Submitted:
21 May 2023
Posted:
22 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Procedures
2.1. Chemicals and Batch Testing
2.2. Preparation of the Rice Husk Biochar and the Composite Adsorbent
3. Results and Discussion
3.1. Adsorbents’ Properties and the Mechanism of Adsorption
3.1.1. Surface Morphology and Elemental Composition Analysis
3.1.2. Fourier Transform Infrared Analysis
3.2. Estimation of the Equilibrium Contact Time and Optimization of the Parameters of Batch Adsorption
3.3. Explanation of the Adsorption Data Using Kinetic Models
3.4. Explanation of the Adsorption Data Using Isotherm Models
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zhou, Q.; Liao, B.; Lin, L.; Qiu, W.; Song, Z. Adsorption of Cu(II) and Cd(II) from Aqueous Solutions by Ferromanganese Binary Oxide–Biochar Composites. Science of The Total Environment 2018, 615, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chai, L.; Li, Q.; Yan, X.; Wang, Q.; Liu, H. Chemical Precipitation Granular Sludge (CPGS) Formation for Copper Removal from Wastewater. RSC Adv. 2016, 6, 114405–114411. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; Gao, W.; Zhang, Y.; Yang, Y. Removal of Copper Ions from Aqueous Solution by a Hydrotalcite-Like Absorbent FeMnMg-LDH. Water Air Soil Pollut 2020, 231, 370. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Wang, C.; Wang, H. Biochar/MnAl-LDH Composites for Cu (ΙΙ) Removal from Aqueous Solution. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2018, 538, 443–450. [Google Scholar] [CrossRef]
- Jayashree, S.; Ramesh, S.T.; Lavanya, A.; Gandhimathi, R.; Nidheesh, P.V. Wastewater Treatment by Microbial Fuel Cell Coupled with Peroxicoagulation Process. Clean Techn Environ Policy 2019, 21, 2033–2045. [Google Scholar] [CrossRef]
- Salazar, R.; Gallardo-Arriaza, J.; Vidal, J.; Rivera-Vera, C.; Toledo-Neira, C.; Sandoval, M.A.; Cornejo-Ponce, L.; Thiam, A. Treatment of Industrial Textile Wastewater by the Solar Photoelectro-Fenton Process: Influence of Solar Radiation and Applied Current. Solar Energy 2019, 190, 82–91. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes (Basel) 2020, 10, 89. [Google Scholar] [CrossRef]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil Pollut 2020, 231, 503. [Google Scholar] [CrossRef]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arabian Journal of Chemistry 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Comparative Removal of Lead and Nickel Ions onto Nanofibrous Sheet of Activated Polyacrylonitrile in Batch Adsorption and Application of Conventional Kinetic and Isotherm Models. Membranes 2021, 11, 10. [Google Scholar] [CrossRef]
- Batool, S.; Idrees, M.; Hussain, Q.; Kong, J. Adsorption of Copper (II) by Using Derived-Farmyard and Poultry Manure Biochars: Efficiency and Mechanism. Chemical Physics Letters 2017, 689, 190–198. [Google Scholar] [CrossRef]
- Rojas, R. Copper, Lead and Cadmium Removal by Ca Al Layered Double Hydroxides. Applied Clay Science 2014, 87, 254–259. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Removal of Copper and Lead Using Banana Biochar in Batch Adsorption Systems: Isotherms and Kinetic Studies. Arab J Sci Eng 2017, 1–12. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, M.; Usman, A.R.A.; Al-Faraj, A.S.; Abduljabbar, A.S.; Al-Wabel, M.I. Biochar Composites with Nano Zerovalent Iron and Eggshell Powder for Nitrate Removal from Aqueous Solution with Coexisting Chloride Ions. Environ Sci Pollut Res Int 2017. [Google Scholar] [CrossRef] [PubMed]
- Kinoti, I.K.; Karanja, E.M.; Nthiga, E.W.; M’thiruaine, C.M.; Marangu, J.M. Review of Clay-Based Nanocomposites as Adsorbents for the Removal of Heavy Metals. Journal of Chemistry 2022, 2022, e7504626. [Google Scholar] [CrossRef]
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Adsorption of Divalent Copper Ions from Synthetic Wastewater Using Layered Double Hydroxides (NiZnFe) and Its Composites with Banana Biochar and Carbon Nanotubes. Water Air Soil Pollut 2020, 231, 346. [Google Scholar] [CrossRef]
- Ethaib, S.; Al-Qutaifia, S.; Al-Ansari, N.; Zubaidi, S.L. Function of Nanomaterials in Removing Heavy Metals for Water and Wastewater Remediation: A Review. Environments 2022, 9, 123. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9. [Google Scholar] [CrossRef]
- Stoian, O.; Covaliu, C.I.; Paraschiv, G.; Catrina (Traistaru), G.-A.; Niță-Lazăr, M.; Matei, E.; Biriş, S. Ștefan; Tudor, P. Magnetite Oxide Nanomaterial Used for Lead Ions Removal from Industrial Wastewater. Materials (Basel) 2021, 14, 2831. [Google Scholar] [CrossRef]
- Do Minh, T.; Song, J.; Deb, A.; Cha, L.; Srivastava, V.; Sillanpää, M. Biochar Based Catalysts for the Abatement of Emerging Pollutants: A Review. Chemical Engineering Journal 2020, 394, 124856. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Utilization of Agro-Industrial and Municipal Waste Materials as Potential Adsorbents for Water Treatment—A Review. Chemical Engineering Journal 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Kumar, P.S.; Gayathri, R.; Rathi, B.S. A Review on Adsorptive Separation of Toxic Metals from Aquatic System Using Biochar Produced from Agro-Waste. Chemosphere 2021, 285, 131438. [Google Scholar] [CrossRef] [PubMed]
- Wijeyawardana, P.; Nanayakkara, N.; Gunasekara, C.; Karunarathna, A.; Law, D.; Pramanik, B.K. Removal of Cu, Pb and Zn from Stormwater Using an Industrially Manufactured Sawdust and Paddy Husk Derived Biochar. Environmental Technology & Innovation 2022, 28, 102640. [Google Scholar] [CrossRef]
- Sanka, P.M.; Rwiza, M.J.; Mtei, K.M. Removal of Selected Heavy Metal Ions from Industrial Wastewater Using Rice and Corn Husk Biochar. Water Air Soil Pollut 2020, 231, 244. [Google Scholar] [CrossRef]
- Han, W.; Hao, H.; Zhang, Q.; Shao, Z. Activated Biochar Loaded CuAl-Layered Double Hydroxide Composite for the Removal of Aniline Aerofloat in Wastewater: Synthesis, Characterization, and Adsorption Mechanism. Journal of Environmental Chemical Engineering 2023, 109293. [Google Scholar] [CrossRef]
- Fang, Q.; Ye, S.; Yang, H.; Yang, K.; Zhou, J.; Gao, Y.; Lin, Q.; Tan, X.; Yang, Z. Application of Layered Double Hydroxide-Biochar Composites in Wastewater Treatment: Recent Trends, Modification Strategies, and Outlook. Journal of Hazardous Materials 2021, 420, 126569. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Meng, L.; Shi, J.; Li, J.; Zhang, X.; Feng, M. Metal-Organic Frameworks/Carbon-Based Materials for Environmental Remediation: A State-of-the-Art Mini-Review. Journal of Environmental Management 2019, 232, 964–977. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Smart Metal-Organic Framework (MOF) Composites and Their Applications in Environmental Remediation. Materials Today Communications 2022, 33, 104823. [Google Scholar] [CrossRef]
- Johnston, A.-L.; Lester, E.; Williams, O.; Gomes, R.L. Understanding Layered Double Hydroxide Properties as Sorbent Materials for Removing Organic Pollutants from Environmental Waters. Journal of Environmental Chemical Engineering 2021, 9, 105197. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Y.; Zhang, R.; Ren, X.; Guo, W. Engineering the Morphology on Layered Double Hydroxides-Induced Persulfate Activation for Enhanced Degradation of Organic Pollutant 2022.
- Chubar, N.; Gilmour, R.; Gerda, V.; Mičušík, M.; Omastova, M.; Heister, K.; Man, P.; Fraissard, J.; Zaitsev, V. Layered Double Hydroxides as the next Generation Inorganic Anion Exchangers: Synthetic Methods versus Applicability. Advances in Colloid and Interface Science 2017, 245, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Ye, S.; Yang, H.; Yang, K.; Zhou, J.; Gao, Y.; Lin, Q.; Tan, X.; Yang, Z. Application of Layered Double Hydroxide-Biochar Composites in Wastewater Treatment: Recent Trends, Modification Strategies, and Outlook. Journal of Hazardous Materials 2021, 420, 126569. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Yin, H.; Li, Y.; Cai, Y.; Yan, C.; Yuan, Y.; Dang, Z. Remediation of Cu and As Contaminated Water and Soil Utilizing Biochar Supported Layered Double Hydroxide: Mechanisms and Soil Environment Altering. Journal of Environmental Sciences 2023, 126, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wan, X.; Zhou, T.; Wang, L.; Yin, X.; Ma, A.; Wang, N. Novel Zn-Fe Engineered Kiwi Branch Biochar for the Removal of Pb(II) from Aqueous Solution. Journal of Hazardous Materials 2022, 424, 127349. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, Y.; Fu, J.; Yuan, L.; Li, Z.; Liu, C.; Zhao, D.; Wang, X. A Novel Magnetic Biochar/MgFe-Layered Double Hydroxides Composite Removing Pb2+ from Aqueous Solution: Isotherms, Kinetics and Thermodynamics. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2019, 567, 278–287. [Google Scholar] [CrossRef]
- Khandaker, S.; Hossain, M.T.; Saha, P.K.; Rayhan, U.; Islam, A.; Choudhury, T.R.; Awual, Md.R. Functionalized Layered Double Hydroxides Composite Bio-Adsorbent for Efficient Copper(II) Ion Encapsulation from Wastewater. Journal of Environmental Management 2021, 300, 113782. [Google Scholar] [CrossRef]
- Saravanan, P.; Josephraj, J.; Thillainayagam, B.P.; Ravindiran, G. Evaluation of the Adsorptive Removal of Cationic Dyes by Greening Biochar Derived from Agricultural Bio-Waste of Rice Husk. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Meng, X. Preparation of a Mg/Al/Fe Layered Supramolecular Compound and Application for Removal of Cr(VI) from Laboratory Wastewater. RSC Adv. 2017, 7, 34984–34993. [Google Scholar] [CrossRef]
- Rashed, S.H.; Abd-Elhamid, A.I.; Abdalkarim, S.Y.H.; El-Sayed, R.H.; El-Bardan, A.A.; Soliman, H.M.A.; Nayl, A.A. Preparation and Characterization of Layered-Double Hydroxides Decorated on Graphene Oxide for Dye Removal from Aqueous Solution. Journal of Materials Research and Technology 2022, 17, 2782–2795. [Google Scholar] [CrossRef]
- Park, M.; Choi, C.L.; Seo, Y.J.; Yeo, S.K.; Choi, J.; Komarneni, S.; Lee, J.H. Reactions of Cu2+ and Pb2+ with Mg/Al Layered Double Hydroxide. Applied Clay Science 2007, 37, 143–148. [Google Scholar] [CrossRef]
- Tan, Y.; Yin, X.; Wang, C.; Sun, H.; Ma, A.; Zhang, G.; Wang, N. Sorption of Cadmium onto Mg-Fe Layered Double Hydroxide (LDH)-Kiwi Branch Biochar. Environmental Pollutants and Bioavailability 2019, 31, 189–197. [Google Scholar] [CrossRef]
- Liang, X.; Zang, Y.; Xu, Y.; Tan, X.; Hou, W.; Wang, L.; Sun, Y. Sorption of Metal Cations on Layered Double Hydroxides. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2013, 433, 122–131. [Google Scholar] [CrossRef]
- Ma, X.; Li, S.; Ren, H.; Zhang, Y.; Ma, Z. Egg White-Mediated Fabrication of Mg/Al-LDH-Hard Biochar Composite for Phosphate Adsorption. Molecules 2022, 27, 8951. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Su, Q.; Xiang, L.; Yuan, Y.; Tu, S. Effect of Pyrolysis Temperature on the Sorption of Cd(II) and Se(IV) by Rice Husk Biochar. Plants 2022, 11, 3234. [Google Scholar] [CrossRef] [PubMed]
- Khitous, M.; Salem, Z.; Halliche, D. Removal of Phosphate from Industrial Wastewater Using Uncalcined MgAl-NO3 Layered Double Hydroxide: Batch Study and Modeling. Desalination and Water Treatment 2016, 57, 15920–15931. [Google Scholar] [CrossRef]
- Palapa, N.R.; Juleanti, N.; Normah; Mohadi, R.; Taher, T.; Rachmat, A.; Lesbani, A. Copper Aluminum Layered Double Hydroxide Modified by Biochar and Its Application as an Adsorbent for Procion Red. J. of Wat. & Envir. Tech. 2020, 18, 359–371. [CrossRef]
- Hoang, L.P.; Nguyen, T.M.P.; Van, H.T.; Yılmaz, M.; Hoang, T.K.; Nguyen, Q.T.; Vi, T.M.H.; Nga, L.T.Q. Removal of Tetracycline from Aqueous Solution Using Composite Adsorbent of ZnAl Layered Double Hydroxide and Bagasse Biochar. Environmental Technology & Innovation 2022, 28, 102914. [Google Scholar] [CrossRef]
- Dalla Nora, F.B.; Lima, V.V.C.; Oliveira, M.L.S.; Hosseini-Bandegharaei, A.; de Lima Burgo, T.A.; Meili, L.; Dotto, G.L. Adsorptive Potential of Zn–Al and Mg–Fe Layered Double Hydroxides for the Removal of 2–Nitrophenol from Aqueous Solutions. Journal of Environmental Chemical Engineering 2020, 8, 103913. [Google Scholar] [CrossRef]
- Karapinar, N.; Donat, R. Adsorption Behaviour of Cu2+ and Cd2+ onto Natural Bentonite. Desalination 2009, 249, 123–129. [Google Scholar] [CrossRef]
- Raji, F.; Pakizeh, M. Study of Hg(II) Species Removal from Aqueous Solution Using Hybrid ZnCl2-MCM-41 Adsorbent. Applied Surface Science 2013, 282, 415–424. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, W.; Zhang, L. Adsorption Isotherms and Kinetics Studies of Malachite Green on Chitin Hydrogels. J. Hazard. Mater. 2012, 209–210, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mishra, R.; Saha, P.; Kushwaha, P. Adsorption Thermodynamics, Kinetics and Isosteric Heat of Adsorption of Malachite Green onto Chemically Modified Rice Husk. Desalination 2011, 265, 159–168. [Google Scholar] [CrossRef]
- Taty-Costodes, V.C.; Fauduet, H.; Porte, C.; Delacroix, A. Removal of Cd(II) and Pb(II) Ions, from Aqueous Solutions, by Adsorption onto Sawdust of Pinus Sylvestris. Journal of Hazardous Materials 2003, 105, 121–142. [Google Scholar] [CrossRef]
- Hameed, B.H.; El-Khaiary, M.I. Batch Removal of Malachite Green from Aqueous Solutions by Adsorption on Oil Palm Trunk Fibre: Equilibrium Isotherms and Kinetic Studies. Journal of Hazardous Materials 2008, 154, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ayranci, E.; Duman, O. Structural Effects on the Interactions of Benzene and Naphthalene Sulfonates with Activated Carbon Cloth during Adsorption from Aqueous Solutions. Chemical Engineering Journal 2010, 156, 70–76. [Google Scholar] [CrossRef]
- Duman, O.; Özcan, C.; Gürkan Polat, T.; Tunç, S. Carbon Nanotube-Based Magnetic and Non-Magnetic Adsorbents for the High-Efficiency Removal of Diquat Dibromide Herbicide from Water: OMWCNT, OMWCNT-Fe3O4 and OMWCNT-κ-Carrageenan-Fe3O4 Nanocomposites. Environmental Pollution 2019, 244, 723–732. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chemical Engineering Journal 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramakrishnan, K.; Gayathri, R. Removal of Nickel(II) from Aqueous Solutions by Ceralite IR 120 Cationic Exchange Resins. Journal of Engineering Science and Technology 2010, 5, 232–243. [Google Scholar]
- Gimbert, F.; Morin-Crini, N.; Renault, F.; Badot, P.-M.; Crini, G. Adsorption Isotherm Models for Dye Removal by Cationized Starch-Based Material in a Single Component System: Error Analysis. Journal of Hazardous Materials 2008, 157, 34–46. [Google Scholar] [CrossRef]
- Abtahi, M.; Mesdaghinia, A.; Saeedi, R.; Nazmara, S. Biosorption of As(III) and As(V) from Aqueous Solutions by Brown Macroalga Colpomenia Sinuosa Biomass: Kinetic and Equilibrium Studies. Desalination and Water Treatment 2013, 51, 3224–3232. [Google Scholar] [CrossRef]
- Naddafi, K.; Rastkari, N.; Nabizadeh, R.; Saeedi, R.; Gholami, M.; Sarkhosh, M. Adsorption of 2,4,6-Trichlorophenol from Aqueous Solutions by a Surfactant-Modified Zeolitic Tuff: Batch and Continuous Studies. Desalination and Water Treatment 2016, 57, 5789–5799. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, İ. Lead Removal from Aqueous Solution by Natural and Pretreated Clinoptilolite: Adsorption Equilibrium and Kinetics. Journal of Hazardous Materials 2007, 146, 362–371. [Google Scholar] [CrossRef]
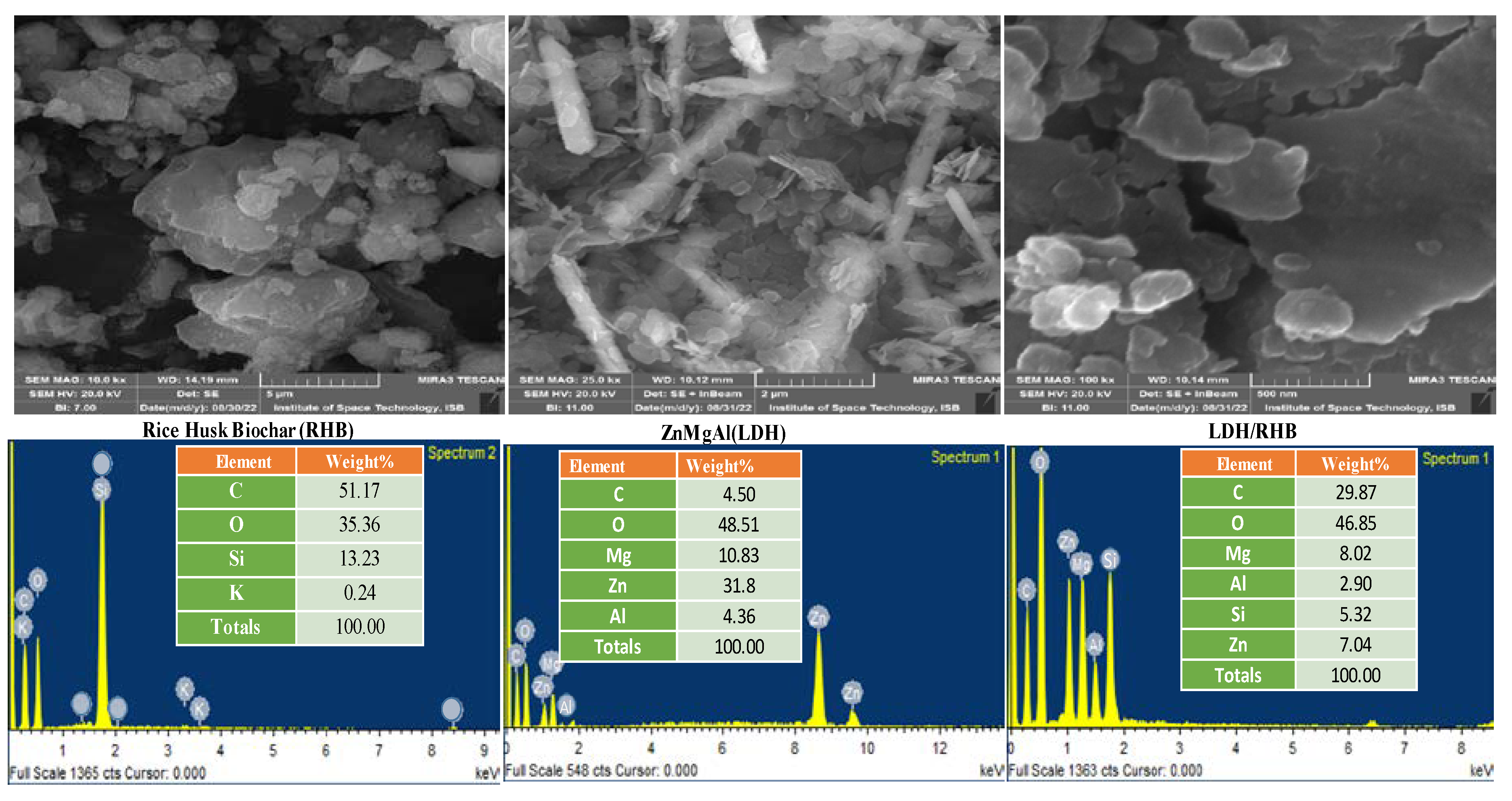
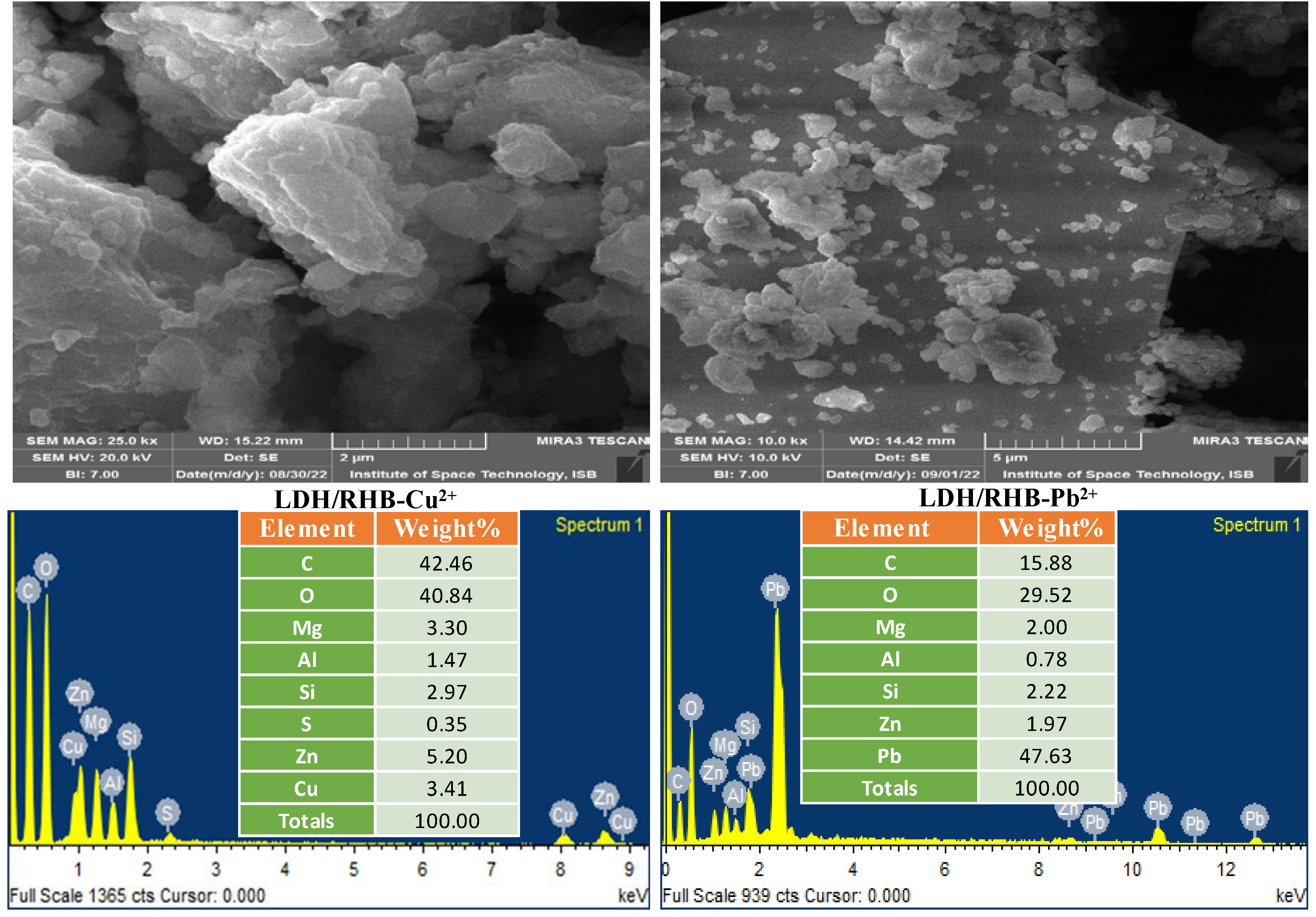
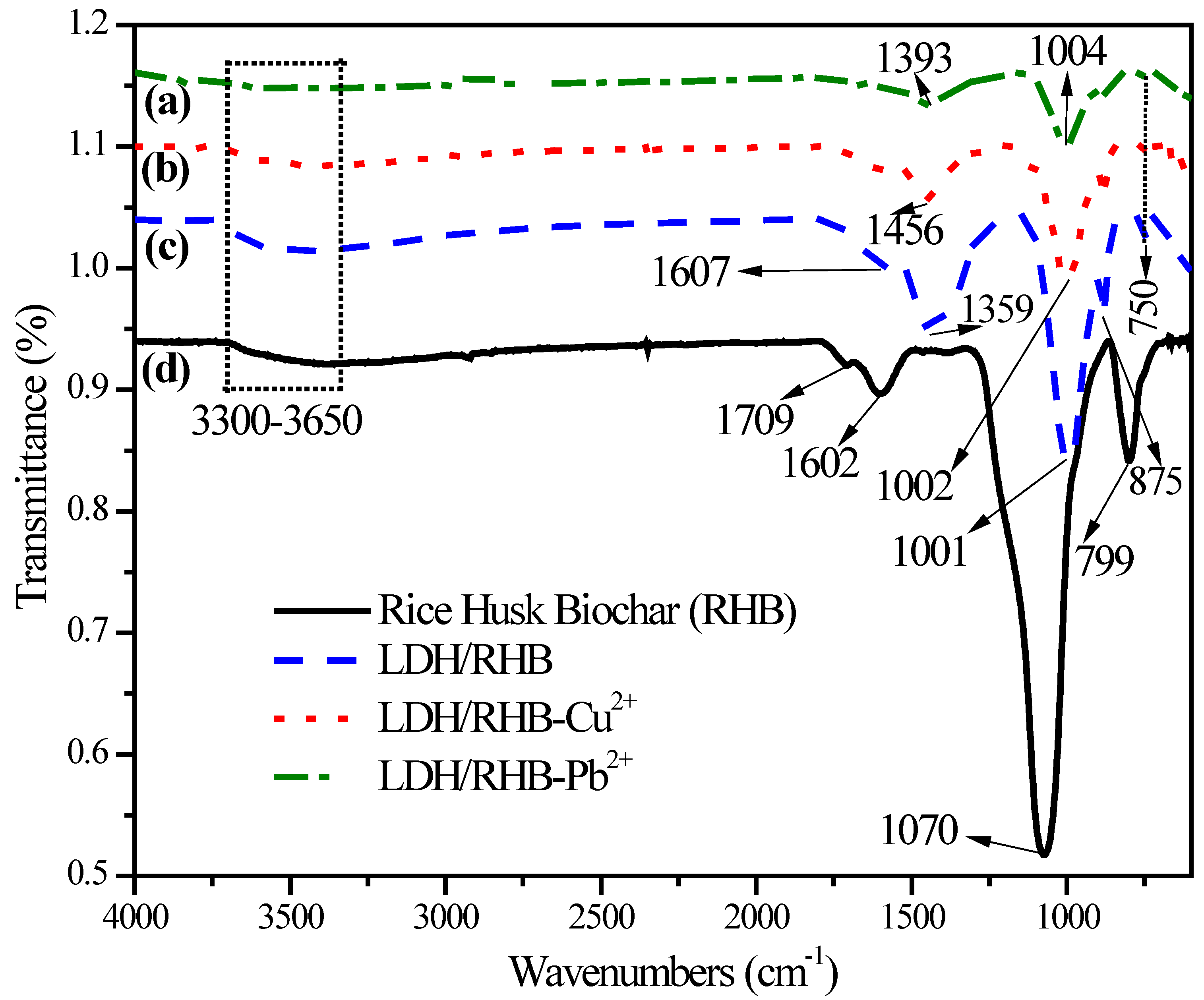
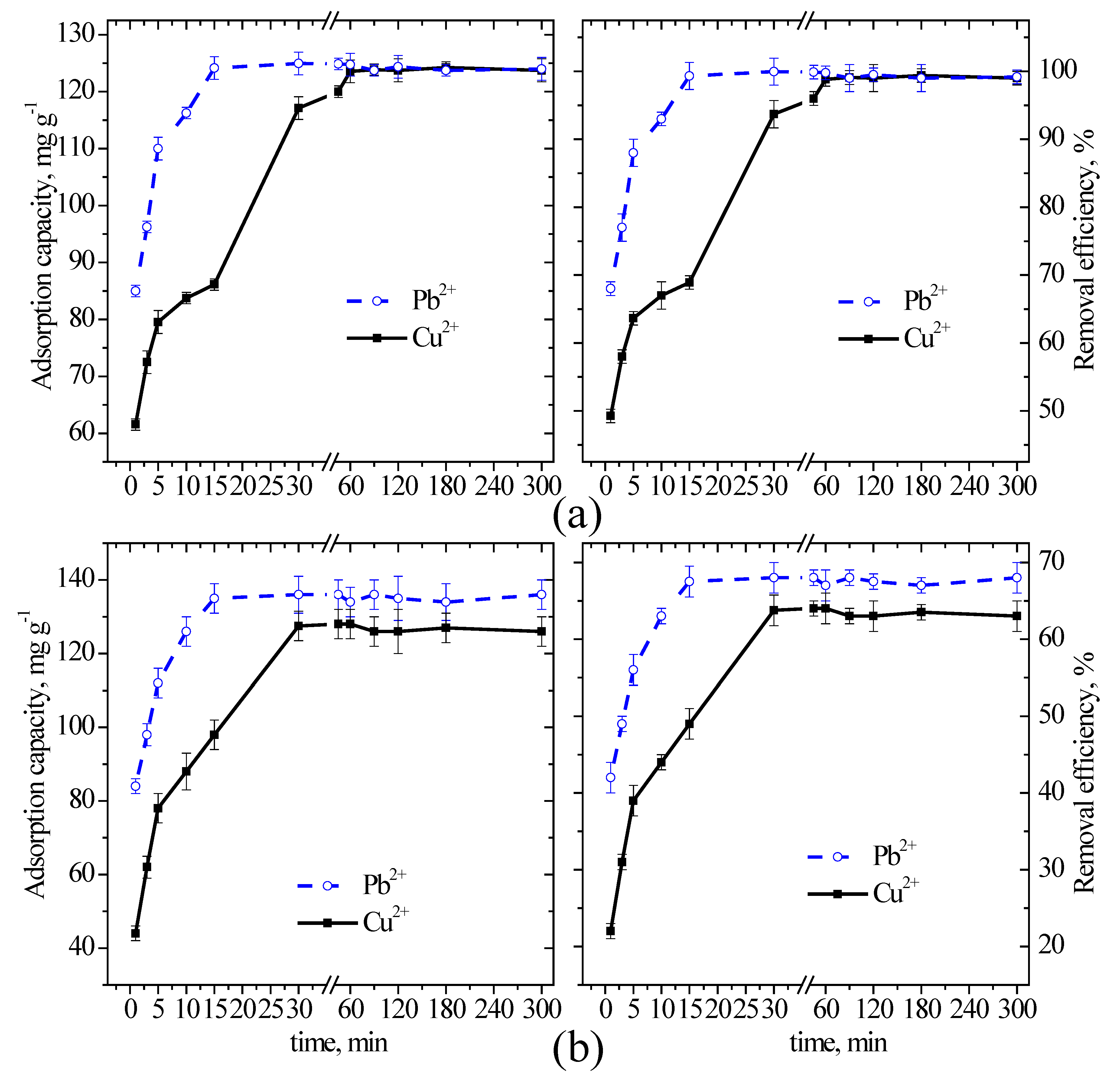
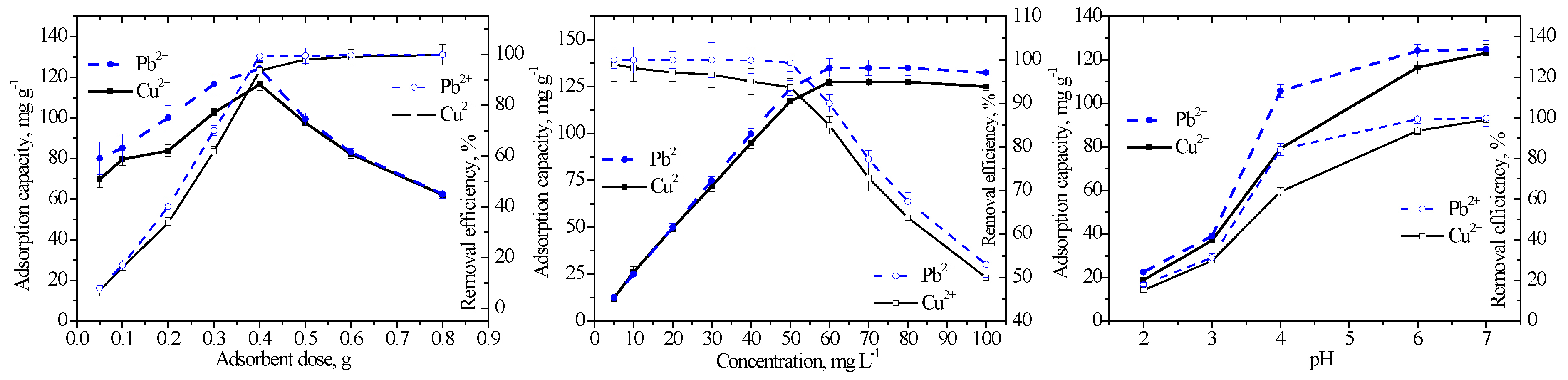
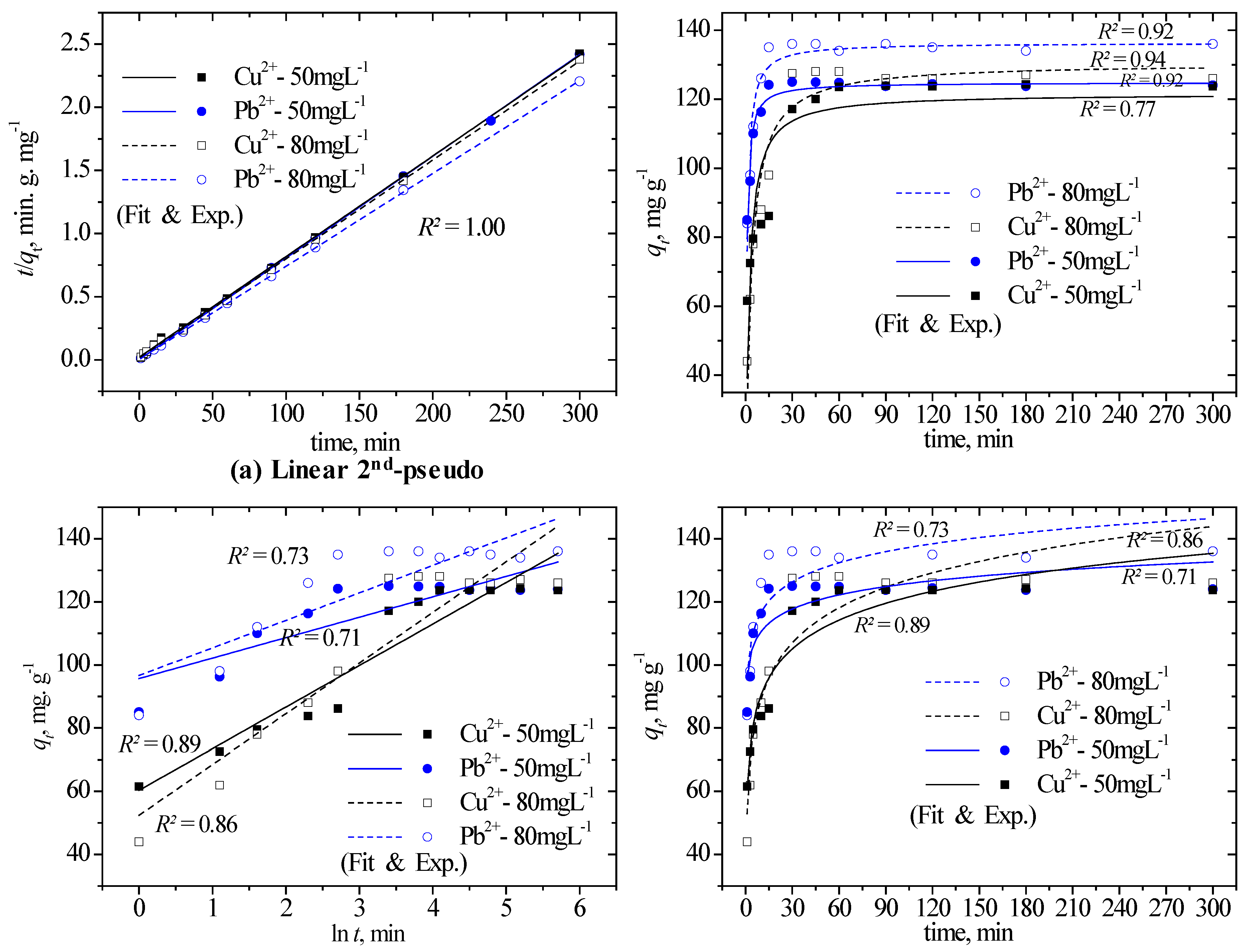
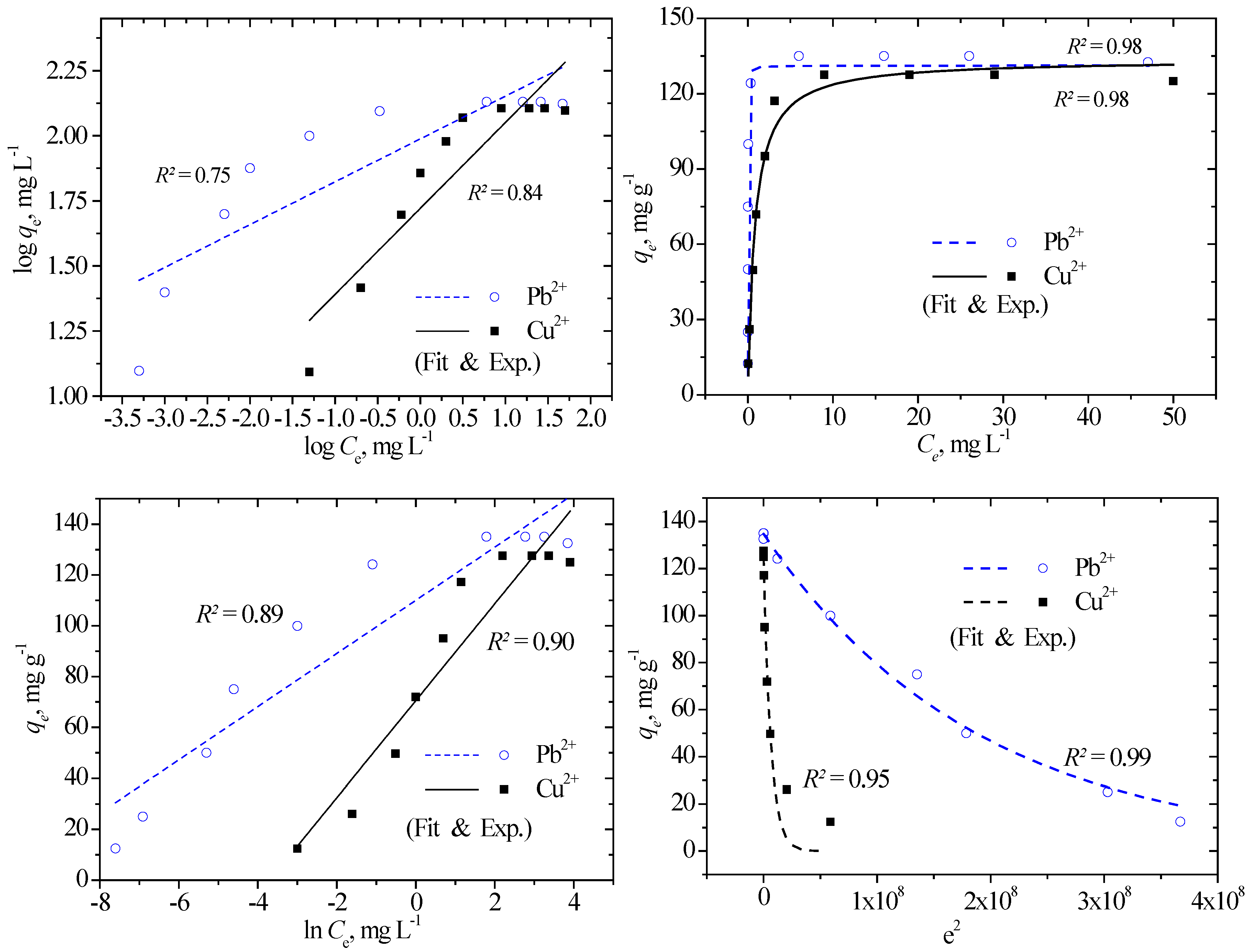
|
Kinetic model |
Parameter | Initial Cu2+ concentrations, mg L-1 | Initial Pb2+ concentrations, mg L-1 | ||||||
| 30 | 50 | 60 | 80 | 30 | 50 | 60 | 80 | ||
| qe exp (mg g−1) | 72.50 | 117.12 | 127.50 | 127.50 | 74.98 | 124.17 | 135.00 | 135.00 | |
| 2nd-pseudo | qe cal (mg g−1) | 71.43 | 125.00 | 129.87 | 128.21 | 74.63 | 123.46 | 135.14 | 135.14 |
| k2 (g mg−1 min−1) | 0.1960 | 0.0030 | 0.0037 | 0.0038 | 0.138 | 0.031 | 0.042 | 0.012 | |
| h (mg g−1 min−1) | 1000.00 | 46.95 | 62.89 | 62.11 | 769.23 | 476.19 | 769.23 | 217.39 | |
| R2 | 0.9999 | 0.9996 | 0.9997 | 0.9996 | 1 | 1 | 0.9999 | 0.9999 | |
| Elovich | α (mg g−1 min−1) | 1.95E+07 | 9.79E+01 | 5.91E+01 | 2.61E+01 | 1.45E+18 | 2.59E+06 | 2.60E+05 | 6.44E+04 |
| β (g mg−1) | 3.37 | 13.16 | 14.66 | 16.06 | 1.62 | 6.48 | 8.03 | 8.73 | |
| R2 | 0.78 | 0.9 | 0.88 | 0.87 | 0.53 | 0.73 | 0.77 | 0.75 | |
| ID-WM | Kip (mg g−1 min1/2) | 0.98 | 4.25 | 4.56 | 4.97 | 0.38 | 1.65 | 2.08 | 2.26 |
| C (mg g−1) | 59.96 | 69.71 | 71.80 | 65.89 | 70.42 | 105.41 | 111.97 | 109.52 | |
| R2 | 0.49 | 0.73 | 0.65 | 0.63 | 0.24 | 0.40 | 0.43 | 0.42 | |
| 1st-pseudo | qe cal (mg g−1) | 3.55 | 18.62 | 14.92 | 13.18 | 1.05 | 5.59 | 5.73 | 5.83 |
| k1 (min−1) | 0.007 | 0.015 | 0.014 | 0.012 | 0.008 | 0.015 | 0.009 | 0.010 | |
| R2 | 0.22 | 0.55 | 0.44 | 0.28 | 0.19 | 0.55 | 0.29 | 0.29 | |
|
Kinetic model |
Parameter | Initial Cu2+ concentrations, mg L-1 | Initial Pb2+ concentrations, mg L-1 | ||||||
| 30 | 50 | 60 | 80 | 30 | 50 | 60 | 80 | ||
| qe exp (mg g−1) | 72.50 | 117.12 | 127.50 | 127.50 | 74.98 | 124.17 | 135.00 | 135.00 | |
| 2nd-pseudo | qe cal (mg g−1) | 71.48 | 121.63 | 129.78 | 130.47 | 75.19 | 124.81 | 136.24 | 136.32 |
| k2 (g mg−1 min−1) | 0.030 | 0.004 | 0.003 | 0.002 | 0.062 | 0.014 | 0.011 | 0.009 | |
| h (mg g−1 min−1) | 153.33 | 57.99 | 51.03 | 40.34 | 350.10 | 219.95 | 202.87 | 172.26 | |
| R2 | 0.90 | 0.77 | 0.89 | 0.94 | 0.96 | 0.92 | 0.89 | 0.92 | |
| Elovich | α (mg g−1 min−1) | 6.60E+07 | 1.28E+03 | 8.62E+02 | 4.20E+02 | 2.10E+18 | 1.69E+07 | 2.08E+06 | 5.65E+05 |
| β (g mg−1) | 0.30 | 0.08 | 0.07 | 0.06 | 0.62 | 0.15 | 0.12 | 0.11 | |
| R2 | 0.76 | 0.89 | 0.87 | 0.86 | 0.48 | 0.71 | 0.74 | 0.73 | |
| ID-WM | Kip (mg g−1 min1/2) | 61.54 | 75.91 | 78.66 | 77.34 | 70.42 | 105.41 | 111.97 | 109.52 |
| C (mg g−1) | 0.87 | 3.96 | 4.19 | 3.05 | 0.38 | 1.65 | 2.08 | 2.26 | |
| R2 | 0.38 | 0.65 | 0.56 | 0.49 | 0.17 | 0.34 | 0.37 | 0.36 | |
| 1st-pseudo | qe cal (mg g−1) | 69.37 | 115.34 | 123.69 | 124.06 | 74.17 | 120.94 | 131.52 | 131.86 |
| k1 (min−1) | 1.23 | 0.30 | 0.23 | 0.18 | 1.74 | 1.00 | 0.88 | 0.69 | |
| R2 | 0.57 | 0.50 | 0.73 | 0.86 | 0.87 | 0.64 | 0.58 | 0.68 | |
| Isotherm model | Mathematical expression | Parameters |
|---|---|---|
| Langmuir |
|
qm, maximum sorption capacity, mg g−1 KL, Langmuir constant, L mg−1 RL, separation factor coefficient |
| Freundlich |
KF, Freundlich constant, L g−1 n, dimensionless constant |
|
| Dubinin–Radushkevich |
E = 1/ |
T, absolute temperature, Kelvin R, universal gas constant, 8.314 J mol−1·K−1 E, mean free energy of adsorption, kJ mol−1 |
| Temkin |
AT, equilibrium binding constant, L g−1 bT, heat of adsorption, kJ mol−1 |
|
| Halsey | nH and kH, Halsey constants | |
| Jovanovic | kj, Jovanovic constant | |
| Redlich–Peterson |
α, L mg−1 β (0–1), dimensionless KRP, R–P constant, L g−1 |
|
| Sips |
ns, degree of heterogeneity, dimensionless KS, energy of adsorption, L g−1 |
| Isotherm | Parameter | Linearized fitting | Nonlinear fit | ||
|---|---|---|---|---|---|
| Cu2+ | Pb2+ | Cu2+ | Pb2+ | ||
| qe exp, mg g−1 | 127.53 | 135 | 127.53 | 135 | |
| Langmuir | qm, mg g−1 | 102.04 | 119.05 | 133.64 | 131.21 |
| KL, L mg−1 | 2.65 | 210.00 | 1.23 | 125.29 | |
| RL | 0.006 | 0.00008 | 0.013 | 0.00013 | |
| R2 | 0.97 | 0.99 | 0.98 | 0.98 | |
| Freundlich | qm, mg g−1 | 238.54 | 197.17 | 153.72 | 154.81 |
| KF, ((mg/g)(L/mg)1/n) | 61.74 | 100.54 | 68.51 | 101.70 | |
| 1/n | 0.330 | 0.165 | 0.197 | 0.103 | |
| R2 | 0.84 | 0.75 | 0.77 | 0.77 | |
| D–R | qm, mg g−1 | 102.51 | 139.49 | 124.64 | 134.71 |
| KDR, (mol kJ−1)2 | 4.0E-08 | 6.0E-09 | 1.54E-07 | 5.3E-09 | |
| E, kJ mol−1 | 3.54 | 7.13 | 1.80 | 7.71 | |
| R2 | 0.85 | 0.98 | 0.95 | 0.99 | |
| Temkin | KT, L mg−1 | 40.46 | 370.33 | 40.46 | 370.53 |
| Hads, kJ mol−1 | 132.23 | 241.05 | 132.22 | 241.04 | |
| R2 | 0.90 | 0.89 | 0.89 | 0.88 | |
| Halsey | qe cal, mg g−1 | 258.10 | 217.21 | 122.50 | 135.17 |
| nH | -3.03 | -6.08 | -5.07 | -9.75 | |
| KH | 0.465 | 0.265 | 0.023 | 0.012 | |
| R2 | 0.84 | 0.75 | 0.77 | 0.77 | |
| Jovanovic | qm, mg g−1 | 53.34 | 58.04 | 126.29 | 127.28 |
| kj, L g−1 | -0.025 | -0.025 | -0.82 | -4.15 | |
| R2 | 0.27 | 0.23 | 0.99 | 0.94 | |
| R–P | KRP, L g−1 | 125.13 | 213.36 | ||
| α, L mg−1 | 0.73 | 1.47 | |||
| β | 1.08 | 0.98 | |||
| R2 | 0.99 | 0.99 | |||
| Sips | qm, mg g−1 | 130.86 | 134.82 | ||
| KS, L g−1 | 1.30 | 2.39 | |||
| nS | 1.17 | 0.70 | |||
| R2 | 0.98 | 1.00 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





