Submitted:
19 May 2023
Posted:
22 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. The Study Group
2.2. Assessment of PD-1, PD-L1
2.3. Statistical Methods
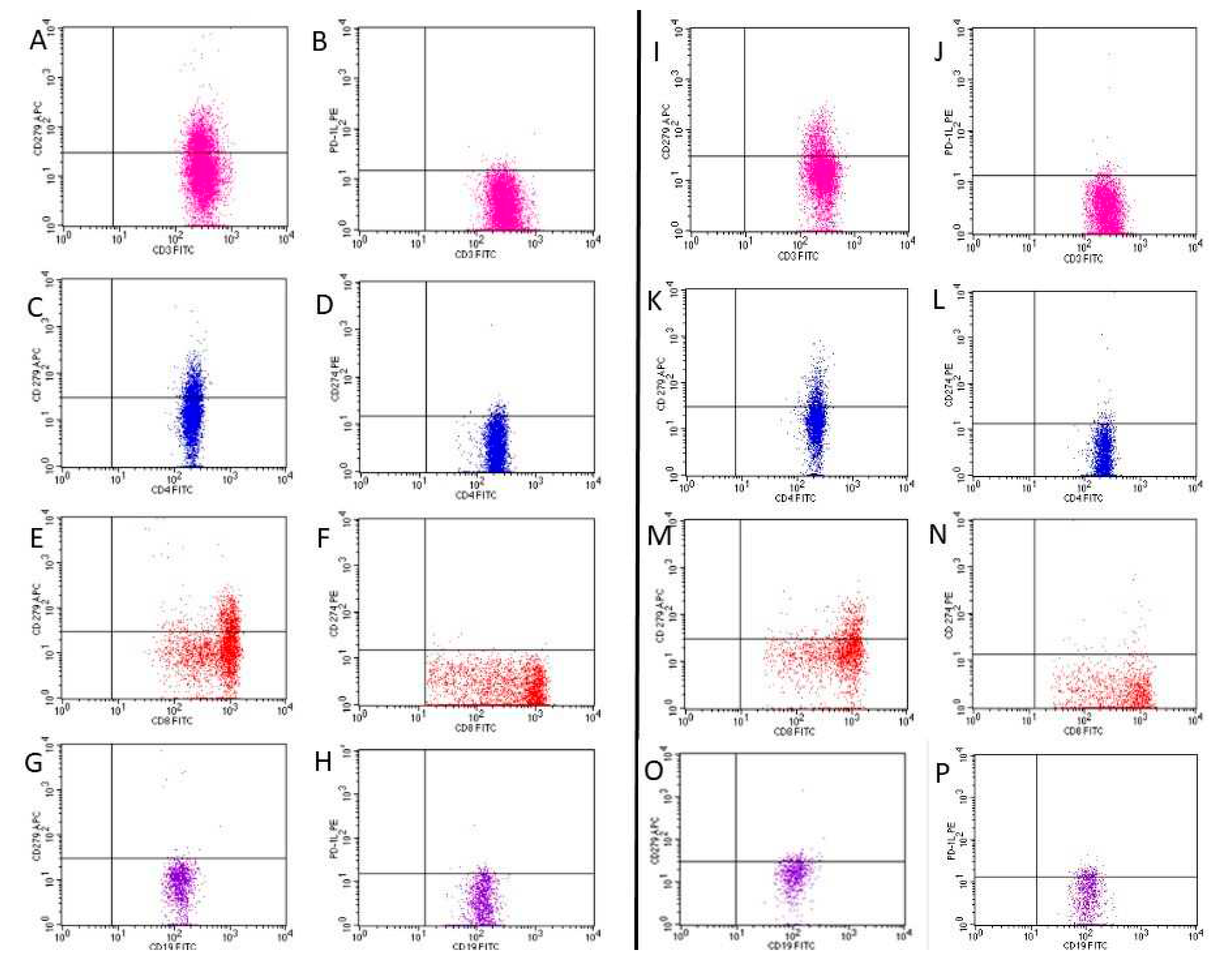
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Armstrong, A.W.; Read, C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, M.; Krasowska, D. PD1/PD-L1 pathway in psoriasis and psoriatic arthritis: a review. Postepy Dermatol Alergol. 2021, 38, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chang, C.; Lu, Q. The inflammatory response in psoriasis: A comprehensive review. Clin Rev Allergy Immunol. 2016, 50, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef] [PubMed]
- Bonigen, J.; Raynaud-Donzel, C.; Hureaux, J.; Kramkimel, N.; Blom, A; Jeudy, G. ; Breton, A.L.; Hubiche, T.; Bedane, C.; Legoupil, D.; et al. Anti-PD1-induced psoriasis: a study of 21 patients. J Eur Acad Dermatol Venereol. 2017, 31, e254–e257. [Google Scholar] [CrossRef] [PubMed]
- Bartosińska, J.; Zakrzewska, E.; Król, A.; Raczkiewicz, D.; Purkot, J.; Majdan, M.; Krasowska, D.; Chodorowska, G.; Giannopoulos, K. Differential expression of programmed death 1 (PD-1) on CD4+ and CD8+ T cells in rheumatoid arthritis and psoriatic arthritis. Pol Arch Intern Med. 2017, 22, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Bartosińska, J.; Purkot, J.; Kowal, M.; Michalak-Stoma, A.; Krasowska, D.; Chodorowska, G.; Giannopoulos, K. The expression of selected molecular markers of immune tolerance in psoriatic patients. Adv Clin Exp Med. 2018, 27, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Mahil, S.K.; Ezejimofor, M.C.; Exton, L.S.; Manounah, L.; Burden, A.D.; Coates, L.C.; de Brito, M.; McGuire, A.; Murphy, R.; et al. Comparing the efficacy and tolerability of biologic therapies in psoriasis: an updated network meta-analysis. Br J Dermatol. 2020, 183, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Wang, J. PD-1/PD-L pathway and autoimmunity. Autoimmunity 2005, 38, 353–357. [Google Scholar] [CrossRef]
- Peled, M.; Strazza, M.; Azoulay-Alfaguter, I.; Silverman, G.J.; Scher, J.U.; Mor, A. Analysis of Programmed Death-1 in Patients with Psoriatic Arthritis. Inflammation. 2015, 38, 1573–1579. [Google Scholar] [CrossRef]
- Raptopoulou, A.P.; Bertsias, G.; Makrygiannakis, D.; Verginis, P.; Kritikos, I.; Tzardi, M.; Klareskog, L; Catrina, A. I.; Sidiropoulos, P.; Boumpas, D.T. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis Rheum. 2010, 62, 1870–1880. [Google Scholar] [CrossRef]
- Sibaud, V.; Meyer, N.; Lamant, L.; Vigarios, E.; Mazieres, J.; Delord, J.P. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016, 28, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Imai. Y.; Ayithan, N.; Wu, X.; Yuan, Y.; Wang, L.; Hwang, S.T. Cutting Edge: PD-1 Regulates Imiquimod-Induced Psoriasiform Dermatitis through Inhibition of IL-17A Expression by Innate γδ-Low T Cells. J Immunol. 2015, 15, 421–425. [Google Scholar] [CrossRef]
- Bommarito, D.; Hall, C.; Taams, L.S.; Corrigall, V.M. Inflammatory cytokines compromise programmed cell death-1 (PD-1)-mediated T cell suppression in inflammatory arthritis through up-regulation of soluble PD-1. Clin Exp Immunol. 2017, 188, 455–466. [Google Scholar] [CrossRef]
- Bartosińska, J.; Zakrzewska, E.; Raczkiewicz, D.; Purkot, J.; Michalak-Stoma, A.; Kowal, M.; Krasowska, D.; Chodorowska, G.; Giannopoulos, K. Suppressed Programmed Death 1 Expression on CD4+ and CD8+ T Cells in Psoriatic Patients. Mediators Inflamm. 2017, 5385102. [Google Scholar] [CrossRef] [PubMed]
- Bartosińska, J.; Zakrzewska, E.; Purkot, J.; Michalak-Stoma, A.; Kowal, M.; Krasowska, D.; Chodorowska, G.; Giannopoulos, K. Decreased blood CD4+PD-1+ and CD8+PD-1+ T cells in psoriatic patients with and without arthritis. Postepy Dermatol Alergol. 2018, 35, 344–350. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Unit or Category | Results |
|---|---|---|
| Age, min–max, M | Years | 18–68, 42.8 |
| Gender, n (%) | Male | 60 (71.4) |
| Female | 24 (28.6) | |
| Weight, min–max, M | kg | 47–125, 84.3 |
| Smoking status, n (%) | Non-smokers | 30 (38.0) |
| Smokers | 54 (62.0) | |
| Psoriasis type, n (%) | I | 72 (88.1) |
| II | 12 (11.9) | |
| Duration of psoriasis, min–max, M | Years | 1–50, 20.5 |
| PASI, min–max, M | 5.5-47.0, 17.5 | |
| BSA, min–max, M | 6-80.0, 28.6 | |
| DLQI, min–max, M | 6-30, 17.4 | |
| PsA, n (%) | Yes | 27 (30.9) |
| Specificity | Fluorochrome | Producer |
|---|---|---|
| Mouse anti human- CD3 | FITC | BD Biosciences, USA |
| Mouse anti human-CD4 | FITC | BD Biosciences, USA |
| Mouse anti human-CD8 | FITC | BD Biosciences, USA |
| Mouse anti human-CD19 | FITC | BD Biosciences, USA |
| Mouse anti human-CD279 | APC | BD Biosciences, USA |
| Mouse anti human -CD274 | PE | BD Biosciences, USA |
| PBMC subtype | Psoriasis | Control | p |
| Median (IQR) | Median (IQR) | ||
| CD3/PD1 | 13.78 (11.03-18.42) | 17.26 (14.51-21.00) | 0.021 |
| CD3/PDL1 | 1.23 (0.74-2.72) | 3.26 (1.67-8.20) | <0.001 |
| CD4/PD1 | 13.58 (9.94-17.46) | 18.10 (13.77-25.25) | 0.002 |
| CD4/PDL1 | 2.14 (1.14-4.49) | 4.30 (3.28-5.28) | 0.001 |
| CD8/PD1 | 13.70 (9.78-17.32) | 14.90 (8.80-18.61) | 0.576 |
| CD8/PDL1 | 0.58 (0.28-1.57) | 1.72 (0.87-3.10) | <0.001 |
| CD19/PD1 | 2.48 (1.44-4.34) | 10.65 (6.14-12.20) | <0.001 |
| CD19/PDL1 | 5.94 (1.39-11.11) | 10.95 (4.67-20.00) | 0.002 |
| PBMC subtype | PASI | BSA | Duration of psoriasis | |||
| r | p | r | p | r | p | |
| CD3/PD-1 | -0.172 | 0.119 | -0.150 | 0.173 | 0.096 | 0.390 |
| CD3/PD-L1 | -0.053 | 0.634 | -0.045 | 0.683 | -0.049 | 0.661 |
| CD4/PD-1 | -0.051 | 0.648 | -0.083 | 0.455 | 0.218 | 0.049 |
| CD4/PD-L1 | -0.013 | 0.908 | 0.035 | 0.750 | -0.106 | 0.343 |
| CD8/PD-1 | -0.150 | 0.174 | -0.126 | 0.253 | 0.148 | 0.186 |
| CD8/PD-L1 | 0.124 | 0.263 | -0.146 | 0.187 | 0.005 | 0.963 |
| CD19/PD-1 | -0.006 | 0.960 | -0.017 | 0.876 | 0.052 | 0.642 |
| CD19/PD-L1 | -0.015 | 0.892 | 0.211 | 0.054 | -0.079 | 0.483 |
| PBMC subtype | Before treatment | During treatment | p |
| Median (IQR) | Median (IQR) | ||
| CD3/PD1 | 13.59 (10.08-20.77) | 13.72 (9.32-16.24) | 0.078 |
| CD3/PDL1 | 1.33 (0.92-2.36) | 3.74 (1.85-11.11) | 0.002 |
| CD4/PD1 | 15.64 (10.00-17.69) | 12.11 (9.78-16.32) | 0.471 |
| CD4/PDL1 | 2.11 (1.43-3.37) | 3.1 (1.42-6.03) | 0.428 |
| CD8/PD1 | 14.73 (10.04-21.10) | 12.84 (7.00-15.73) | 0.041 |
| CD8/PDL1 | 0.59 (0.28-1.36) | 1.65 (0.63-5.84) | 0.006 |
| CD19/PD1 | 3.74 (1.79-4.36) | 2.91 (1.14-6.11) | 0.737 |
| CD19/PDL1 | 4.77 (1.77-10.00) | 9.6 (2.67-13.39) | 0.302 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





