Submitted:
22 May 2023
Posted:
23 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
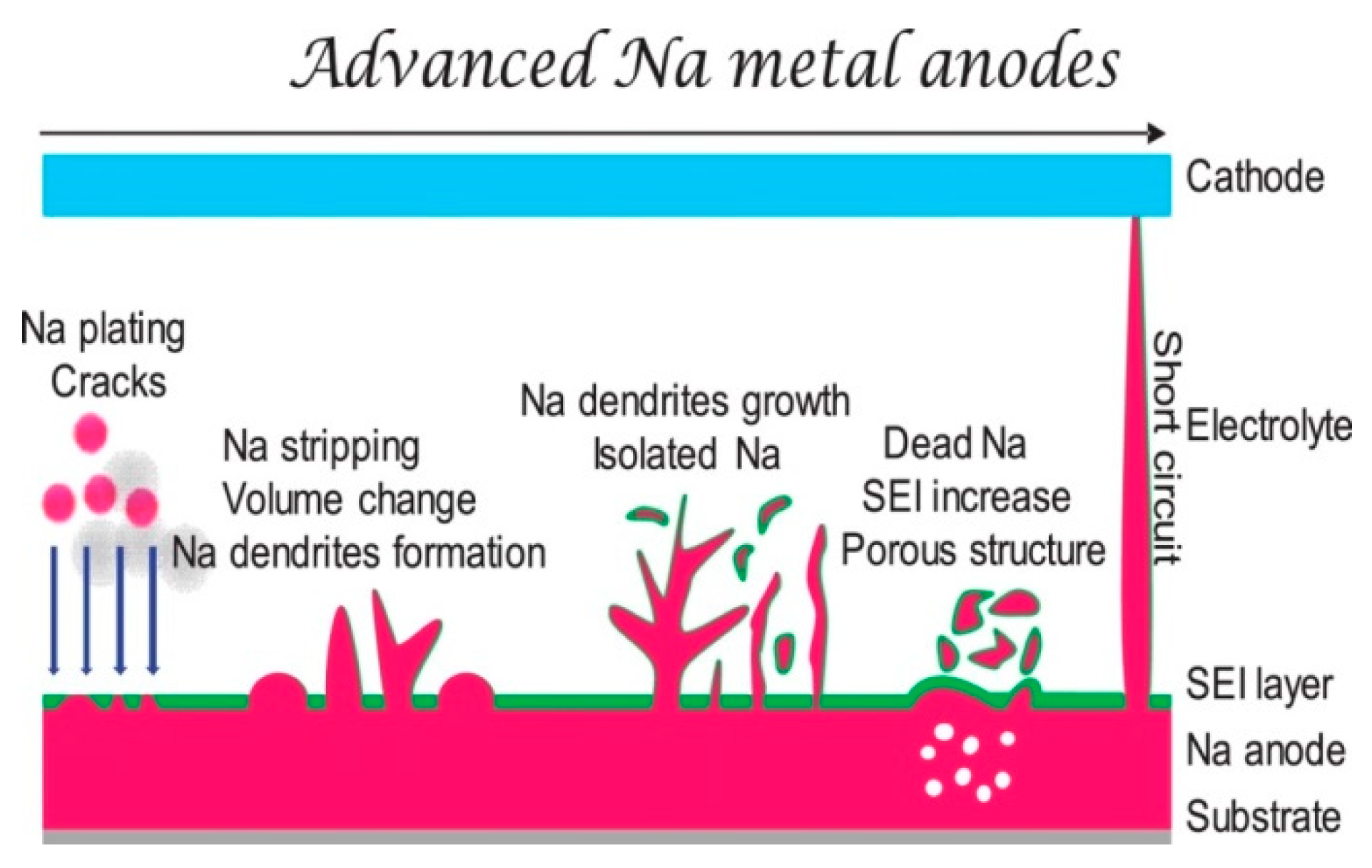
2. Challenges for Dendrite Free Na Metal Anodes
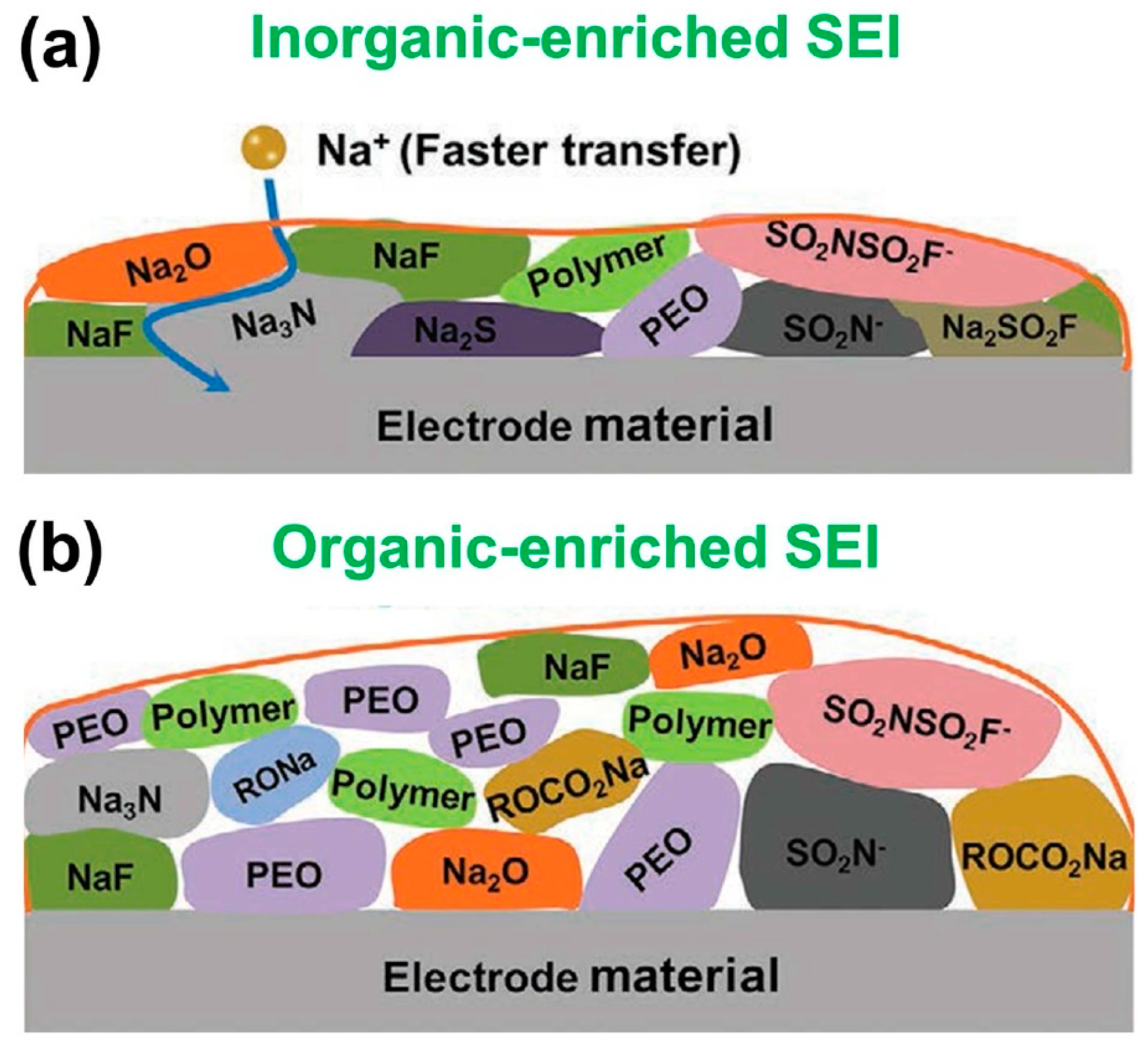
2.1. Unstable SEI
2.2. Uncontrollable Dendritic Na formation
2.3. Severe Volume Expansion
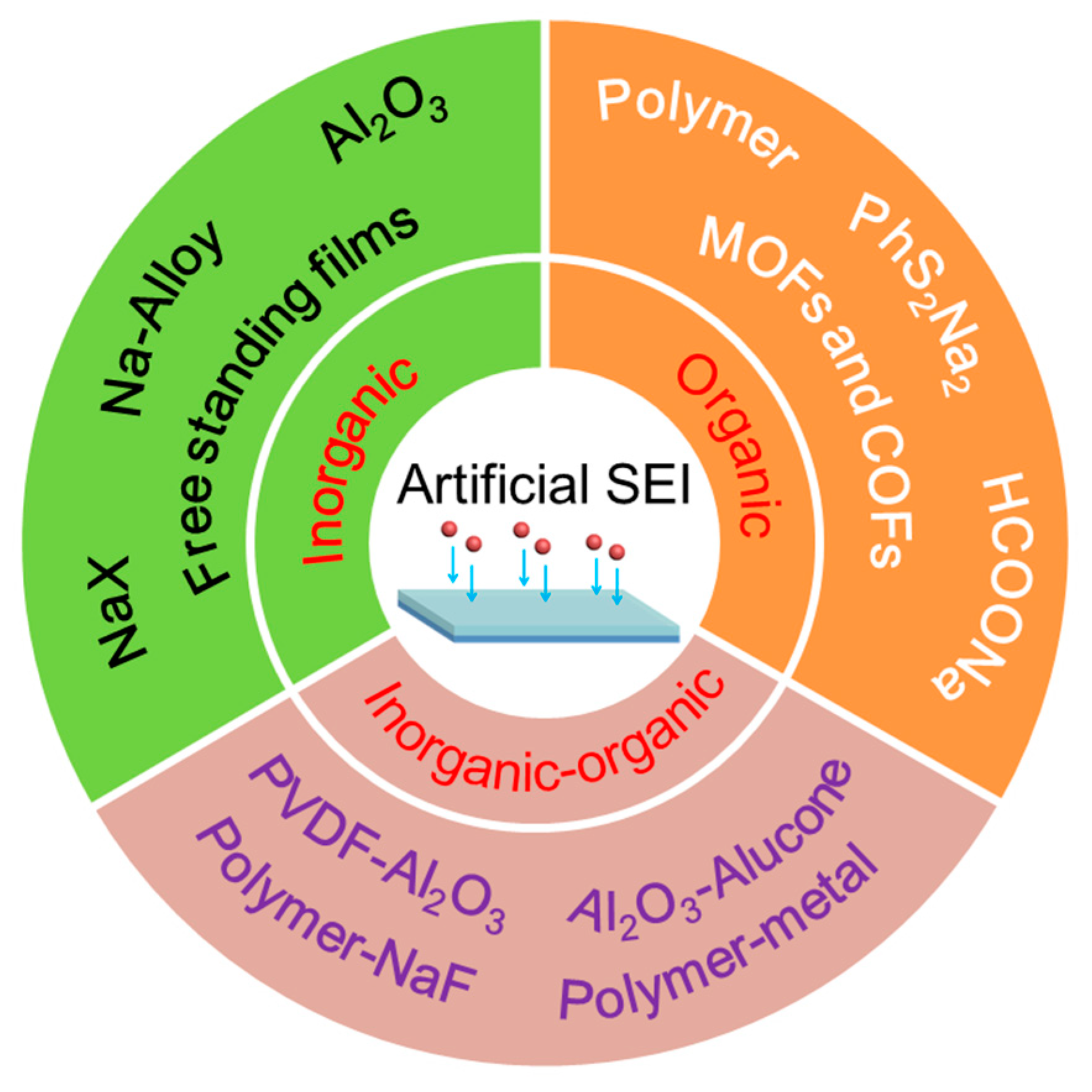
3. Advances in Artificial SEI Interphase
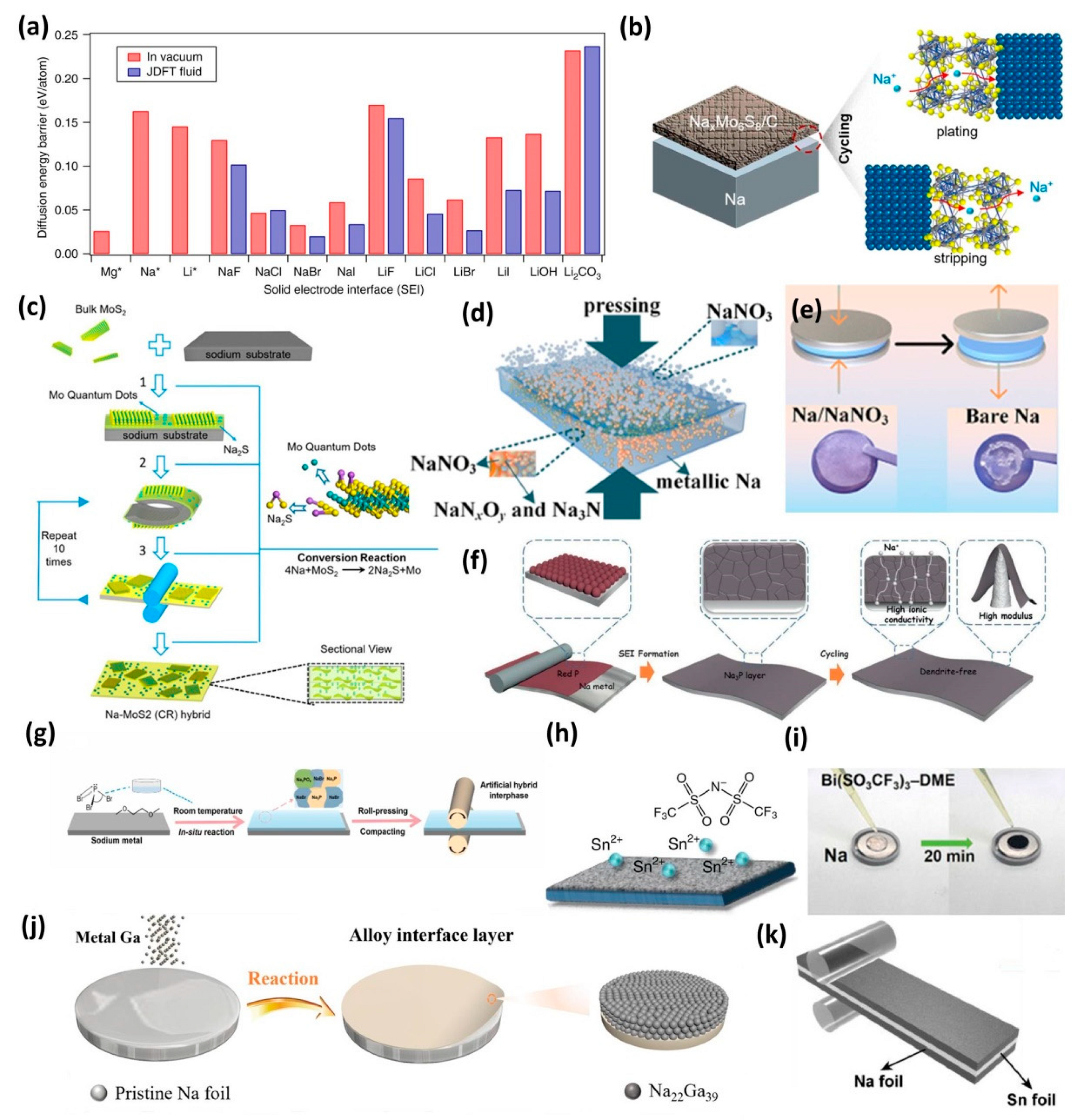
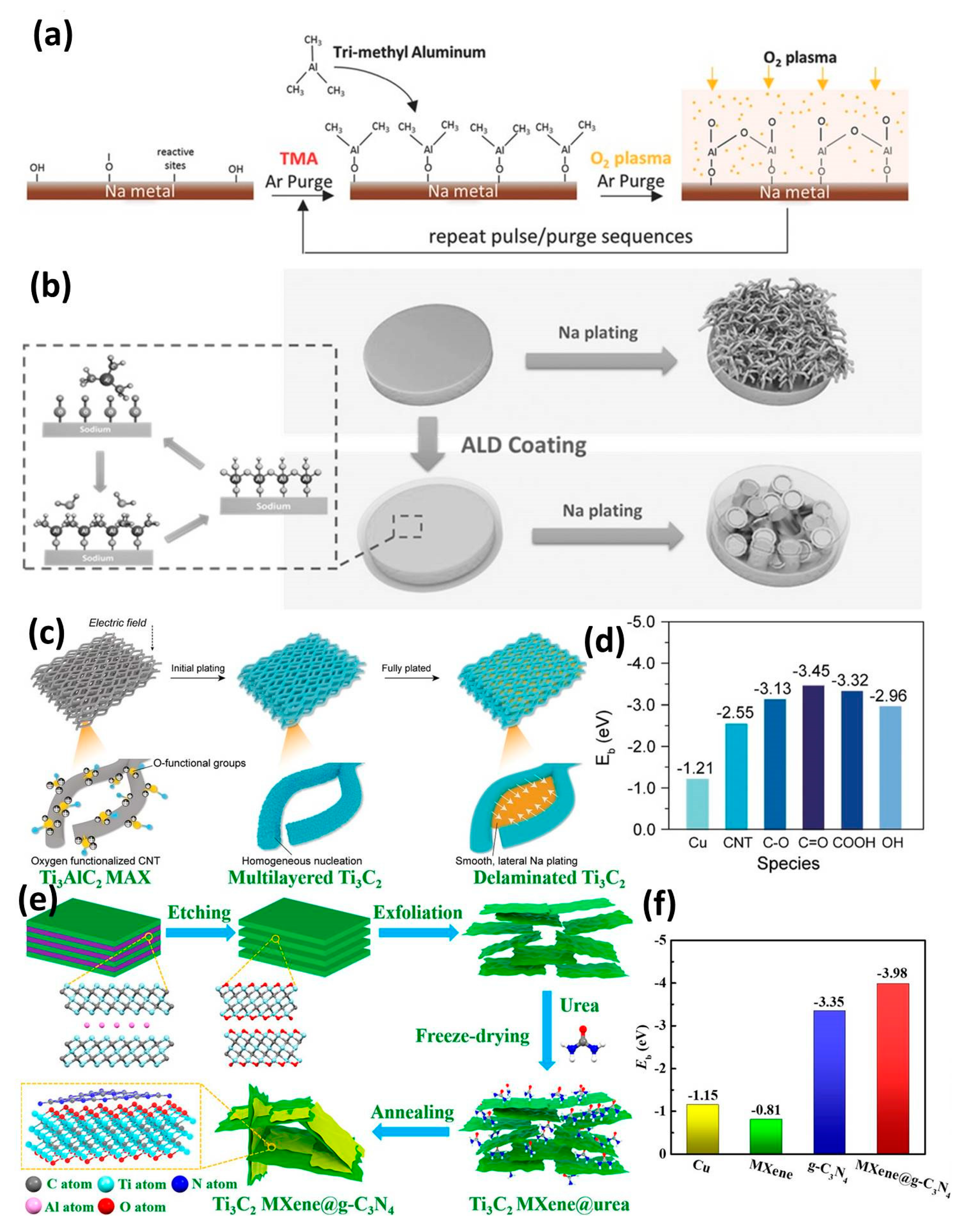
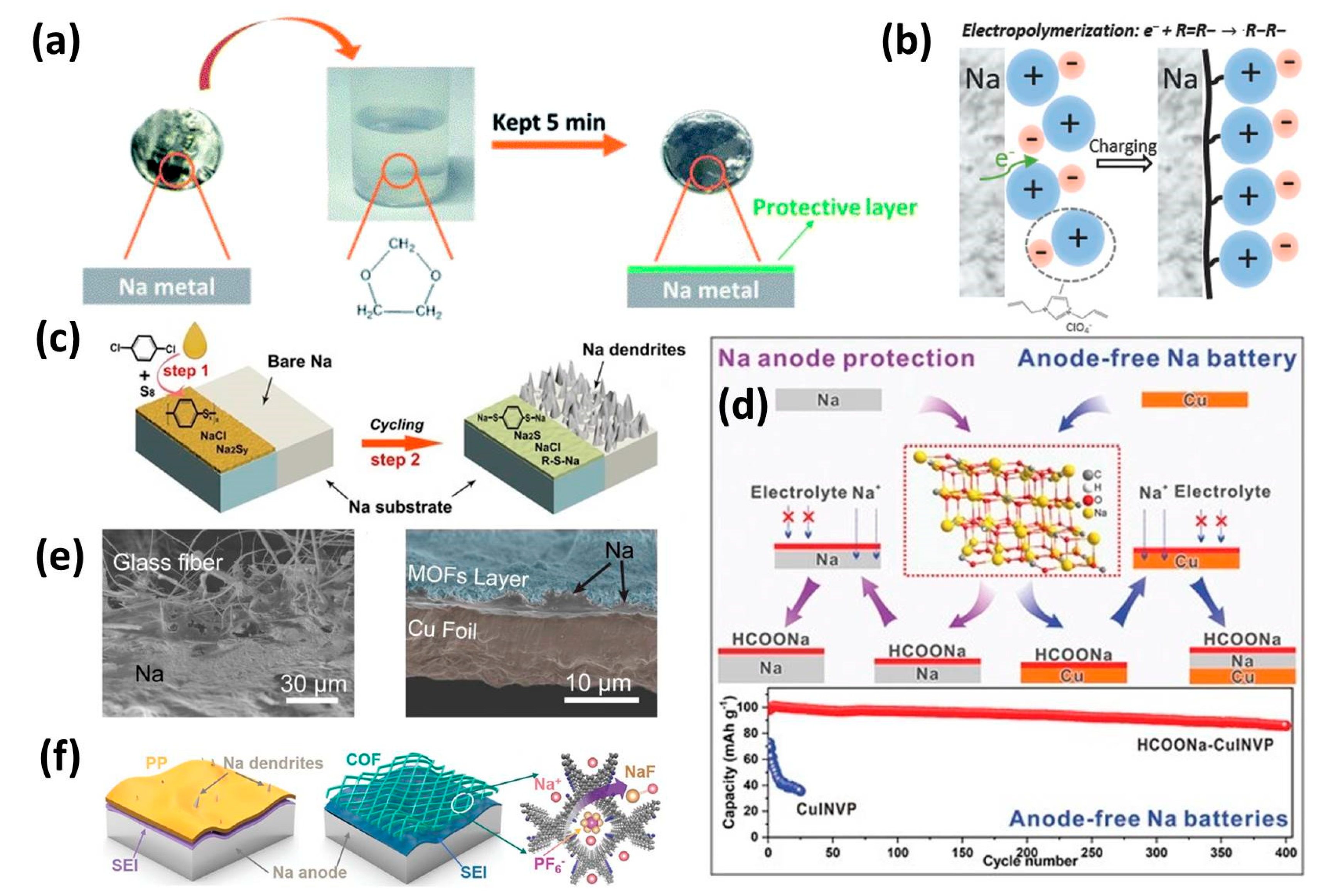
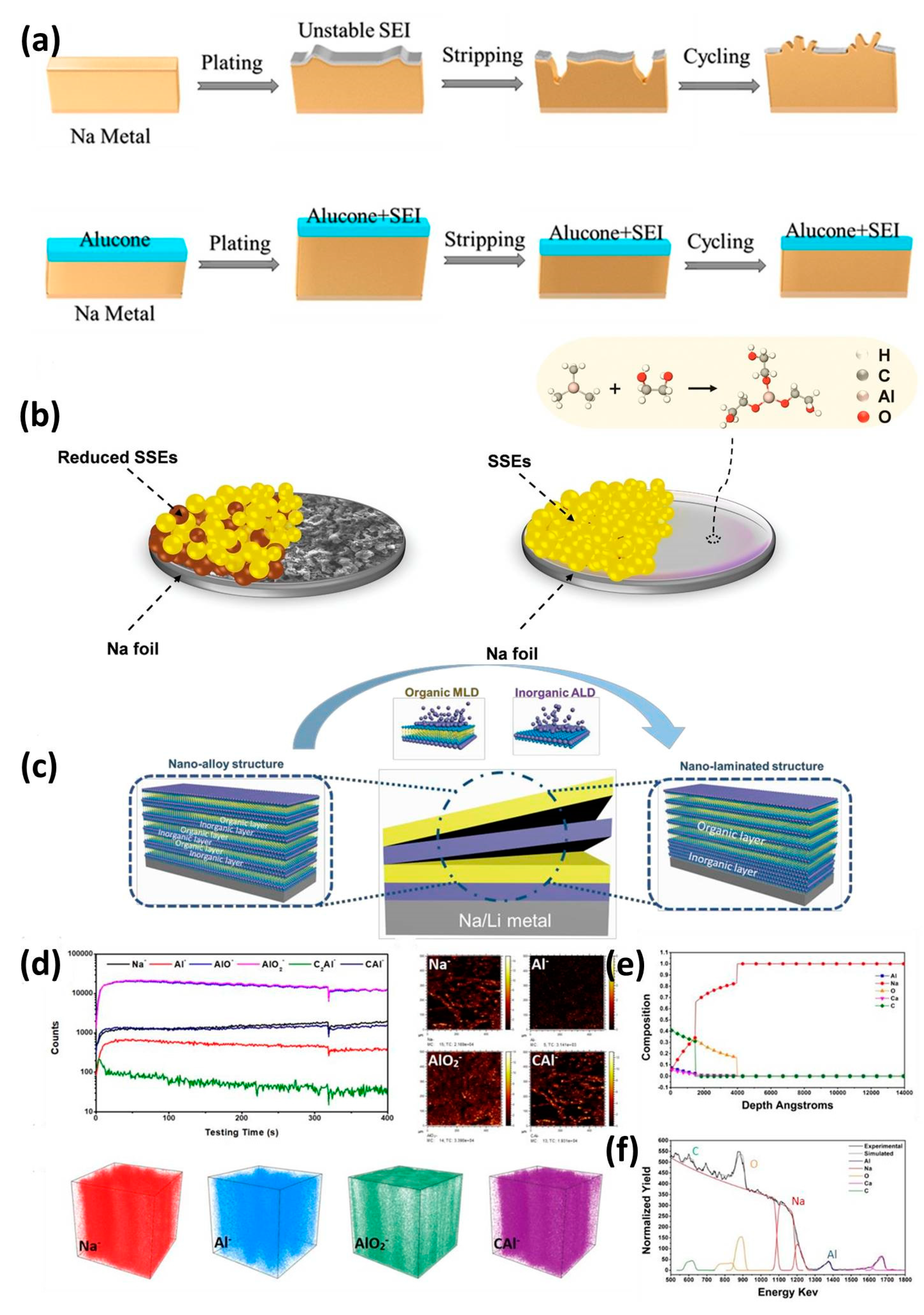
3.1. Inorganic Interphase
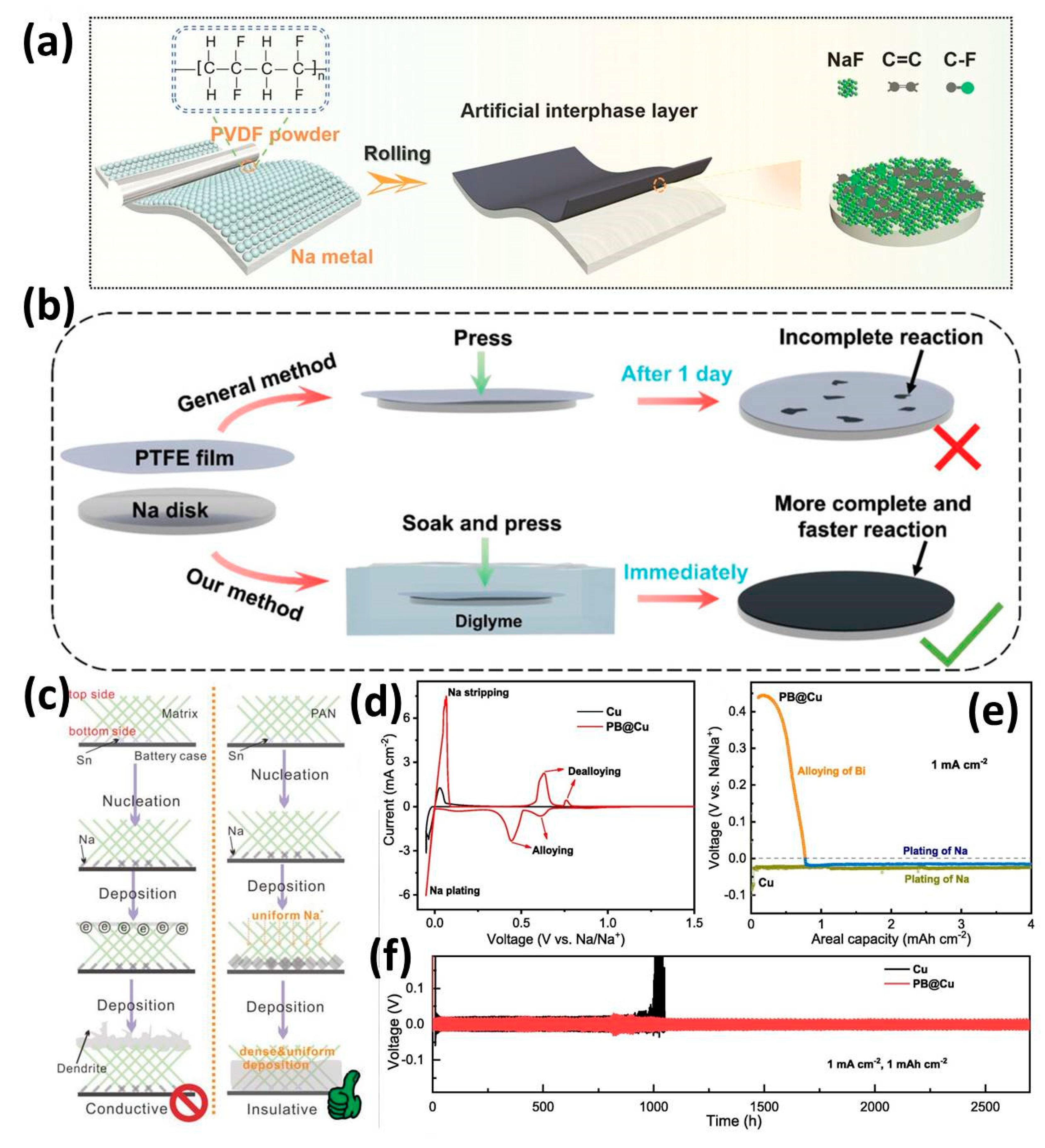
3.2. Organic Interphase
3.3. Hybrid Interphase
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hwang, J. -Y.; Myung, S. -T.; Sun, Y. -K. Sodium-ion batteries: present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K. M. How Comparable are sodium-ion batteries to lithium-ion counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Hirsh, H. S.; Li, Y. X.; Tan, D. H. S.; Zhang, M. H.; Zhao, E. Y.; Meng, Y. S. Sodium-ion batteries paving the way for grid energy storage. Adv. Energy Mater. 2020, 10, 2001274. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.; Jiang, Z.; Zeng, X.; Ji, J.; Li, Z.; Gao, X.; Sun, M.; Lin, Z.; Ling, M.; Zheng, J. C.; Liang, C. D. Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage. Energy Environ. Sci. 2019, 12, 1512–1533. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X. F.; Kuo, L. Y. Kaghazchi, P.; Sun, Q.; Liang, J. N.; Wang, B. Q.; Lushington, A.; Li, R. Y.; Zhang, H. M.; Sun, X. L. High capacity, dendrite-free growth, and minimum volume change Na metal anode. Small 2018, 14, 1703717. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xiong, P.; Maitra, U.; Langsdorf, D.; Yan, K.; Wang, C. Y.; Janek, J.; Schröder, D.; Wang, G. X. Design strategies to enable the efficient use of sodium metal anodes in high-energy batteries. Adv. Mater. 2019, 32, 1903891. [Google Scholar] [CrossRef] [PubMed]
- Cao, R. G.; Mishra, K.; Li, X. L.; Qian, J. F.; Engelhard M., H.; Bowden, M. E.; Han, S. H.; Mueller K., T.; Henderson, W. A.; Zhang, J. -G. Enabling room temperature sodium metal batteries. Nano Energy 2016, 30, 825–830. [Google Scholar] [CrossRef]
- Zheng, J. M.; Chen, S. R.; Zhao, W. G.; Song, J. H.; Mueller K., T.; Zhang, J. -G. Extremely stable sodium metal batteries enabled by localized high-concentration electrolytes. ACS Energy Lett. 2018, 3, 315–321. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Lee, J. M.; Kim, K.; Cha, A.; Kang, S. J.; Wi, T.; Kang, S. J.; Lee, H. -W.; Choi, N. -S. Fluoroethylene carbonate-based electrolyte with 1 M sodium bis (fluorosulfonyl) imide enables high-performance sodium metal electrodes. ACS Appl. Mater. Interfaces 2018, 10, 15270–15280. [Google Scholar] [CrossRef]
- Ma, B.; Bai, P. Fast charging limits of ideally stable metal anodes in liquid electrolytes. Adv. Energy Mater. 2022, 12, 2102967. [Google Scholar] [CrossRef]
- Ji, Y. Y.; Li, J. B.; Li, J. L. Recent development of electrolyte engineering for sodium metal batteries. Batteries 2022, 8, 157. [Google Scholar] [CrossRef]
- Zheng, X. Y.; Cao, Z.; Gu, Z. Y.; Huang, L. Q.; Sun, Z. H.; Zhao, T.; Yu, S. J.; Wu, X. L.; Huang, Y. H. Toward high temperature sodium metal batteries via regulating the electrolyte/electrode interfacial chemistries. ACS Energy Lett. 2022, 7, 2032–2042. [Google Scholar] [CrossRef]
- Xia, X. M.; Lv, X.; Yao, Y.; Chen, D.; Tang, F.; Liu, N.; Feng, Y. Z.; Rui, X. H.; Yu, Y. A sodiophilic VN interlayer stabilizing a Na metal anode. Nanoscale Horiz. 2022, 7, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Shi, H. D.; Yue, M.; Zhang, C. F.; Dong, Y. F.; Lu, P. F.; Zheng, S. H.; Huang, H. J.; Chen, J.; Wen, P. C.; Xu, Z. C.; Zheng, Q.; Li, X. F.; Yu, Y.; Wu, Z. S. 3D flexible, conductive and recyclable Ti3C2Tx MXene-melamine foam for high areal capacity and long lifetime alkali-metal anode. ACS Nano. 2020, 14, 8678. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bai, W. L.; Wang, H.; Kong, D. Z.; Xu, T. Q.; Zhang, Z. F.; Zang, J. H.; Wang, X. C.; Zhang, S.; Tian, Y. T.; Li, X. J.; Lee, C. S.; Wang, Y. 3D printed Au/rGO microlattice host for dendrite-free sodium metal anode. Energy Storage Mater. 2023, 55, 631–641. [Google Scholar] [CrossRef]
- Kang, T.; Sun, C.; Li, Y.; Song, T.; Guan, Z.; Tong, Z.; Nan, J.; Lee, C. S. Dendrite-free sodium metal anodes via solid electrolyte interphase engineering with a covalent organic framework separator. Adv. Energy Mater. 2023, 13, 2204083. [Google Scholar] [CrossRef]
- Li, M. H.; Lu, G. J.; Zheng, W. K.; Zhao, Q. N.; Li, Z. P.; Jiang, X. P.; Yang, Z. G.; Li, Z. Y.; Qu, B. H.; Xu, C. H. Multifunctionalized safe separator toward practical sodium-metal batteries with high-performance under high mass loading. Adv. Funct. Mater. 2023, 33, 2214759. [Google Scholar] [CrossRef]
- Wang, Y. X.; Dong, H.; Katyal, N.; Hao, H. C.; Liu, P. C.; Celio, H.; Henkelman, G.; Watt, J.; Mitlin, D. Sodium-antimony-telluride intermetallic allows sodium metal cycling at 100% depth of discharge and as anode-free metal battery. Adv. Mater. 2022, 34, 2106005. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X. B.; Huang, J. -Q.; Kaskel, S.; Chou, S. L.; Park, H. S.; Zhang, Q. Alloy anodes for rechargeable alkali-metal batteries: progress and challenge. ACS Matterials Lett. 2019, 1, 217. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, Y. Y.; Zhang, X. G.; Li, J. T.; Wang, X. Y.; Yan, J. W.; Wu, D. Y.; Zheng, M. S.; Dong, Q. F.; Mao, B. W. Stable Na plating and stripping electrochemistry promoted by in situ construction of an alloy-based sodiophilic interphase. Adv. Mater. 2019, 31, 1807495. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C. L.; Matios, E.; Luo, J. M.; Lu, X.; Zhang, Y. W.; Hu, X. F.; Li, W. Y. Enabling ultrahigh rate and capacity sodium metal anodes with lightweight solid additives. Energy Storage Mater. 2020, 32, 244–252. [Google Scholar] [CrossRef]
- Bao, C. Y.; Wang, B.; Liu, P.; Wu, H.; Zhou, Y.; Wang, D. L.; Liu, H. K.; Dou, S. X. Solid electrolyte interphases on sodium metal anodes. Adv. Funct. Mater. 2020, 30, 2004891. [Google Scholar] [CrossRef]
- Gao, L.; Chen, J.; Chen, Q. L.; Kong, X. Q. The chemical evolution of solid electrolyte interface in sodium metal batteries. Sci. Adv. 2022, 8, eabm4606. [Google Scholar] [CrossRef]
- Wang, T.; Hua, Y. B.; Xu, Z. W.; Yu, J. S. Recent advanced development of artificial interphase engineering for stable sodium metal anodes. Small 2022, 18, 2102250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C. L.; Lu, Y. X.; Yue, J. M.; Pan, D.; Qi, Y. R.; Hu, Y. S.; Chen, L. Q. Advanced Na metal anodes. J. Energy Chem. 2018, 27, 1584–1596. [Google Scholar] [CrossRef]
- Li, Z. P.; Zhu, K. J.; Liu, P.; Jiao, L. F. 3D confinement strategy for dendrite-free sodium metal batteries. Adv. Energy Mater. 2022, 12, 2100359. [Google Scholar] [CrossRef]
- Chen, X.; Bai, Y. K.; Shen, X.; Peng, H. -J.; Zhang, Q. Sodiophilicity/potassiophilicity chemistry in sodium/potassium metal anodes. J. Energy Chem. 2020, 51, 1–6. [Google Scholar] [CrossRef]
- Ma, B. Y.; Lee, Y. J.; Bai, P. Dynamic interfacial stability confirmed by microscopic optical operando experiments enables high-retention-rate anode-free Na metal full cells. Adv. Sci. 2021, 8, 2005006. [Google Scholar] [CrossRef]
- Wang, S. Y.; Cai, W. B.; Sun, Z. H.; Huang, F. Y.; Jie, Y. L.; Liu, Y.; Chen, Y. W.; Peng, B.; Cao, R. G.; Zhang, G. Q.; Jiao, S. H. Stable cycling of Na metal anodes in a carbonate electrolyte. Chem. Commun. 2019, 55, 14375–14378. [Google Scholar] [CrossRef]
- Mandl, M.; Becherer, J.; Kramer, D.; Mönig, R.; Diemant, T.; Diemant, T.; Behm, J.; Hahn, M.; Böse, O.; Danzer, M. A. Sodium metal anodes: deposition and dissolution behaviour and SEI formation. Electrochim. Acta 2020, 354, 136698. [Google Scholar] [CrossRef]
- Lee, B.; Paek, E.; Mitlin, D.; Lee, S. W. Sodium metal anodes: Emerging solutions to dendrite growth. Chem. Rev. 2019, 119, 5416–5460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Adair, K. R.; Sun, X. L. ; Recent developments and insights into the understanding of Na metal anodes for Na-metal batteries. Energy Environ. Sci. 2018, 11, 2673–2695. [Google Scholar] [CrossRef]
- Lei, D. N.; He, Y. B.; Huang, H. J.; Yuan, Y. F.; Zhong, G. M.; Zhao, Q.; Hao, X. G.; Zhang, D. F.; Lai, C.; Zhang, S. W.; Ma, J. B.; Wei, Y. P.; Yu, Q. P.; Lv, W.; Yu, Y.; Li, B. H.; Yang, Q. H.; Yang, Y.; Lu, J.; Kang, F. Y. Cross-linked beta alumina nanowires with compact gel polymer electrolyte coating for ultra-stable sodium metal battery. Nat. Commun. 2019, 10, 4244. [Google Scholar] [CrossRef] [PubMed]
- Liu, T. F.; Yang, X.K.; Nai, J. W.; Wang, Y.; Liu, Y. J.; Liu, C. T.; Tao, X. Y. Recent development of Na metal anodes: interphase engineering chemistries determine the electrochemical performance. Chem. Eng. J. 2021, 409, 127943. [Google Scholar] [CrossRef]
- Ji, Y. Y.; Sun, H. C.; Li, Z. B.; Ma, L.; Zhang, W. G.; Liu, Y. M.; Pan, L. K. Salt engineering toward stable cation migration of Na metal anodes. J. Mater. Chem. A 2022, 10, 25539–25545. [Google Scholar] [CrossRef]
- Liu, W.; Liu, P. C.; Mitlin, D. Review of emerging concepts in SEI analysis and artificial SEI membranes for lithium, sodium, and potassium metal battery anodes. Adv. Energy Mater. 2020, 10, 2002297. [Google Scholar] [CrossRef]
- Matios, E.; Wang, H.; Wang, C. L.; Li, W. Y. Enabling safe sodium metal batteries by solid electrolyte interphase engineering: a review. Ind. Eng. Chem. Res. 2019, 58, 9758–9780. [Google Scholar] [CrossRef]
- Xia, X. M.; Du, C. F.; Zhong, S. E.; Jiang, Y.; Yu, H.; Sun, W. P.; Pan, H. G.; Rui, X. H. , Yu, Y. Homogeneous Na deposition enabling high-energy Na-metal batteries. Adv. Funct. Mater. 2022, 32, 2110280. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, S.; Jo, C. S.; Lee, J. A review on recent approaches for designing the SEI layer on sodium metal anodes. Mater. Adv. 2020, 1, 3143–3166. [Google Scholar] [CrossRef]
- Liu, W.; Liu, P. C.; Mitlin, D. Tutorial review on structure-dendrite growth relations in metal battery anode supports. Chem. Soc. Rev. 2020, 49, 7284–7300. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, T. F.; Ai, X. P.; Liang, C. D. Aligning academia and industry for unified battery performance metrics. Nat. Commun. 2018, 9, 5262. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, Y. J.; Lee, M. J.; Han, J. H.; Lim, J.; Ryu, K.; Yoon, H.; Kim, B. H.; Kim, B. J.; Lee, S. W. A 3D hierarchical host with enhanced sodiophilicity enabling anode-free sodium-metal batteries. Adv. Mater. 2022, 34, 2109767. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, W.; Dong, L. B.; Shao, G. J.; Wang, G. X.; Peng, X. W. Hierarchical ZnO nanorod arrays grown on copper foam as an advanced three-dimensional skeleton for dendrite-free sodium metal anodes. Nano Energy 2021, 80, 105563. [Google Scholar] [CrossRef]
- Wang, C. L.; Wang, H.; Matios, E.; Hu, X. F.; Li, W. Y. A chemically engineered porous copper matrix with cylindrical core-shell skeleton as a stable host for metallic sodium anodes. Adv. Funct. Mater. 2018, 28, 1802282. [Google Scholar] [CrossRef]
- Ma, Y.; Gu, Y. T.; Yao, Y. Z.; Jin, H. D.; Zhao, X. H.; Yuan, X. T.; Lian, Y. L.; Qi, P. W.; Shah, R.; Peng, Y.; Deng, Z. Alkaliphilic Cu2O nanowires on copper foam for hosting Li/Na as ultrastable alkali-metal anodes. J. Mate. Chem. A 2019, 7, 20926–20935. [Google Scholar] [CrossRef]
- Mubarak, N.; Ihsan-Ul-Haq, M.; Huang, H.; Cui, J.; Yao, S. S.; Susca, A.; Wu, J. X.; Wang, M. Y.; Zhang, X. H.; Huang, B. L.; Kim, J. K. Metal organic framework-induced mesoporous carbon nanofibers as ultrastable Na metal anode host. J. Mate. Chem. A 2020, 8, 10269–10282. [Google Scholar] [CrossRef]
- Yue, L.; Qi, Y. R.; Niu, Y. B.; Bao, S. J.; Xu, M. W. Low-barrier, dendrite-free, and stable Na plating/stripping enabled by gradient sodiophilic carbon skeleton. Adv. Energy Mater. 2021, 11, 2102497. [Google Scholar] [CrossRef]
- Wang, Z. X.; Huang, Z. X.; Wang, H.; Li, W. D.; Wang, B. Y.; Xu, J. M.; Xu, T. T.; Zang, J. H.; Kong, D. Z.; Li, X. J.; Yang, H. Y.; Wang, Y. 3D-printed sodiophilic V2CTx/rGO-CNT MXene microgrid aerogel for stable Na metal anode with high areal capacity. ACS. Nano. 2022, 16, 9105–9116. [Google Scholar] [CrossRef]
- Shi, H. D.; Yue, M.; John Zhang, C. F.; Dong, Y. F.; Liu, P. F.; Zheng, S. H.; Huang, H. J.; Chen, J.; Wen, P. C.; Xu, Z. C.; Zheng, Q.; Li, X. F.; Yu, Y.; Wu, Z. -S. 3D flexible, conductive, and recyclable Ti3C2Tx MXene-melamine foam for high-areal-capacity and long-lifetime alkali-metal anode. ACS Nano 2020, 14, 8678–8688. [Google Scholar] [CrossRef]
- Chu, C. X.; Li, R.; Cai, F. P.; Bai, Z. C.; Wang, Y. X.; Xu, X.; Wang, N.; Yang, J.; Dou, S. X. Recent advanced skeletons in sodium metal anodes. Energy Environ. Sci. 2021, 14, 4318–4340. [Google Scholar] [CrossRef]
- Yang, C.; Xin, S.; Mai, L. Q.; You, Y. Materials design for high-safety sodium-ion battery. Adv. Energy Mater. 2021, 11, 2000974. [Google Scholar] [CrossRef]
- Wang, H.; Yu, D.; Kuang, C.; Cheng, L.; Li, W.; Feng, X.; Zhang, Z.; Zhang, X.; Zhang, Y. Alkali metal anodes for rechargeable batteries. Chem. 2019, 5, 313–338. [Google Scholar] [CrossRef]
- Zhang, X. Q.; Cheng X., B.; Zhang, Q. Advances in interfaces between Li metal anode and electrolyte. Adv. Mater. Interfaces 2018, 5, 1701097. [Google Scholar] [CrossRef]
- Xu, Z. X.; Yang, J.; Zhang, T.; Sun, L. M.; Nuli, Y. N.; Wang, J. L.; Hirano, S. Stable Na metal anode enabled by a reinforced multistructural SEI layer. Adv. Funct. Mater. 2019, 29, 1901924. [Google Scholar] [CrossRef]
- Fang, W.; Jiang, H.; Zheng, Y.; Zheng, H.; Liang, X.; Chen, C. H.; Xiang, H. F. A bilayer interface formed in high concentration electrolyte with SbF3 additive for long-cycle and high-rate sodium metal battery. J. Power Sources 2020, 455, 227956. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, X. H.; Xie, D.; Fu, X. X.; Lin, Z. H.; Yang, C. H.; Zhang, K. L.; Liu, M. L. A scalable approach for dendrite-free alkali metal anodes via room-temperature facile surface fluorination. ACS Appl. Mater. Interfaces 2019, 11, 4962–4968. [Google Scholar] [CrossRef]
- Zhou, X. F.; Liu, F. F.; Wang, Y. J.; Yao, Y.; Shao, Y.; Rui, X. H.; Wu, F. X.; Yu, Y. Heterogeneous interfacial layers derived from the in situ reaction of CoF2 nanoparticles with sodium metal for dendrite-free Na metal anodes. Adv. Energy Mater. 2022, 12, 2202323. [Google Scholar] [CrossRef]
- Miao, X. G.; Di, H. X.; Ge, X. L.; Zhao, D. Y.; Wang, P.; Wang, R. T.; Wang, C. X.; Yin, L. W. AlF3-modified anode-electrolyte interface for effective Na dendrites restriction in NASICON-based solid-state electrolyte. Energy Storage Mater. 2020, 30, 170–178. [Google Scholar] [CrossRef]
- Cheng, Y. F.; Yang, X. M.; Li, M. H.; Li, X. Y.; Lu, X. Z.; Wu, D. J.; Han, B.; Zhang, Q.; Zhu, Y. M.; Gu, M. Enabling ultrastable alkali metal anodes by artificial solid electrolyte interphase fluorination. Nano Lett. 2022, 22, 4347–4353. [Google Scholar] [CrossRef]
- Xie, Y. Y.; Hu, J. X.; Zhang, L. Y.; Wang, A. N.; Zheng, J. Q.; Li, H. X.; Lai, Y. Q.; Zhang, Z. A. Stabilizing Na metal anode with NaF interface on spent cathode carbon from aluminum electrolysis. Chem. Commun. 2021, 57, 7561–7564. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X. Y.; Fu, H. Y.; Hu, C. C.; Xu, H.; Huang, Y.; Wen, J. Y.; Sun, H. B.; Luo, W.; Huang, Y. H. Toward a stable sodium metal anode in carbonate electrolyte: a compact, inorganic alloy interface. J. Phys. Chem. Lett. 2019, 10, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Eng, A. Y. S.; Wang, Y.; Nguyen, D. T.; Ng, M. F.; Seh, Z. W. An artificial metal-alloy interphase for high-rate and long-life sodium-sulfur batteries. Energy Storage Mater. 2020, 19, 1–8. [Google Scholar] [CrossRef]
- Lu, Q. Q.; Omar, A.; Hantusch, M.; Oswald, S.; Ding, L.; Nielsch, K.; Mikhailova, D. Dendrite-free and corrosion-resistant sodium metal anode for enhanced sodium batteries. Appl. Surf. Sci. 2022, 600, 154168. [Google Scholar] [CrossRef]
- Chen, Q. W.; He, H.; Hou, Z.; Zhuang, W. M.; Zhang, T. X.; Sun, Z. Z.; Huang, L. M. Building an artificial solid electrolyte interphase with high-uniformity and fast ion diffusion for ultralong-life sodium metal anodes. J. Mate. Chem. A 2020, 8, 16232–16237. [Google Scholar] [CrossRef]
- Tian, H. J.; Shao, H. Z.; Chen, Y.; Fang, X. Q.; Xiong, P.; Sun, B.; Nottenc, P. H. L.; Wang, G. X. Ultra-stable sodium metal-iodine batteries enabled by an in-situ solid electrolyte interphase. Nano Energy 2019, 57, 692–702. [Google Scholar] [CrossRef]
- Choudhury, S.; Wei, S. Y.; Ozhabes, Y.; Gunceler, D.; Zachman, M. J.; Tu, Z. Y.; Shin, J. H.; Nath, P.; Agrawall, A.; Kourkoutis, L. F.; Arias, T. A.; Archer, L. A. Designing solid-liquid interphases for sodium batteries. Nat. Commun. 2017, 8, 898. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liang, J. W.; Sun, Q.; Goncharova, L. V.; Wang, J. W.; Wang, C. H.; Adair, K. R.; Li, X. N.; Zhao, F. P.; Sun, Y. P.; Li, R. Y.; Sun, X. L. In-situ formation of highly controllable and stable Na3PS4 as protective layer for Na metal anode. J. Mate. Chem. A 2019, 47, 4119–4125. [Google Scholar] [CrossRef]
- Lu, K.; Gao, S. Y.; Li, G. S.; Kaelin, J.; Zhang, Z. C.; Cheng, Y. W. Regulating interfacial Na-ion flux via artificial layers with fast ionic conductivity for stable and high-rate Na metal batteries. ACS Materials Lett. 2019, 1, 303–309. [Google Scholar] [CrossRef]
- Zhang, D.; Li, B.; Wang, S.; Yang, S. B. Simultaneous formation of artificial SEI film and 3D host for stable metallic sodium anodes. ACS Appl. Mater. Interfaces 2017, 9, 40265–40272. [Google Scholar] [CrossRef]
- Wang, X. C.; Fu, L.; Zhan, R. M.; Wang, L. Y.; Li, G. C.; Wan, M. T.; Wu, X. L.; Seh, Z. W.; Wang, L.; Sun, Y. M. Addressing the low solubility of a solid electrolyte interphase stabilizer in an electrolyte by composite battery anode design. ACS Appl. Mater. Interfaces 2021, 13, 13354–13361. [Google Scholar] [CrossRef]
- Shi, P. C.; Zhang, S. P.; Lu, G. X.; Wang, L. F.; Jiang, Y.; Liu, F. F.; Yao, Y.; Yang, H.; Ma, M. Z.; Ye, S. F.; Tao, X. Y.; Feng, Y. Z.; Wu, X. J.; Rui, X. H.; Yu, Y. Red phosphorous-derived protective layers with high ionic conductivity and mechanical strength on dendritefree sodium and potassium metal anodes. Adv. Energy Mater. 2021, 11, 2003381. [Google Scholar] [CrossRef]
- Yang, H.; He, F. X.; Li, M. H.; Huang, F. Y.; Chen, Z. H.; Shi, P. C.; Liu, F. F.; Jiang, Y.; He, L. X.; Gu, M.; Yu, Y. Design principles of sodium/potassium protection layer for high-power high-energy sodium/potassium-metal batteries in carbonate electrolytes: a case study of Na2Te/K2Te. Adv. Mater. 2021, 33, 2106353. [Google Scholar] [CrossRef]
- Luo, Z.; Tao, S. S.; Tian, Y.; Xu, L. Q.; Wang, Y.; Cao, X. Y.; Wang, Y. P.; Deng, W. T.; Zou, G. Q.; Liu, H.; Hou, H. S.; Ji, X. B. Robust artificial interlayer for columnar sodium metal anode. Nano Energy 2022, 97, 107203. [Google Scholar] [CrossRef]
- Zhang, Y. J.; Huang, Z. Y.; Liu, H. X.; Chen, H. F.; Wang, Y. Y.; Wu, K.; Wang, G. Y.; Wu, C. Amorphous phosphatized hybrid interfacial layer for dendrite-free sodium deposition. J. Power Sources 2023, 569, 233023. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Y.; Ling, F. X.; Lu, G. X.; Huang, F. Y.; Tao, X. Y.; Wu, S. F.; Cheng, X. L.; Liu, F. F.; Li, D. J.; Yang, H.; Yao, Y.; Shi, P. C.; Chen, Q. W.; Rui, X. H.; Yu, Y. Artificial heterogeneous interphase layer with boosted ion affinity and diffusion for Na/K-metal batteries. Adv. Mater. 2022, 34, 2109439. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. M.; Xu, S. T.; Tang, F.; Yao, Y.; Wang, L. F.; Liu, L.; He, S. N.; Yang, Y. X.; Sun, W. P.; Xu, C.; Feng, Y. Z.; Pan, H. G.; Rui, X. H.; Yu, Y. A multifunctional interphase layer enabling superior sodium-metal batteries under ambient temperature and -40oC. Adv. Mater. 2023, 35, 2209511. [Google Scholar] [CrossRef]
- Xia, X. M.; Yang, Y.; Chen, K. Z.; Xu, S. T.; Tang, F.; Liu, L.; Xu, C.; Rui, X. H. Enhancing interfacial strength and wettability for wide-temperature sodium metal batteries. Small. 2023, 19, 2300907. [Google Scholar] [CrossRef]
- Li, D. J.; Sun, Y. J.; Li, M. H.; Cheng, X. L.; Yao, Y.; Huang, F. Y.; Jiao, S. H.; Gu, M.; Rui, X. H.; Ali, Z.; Ma, C.; Wu, Z. -S. Yu, Y. Rational design of an artificial SEI: alloy/solid electrolyte hybrid layer for a highly reversible Na and K metal anode. ACS Nano. 2022, 16, 16966–16975. [Google Scholar] [CrossRef]
- Chu, F. L.; Hu, J. L.; Tian, J.; Zhou, X. J.; Li, Z.; Li, C. L. In situ plating of porous Mg network layer to reinforce anode dendrite suppression in Li-metal batteries. ACS Appl. Mater. Interfaces 2018, 10, 12678–12689. [Google Scholar] [CrossRef]
- Tu, Z. Y.; Choudhury, S.; Zachman, M. J.; Wei, S. Y.; Zhang, K. H.; Kourkoutis, L. F.; Archer, L. A. Fast ion transport at solid-solid interfaces in hybrid battery anodes. Nature Energy 2018, 3, 310–316. [Google Scholar] [CrossRef]
- Ma, M. Y.; Lu, Y.; Yan, Z. H.; Chen, J. In situ synthesis of Bi layer on Na metal anode for fast nterfacial transport in Na-O2 batteries. Batteries & Supercaps 2019, 2, 663–667. [Google Scholar]
- Peng, Z.; Song, J. H.; Huai, L. Y.; Jia, H. P.; Xiao, B. W.; Zou, L. F.; Zhu, G. M.; Martinez, A.; Roy, S.; Murugesan, V.; Lee, H.; Ren, X. D.; Li, Q. Y.; Liu, B.; Li, X. L.; Wang, D. Y.; Wu, X.; Zhang, J. G. Enhanced stability of Li metal anodes by synergetic control of nucleation and the solid electrolyte interphase. Adv. Energy Mater. 2019, 9, 1901764. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q. Z.; Lei, Y. Y.; Zhou, D. L.; Wu, W. W.; Wu, X. H. Stabilizing sodium metal anode by in-situ formed Ag metal layer. J. Alloy Compd. 2022, 926, 166850. [Google Scholar] [CrossRef]
- Liu, C. Y.; Xie, Y. Y.; Li, H. X.; Xu, J. Y.; Zhang, Z. A. In situ construction of sodiophilic alloy interface enabled homogenous Na nucleation and deposition for sodium metal anode. J. Electrochem. Soc. 2022, 169, 080521. [Google Scholar] [CrossRef]
- Lv, X.; Tang, F.; Yao, Y.; Xu, C.; Chen, D.; Liu, L.; Feng, Y. Z.; Rui, X. H.; Yu, Y. Sodium-gallium alloy layer for fast and reversible sodium deposition. SusMat 2022, 2, 699–707. [Google Scholar] [CrossRef]
- Li, G. C.; Yang, Q. P.; Chao, J.; Zhang, B.; Wan, M. T.; Liu, X. X.; Mao, E.; Wang, L.; Yang, H.; Seh, Z. W.; Jiang, J. J.; Sun, Y. M. Enhanced processability and electrochemical cyclability of metallic sodium at elevated temperature using sodium alloy composite. Energy Storage Mater. 2021, 35, 310–316. [Google Scholar] [CrossRef]
- Luo, W.; Lin, C. -F.; Zhao, O.; Noked, M.; Zhang, Y.; Rubloff, G. W.; Hu, L. B. Ultrathin surface coating enables the stable sodium metal anode. Adv. Energy Mater. 2017, 7, 1601526. [Google Scholar] [CrossRef]
- Zhao, Y.; Goncharova, L. V.; Lushington, A.; Sun, Q.; Yadegari, H.; Wang, B. Q.; Xiao, W.; Li, R. Y.; Sun, X. L. Superior stable and long life sodium metal anodes achieved by atomic layer deposition. Adv. Mater. 2017, 29, 1606663. [Google Scholar] [CrossRef]
- Jin, E.; Tantratian, K.; Zhao, C.; Codirenzi, A.; Goncharova, L. V.; Wang, C. H.; Yang, F. P.; Wang, Y. J.; Pirayesh, P.; Guo, J. H.; Chen, L.; Sun, X. L.; Zhao, Y. Ionic conductive and highly-stable interface for alkali metal anodes. Small 2022, 18, 2203045. [Google Scholar] [CrossRef]
- Sullivan, M.; Tang, P.; Meng, X. B. Atomic and molecular layer deposition as surface engineering techniques for emerging alkali metal rechargeable batteries. Molecules 2022, 27, 6170. [Google Scholar] [CrossRef]
- Ye, L.; Liao, M.; Zhao, T. C.; Sun, H.; Zhao, Y.; Sun, X. M.; Wang, B. J.; Peng, H. S. A sodiophilic interphase-mediated, dendrite-free anode with ultrahigh specific capacity for sodium-metal batteries. Angew. Chem. Int. Ed. 2019, 131, 2–9. [Google Scholar] [CrossRef]
- Liu, P.; Yi, H. T.; Zheng, S. Y.; Li, Z. P.; Zhu, K. J.; Sun, Z. Q.; Jin, T.; Jiao, L. F. Regulating deposition behavior of sodium ions for dendrite-free sodium-metal anode. Adv. Energy Mater. 2021, 11, 2101976. [Google Scholar] [CrossRef]
- Zhang, S. J.; You, J. H.; He, Z. W.; Zhong, J. J.; Zhang, P. F.; Yin, Z. W.; Pan, F.; Ling, M.; Zhang, B. K.; Lin, Z. Scalable lithiophilic/sodiophilic porous buffer layer fabrication enables uniform nucleation and growth for lithium/sodium metal batteries. Adv. Funct. Mater. 2022, 32, 2200967. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C. L.; Matios, E.; Li, W. Y. ; Critical role of ultrathin graphene films with tunable thickness in enabling highly stable sodium metal anodes. Nano Lett. 2017, 17, 6808–6815. [Google Scholar] [CrossRef] [PubMed]
- Tian, H. Z.; Seh, Z. W.; Yan, K.; Fu, Z. H.; Tang, P.; Lu, Y. Y.; Zhang, R. F.; Legut, D.; Cui, Y.; Zhang, Q. F. Theoretical investigation of 2D layered materials as protective films for lithium and sodium metal anodes. Adv. Energy Mater. 2017, 7, 1602528. [Google Scholar] [CrossRef]
- He, X.; Jin, S.; Miao, L. C.; Cai, Y. C.; Hou, Y. P.; Li, H. X.; Zhang, K.; Yan, Z. H.; Chen, J. A 3D hydroxylated MXene/carbon nanotubes composite as a scaffold for dendrite-free sodium-metal Electrodes. Angew Chem. Int. Ed. 2020, 59, 16705–16711. [Google Scholar] [CrossRef]
- Luo, J. M.; Wang, C. L.; Wang, H.; Hu, X. F.; Matios, E.; Lu, X.; Zhang, W. K.; Tao, X. Y.; Li, W. Y. Pillared MXene with ultralarge interlayer spacing as a stable matrix for high performance sodium metal anodes. Adv. Funct. Mater. 2019, 29, 1805946. [Google Scholar] [CrossRef]
- Jiang, H. Y.; Lin, X. H.; Wei, C. L.; Zhang, Y. C.; Feng, J. K.; Tian, X. L. Sodiophilic Mg2+-decorated Ti3C2 MXene for dendrite-free sodium metal batteries with carbonate-based electrolytes. Small 2022, 18, 2107637. [Google Scholar] [CrossRef]
- Bao, C. Y.; Wang, J. H.; Wang, B.; Sun, J. G.; He, L. C.; Pan, Z. H.; Jiang, Y. P.; Wang, D. L.; Liu, X. M.; Dou, S. X.; Wang, J. 3D sodiophilic Ti3C2 MXene@g-C3N4 hetero-interphase raises the stability of sodium metal anodes. ACS Nano 2022, 16, 17197–17209. [Google Scholar] [CrossRef]
- Shi, R. J.; Shen, Z.; Yue, Q. Q.; Zhao, Y. Advances in functional organic material-based interfacial engineering on metal anodes for rechargeable secondary batteries. Nanoscale. 2023. [Google Scholar] [CrossRef]
- Li, N. W.; Shi, Y.; Yin, Y. X.; Zeng, X. X.; Li, J. Y.; Li, C. J.; Wan, L. J.; Wen, R.; Guo, Y. G. A flexible solid electrolyte interphase layer for long-life lithium metal anodes. Angew Chem. Int. Ed. 2018, 130, 1521–1525. [Google Scholar] [CrossRef]
- Ma, J. L.; Yin, Y. B.; Liu, T.; Zhang, X. B.; Yan, J. M.; Jiang, Q. Suppressing sodium dendrites by multifunctional polyvinylidene fluoride (PVDF) interlayers with nonthrough pores and high flux/affinity of sodium ions toward long cycle life sodium oxygen-batteries. Adv. Funct. Mater. 2018, 28, 1703931. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, W. H.; Yu, Y. K.; Zhao, X. X.; Chen, Q. W.; Zhao, L. F.; Di, Q.; Ju, H. X.; Quan, Z. W. Poly(vinylidene difluoride) coating on Cu current collector for high-performance Na metal anode. Energy Storage Mater. 2020, 24, 588–593. [Google Scholar] [CrossRef]
- Shuai, Y.; Lou, J.; Pei, X. L.; Su, C. Q.; Ye, X. S.; Zhang, L. M.; Wang, Y.; Xu, Z. X.; Gao, P. P.; He, S. J.; Wang, Z. L.; Chen, K.H. Constructing an in situ polymer electrolyte and a Na-rich artificial SEI layer toward practical solid-state Na metal batteries. ACS Appl. Mater. Interfaces 2022, 14, 45382–45391. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q. Q.; Omar, A.; Ding, L.; Oswald, S.; Hantusch, M.; Giebeler, L.; Nielsch, K.; Mikhailova, D. A facile method to stabilize sodium metal anodes towards high-performance sodium batteries. J. Mater. Chem. A 2021, 9, 9038–9047. [Google Scholar] [CrossRef]
- Wei, S. Y.; Choudhury, S.; Xu, J.; Nath, P.; Tu, Z. Y.; Archer, L. A. Highly stable sodium batteries enabled by functional ionic polymer membranes. Adv. Mater. 2017, 29, 1605512. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, G.Y.; Liu, X.; Guo, B. K.; Xu, G.; Huang, Z. Y.; Wu, M. H.; Liu, H, K.; Dou, S. X.; Wu, C. Dendrite-free sodium metal anodes enabled by a sodium benzenedithiolate-rich protection layer. Angew Chem. Int. Ed. 2020, 132, 6658–6662. [Google Scholar] [CrossRef]
- Wang, C. Z.; Zheng, Y.; Chen, Z. N.; Zhang, R. R.; He, W.; Li, K. X.; Yan, S.; Cui, J. Q.; Fang, X. L.; Yan, J. W.; Xu, G.; Peng, D. L.; Ren, B.; Zheng, N. F. ; Robust anode-free sodium metal batteries enabled by artificial sodium formate interface. Adv. Energy Mater. 2023, 13, 2204125. [Google Scholar] [CrossRef]
- Qian, J.; Li, Y.; Zhang, M. L.; Luo, R.; Wang, F. J.; Ye, Y. S.; Xing, Y.; Li, W. L.; Qu, W. J.; Wang, L. L.; Li, L.; Li, Y. J.; Wu, F.; Chen, R. J. Protecting lithium/sodium metal anode with metal-organic framework based compact and robust shield. Nano Energy 2019, 60, 866–874. [Google Scholar] [CrossRef]
- Zhu, M. Q.; Li, S. M.; Li, B.; Gong, Y. J.; Du, Z. G.; Yang, S. B. Homogeneous guiding deposition of sodium through main group II metals toward dendrite-free sodium anodes. Sci. Adv. 2019, 5, eaau6264. [Google Scholar] [CrossRef]
- Miao, X. G.; Wang, P.; Sun, R.; Li, J. F.; Wang, Z. X.; Zhang, T.; Wang, R. T.; Li, Z. Q.; Bai, Y. J.; Hao, R.; Yin, L. W. Liquid metal-organic frameworks in-situ derived interlayer for high-performance solid-state Na-metal batteries. Adv. Energy Mater. 2021, 11, 2102396. [Google Scholar] [CrossRef]
- Hu, Y. Y.; Han, R. X.; Mei, L.; Liu, J. L.; Sun, J. C.; Yang, K.; Zhao, J. W. Design principles of MOF-related materials for highly stable metal anodes in secondary metal-based batteries. Mater. Today Energy 2021, 19, 100608. [Google Scholar] [CrossRef]
- He, Y. B.; Qiao, Y.; Chang, Z.; Zhou, H. S. The potential of electrolyte filled MOF membranes as ionic sieves in rechargeable batteries. Energy Environ. Sci. 2019, 12, 2327–2344. [Google Scholar] [CrossRef]
- Kim, Y. J.; Lee, H.; Noh, H.; Lee, J.; Kim, S.; Ryou, M. H.; Lee, Y. M.; Kim, H. T. Enhancing the cycling stability of sodium metal electrode by building an inorganic/organic composite protective layer. ACS Appl. Mater. Interfaces 2017, 9, 6000–6006. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Y.; Jie, Y. L.; Sun, Z. H.; Cai, W. B.; Chen, Y. W.; Huang, F. Y.; Liu, Y.; Li, X. P.; Du, R. Q.; Cao, R. G.; Zhang, G. Q.; Jiao, S. H. An implantable artificial protective layer enables stable sodium metal anodes. ACS Appl. Energy Mater. 2020, 3, 8688–8694. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, W. H.; Chen, Q. W.; Yu, Y. K.; Zhao, X. X.; Tang, M.; Zheng, Y. Y.; Quan, Z. W. Hybrid protective layer for stable sodium metal anodes at high utilization. ACS Appl. Mater. Interfaces 2019, 11, 37693–37700. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Tang, F.; Xu, S. T.; Yao, Y.; Yuan, Z. S.; Liu, L.; He, S. N.; Yang, Y. X.; Sun, W. P.; Pan, H. G.; Rui, X. H.; Yu, Y. Construction of inorganic/organic hybrid layer for stable Na metal anode operated under wide temperatures. Small 2023, 19, 2300215. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y. Y.; Liu, C. Y.; Zheng, J. Q.; Li, H. X.; Zhang, L. Y.; Zhang, Z. A. NaF-rich protective layer on PTFE coating microcrystalline graphite for highly stable Na metal anodes. Nano Res. 2023, 16, 2436–2444. [Google Scholar] [CrossRef]
- Xu, M. Y.; Li, Y.; Ihsan-Ul-Haq, M.; Mubarak, N.; Liu, Z. J.; Wu, J. X.; Luo, Z. T.; Kim, J. K. NaF-rich solid electrolyte interphase for dendrite-free sodium metal batteries. Energy Storage Mater. 2022, 44, 477–486. [Google Scholar] [CrossRef]
- Tai, Z. X.; Liu, Y. J.; Yu, Z. P.; Lu, Z. Y.; Bondarchuk, O.; Peng, Z. J.; Liu, L. F. Non-collapsing 3D solid-electrolyte interphase for high-rate rechargeable sodium metal batteries. Nano Energy 2022, 94, 106947. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, X. K.; Wang, J. C.; Zheng, J. L.; Yue, K.; Liu, T. F.; Wang, Y.; Nai, J. W.; Liu, Y. J.; Tao, X. Y. Rapidly constructing sodium fluoride-rich interface by pressure and diglyme-induced defluorination reaction for stable sodium metal anode. Small 2023, 19, 2207540. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q. W.; Hou, Z.; Sun, Z. Z.; Pu, Y. Y.; Jiang, Y. B.; Zhao, Y.; He, H.; Zhang, T. X.; Huang, L. M. Polymer-inorganic composite protective layer for stable Na metal anodes. ACS Appl. Energy Mater. 2020, 3, 2900–2906. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C. L.; Matios, E.; Luo, J. M.; Hu, X. F.; Yue, Q.; Kang, Y. J.; Li, W. Y. Sodium deposition with a controlled location and orientation for dendrite-free sodium metal batteries. Adv. Energy Mater. 2020, 10, 2002308. [Google Scholar] [CrossRef]
- Zhang, J. L.; Wang, S.; Wang, W. H.; Li, B. H. Stabilizing sodium metal anode through facile construction of organic-metal interface. J. Energy Chem. 2022, 66, 133–139. [Google Scholar] [CrossRef]
- Zhao, Y.; Goncharova, L. V.; Zhang, Q.; Kaghazchi, P.; Sun, Q.; Lushington, A.; Wang, B. Q.; Li, R. Y.; Sun, X. L. Inorganic-organic coating via molecular layer deposition enables long life sodium metal anode. Nano Lett. 2017, 17, 5653–5659. [Google Scholar] [CrossRef]
- Zhang, S. M.; Zhao, Y.; Zhao, F. P.; Zhang, L.; Wang, C. H.; Li, X. N.; Liang, J. W.; Li, W. H.; Sun, Q.; Yu, C.; Luo, J.; Doyle-Davis, K.; Li, R. Y.; Sham, T. K.; Sun, X. L. Gradiently sodiated alucone as an interfacial stabilizing strategy for solid-state Na metal batteries. Adv. Funct. Mater. 2020, 30, 2001118. [Google Scholar] [CrossRef]
- Pirayesh, P.; Tantratian, K.; Amirmaleki, M.; Yang, F. P.; Jin, E. Z.; Wang, Y. J.; Goncharova, L. V.; Guo, J. H.; Filleter, T.; Chen, L.; Zhao, Y. From nano-alloy to nano-laminated interfaces for highly stable alkali metal anodes. Adv. Mater. 2023, 35, 2301414. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




