Submitted:
20 May 2023
Posted:
23 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1 Animals
2.2 Experimental ulcerative colitis
2.3 Immunofluorescence
2.4 Qualitative analysis
2.5 Quantitative analysis
2.6 Histological analysis
2.7 Statistical analysis
3. Results
3.1. Experimental ulcerative colitis
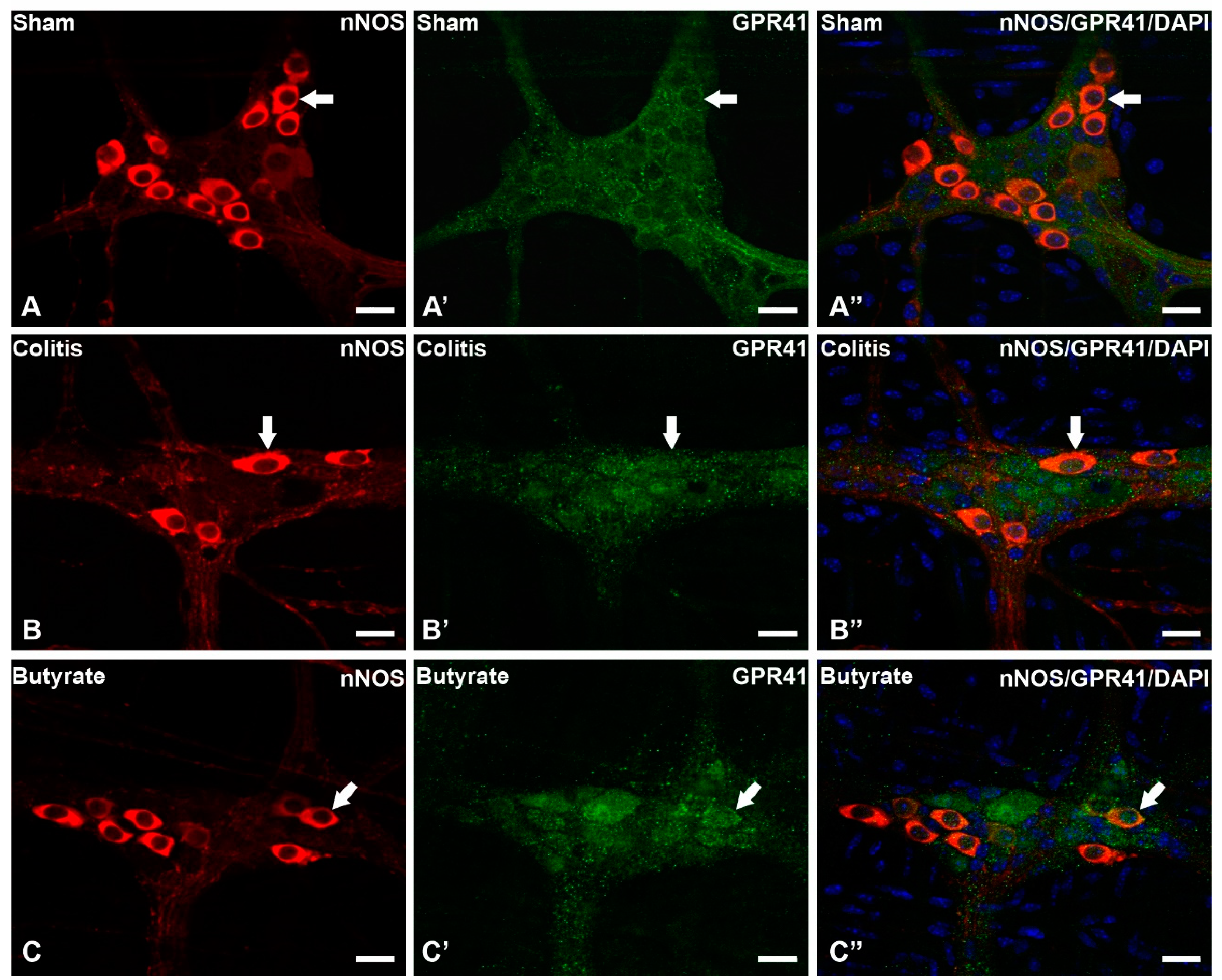
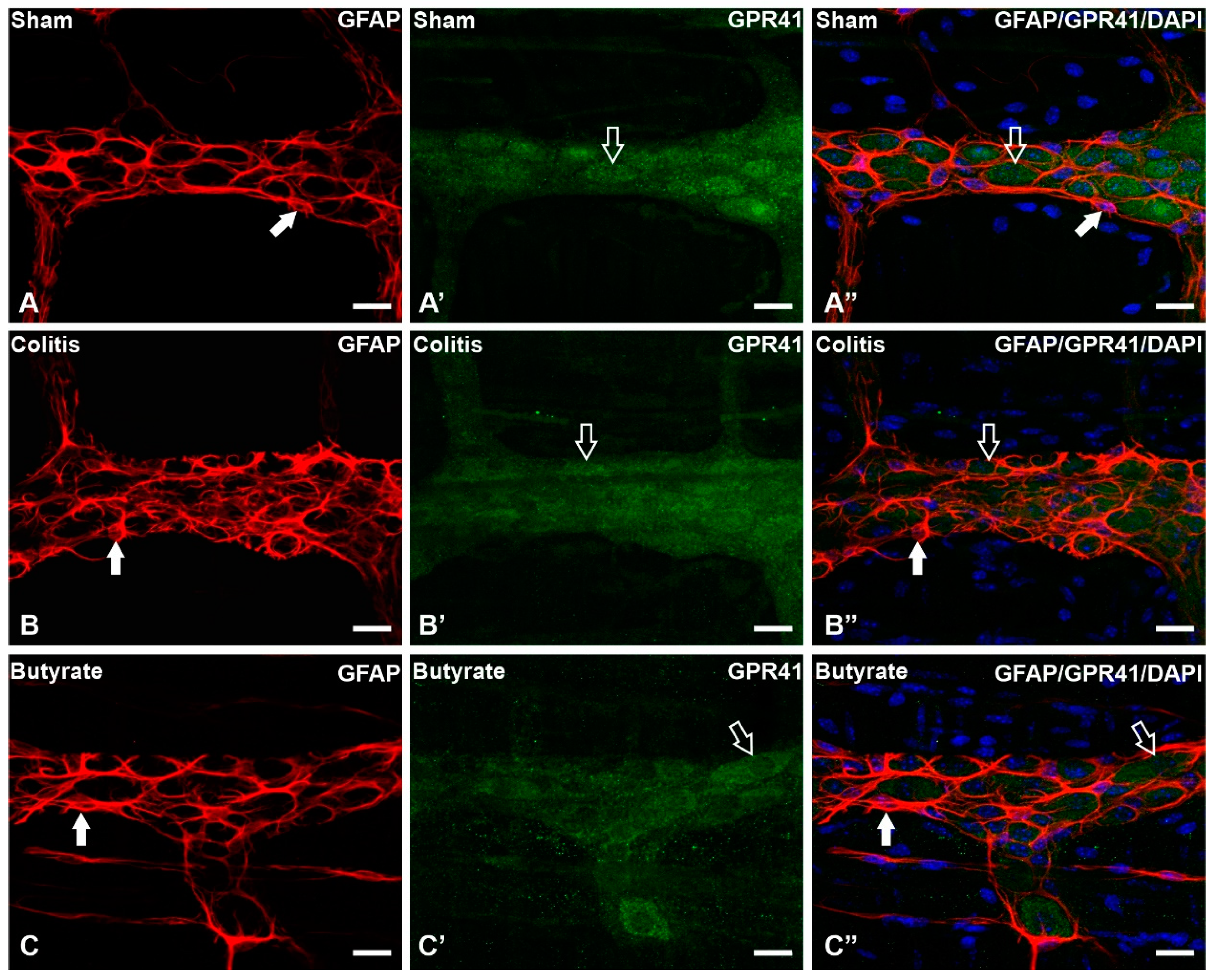
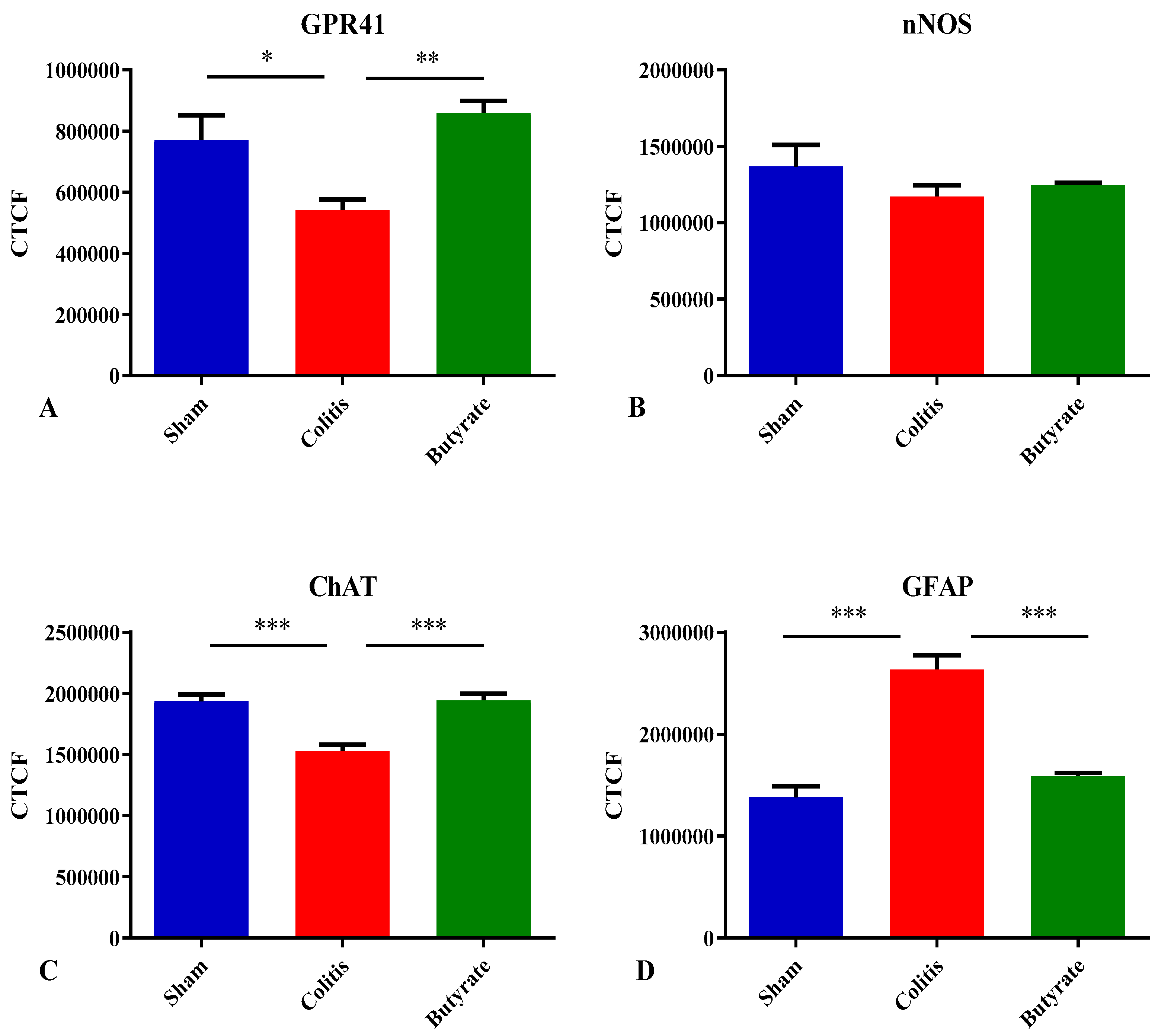
3.2. Qualitative analysis
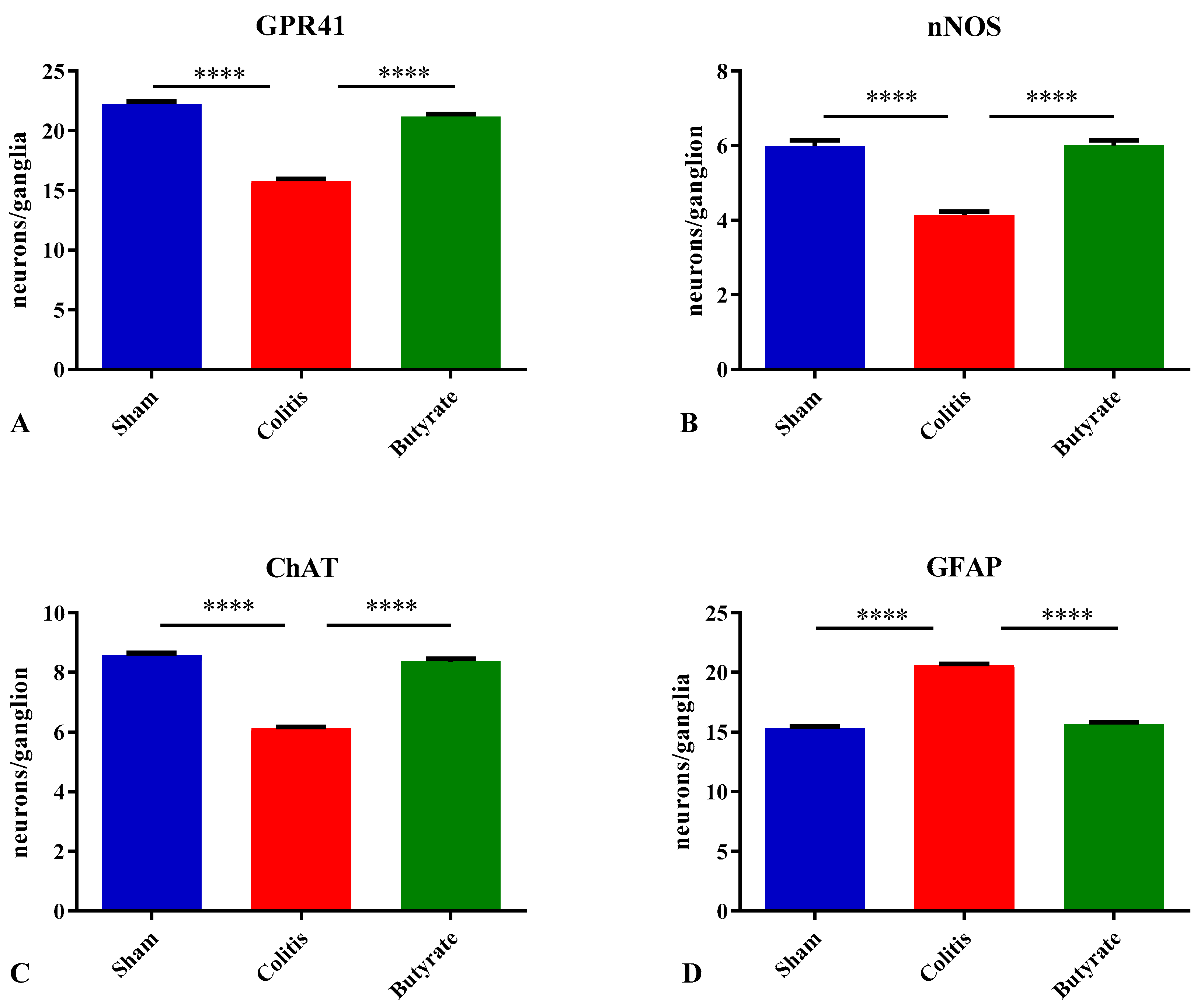
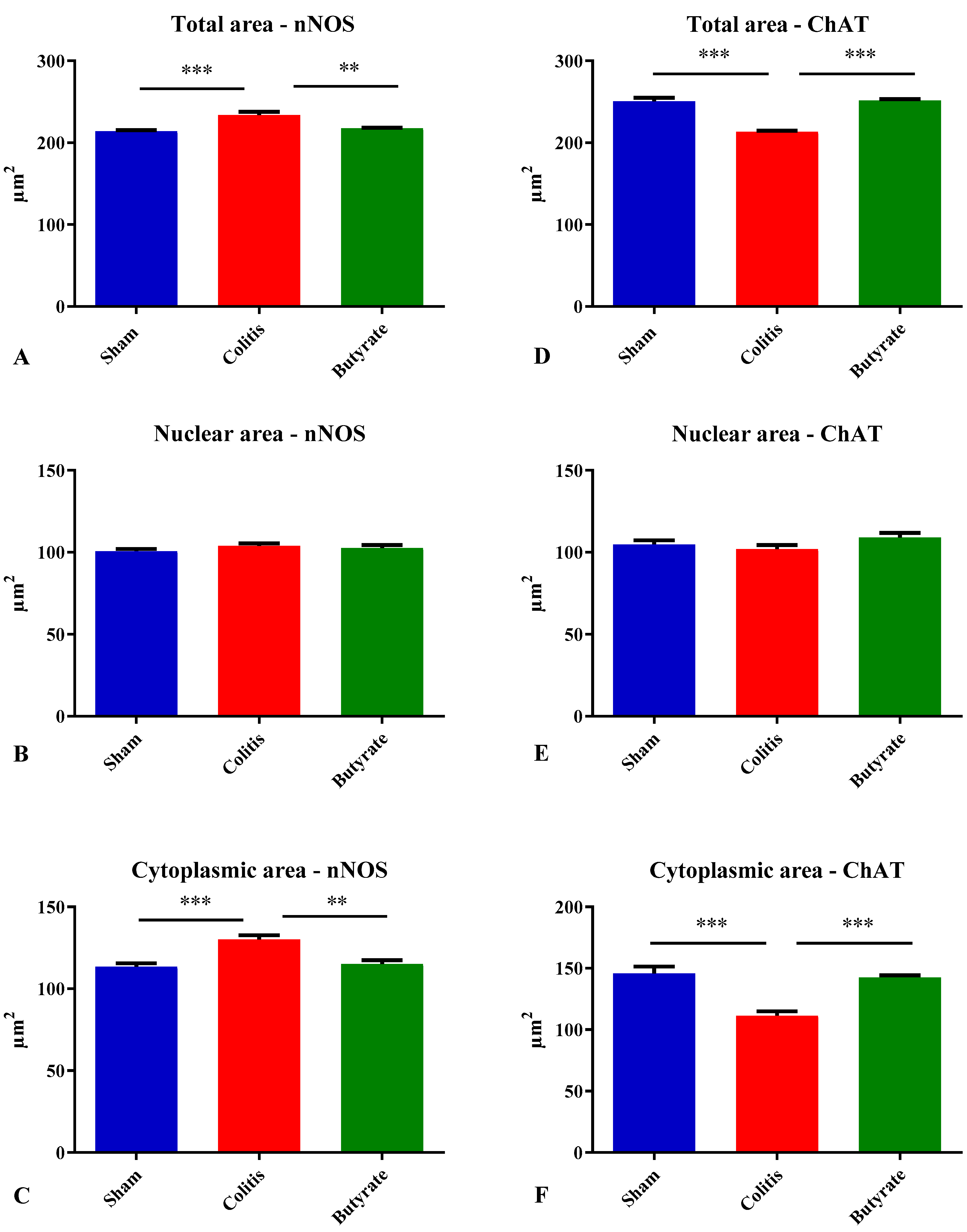
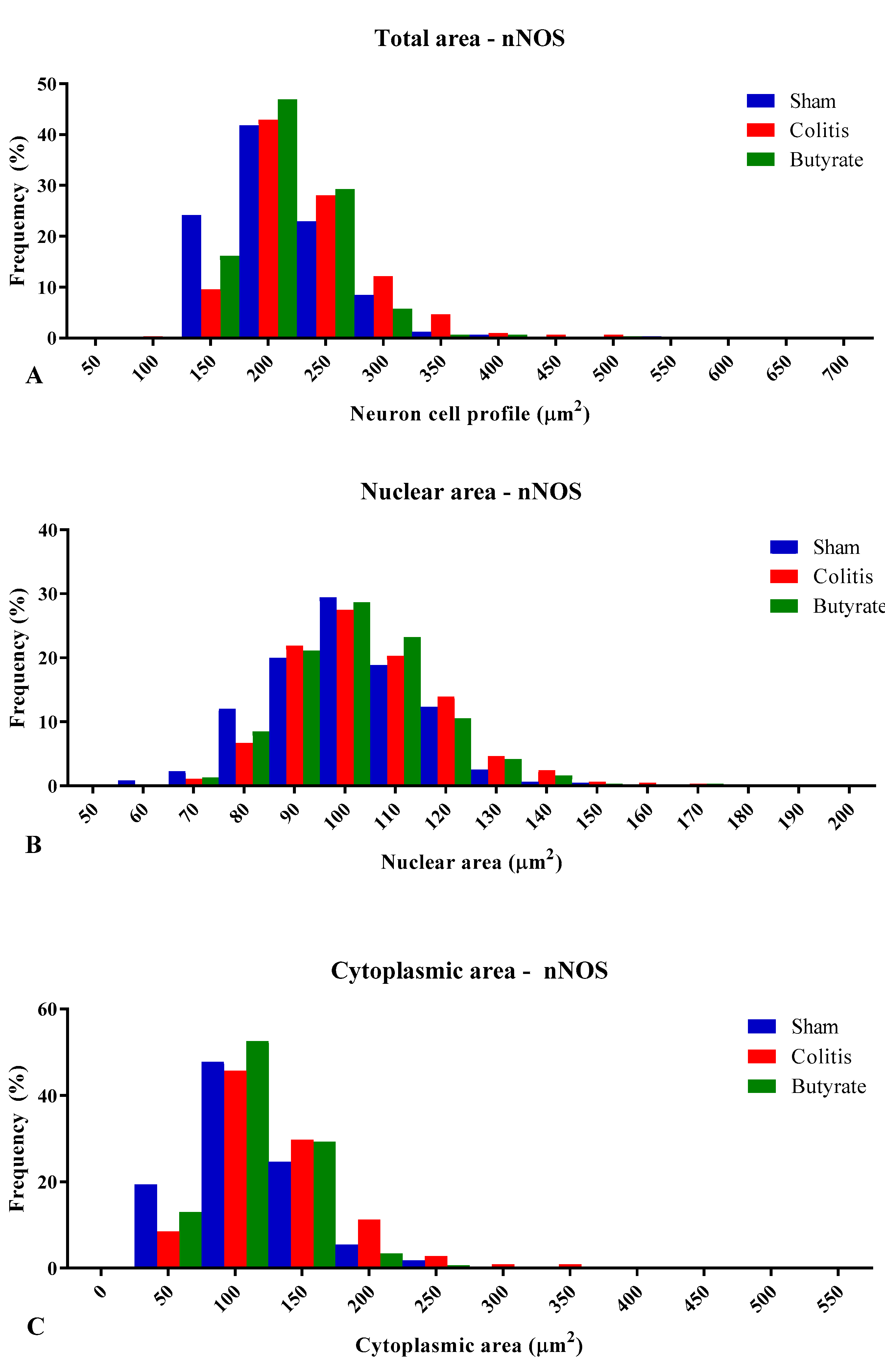
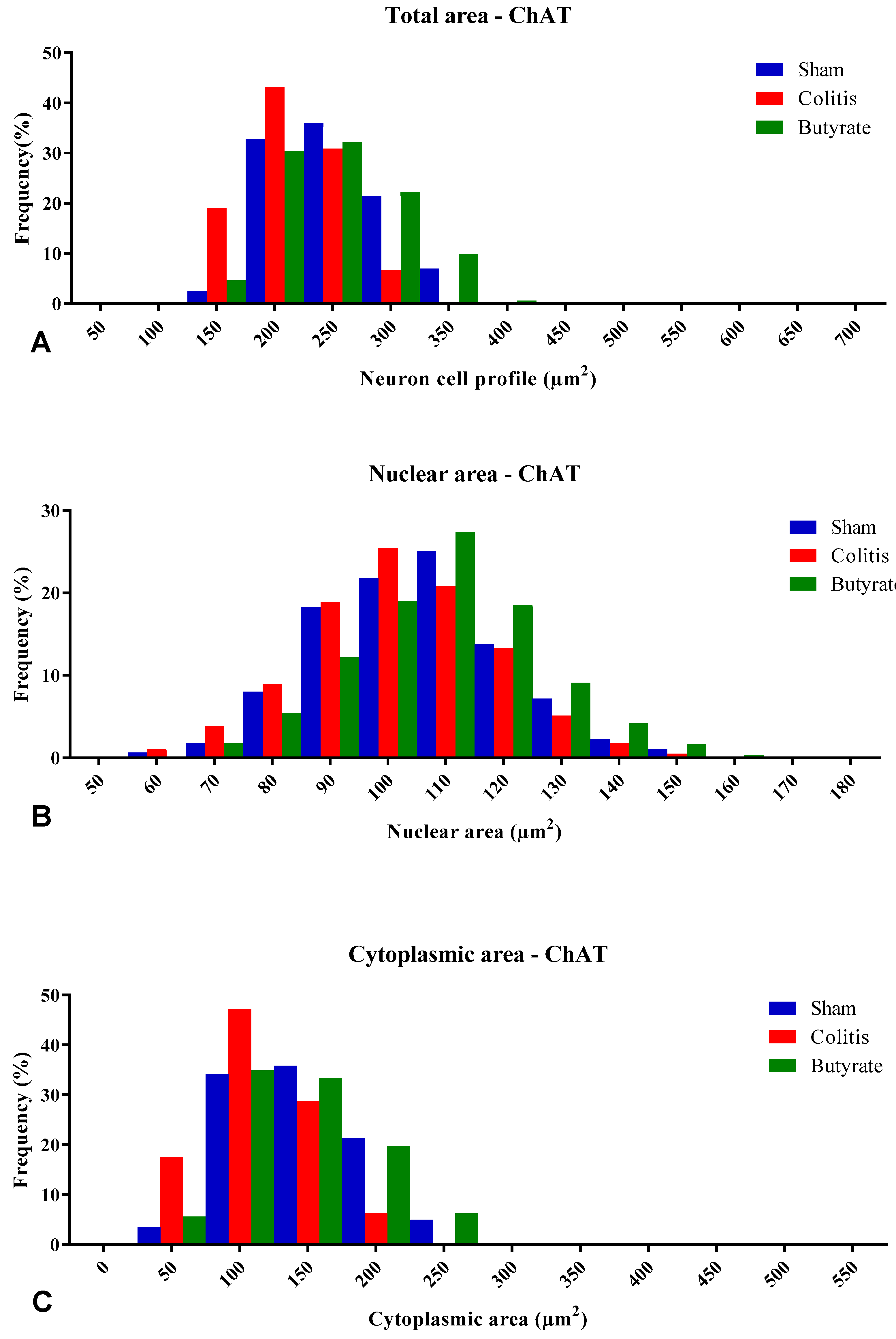
3.3. Quantitative analysis
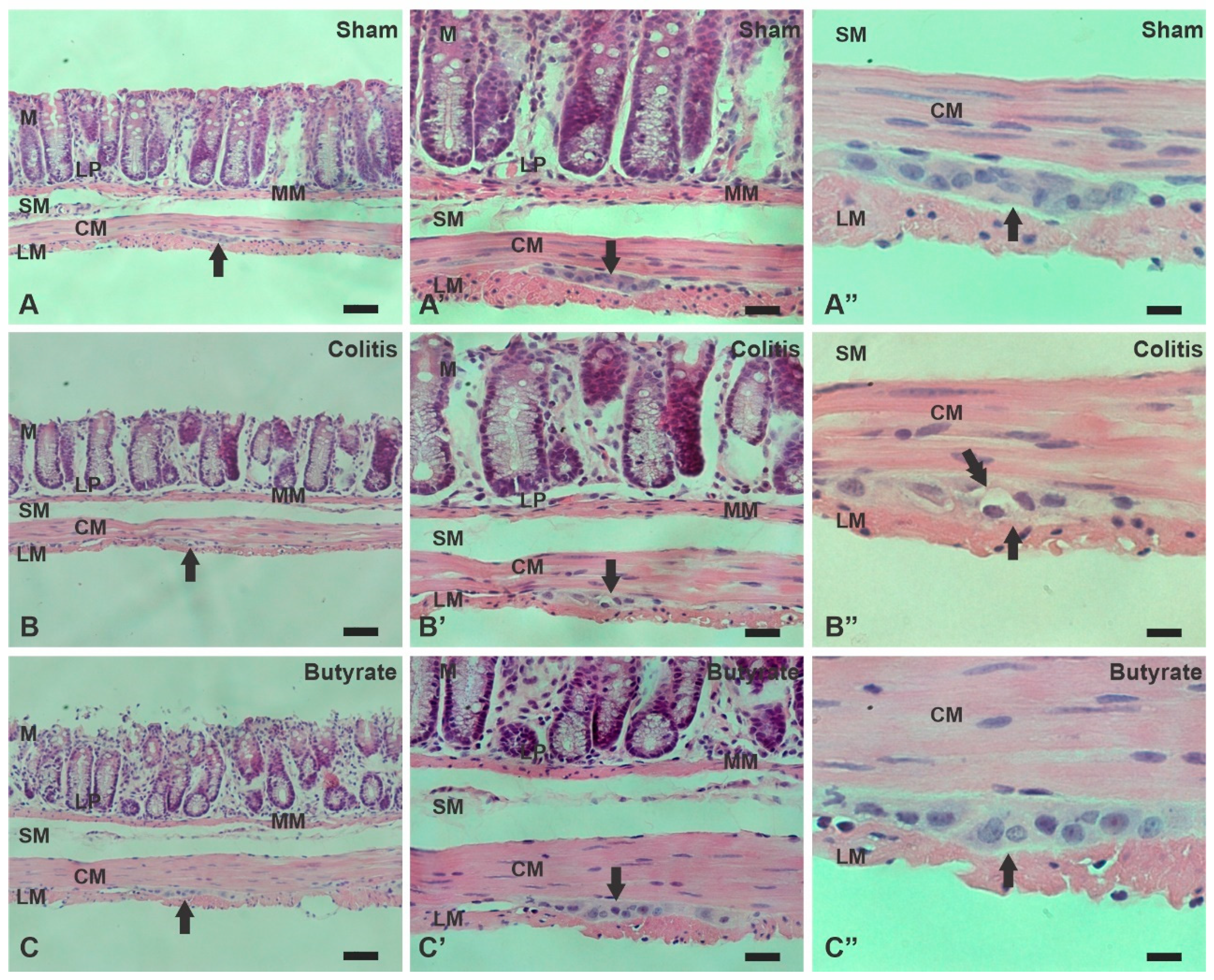
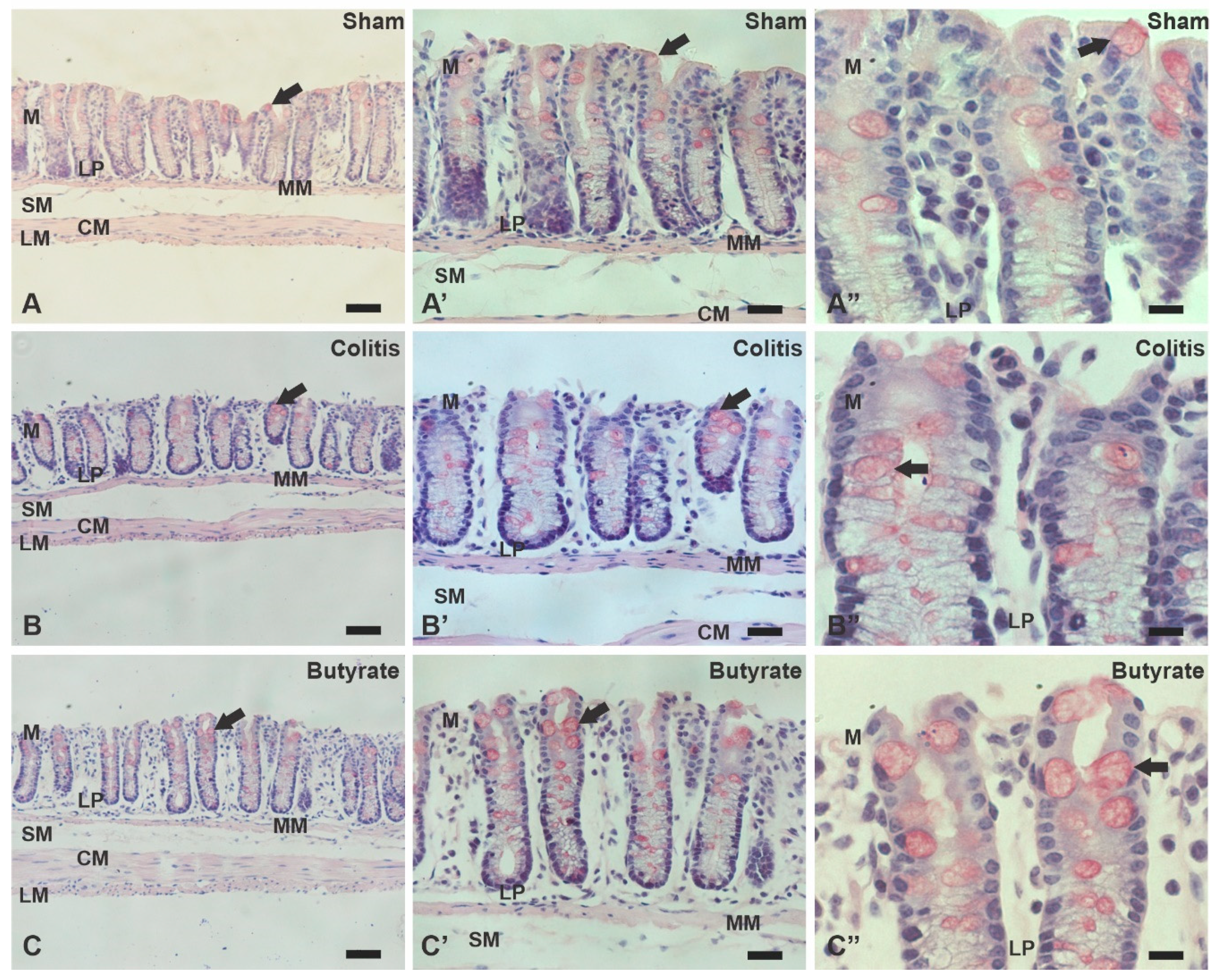
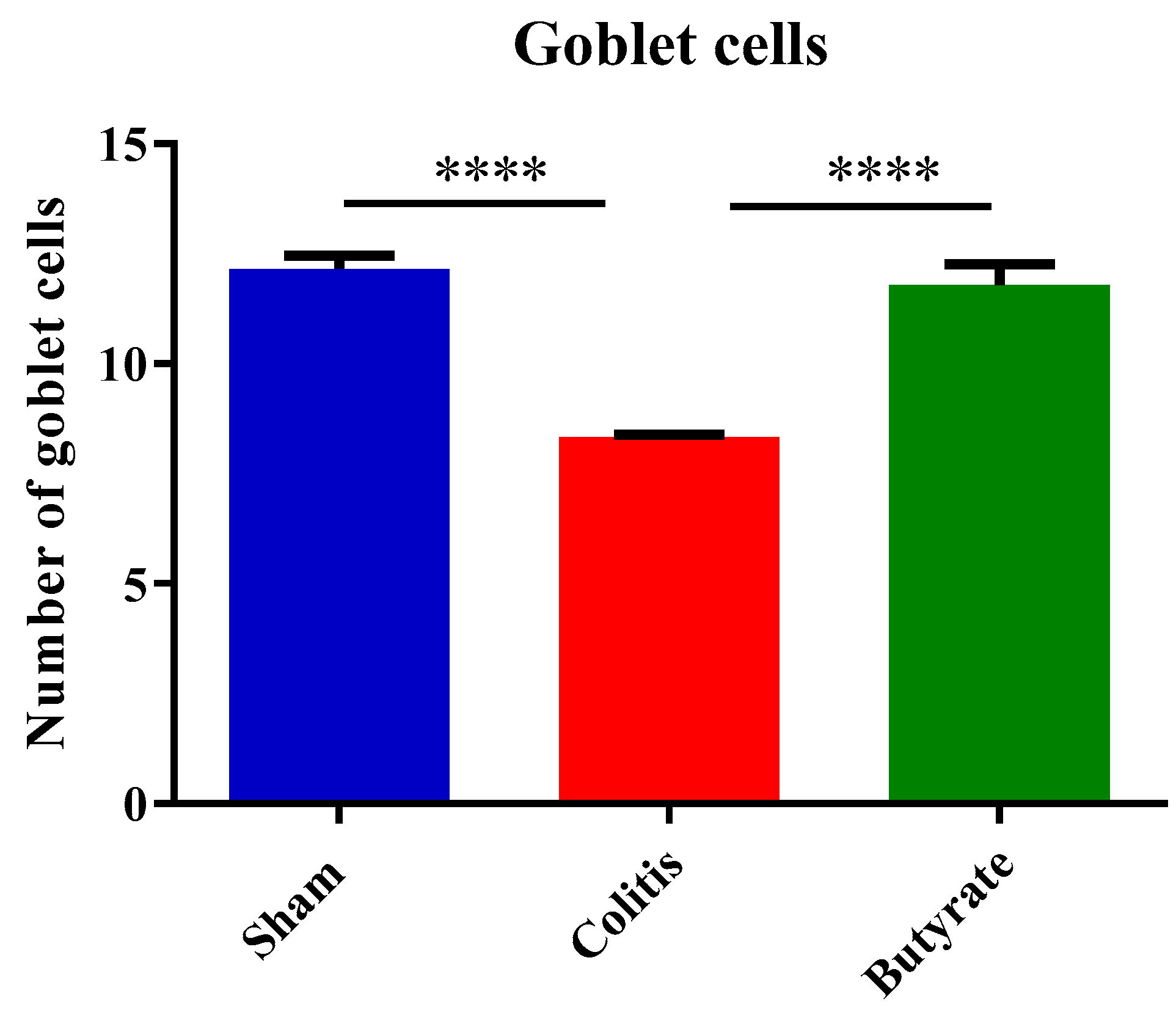
3.4. Histological analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furness, J.B. The Enteric Nervous System; 1st ed.; Blackwell Publishing Ltd: Malden, Massachusetts, USA, 2006.
- Furness, J.B. The Enteric Nervous System and Neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [CrossRef]
- Sairenji, T.; Collins, K.L.; Evans, D. V. An Update on Inflammatory Bowel Disease. Prim. Care - Clin. Off. Pract. 2017, 44, 673–692. [CrossRef]
- Malik, T.A. Inflammatory Bowel Disease. Historical Perspective, Epidemiology, and Risk Factors. Surg. Clin. North Am. 2015, 95, 1105–1122.
- Abraham, C.; Cho, J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009, 361, 2066–2078.
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The Gut Microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [CrossRef]
- Stange, E.F.; Schroeder, B.O. Microbiota and Mucosal Defense in IBD: An Update. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 963–976. [CrossRef]
- da Silva, M.V.; Marosti, A.R.; Mendes, C.E.; Palombit, K.; Castelucci, P. Differential Effects of Experimental Ulcerative Colitis on P2X7 Receptor Expression in Enteric Neurons. Histochem. Cell Biol. 2015, 143, 171–184. . [CrossRef]
- da Silva, M.V.; Marosti, A.R.; Mendes, C.E.; Palombit, K.; Castelucci, P. Submucosal Neurons and Enteric Glial Cells Expressing the P2X7 Receptor in Rat Experimental Colitis. Acta Histochem. 2017, 119, 481–494. [CrossRef]
- Evangelinellis, M.M.; Souza, R.F.; Mendes, C.E.; Castelucci, P. Effects of a P2X7 Receptor Antagonist on Myenteric Neurons in the Distal Colon of an Experimental Rat Model of Ulcerative Colitis. Histochem. Cell Biol. 2022, 157, 65–81. [CrossRef]
- Makowska, K. Changes in The Expression of Somatostatin (SOM) in Nerve Fibers of Gastrointestinal Mucosa in Dogs with Inflammatory Bowel Disease (IBD). Med. Case Rep. 2019,5, 90.
- de Fontgalland, D.; Brookes, S.J.; Gibbins, I.; Sia, T.C.; Wattchow, D.A. The Neurochemical Changes in the Innervation of Human Colonic Mesenteric and Submucosal Blood Vessels in Ulcerative Colitis and Crohn’s Disease. Neurogastroenterol. Motil. 2014, 26, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.R. Colitis Is Associated with a Loss of Intestinofugal Neurons. Am. J. Physiol. - Gastrointest. Liver Physiol. 2012, 303, G1096. [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of Short-Chain Fatty Acid Production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Ganapathy, V.; Thangaraju, M.; Gopal, E.; Martin, P.M.; Itagaki, S.; Miyauchi, S.; Prasad, P.D. Sodium-Coupled Monocarboxylate Transporters in Normal Tissues and in Cancer. AAPS J. 2008, 10, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Sepponen, K.; Ruusunen, M.; Pakkanen, J.A.; Pösö, A.R. Expression of CD147 and Monocarboxylate Transporters MCT1, MCT2 and MCT4 in Porcine Small Intestine and Colon. Vet. J. 2007, 174, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and Medium-Chain Fatty Acids in Energy Metabolism: The Cellular Perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, J.G.; Venema, K.; van de Poll, M.C.; Olde Damink, S.W.; Buurman, W.A.; Dejong, C.H. Short Chain Fatty Acids Exchange across the Gut and Liver in Humans Measured at Surgery. Clin. Nutr. 2009, 28, 657–661. [Google Scholar] [CrossRef]
- Boets, E.; Gomand, S. V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic Availability and Metabolism of Colonic-Derived Short-Chain Fatty Acids in Healthy Subjects: A Stable Isotope Study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef]
- Boets, E.; Deroover, L.; Houben, E.; Vermeulen, K.; Gomand, S. V.; Delcour, J.A.; Verbeke, K. Quantification of in Vivo Colonic Short Chain Fatty Acid Production from Inulin. Nutrients 2015, 7, 8916–8929. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G Protein-Coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Caetano, M.A.F.; Castelucci, P. Role of Short Chain Fatty Acids in Gut Health and Possible Therapeutic Approaches in Inflammatory Bowel Diseases. World J. Clin. Cases 2022, 10, 9985–10003. [Google Scholar] [CrossRef] [PubMed]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review Article: The Role of Butyrate on Colonic Function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Kaji, I.; Akiba, Y.; Furuyama, T.; Adelson, D.W.; Iwamoto, K.; Watanabe, M.; Kuwahara, A.; Kaunitz, J.D. Free Fatty Acid Receptor 3 Activation Suppresses Neurogenic Motility in Rat Proximal Colon. Neurogastroenterol. Motil. 2018, 30, e13157. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Costanzo, M. Di; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential Beneficial Effects of Butyrate in Intestinal and Extraintestinal Diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Hertati, A.; Hayashi, S.; Ogata, H.; Miyata, K.; Kato, R.; Yamamoto, T.; Kadowaki, M. Morphological Elucidation of Short-Chain Fatty Acid Receptor GPR41-Positive Enteric Sensory Neurons in the Colon of Mice with Dextran Sulfate Sodium-Induced Colitis. Heliyon 2020, 6, e05647. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Pachnis, V. The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology 2016, 151, 836–844. [Google Scholar] [CrossRef]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium Butyrate Inhibits the NF-Kappa B Signaling Pathway and Histone Deacetylation, and Attenuates Experimental Colitis in an IL-10 Independent Manner. Int. Immunopharmacol. 2017, 51, 47–56. [Google Scholar] [CrossRef]
- Bell, C.J.; Gall, D.G.; Wallace, J.L. Disruption of Colonic Electrolyte Transport in Experimental Colitis. Am. J. Physiol. - Gastrointest. Liver Physiol. 1995, 268. [CrossRef]
- Cooper, H.S.; Murthy, S.N.S.; Shah, R.S.; Sedergran, D.J. Clinicopathologic Study of Dextran Sulfate Sodium Experimental Murine Colitis. Lab. Investig. 1993, 69, 238–250. [Google Scholar] [CrossRef]
- Nooh, H.Z.; Nour-Eldien, N.M. The Dual Anti-Inflammatory and Antioxidant Activities of Natural Honey Promote Cell Proliferation and Neural Regeneration in a Rat Model of Colitis. Acta Histochem. 2016, 118, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Schwertassek, U.; Seydel, A.; Weber, K.; Falk, W.; Hauschildt, S.; Lehmann, J. A Refined and Translationally Relevant Model of Chronic DSS Colitis in BALB/c Mice. Lab. Anim. 2018, 52, 240–252. [Google Scholar] [CrossRef]
- Vieira, C.; Ferreirinha, F.; Magalhães-Cardoso, M.T.; Silva, I.; Marques, P.; Correia-de-Sá, P. Post-Inflammatory Ileitis Induces Non-Neuronal Purinergic Signaling Adjustments of Cholinergic Neurotransmission in the Myenteric Plexus. Front. Pharmacol. 2017, 8, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Isiksoy, S.; Dundar, E.; Pasaoglu, O.; Bal, C. The effects of sodium phosphate and polyethylene glycol-electrolyte bowel preparation solutions on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in the rat. Experimental and Toxicologic Pathology. 2003, 55, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Fabia, R.; Ar’rajab, A.; Johansson, M.L.; Willén, R.; Andersson, R.; Molin, G.; Bengmark, S. The Effect of Exogenous Administration of Lactobacillus Reuteri R2LC and Oat Fiber on Acetic Acid-Induced Colitis in the Rat. Scand. J. Gastroenterol. 1993, 28, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Trevizan, A.R.; Vicentino-Vieira, S.L.; da Silva Watanabe, P.; Góis, M.B.; de Melo, G. de A.N.; Garcia, J.L.; José de Almeida Araújo, E.; Sant’Ana, D. de M.G. Kinetics of Acute Infection with Toxoplasma Gondii and Histopathological Changes in the Duodenum of Rats. Exp. Parasitol. 2016, 165, 22–29.
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R.; et al. Microbiota Changes Induced by Microencapsulated Sodium Butyrate in Patients with Inflammatory Bowel Disease. Neurogastroenterol. Motil. 2020, 32, 13–25. [Google Scholar] [CrossRef]
- Simeoli, R.; Mattace Raso, G.; Pirozzi, C.; Lama, A.; Santoro, A.; Russo, R.; Montero-Melendez, T.; Berni Canani, R.; Calignano, A.; Perretti, M.; et al. An Orally Administered Butyrate-Releasing Derivative Reduces Neutrophil Recruitment and Inflammation in Dextran Sulphate Sodium-Induced Murine Colitis. Br. J. Pharmacol. 2017, 174, 1484–1496. [Google Scholar] [CrossRef]
- Nøhr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Møller, M. Expression of the Short Chain Fatty Acid Receptor GPR41/FFAR3 in Autonomic and Somatic Sensory Ganglia. Neuroscience 2015, 290, 126–137. [Google Scholar] [CrossRef]
- Kaji, I.; Iwanaga, T.; Watanabe, M.; Guth, P.H.; Engel, E.; Kaunitz, J.D.; Akiba, Y. SCFA Transport in Rat Duodenum. Am. J. Physiol. - Gastrointest. Liver Physiol. 2015, 308, G188–G197. [CrossRef]
- Lakhan, S.E.; Kirchgessner, A. Neuroinflammation in Inflammatory Bowel Disease. J. Neuroinflammation 2010, 7, 37. [Google Scholar] [CrossRef]
- Magalhães, H.I.R.; Castelucci, P. Enteric Nervous System and Inflammatory Bowel Diseases: Correlated Impacts and Therapeutic Approaches through the P2X7 Receptor. World J. Gastroenterol. 2021, 27, 7909–7924. [Google Scholar] [CrossRef]
- Dong, Z.; Saikumar, P.; Weinberg, J.M.; Venkatachalam, M.A. Calcium in Cell Injury and Death. Annu. Rev. Pathol. 2006, 1, 405–434. [Google Scholar] [CrossRef]
- Vanden Berghe, P.; Tack, J.; Andrioli, A.; Missiaen, L.; Janssens, J. Receptor-Induced Ca2+ Signaling in Cultured Myenteric Neurons. Am. J. Physiol. - Gastrointest. Liver Physiol. 2000, 278, 905–914. [CrossRef]
- Rivera, L.R.; Poole, D.P.; Thacker, M.; Furness, J.B. The Involvement of Nitric Oxide Synthase Neurons in Enteric Neuropathies. Neurogastroenterol. Motil. 2011, 23, 980–988. [Google Scholar] [CrossRef]
- Von Boyen, G.B.T.; Schulte, N.; Pflüger, C.; Spaniol, U.; Hartmann, C.; Steinkamp, M. Distribution of Enteric Glia and GDNF during Gut Inflammation. BMC Gastroenterol. 2011, 11, 2–8. [Google Scholar] [CrossRef]
- Von Boyen, G.B.T.; Steinkamp, M.; Reinshagen, M.; Schäfer, K.H.; Adler, G.; Kirsch, J. Proinflammatory Cytokines Increase Glial Fibrillary Acidic Protein Expression in Enteric Glia. Gut 2004, 53, 222–228. [Google Scholar] [CrossRef]
- Stavely, R.; Abalo, R.; Nurgali, K. Targeting Enteric Neurons and Plexitis for the Management of Inflammatory Bowel Disease. Curr. Drug Targets 2020, 21, 1428–1439. [Google Scholar] [CrossRef]
- Yakovlev, A.G.; Faden, A.I. Mechanisms of Neural Cell Death: Implications for Development of Neuroprotective Treatment Strategies. NeuroRx 2004, 1, 5–16. [Google Scholar] [CrossRef]
- Carretta, M.D.; Quiroga, J.; López, R.; Hidalgo, M.A.; Burgos, R.A. Participation of Short-Chain Fatty Acids and Their Receptors in Gut Inflammation and Colon Cancer. Front. Physiol. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Bolognini, D.; Tobin, A.B.; Milligan, G.; Moss, C.E. The Pharmacology and Function of Receptors for Short-Chain Fatty Acids. Mol. Pharmacol. 2016, 89, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Davie, J.R. Nutritional Proteomics in Cancer Prevention Inhibition of Histone Deacetylase Activity. J. Nutr 2003, 133, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.B.; Navegantes-Lima, K.C.; Oliveira, A.L.B.; Rodrigues, D.V.S.; Gaspar, S.L.F.; Monteiro, V.V.S.; Moura, D.P.; Monteiro, M.C. Protective Mechanisms of Butyrate on Inflammatory Bowel Disease. Curr. Pharm. Des. 2018, 24, 4154–4166. [Google Scholar] [CrossRef] [PubMed]
- Kazemi Sefat, N.A.; Mohammadi, M.M.; Hadjati, J.; Talebi, S.; Ajami, M.; Daneshvar, H. Sodium Butyrate as a Histone Deacetylase Inhibitor Affects Toll-Like Receptor 4 Expression in Colorectal Cancer Cell Lines. Immunol. Invest. 2019, 48, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Soret, R.; Chevalier, J.; De Coppet, P.; Poupeau, G.; Derkinderen, P.; Segain, J.P.; Neunlist, M. Short-Chain Fatty Acids Regulate the Enteric Neurons and Control Gastrointestinal Motility in Rats. Gastroenterology 2010, 138. [Google Scholar] [CrossRef] [PubMed]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A Review of the Diagnosis, Prevention, and Treatment Methods of Inflammatory Bowel Disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-Induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Nielsen, D.S.G.; Jensen, B.B.; Theil, P.K.; Nielsen, T.S.; Knudsen, K.E.B.; Purup, S. Effect of Butyrate and Fermentation Products on Epithelial Integrity in a Mucus-Secreting Human Colon Cell Line. J. Funct. Foods 2018, 40, 9–17. [Google Scholar] [CrossRef]
- Miao, W.; Wu, X.; Wang, K.; Wang, W.; Wang, Y.; Li, Z.; Liu, J.; Li, L.; Peng, L. Sodium Butyrate Promotes Reassembly of Tight Junctions in Caco-2 Monolayers Involving Inhibition of MLCK/MLC2 Pathway and Phosphorylation of PKCβ2. Int. J. Mol. Sci. 2016, 17, 1–12. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor–Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef]
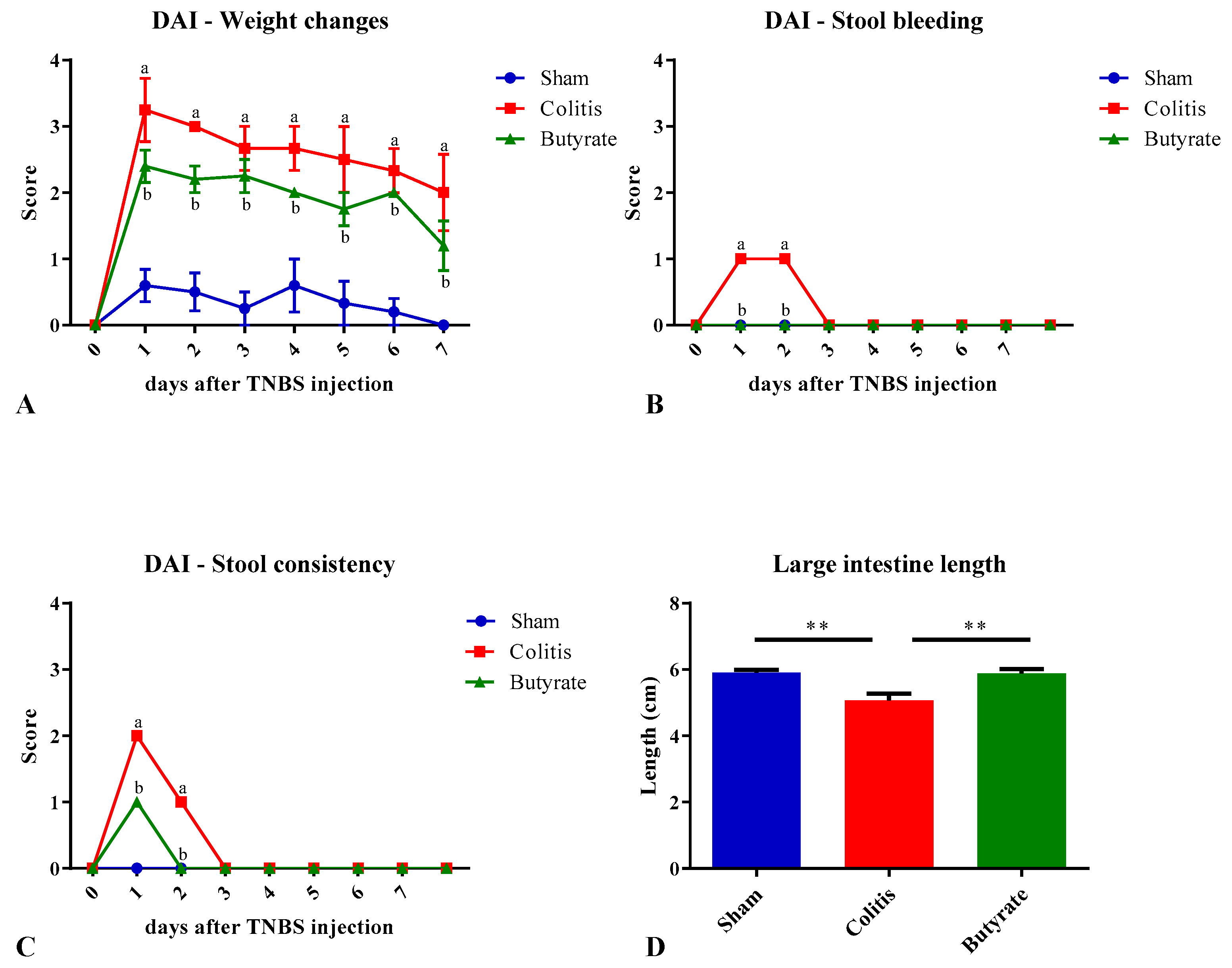


| Score | Weight change | Stool consistency | Stool and/or rectal bleeding |
|---|---|---|---|
| 0 | <1% | Normal stools | No bleeding |
| 1 | 1-2% | Soft stools | Mild bleeding |
| 2 | 2-4% | Soft stools that did not stick to the anus | Moderate bleeding |
| 3 | 4-6% | Soft stools that stick to the anus | Severe bleeding |
| 4 | >6% | Diarrhea | Gross bleeding |
| Antigen | Host | Dilution | Source |
|---|---|---|---|
| GPR41 | Rabbit | 1:200 | Sigma |
| nNOS | Sheep | 1:1000 | Millipore |
| ChAT | Goat | 1:100 | Millipore |
| GFAP | Goat | 1:1000 | Sigma |
| PGP9.5 | Guinea pig | 1:200 | Sigma |
| Secondary antibodies | |||
| Alexa Fluor 488-conjugated donkey anti-rabbit IgG 488 | 1:100 | Molecular Probes | |
| Alexa Fluor 594-conjugated donkey anti-sheep IgG 594 | 1:500 | Molecular Probes | |
| Alexa Fluor-594 conjugated donkey anti-guinea pig IgG 594 | 1:100 | Molecular Probes | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





