1. Introduction
The search for efficiency in the field of training and health is one of the goals of trainers and therapists. New methods and technologies are constantly being applied for this purpose. In recent years, blood flow restriction (BFR) training (BFRT) is being studied for its effects in different populations (1,2). BFRT is based on low-intensity (usually between 20-50% of one-repetition maximum [1-RM]) exercise performed whit local hypoxia induced by an inflatable tourniquet located on the proximal limb, which restricts arterial inflow (50-80% commonly) and venous return from the extremity (3). BFRT is an interesting alternative to high-intensity resistance training for improving muscle strength (4).
BFR applied with moderate vascular occlusion has been proven to enhance muscle hypertrophy with low exercise intensity (i.e., 20% of 1-RM) (5). Scientific literature also supports that BFRT can lead to muscle strength improvements in healthy participants (6). In addition, BFRT has been postulated to provide benefits not only in muscle mass but also in tendon structure. In fact, some studies have reported similar gains after a resistance training program with low intensities (20-35% 1-RM) combined with BFRT to those reported by high intensity resistance training (70-85% 1-RM) (7). A greater resistance to fatigue and a lower perception of effort have also been reported after a period of training with this methodology (8). Indeed, the ideal occlusive pressure to maximize these improvements is the greatest challenge of BFRT research nowadays (9). Despite the underpinning physiological mechanisms that promote these adaptations after low-intensity resistance exercise training combined with BFRT remains unkonwn (10), it is highly used in clincial contexts (11).
In addition to these training-induced effects, BFR can acutely affect to the neuromuscular function. Higher decreases in muscle force and electromyography (EMG) amplitude have been showed during BFRT compared to traditional weight training CON. Those results indicate that the fatigue caused by BFRT may be due to a combination of peripheral (enhanced twitch reduction) and central fatigue (maximal isometric voluntary contration [MVIC] and EMG amplitude reduction) (12). Moreover, is widely known that no differences were found in the recruitment of type II fibres, with type I fibres being preferentially recruited during BFRT (13). However, it is also known that corticospinal excitability increases after BFRT, showing higher activation levels in comparison with traditional training (2). These findings suggests that BFRT may lead to a better neural performance (14), which is usually monitorized during training through velocity movement control (15). Since it allows to load quantification (16), 1-RM estimation (17) and neuromuscular fatigue monitorization (18,19).
Recently, Wilk and colleagues (20) showed that BFRT combined with low intensity resistance exercise (20 to 50% of the estimated 1-RM) led to higher peak velocities during the bench press exercise in comparisson with the control condition (i.e., weight training without occlusion). In addition, they observed similar mean concentric velocities between both conditions. This appears to indicate that neuromuscular function is not affected by BFRT when low loads are used, despite contradictory results. However, to the best of our knowledge, it is unknown whether BFRT can lead to less activation of the involved musculature and to greater intra- and inter-set neuromuscular fatigue. Therefore, this study aimed to analyze the effects of BFRT on neuromuscular performance (i.e., movement velocity and effort index [EI]) and muscle activity in well-trained healthy participants. We hypothesized that the application of BFRT will result in similar kinematic parameters but with some differences in the muscle activity and EI during the back-squat exercise.
2. Materials and Methods
2.1. Participants
24 healthy volunteers (male [n=10, 25.0 ± 1.5 years; 178 ± 7.6 cm; 76.8 ± 11.2 kg; 26.8 ± 4.2 kg·m-2; female [n=11], 24.4 ± 2.3 years; 168 ± 3.6 cm; 61.3 ± 6.4 kg; 21.6 ± 3.9 kg·m-2] participated in this study. All participants had no history of lower limb injuries in the past six months and had at least two years of resistance training experience. In addition to the PARQ questionnaire, all participants underwent a physical examination and were assessed through a physical activity habits questionnaire (IPAQ) (21). Three participants dropped out of the study due to personal reasons, leaving 21 participants who completed the study and were randomized, analysed, and included in this study. The participants were told to avoid taking pain relievers (6 hours before), alcohol (48 hours before), caffeine (6 hours before), and strenuous exercise (48 hours before) each session. They were also informed about the general experimental procedures, potential risks, and the research objectives and hypotheses. The research protocol was reviewed and approved by the University Ethics Committee (reference number: EADECAFYD2020-3), in accordance with the ethical guidelines of the Helsinki declaration (World Medical Association Declaration of Helsinki, 2018) (22).
2.2. Trial Design
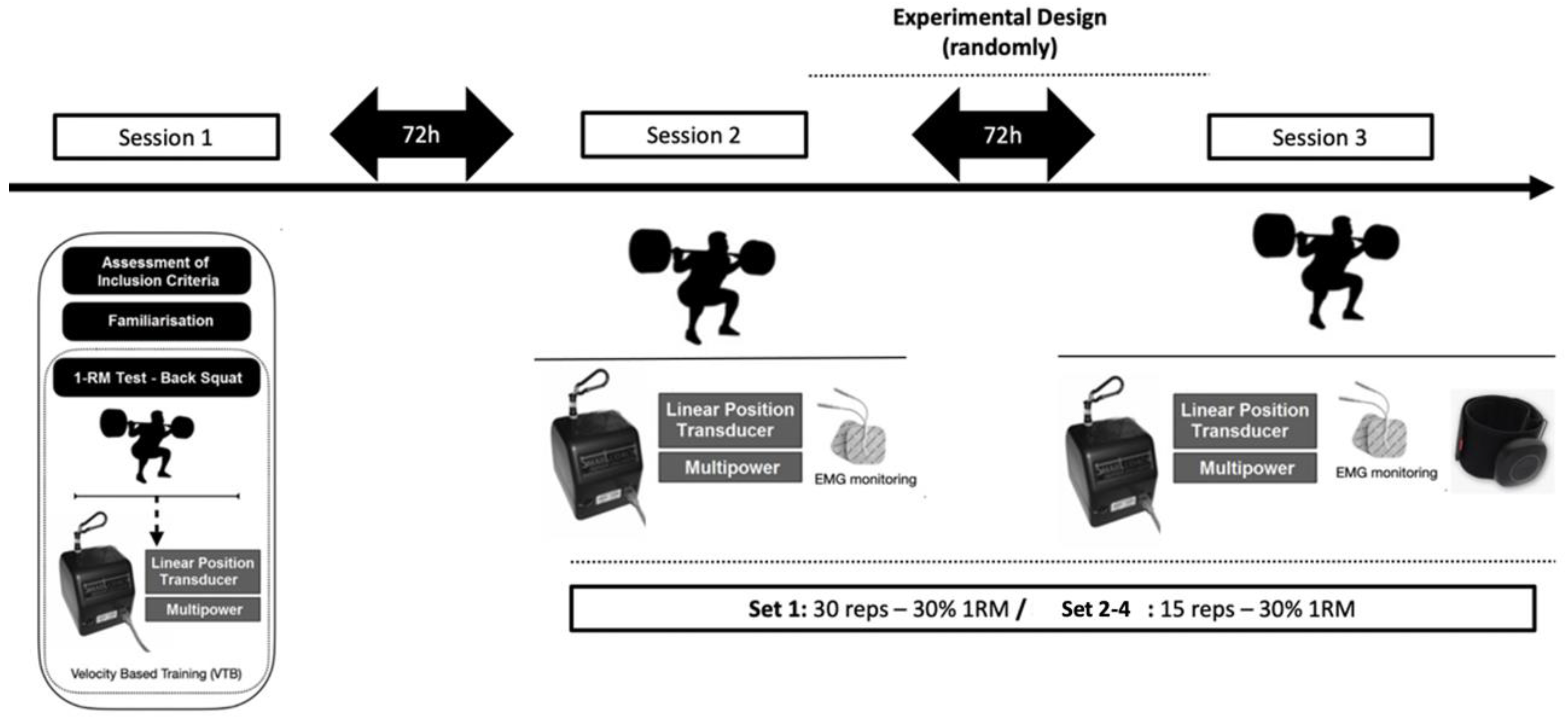
A randomised clinical trial was performed to examine the impact of BFRT on muscle strength-related variables. The participants visited the laboratory on three separate times. During their first visit, their height (SECA 220, Hamburg, Germany), and body composition (Tanita RD-545, Tokyo,Japan), were measured, and they underwent a warm-up and a back-squat 1-RM test. The second and third visits involved either the BFRT or control (non-BFRT) protocols in a randomized crossover design, with a 72-hour break in between (
Figure 1).
2.3. Procedures
2.3.1. Back Squat One-Repetition Maximum Test
After preparing with a warm-up routine that involved 5 minutes of cycling at a comfortable pace on a stationary cycling (Technogym, Barcelona, Spain) 5 minutes of exercises to mobilize lower limb joints, 3 sets of 30-meter sprints, and 3 sets of 5 half squats using weights of 20, 30, and 40 kg, the 1-RM test was conducted on a multipower device (Technogym Trade Company 2017, Barcelona, Spain) using a progressive loading protocol (17).
The protocol that involved gradual changes in velocity as the load increases was previously described (23). The best repetition at each weight was selected based on the mean propulsion velocity (24). In addition, the EI as an indicator of accumulated fatigue and perception of effort were measured for both BFRT and non-BFRT conditions according to previous studies (19).
2.3.2. Back-Squat Exercise Protocol
The back-squat exercise was performed using the same multipower device (Technogym Trade Company 2017, Barcelona, Spain) that was utilized during the 1-RM test. A highly-used protocol to prescribe BFRT was used (25). It consisted on 4 sets of 30, 15, 15 and 15 repetitions performed by each participant with 60 seconds rest between sets (25). The participants started the exercise standing straight, with their knees and hips fully extended (0 degrees knee flexion), feet positioned at shoulder width apart, and both feet flat on the ground parallel to each other. They were instructed to perform each repetition with maximum effort. Encouragement and feedback on velocity were given to motivate participants to put forth their best effort during each repetition. They were required to keep their feet on the ground, but lifting their heels at the end of the lifting phase was allowed. The same warm-up routine used before the 1-RM test was also used before each experimental session.
Mean propulsive velocity (MPV), and peak velocity (Vmax) for the concentric phase of the movement were collected from a linear position transducer (1,000 Hz sampling rate, (SmartCoach Europe AB, Stockholm, Sweden; SC). The linear position transducer was the criterion measure to calculate (23). It was attached to the barbell that participants used to perform the back-squat exercise and interfaced with a personal computer to digital data acquisition. During each lift, instantaneous kinematic data was collected with a sample frequency of 1000 Hz. Previous research reported the highly reliable data of the linear position transducer used (26).
2.3.3. BFRT Protocol
During the BFR session, participants wore pressure cuffs at the most proximal region of each leg. The cuffs used were Airbands (Vald Performance, Albion, Australia) with dimensions of 45-64 cm. To establish the specific pressure value, after a 5-minute rest, the value for full arterial occlusion pressure was determined. The exercise protocol prescribed 80% of the individual arterial occlusion pressure (AOP) as previously used (23) to enhance maximum strength and muscle thickness. The cuffs were inflated before starting the exercise.
2.3.4. Surface EMG Protocol
The EMG signals were collected from the dominant leg's quadriceps muscles (rectus femoris [RF] and vastus lateralis [VL]). The recommended procedure from the SENIAM project was followed for preparing the skin and placing the electrodes (27). The hair was shaved and the skin cleaned with alcohol, then rubbed until a reddish colour appeared to improve electrode adherence. The electrodes were placed with the subject seated and the knee slightly bent (distance between electrodes: 20 mm), and data was collected using a wireless EMG system (mDurance Solutions SL, Granada, Spain) with 4 EMG channels, a sampling frequency of 1024 Hz, and a bandwidth of 8.4 kHz. The resolution of the EMG signal was 24 bits, and the amplification was 100-10,000 V/V. The electrodes were pre-gelled Ag/AgCl with a diameter of 10 mm, and the location of each motor point was determined using a low-voltage motor point pencil unit (28). A reference electrode was placed on the head of the same leg's fibula (29).
After electrode placement, participants sat down and performed a maximum voluntary isometric contraction (MVIC) of the quadriceps with the knee at 90° (30). Two repetitions of the isometric knee extension test were performed for 5 seconds, separated by a rest period of 2 minutes (31). Participants were instructed to gradually increase the force of the muscle contraction for up to a maximum of 2 seconds, holding the resulting MVIC for 3 seconds (32). The root mean square EMG (EMGrms) signal was recorded.
2.3.5. Statistical Analysis
All statistical analysis was performed using the Jamovi software package (The Jamovi Project, v.1.6.23.0; downloadable at
https://www.jamovi.org). Normality was checked by the Shapiro-Wilk normality test. Then, a repeated measures linear-mixed model fitted with a restricted maximum likelihood method and unstructured covariates was used to compare outcomes between sets (sets 1-4) and conditions (BFRT and non-BFRT). The main outcomes used in statistical analyses were peak and mean concentric velocity, and EMG
RMS and peak normalized EMG activation for the RF and VM muscles. The level of significance for all tests was set to
α = 0.05. Mean, standard error (SE) and the t value were reported for all statistical analyses.
Sample size was estimated
a priori for an ANOVA repeated measures using G*power (G*Power 3.1.9.2, Heinrich Heine-Universitat Dusseldorf, Dusseldorf, Germany;
http://www.gpower.hhu.de/). The effect size was computed using the means and between-subject standard deviation (SD)’s from a previously published study (33) that analyzed kinematic data during the back-squat exercise with and without BFR. The means and SDs were 1.57 ± 0.12 and 1.45 ± 0.15 m·s
-1 of peak concentric velocity, respectively. The 95% confidence intervals were therefore 1.58-1.66 and 1.35-1.56 m·s
-1 respectively, resulting in a Cohens
dz effect size of 0.88, which can be classified as and so equivalent to an f = 0.3 (moderate). The average SD was used to compute the effect size. Alpha was set to 5%, while power was set at 80% (1−
β). The estimated sample size was 12 participants (actual power = 0.846), but considering possible dropouts, we enrolled 24 participants in this study.
3. Results
Regarding movement velocity, significant effects were only observed for exercise set (p<0.001, F=13.1; i.e., Set 1 vs Set 2: p<0.001, t=4.2; and Set 1 vs Set 3; p<0.001, t=4.1) for EI. However, no significant interactions were observed between exercise set and condition (p=0.718, F=0.45). No significant interactions between sets, conditions and set*condition were observed for VM and Vmax. As shown in
Table 1, Set 1 in the BFRT condition manifested higher (p=0.033) IE compared with Set 2 (mean [SE and t]: 8.57 [SE=2.5, t=3.5]). Similarly, Set 1 in the BFRT condition showed higher (p=0.037) IE compared with Set 3 in the control condition (9.96 [SE=2.9, t=3.5]). Set 2, Set 3 and Set 4 did not show significant differences between them in the BFRT condition nor in the non-BFRT condition. No between-group differences were observed for any set. Regarding peak and mean concentric velocity, similar results were observed (
Table 1).
An individual analysis of EMGmax and EMGrms of each muscle was performed (
Table 1). Regarding RF, significant effects for exercise set in EMGmax (p=0.003, F=5.0; Set 1 vs Set 2: p<0.001, t=4.6) and EMGrms (p<0.001, F=12.6; Set 1 vs Set 2: p<0.001, t=4.9; and Set 1 vs Set 3; p=0.002, t=4.0) were observed. As shown in
Table 1, higher RF EMGmax (9.3 μV, p=0.004, SE=2.2, t=4.2) and EMGrms (9.3 μV, p=0.05, SE=23.3, t=2.7) values were observed when compared Set 1 and Set 2 in the BFRT condition. However, no other significant interaction between sets or between conditions were observed. Similarly, regarding VL, significant effects were shown for exercise set in EMGmax (p=0.002, F=5.4; Set 1 vs Set 2: p=0.005, t=3.6; and Set 1 vs Set 3; p=0.042, t=2.8) and EMGrms (p<0.001, F=7.0; Set 1 vs Set 2: p=0.01, t=3.4; and Set 1 vs Set 3; p=0.042, t=2.8) were observed. However, the results were similar between series and between conditions. No significant differences were observed.
4. Discussion
This study aimed to analyze the effects of BFRT with an 80% of AOP on kinematic and EMG parameters during low intensity (i.e., 30% of 1-RM) back-squat exercise. The main result of this study was that no significant differences on MPV and Vmax were observed between sets of the back-squat exercise during both BFRT and non-BFRT conditions. However, higher intra-set EI were observed in Set 1 compared to Set 2 when BFRT was applied. Similarly, higher RF, EMGpeak and EMGrms were observed in Set 1 compared to Set 2 after BFRT. Therefore, BFRT did not result in significant changes in neuromuscular performance during low-intensity resistance training. However, it may lead to greater intra-set velocity loss and lower activation of involved musculature when high volumes (i.e., more than 15 repetitions) were prescribed but not when lower volumes (i.e., until 15 repetitions) were used.
To date, only few studies have investigated the acute effects of BFRT on movement velocity during the back-squat exercise (33,34). Recent studies (35), compared different AOP (from 40 to 100%) during the squat and bench press exercises performed at 60% 1-RM. They showed an increase on mean propulsive velocity during the squat with 100% of AOP. Similarly, another research (33) showed that the back-squat exercise performed at 70% 1-RM with 150% of AOP had a significantly higher peak concentric velocity and power output. On the other hand, as it was observed in our results, when lower cuff pressure (less AOP than 100%) was applied, no differences were observed between BFRT and traditional resistance exercise on kinematic parameters regardless of load used (30% 1-RM). According to previous studies (33,34), it seems that the higher AOP, the greater enhancements in kinematic parameters during multi-joint exercises. Therefore, using a lower AOP in combination with low loads during BFRT could be an effective strategy to enhance performance without increasing intensity or AOP, as our results demonstrated. Therefore, implementing low-intensity BFRT could be an interesting approach for rehabilitation purposes or any other scenario where it is not possible to prescribe high-intensity training.
BFRT has been usually prescribed with low intensity exercises (36). Indeed, some studies (37,38) have shown that BFRT with heavy loads did not lead to additional benefits. In addition, low loads (between 20 and 50% 1-RM) are used because they do not compromise performance during exercise. A study (20) that combined continuous and intermittent BFRT using both low and high intensities, showed that BFRT used during resistance exercise training increases peak concentric velocity. Which in turn, validates this method within performance purposes (i.e., enhance explosiveness). In addition, it has been proposed that BFRT using loads above of 60% 1-RM leads to higher intramuscular pressure due to the increased mechanical tension, which results in stemmed blood flow during exercise (39). Additionally, blood flow during recovery can be compromised due to muscle inflammation, as a result of osmotic and fluid changes that occur in the muscle (40–42). Therefore, since blood flow during exercise and recovery is reduced due to muscle tension and inflammation, as it occurs with heavy weightlifting, additional hypoxia induced by BFRT does not cause further metabolic effects (20). Thus, low loading patterns during BFRT are usually employed to enhance the metabolic response that we could achieve at the same intensity with traditional exercises (3). This increased metabolic response and the acidic environment promoted by hypoxia greatly influences muscle protein synthesis, altering the genetic regulation of satellite muscle cells, increasing muscle fiber recruitment and improving muscle endurance (3). Hypoxia and the accumulation of metabolites are thought to promote muscle fiber recruitment. Activation of fast twitch fibers is essential for improving athletic performance. Several studies that measure muscle activation through EMG during non-fatigued contractions have shown significantly greater responses with the application of BFRT (43). However, other studies (42) did not show an increase on EMG activity during low-load BFRT compared to traditional heavy-load RT. These results are in line with our data, as no differences were observed between the BFRT condition and the control condition in muscle activity. In our case we have observed that performing a high volume (i.e., number of repetitions) can affect the activation of the involved muscles in the subsequent set (
Table 1). Therefore, another aspect that should be further considered is the effect of volume and neuromuscular fatigue on muscle activation, since it can influence the effects of BFRT.
Another important aspect to consider in the application of BFRT is the possible differences of applying it only in a single training session or over a longer period. There are limited studies that have specifically compared the acute and chronic effects of BFRT. A study (45) showed that two sessions per day of training with the same volume do not necessarily result in larger responses in all hormones than one session per day of training. Another study found, after applying a 7-week intervention with BFRT and loads at 20% of 1-RM, similar magnitudes and mechanisms for strength adaptation and intramuscular anabolic activity as those found with heavy-load (70% 1-RM). Similarly, and with applicability in performance sports, periodic restriction of local blood flow followed by reperfusion has been shown to improve performance in cycling, running, and swimming (46). In relation to chronic effects, ischemic preconditioning has been proposed as a possible explanation for the increase in improvement in sports performance with a high AOP (47). After a brief ischemia, produced during a certain period of time, they can perform an ergogenic effect, because the hyperemia experienced after the occlusion, seems to produce an increase in the production of nitric oxide, in addition to an increase in the resynthesis of phosphocreatine, an altered kinetics of oxi-desoxyhemoglobin and a greater oxygen consumption (48,49). Therefore, it seems that applying BFRT with low-intensity, could be effective and similar to training with high loads without restriction, and that both acute and chronic effects could be obtained by this methodology. Specially, the results of this study are interesting for both sports performance and rehabilitation context when lifting heavy training loads is contraindicated.
5. Limitations
Our study presented some concerns that should be noted. This study might be considered as an exploratory study due to the low sample size; however, the calculation of statistical power helped to draw accurate conclusions in the population used. Therefore, readers should interpret and generalize our results with caution, since further research is needed to provide an accurate and reliable BFRT application pathway. Moreover, we cannot extrapolate our results to patients undergoing rehabilitation or physical reconditioning, as only healthy and well-trained participants volunteered in this study. Finally, more studies are warren to understand the time-course effects and chronic adaptations on neuromuscular performance of velocity-based BFRT.
6. Conclusion
In conclusion, low intensity BFRT performed with 80% AOP did not induce changes in neuromuscular performance (kinematic and EMG parameters) during the back-squat exercise in well-trained young men and women. However, high volumes (i.e., more than 15 repetitions) induced greater intra-set velocity loss and lower activation of the involved musculature compared to lower volumes. Therefore, the implementation of BFRT is an effective and safe strategy in healthy populations to improve their performance without increasing training intensity.
Author Contributions
Conceptualization, .M.G.S. and J.M.J.-C.,; Data curation, M.G.S. and M.G.G.; Formal analysis, J.M.J.-C., and SM.I.; Funding Acquisition, A.S.-T.L.; Investigation, M.G.S and S.M.I and J J.M.J.-C.; Methodology, J.M.J.-C.; Project Administration, M.G.S.; Resources M.G.S..; Supervision, M.G.S. and S.V.M. and A.R.-S.; Validation, J.M.J.-C., and S.M.I and M.G.S.; Visualization, J.M.J.-C., Writing—Original Draft Preparation, M.G.S. and J.M.J.-C., and S.M.I.; Writing—Review and Editing, J.M.J.-C., M.G.S., M.G.G., S.M.I., S.V.M. and J.B.P. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferraz RB, Gualano B, Rodrigues R, Kurimori CO, Fuller R, Lima FR, et al. Benefits of Resistance Training with Blood Flow Restriction in Knee Osteoarthritis. Med Sci Sports Exerc. 2018 May 1;50(5):897–905. [CrossRef]
- Kjeldsen SS, Næss-Schmidt ET, Hansen GM, Nielsen JF, Stubbs PW. Neuromuscular effects of dorsiflexor training with and without blood flow restriction. Heliyon. 2019;5(8). [CrossRef]
- Bowman EN, Elshaar R, Milligan H, Jue G, Mohr K, Brown P, et al. Proximal, Distal, and Contralateral Effects of Blood Flow Restriction Training on the Lower Extremities: A Randomized Controlled Trial. Sports Health. 2019;11(2):149–56. [CrossRef]
- Labata-Lezaun N, Llurda-Almuzara L, González-Rueda V, López-de-Celis C, Cedeño-Bermúdez S, Bañuelos-Pago J, et al. Effectiveness of Blood Flow Restriction Training on Muscle Strength and Physical Performance in Older Adults: A Systematic Review and Meta-analysis. Arch Phys Med Rehabil. 2022;103(9):1848–57. [CrossRef]
- Pant G, Education UB-J of P, and S, 2017 undefined. Effect of restricted blood flow on muscle hypotrophy & O2 saturation level on weight training. AcademiaEdu [Internet]. 2017;(May):2–4. Available from: http://www.academia.edu/download/52932499/4-2-50-850.pdf.
- Flocco P, Galeoto G. Effect of blood flow restriction training on physiological outcomes in healthy athletes: A systematic review and meta-analysis. Muscles Ligaments Tendons J. 2021;11(1):101–17. [CrossRef]
- Centner C, Jerger S, Lauber B, Seynnes O, Friedrich T, Lolli D, et al. Low-Load Blood Flow Restriction and High-Load Resistance Training Induce Comparable Changes in Patellar Tendon Properties. Vol. Publish Ah, Medicine & Science in Sports & Exercise. 2021. [CrossRef]
- Schwiete C, Franz A, Roth C, Behringer M. Effects of Resting vs. Continuous Blood-Flow Restriction-Training on Strength, Fatigue Resistance, Muscle Thickness, and Perceived Discomfort. Front Physiol. 2021;12(March). [CrossRef]
- Wortman RJ, Brown SM, Savage-Elliott I, Finley ZJ, Mulcahey MK. Blood Flow Restriction Training for Athletes: A Systematic Review. Am J Sports Med. 2021;49(7):1938–44. [CrossRef]
- Yasuda T, Loenneke JP, Thiebaud RS, Abe T. Effects of Blood Flow Restricted Low-Intensity Concentric or Eccentric Training on Muscle Size and Strength. PLoS One. 2012;7(12):1–7. [CrossRef]
- Chulvi-Medrano I, Picón-Martínez M, Cortell-Tormo JM, Tortosa-Martínez J, Alonso-Aubin DA, Alakhdar Y. Different time course of recovery in achilles tendon thickness after low-load resistance training with and without blood flow restriction. J Sport Rehabil. 2021;30(2):300–5. [CrossRef]
- Karabulut M, Cramer JT, Abe T, Sato Y, Bemben MG. Neuromuscular fatigue following low-intensity dynamic exercise with externally applied vascular restriction. J Electromyogr Kinesiol. 2010 Jun 1;20(3):440–7. [CrossRef]
- Centner C, Ritzmann R, Schur S, Gollhofer A, König D. Blood flow restriction increases myoelectric activity and metabolic accumulation during whole-body vibration. Eur J Appl Physiol [Internet]. 2019;119(6):1439–49. [CrossRef]
- Queiros VS De, França IM De, Trybulski R. Myoelectric Activity and Fatigue in Low-Load Resistance Exercise With Different Pressure of Blood Flow Restriction : A Systematic Review and Meta-Analysis. 2021;12(November).
- González-Badillo JJ, Sánchez-Medina L, Ribas-Serna J, Rodríguez-Rosell D. Toward a New Paradigm in Resistance Training by Means of Velocity Monitoring: A Critical and Challenging Narrative. Sport Med - open [Internet]. 2022;8(1):118. Available from: http://www.ncbi.nlm.nih.gov/pubmed/36114395 . [CrossRef]
- García-Ramos A, Suzovic D, Pérez-Castilla A. The load-velocity profiles of three upper-body pushing exercises in men and women. Sport Biomech. 2019;(March). [CrossRef]
- Sánchez-Medina L, Pallarés J, Pérez C, Morán-Navarro R, González-Badillo J. Estimation of Relative Load From Bar Velocity in the Full Back Squat Exercise. Sport Med Int Open. 2017;01(02):E80–8.
- Sánchez-Medina L, González-Badillo JJ. Velocity loss as an indicator of neuromuscular fatigue during resistance training. Med Sci Sports Exerc. 2011;43(9):1725–34. [CrossRef]
- Rodríguez-Rosell D, Yáñez-García JM, Torres-Torrelo J, Mora-Custodio R, Marques MC, González-Badillo JJ. Effort index as a novel variable for monitoring the level of effort during resistance exercises. J Strength Cond Res. 2018;32(8):2139–53. [CrossRef]
- Wilk M, Gepfert M, Krzysztofik M, Stastny P, Zajac A, Bogdanis GC. Acute Effects of Continuous and Intermittent Blood Flow Restriction on Movement Velocity During Bench Press Exercise Against Different Loads. Front Physiol. 2020;11(November):1–9. [CrossRef]
- Cancela J, Ayán C, Vila H, Gutiérrez J, Gutiérrez-Santiago A. Validez de Constructo del Cuestionario Internacional de Actividad Física en Universitarios Españoles. Rev Iberoam Diagnóstico y Evaluación – e Avaliação Psicológica. 2019;52(3):5–14. [CrossRef]
- World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects | Enhanced Reader [Internet]. [cited 2021 Apr 22]. Available from: chrome-extension://dagcmkpagjlhakfdhnbomgmjdpkdklff/enhanced-reader.html?openApp&pdf=https%3A%2F%2Fjamanetwork.com%2Fjournals%2Fjama%2Farticlepdf%2F1760318%2Fjsc130006.pdf.
- Sanchez-Medina L, Perez CE, Gonzalez-Badillo JJ. Importance of the propulsive phase in strength assessment. Int J Sports Med. 2010;31(2):123–9. [CrossRef]
- Schoenfeld BJ, Pope ZK, Benik FM, Hester GM, Sellers J, Nooner JL, et al. Longer interset rest periods enhance muscle strength and hypertrophy in resistance-trained men. J Strength Cond Res. 2016;30(7):1805–12. [CrossRef]
- Garcia-Sillero M, Chulvi-Medrano I, Maroto-Izquierdo S, Bonilla DA, Vargas-Molina S, Benítez-Porres J. Effects of Preceding Transcranial Direct Current Stimulation on Movement Velocity and EMG Signal during the Back Squat Exercise. J Clin Med. 2022;11(17). [CrossRef]
- García-Sillero M, Jurado-Castro JM, Benítez-Porres J, Vargas-Molina S. Acute effects of a percussive massage treatment on movement velocity during resistance training. Int J Environ Res Public Health. 2021;18(15). [CrossRef]
- Stegeman D, Hermens H. Standards for surface electromyography: The European project Surface EMG for non-invasive assessment of muscles (SENIAM). Línea) Dispon en http//www med … [Internet]. 2007;(January):108–12. Available from: http://www.seniam.org/%5Cnhttp://www.med.uni-jena.de/motorik/pdf/stegeman.pdf.
- Hermens HJ, Merletti R, Rix H, Freriks B. The State of the Art on Signal Processing Methods for Surface ElectroMyoGraphy. Seniam. 1998;7–9.
- Napoli NJ, Mixco AR, Bohorquez JE, Signorile JF. An EMG comparative analysis of quadriceps during isoinertial strength training using nonlinear scaled wavelets. Hum Mov Sci. 2015 Apr 1;40:134–53. [CrossRef]
- Park J, Ty Hopkins J. Quadriceps activation normative values and the affect of subcutaneous tissue thickness. J Electromyogr Kinesiol. 2011 Feb 1;21(1):136–40.
- Roberts D, Kuenze C, Saliba S, Hart JM. Accessory muscle activation during the superimposed burst technique. J Electromyogr Kinesiol. 2012 Aug 1;22(4):540–5. [CrossRef]
- Jakobsen MD, Sundstrup E, Andersen CH, Aagaard P, Andersen LL. Muscle activity during leg strengthening exercise using free weights and elastic resistance: Effects of ballistic vs controlled contractions. Hum Mov Sci. 2013 Feb;32(1):65–78. [CrossRef]
- Gepfert M, Krzysztofik M, Kostrzewa M, Jarosz J, Trybulski R, Zajac A, et al. The acute impact of external compression on back squat performance in competitive athletes. Int J Environ Res Public Health. 2020;17(13):1–11. [CrossRef]
- Wilk M, Trybulski R, Krzysztofik M, Wojdala G, Campos Y, Zajac A, et al. Acute Effects of Different Blood Flow Restriction Protocols on Bar Velocity During the Squat Exercise. Front Physiol. 2021;12(June):1–8. [CrossRef]
- Serrano-Ramon J, Cortell-Tormo J, Bautista I, García Jaén M, Chulvi-Medrano I. Acute effects of different external compression with blood flow restriction on force-velocity profile during squat and bench press exercises. Biol Sport. 2023;209–16. [CrossRef]
- Farup J, de Paoli F, Bjerg K, Riis S, Ringgard S, Vissing K. Blood flow restricted and traditional resistance training performed to fatigue produce equal muscle hypertrophy. Scand J Med Sci Sport. 2015;25(6):754–63. [CrossRef]
- Teixeira EL, Painelli V de S, Schoenfeld BJ, Silva-Batista C, Longo AR, Aihara AY, et al. Perceptual and Neuromuscular Responses Adapt Similarly Between High-Load Resistance Training and Low-Load Resistance Training With Blood Flow Restriction. J Strength Cond Res. 2020;Publish Ah(May). [CrossRef]
- Laurentino G, Ugrinowitsch C, Aihara AY, Fernandes AR, Parcell AC, Ricard M, et al. Effects of strength training and vascular occlusion. Int J Sports Med. 2008;29(8):664–7. [CrossRef]
- Gołaś A, Maszczyk A, Petr M, Statsny P, Wilk M, Wróbel G. Changes in Bar Velocity and Muscular Activity During the Bench Press in Relation to the Load Lifted. Cent Eur J Sport Sci Med. 2015;11(3):95–101. [CrossRef]
- Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: Mechanisms and recommendations for training practices. Sport Med. 2006;36(2):133–49.
- Moore DR, Burgomaster KA, Schofield LM, Gibala MJ, Sale DG, Phillips SM. Neuromuscular adaptations in human muscle following low intensity resistance training with vascular occlusion. Eur J Appl Physiol. 2004;92(4–5):399–406. [CrossRef]
- Neto GR, Santos HH, Sousa JBC, Júnior ATA, Araújo JP, Aniceto RR, et al. Effects of high-intensity blood flow restriction exercise on muscle fatigue. J Hum Kinet. 2014;41(1):163–72. [CrossRef]
- Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol. 2000;88(6):2097–106. [CrossRef]
- Pope ZK, Willardson JM, Schoenfeld BJ. Exercise and blood flow restriction. Vol. 27, Journal of Strength and Conditioning Research. 2013. 2914–2926 p.
- Sharifi S, Monazzami A, Nikousefat Z, Heyrani A, Yari K. The acute and chronic effects of resistance training with blood flow restriction on hormonal responses in untrained young men: A comparison of frequency. Cell Mol Biol. 2020;66(1):1–8. [CrossRef]
- Incognito A V., Burr JF, Millar PJ. The Effects of Ischemic Preconditioning on Human Exercise Performance. Sport Med. 2016;46(4):531–44.
- de Souza HLR, Arriel RA, Hohl R, da Mota GR, Marocolo M. Is Ischemic Preconditioning Intervention Occlusion-Dependent to Enhance Resistance Exercise Performance? J Strength Cond Res. 2021;35(10):2706–12.
- Bailey TG, Birk GK, Timothy Cable N, Atkinson G, Green DJ, Jones H, et al. Remote ischemic preconditioning prevents reduction in brachial artery flow-mediated dilation after strenuous exercise. Am J Physiol - Hear Circ Physiol. 2012;303(5):533–8. [CrossRef]
- Andreas M, Schmid AI, Keilani M, Doberer D, Bartko J, Crevenna R, et al. Effect of ischemic preconditioning in skeletal muscle measured by functional magnetic resonance imaging and spectroscopy: A randomized crossover trial. J Cardiovasc Magn Reson [Internet]. 2011;13(1):32. Available from: http://www.jcmr-online.com/content/13/1/32 . [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).