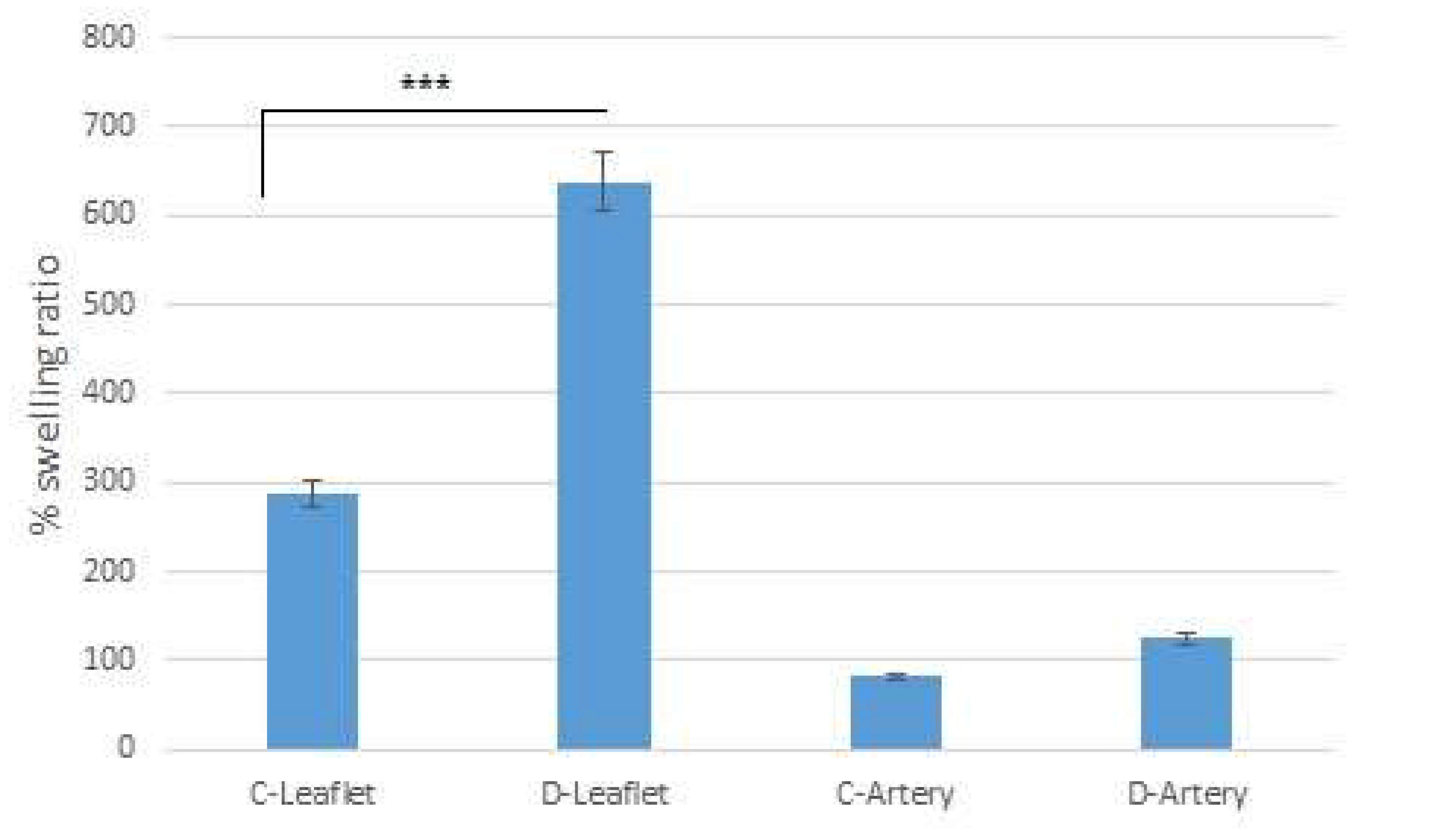
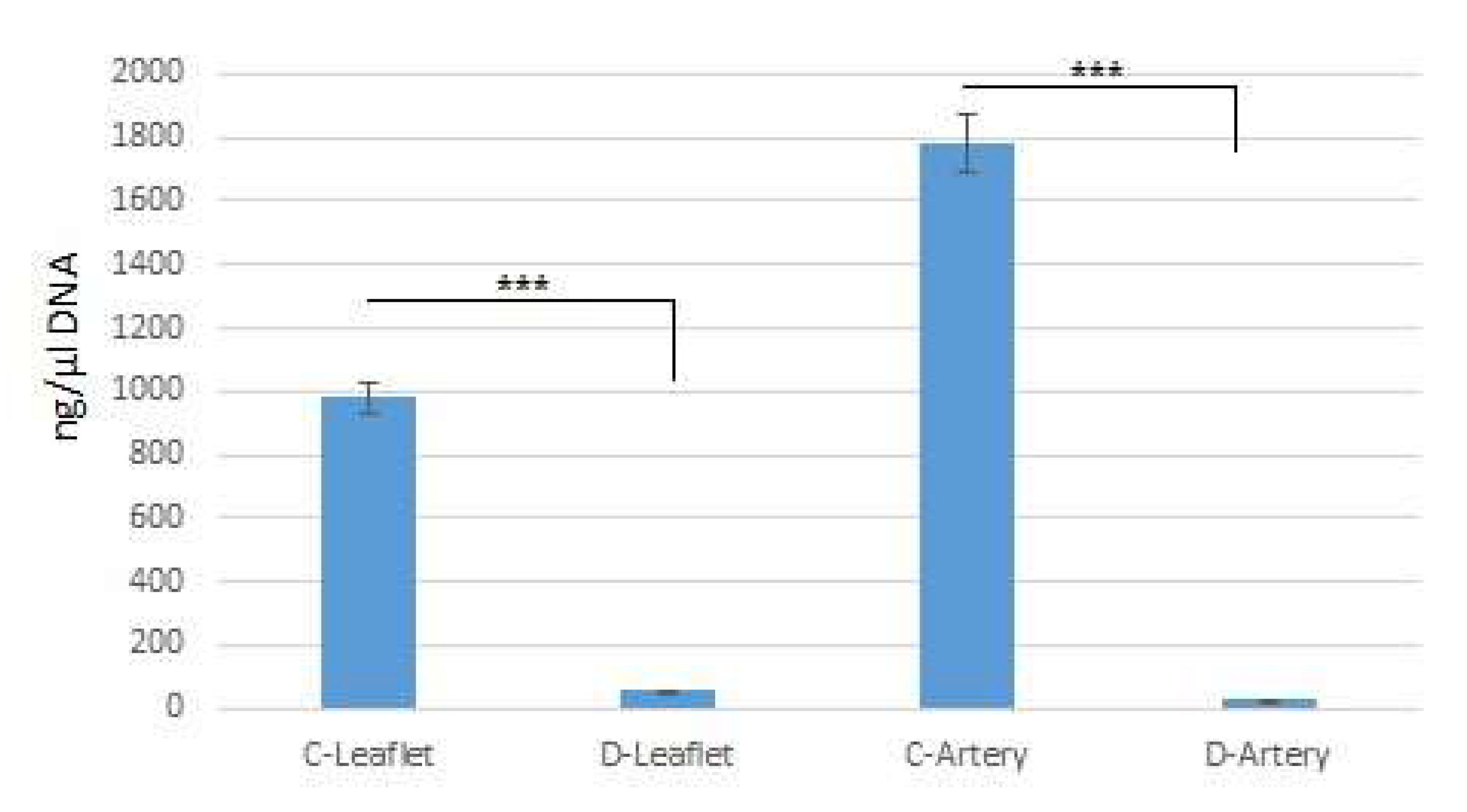

| UTS (MPa) | Modulus of Elasticity (MPa) | |
|---|---|---|
| Control | 0,033 ± 0,02 | 0,0037 ± 0,002 |
| Decellularized | 0,047 ± 0,015 | 0,011 ± 0,003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





