Submitted:
22 May 2023
Posted:
23 May 2023
You are already at the latest version
Abstract
Keywords:
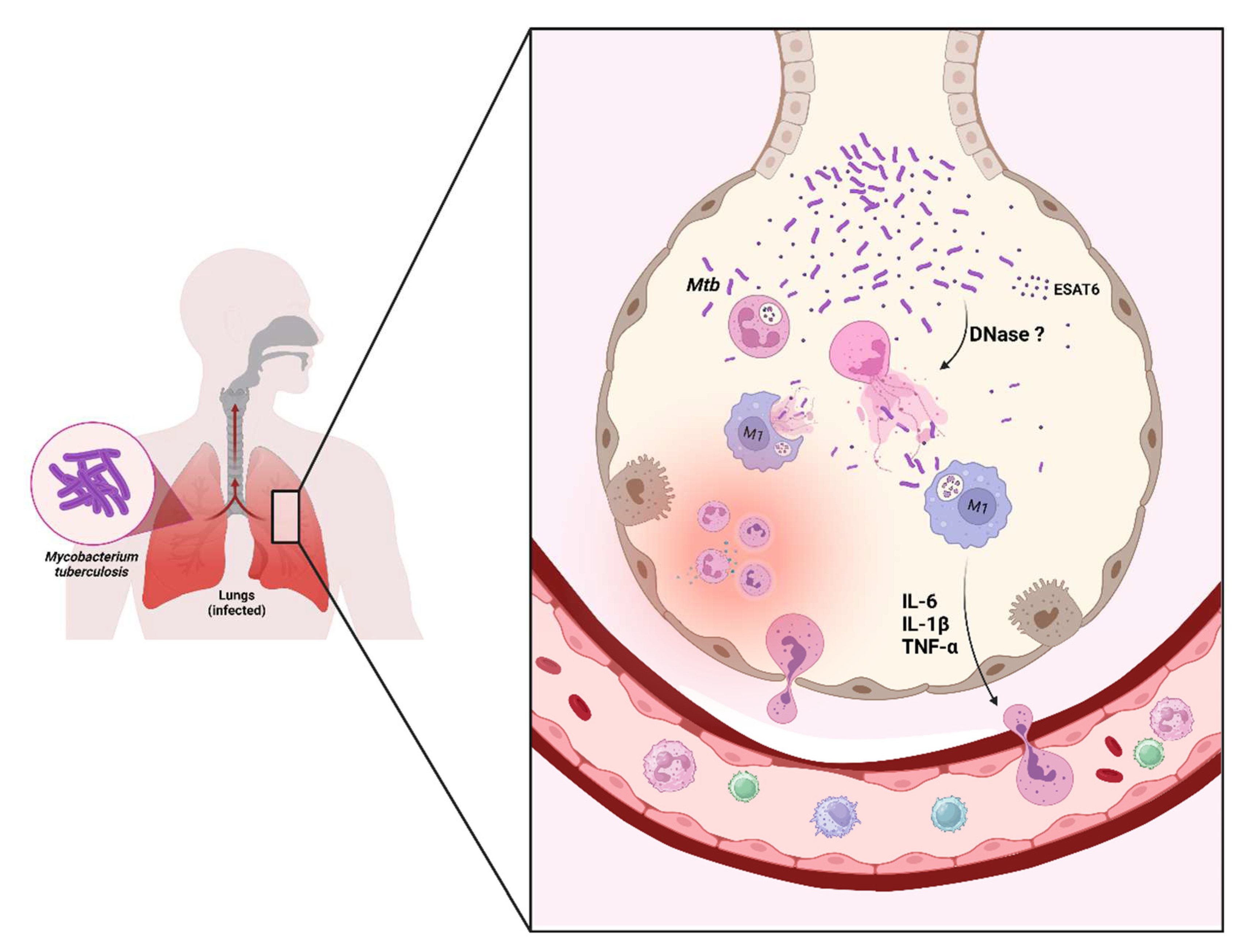
1. Introduction
2. Tuberculosis
3. Neutrophils
4. Neutrophil extracellular trap in tuberculosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garrido-Cardenas, J.A.; de Lamo-Sevilla, C.; Cabezas-Fernández, M.T.; Manzano-Agugliaro, F.; Martínez-Lirola, M. Global tuberculosis research and its future prospects. Tuberculosis (Edinb). 2020, 121. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, B.M.F.; Krishnan, S.; Barreto-Duarte, B.; Araújo-Pereira, M.; Queiroz, A.T.L.; Ellner, J.J.; Salgame, P.; Scriba, T.J.; Sterling, T.R.; Gupta, A.; et al. Diagnostic biomarkers for active tuberculosis: progress and challenges. EMBO Mol. Med. 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Dicks, K. V.; Stout, J.E. Molecular Diagnostics for Mycobacterium tuberculosis Infection. Annu. Rev. Med. 2019, 70, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Lucena, L.A. de; Dantas, G.B. da S.; Carneiro, T.V.; Lacerda, H.G. Factors Associated with the Abandonment of Tuberculosis Treatment in Brazil: A Systematic Review. Rev. Soc. Bras. Med. Trop. 2023, 56. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P. Challenges & Solutions for Recent Advancements in Multi-Drugs Resistance Tuberculosis: A Review. Microbiol. insights 2023, 16, 117863612311524. [Google Scholar] [CrossRef]
- Singh, S.; Saavedra-Avila, N.A.; Tiwari, S.; Porcelli, S.A. A century of BCG vaccination: Immune mechanisms, animal models, non-traditional routes and implications for COVID-19. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Pai, M.; Kasaeva, T.; Swaminathan, S. Covid-19’s Devastating Effect on Tuberculosis Care - A Path to Recovery. N. Engl. J. Med. 2022, 386, 1490–1493. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Global Tuberculosis Report 2022. Geneva World Heal. Organ. 2022.
- Kanabalan, R.D.; Lee, L.J.; Lee, T.Y.; Chong, P.P.; Hassan, L.; Ismail, R.; Chin, V.K. Human tuberculosis and Mycobacterium tuberculosis complex: A review on genetic diversity, pathogenesis and omics approaches in host biomarkers discovery. Microbiol. Res. 2021, 246. [Google Scholar] [CrossRef]
- Scriba, T.J.; Coussens, A.K.; Fletcher, H.A. Human Immunology of Tuberculosis. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Borkute, R.R.; Woelke, S.; Pei, G.; Dorhoi, A. Neutrophils in Tuberculosis: Cell Biology, Cellular Networking and Multitasking in Host Defense. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef] [PubMed]
- Burn, G.L.; Foti, A.; Marsman, G.; Patel, D.F.; Zychlinsky, A. The Neutrophil. Immunity 2021, 54, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Loddenkemper, R.; Murray, J.F. History of Tuberculosis. In Essential Tuberculosis; Springer, Cham: Cham, 2021; pp. 3–9. [Google Scholar]
- Guerra-De-Blas, P.D.C.; Torres-González, P.; Bobadilla-Del-Valle, M.; Sada-Ovalle, I.; Ponce-De-León-Garduño, A.; Sifuentes-Osornio, J. Potential Effect of Statins on Mycobacterium tuberculosis Infection. J. Immunol. Res. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Prim. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, D.W.; Raviglione, M.C. Basic and Descriptive Epidemiology of Tuberculosis. In Essential Tuberculosis; Springer, Cham, 2021; pp. 29–36.
- Campaniço, A.; Harjivan, S.G.; Warner, D.F.; Moreira, R.; Lopes, F. Addressing Latent Tuberculosis: New Advances in Mimicking the Disease, Discovering Key Targets, and Designing Hit Compounds. Int. J. Mol. Sci. 2020, Vol. 21, Page 8854 2020, 21, 8854. [Google Scholar] [CrossRef]
- Ferluga, J.; Yasmin, H.; Al-Ahdal, M.N.; Bhakta, S.; Kishore, U. Natural and trained innate immunity against Mycobacterium tuberculosis. Immunobiology 2020, 225. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.D.; Stein, C.M.; Seshadri, C.; Campo, M.; Alter, G.; Fortune, S.; Schurr, E.; Wallis, R.S.; Churchyard, G.; Mayanja-Kizza, H.; et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat. Rev. Immunol. 2018, 18, 575–589. [Google Scholar] [CrossRef]
- Warner, D.F.; Koch, A.; Mizrahi, V. Diversity and disease pathogenesis in Mycobacterium tuberculosis. Trends Microbiol. 2015, 23, 14–21. [Google Scholar] [CrossRef]
- Bruchfeld, J.; Forsman, L.D.; Fröberg, G.; Niward, K. Extrapulmonary Tuberculosis. In Essential Tuberculosis; Springer, Cham: Cham, 2021; pp. 259–266. [Google Scholar]
- Rodriguez-Takeuchi, S.Y.; Renjifo, M.E.; Medina, F.J. Extrapulmonary Tuberculosis: Pathophysiology and Imaging Findings. Radiographics 2019, 39, 2023–2037. [Google Scholar] [CrossRef]
- Wilkinson, R.J.; Rohlwink, U.; Misra, U.K.; Van Crevel, R.; Mai, N.T.H.; Dooley, K.E.; Caws, M.; Figaji, A.; Savic, R.; Solomons, R.; et al. Tuberculous meningitis. Nat. Rev. Neurol. 2017 1310 2017, 13, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S. Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection. Clin. Dev. Immunol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A.; Bustin, S.; Alsayed, S.S.R.; Gunosewoyo, H. Tuberculosis: Pathogenesis, Current Treatment Regimens and New Drug Targets. Int. J. Mol. Sci. 2023, Vol. 24, Page 5202 2023, 24, 5202. [Google Scholar] [CrossRef]
- Palucci, I.; Delogu, G. Host Directed Therapies for Tuberculosis: Futures Strategies for an Ancient Disease. Chemotherapy 2018, 63, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Gonzalez, R.; Prince, O.; Cooper, A.; Khader, S.A. Cytokines and Chemokines in Mycobacterium tuberculosis Infection. Microbiol. Spectr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mundra, A.; Yegiazaryan, A.; Karsian, H.; Alsaigh, D.; Bonavida, V.; Frame, M.; May, N.; Gargaloyan, A.; Abnousian, A.; Venketaraman, V. Pathogenicity of Type I Interferons in Mycobacterium tuberculosis. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.D. Mechanisms of M. tuberculosis Immune Evasion as Challenges to TB Vaccine Design. Cell Host Microbe 2018, 24, 34–42. [Google Scholar] [CrossRef]
- Sia, J.K.; Rengarajan, J. Immunology of Mycobacterium tuberculosis Infections. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Kim, H.; Shin, S.J. Pathological and protective roles of dendritic cells in Mycobacterium tuberculosis infection: Interaction between host immune responses and pathogen evasion. Front. Cell. Infect. Microbiol. 2022, 12, 891878. [Google Scholar] [CrossRef]
- Zumla, A.; Rao, M.; Parida, S.K.; Keshavjee, S.; Cassell, G.; Wallis, R.; Axelsson-Robertsson, R.; Doherty, M.; Andersson, J.; Maeurer, M.J. Inflammation and tuberculosis: host-directed therapies. J. Intern. Med. 2015, 277, 373–387. [Google Scholar] [CrossRef]
- Domingo-Gonzalez, R.; Prince, O.; Cooper, A.; Khader, S.A. Cytokines and Chemokines in Mycobacterium tuberculosis Infection. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P. Adult pulmonary tuberculosis as a pathological manifestation of hyperactive antimycobacterial immune response. Clin. Transl. Med. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.C.K.; Noursadeghi, M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat. Rev. Microbiol. 2018, 16, 80–90. [Google Scholar] [CrossRef]
- Ssekamatte, P.; Sande, O.J.; van Crevel, R.; Biraro, I.A. Immunologic, metabolic and genetic impact of diabetes on tuberculosis susceptibility. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Jasenosky, L.D.; Scriba, T.J.; Hanekom, W.A.; Goldfeld, A.E. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol. Rev. 2015, 264, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Lu, Z.; Liu, C.H. Host defense mechanisms against Mycobacterium tuberculosis. Cell. Mol. Life Sci. 2020, 77, 1859–1878. [Google Scholar] [CrossRef]
- Urdahl, K.B. Understanding and overcoming the barriers to T cell-mediated immunity against tuberculosis. Semin. Immunol. 2014, 26, 578–587. [Google Scholar] [CrossRef]
- Jiang, J.; Cao, Z.; Xiao, L.; Su, J.; Wang, J.; Liang, J.; Yang, B.; Liu, Y.; Zhai, F.; Wang, R.; et al. Single-cell profiling identifies T cell subsets associated with control of tuberculosis dissemination. Clin. Immunol. 2023, 248. [Google Scholar] [CrossRef]
- Ehlers, S.; Schaible, U.E. The granuloma in tuberculosis: Dynamics of a host-pathogen collusion. Front. Immunol. 2012, 3, 411. [Google Scholar] [CrossRef]
- Gideon, H.P.; Hughes, T.K.; Tzouanas, C.N.; Wadsworth, M.H.; Tu, A.A.; Gierahn, T.M.; Peters, J.M.; Hopkins, F.F.; Wei, J.R.; Kummerlowe, C.; et al. Multimodal profiling of lung granulomas in macaques reveals cellular correlates of tuberculosis control. Immunity 2022, 55, 827–846. [Google Scholar] [CrossRef]
- Parker, H.A.; Forrester, L.; Kaldor, C.D.; Dickerhof, N.; Hampton, M.B. Antimicrobial Activity of Neutrophils Against Mycobacteria. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, V.; Turk, M.; Jenne, C.N.; Kim, S.J. Neutrophils in viral infection. Cell Tissue Res. 2018, 371, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante-Silva, L.H.A.; Carvalho, D.C.M.; Lima, É. de A.; Galvão, J.G.F.M.; da Silva, J.S. d. F.; Sales-Neto, J.M. de; Rodrigues-Mascarenhas, S. Neutrophils and COVID-19: The road so far. Int. Immunopharmacol. 2021, 90. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Galani, I.E.; Andreakos, E. Neutrophils in viral infections: Current concepts and caveats. J. Leukoc. Biol. 2015, 98, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Backman, E. Eradicating, retaining, balancing, swarming, shuttling and dumping: a myriad of tasks for neutrophils during fungal infection. Curr. Opin. Microbiol. 2020, 58, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Newburger, P.E. Autoimmune and other acquired neutropenias. Hematol. Am. Soc. Hematol. Educ. Progr. 2016, 2016, 38–42. [Google Scholar] [CrossRef]
- Kumar, S.; Dikshit, M. Metabolic Insight of Neutrophils in Health and Disease. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Shim, H.B.; Deniset, J.F.; Kubes, P. Neutrophils in homeostasis and tissue repair. Int. Immunol. 2022, 34, 399–407. [Google Scholar] [CrossRef]
- Palomino-Segura, M.; Sicilia, J.; Ballesteros, I.; Hidalgo, A. Strategies of neutrophil diversification. Nat. Immunol. 2023, 24, 575–584. [Google Scholar] [CrossRef]
- Hidalgo, A.; Chilvers, E.R.; Summers, C.; Koenderman, L. The Neutrophil Life Cycle. Trends Immunol. 2019, 40, 584–597. [Google Scholar] [CrossRef]
- Grieshaber-Bouyer, R.; Radtke, F.A.; Cunin, P.; Stifano, G.; Levescot, A.; Vijaykumar, B.; Nelson-Maney, N.; Blaustein, R.B.; Monach, P.A.; Nigrovic, P.A.; et al. The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Hoffman, H.M.; Kubes, P.; Cassatella, M.A.; Zychlinsky, A.; Hedrick, C.C.; Catz, S.D. Neutrophils: New insights and open questions. Sci. Immunol. 2018, 3, eaat4579. [Google Scholar] [CrossRef] [PubMed]
- Deniset, J.F.; Kubes, P. Neutrophil heterogeneity: Bona fide subsets or polarization states? J. Leukoc. Biol. 2018, 103, 829–838. [Google Scholar] [CrossRef]
- Cassatella, M.A.; Östberg, N.K.; Tamassia, N.; Soehnlein, O. Biological Roles of Neutrophil-Derived Granule Proteins and Cytokines. Trends Immunol. 2019, 40, 648–664. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, M.; Kubes, P. The Healing Power of Neutrophils. Trends Immunol. 2019, 40, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Chatfield, S.M.; Thieblemont, N.; Witko-Sarsat, V. Expanding Neutrophil Horizons: New Concepts in Inflammation. J. Innate Immun. 2018, 10, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. Mechanisms of Leukocyte Transendothelial Migration. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 323–344. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.; Hong, C. Deep insight into neutrophil trafficking in various organs. J. Leukoc. Biol. 2017, 102, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.R.; Huttenlocher, A. Neutrophils in the Tumor Microenvironment. Trends Immunol. 2016, 37, 41–52. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Hilda, J.N.; Das, S.; Tripathy, S.P.; Hanna, L.E. Role of neutrophils in tuberculosis: A bird’s eye view. Innate Immun. 2020, 26, 240–247. [Google Scholar] [CrossRef]
- Panteleev, A. V.; Nikitina, I.Y.; Burmistrova, I.A.; Kosmiadi, G.A.; Radaeva, T. V.; Amansahedov, R.B.; Sadikov, P. V.; Serdyuk, Y. V.; Larionova, E.E.; Bagdasarian, T.R.; et al. severe tuberculosis in humans correlates best with neutrophil abundance and lymphocyte deficiency and does not correlate with antigen-specific CD4 T-cell response. Front. Immunol. 2017, 8, 963. [Google Scholar] [CrossRef]
- Lowe, D.M.; Bandara, A.K.; Packe, G.E.; Barker, R.D.; Wilkinson, R.J.; Griffiths, C.J.; Martineau, A.R. Neutrophilia independently predicts death in tuberculosis. Eur. Respir. J. 2013, 42, 1752–1757. [Google Scholar] [CrossRef]
- van der Meer, A.J.; Zeerleder, S.; Blok, D.C.; Kager, L.M.; Lede, I.O.; Rahman, W.; Afroz, R.; Ghose, A.; Visser, C.E.; Zahed, A.S.M.; et al. Neutrophil extracellular traps in patients with pulmonary tuberculosis. Respir. Res. 2017, 18. [Google Scholar] [CrossRef]
- Poh, X.Y.; Loh, F.K.; Friedland, J.S.; Ong, C.W.M. Neutrophil-Mediated Immunopathology and Matrix Metalloproteinases in Central Nervous System - Tuberculosis. Front. Immunol. 2022, 12. [Google Scholar] [CrossRef]
- Arcos, J.; Diangelo, L.E.; Scordo, J.M.; Sasindran, S.J.; Moliva, J.I.; Turner, J.; Torrelles, J.B. Lung Mucosa Lining Fluid Modification of Mycobacterium tuberculosis to Reprogram Human Neutrophil Killing Mechanisms. J. Infect. Dis. 2015, 212, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Monin, L.; Torres, D.; Slight, S.; Mehra, S.; McKenna, K.C.; Junecko, B.A.F.; Reinhart, T.A.; Kolls, J.; Báez-Saldańa, R.; et al. S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis. Am. J. Respir. Crit. Care Med. 2013, 188, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Lovewell, R.R.; Baer, C.E.; Mishra, B.B.; Smith, C.M.; Sassetti, C.M. Granulocytes act as a niche for Mycobacterium tuberculosis growth. Mucosal Immunol. 2020 141 2020, 14, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science (80-.). 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Brinkmann, V. Neutrophil Extracellular Traps in the Second Decade. J. Innate Immun. 2018, 10, 414–421. [Google Scholar] [CrossRef]
- Narasaraju, T.; Tang, B.M.; Herrmann, M.; Muller, S.; Chow, V.T.K.; Radic, M. Neutrophilia and NETopathy as Key Pathologic Drivers of Progressive Lung Impairment in Patients With COVID-19. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Hahn, J.; Knopf, J.; Maueröder, C.; Kienhöfer, D.; Leppkes, M.; Herrmann, M. Neutrophils and neutrophil extracellular traps orchestrate initiation and resolution of inflammation. Clin. Exp. Rheumatol. 2016, 34, 6–8. [Google Scholar]
- Porto, B.N.; Stein, R.T. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front. Immunol. 2016, 7. [Google Scholar] [CrossRef]
- Dang, G.; Cui, Y.; Wang, L.; Li, T.; Cui, Z.; Song, N.; Chen, L.; Pang, H.; Liu, S. Extracellular Sphingomyelinase Rv0888 of Mycobacterium tuberculosis Contributes to Pathological Lung Injury of Mycobacterium smegmatis in Mice via Inducing Formation of Neutrophil Extracellular Traps. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: how vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rizo, V.; Martínez-Guzmán, M.A.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Hiroki, C.H.; Toller-Kawahisa, J.E.; Fumagalli, M.J.; Colon, D.F.; Figueiredo, L.T.M.; Fonseca, B.A.L.D.; Franca, R.F.O.; Cunha, F.Q. Neutrophil Extracellular Traps Effectively Control Acute Chikungunya Virus Infection. Front. Immunol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Nett, J.E. Neutrophil extracellular traps in fungal infection. Semin. Cell Dev. Biol. 2019, 89, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Guimarães-Costa, A.B.; Nascimento, M.T.C.; Froment, G.S.; Soares, R.P.P.; Morgado, F.N.; Conceição-Silva, F.; Saraiva, E.M. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 6748–6753. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Kichik, V.; Mondragón-Flores, R.; Mondragón-Castelán, M.; Gonzalez-Pozos, S.; Muñiz-Hernandez, S.; Rojas-Espinosa, O.; Chacón-Salinas, R.; Estrada-Parra, S.; Estrada-García, I. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis (Edinb). 2009, 89, 29–37. [Google Scholar] [CrossRef]
- Filio-Rodríguez, G.; Estrada-García, I.; Arce-Paredes, P.; Moreno-Altamirano, M.M.; Islas-Trujillo, S.; Ponce-Regalado, M.D.; Rojas-Espinosa, O. In vivo induction of neutrophil extracellular traps by Mycobacterium tuberculosis in a guinea pig model. Innate Immun. 2017, 23, 625–637. [Google Scholar] [CrossRef]
- Hahn, S.; Giaglis, S.; Chowdury, C.S.; Hösli, I.; Hasler, P. Modulation of neutrophil NETosis: interplay between infectious agents and underlying host physiology. Semin. Immunopathol. 2013, 35, 439–453. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakayama, H.; Sasaki, S.; Takahashi, K.; Iwabuchi, K. Mycobacterium avium-intracellulare complex promote release of pro-inflammatory enzymes matrix metalloproteinases by inducing neutrophil extracellular trap formation. Sci. Rep. 2022, 12. [Google Scholar] [CrossRef]
- Mendoza-Aguilar, M.D.; Arce-Paredes, P.; Aquino-Vega, M.; Rodríguez-Martínez, S.; Rojas-Espinosa, O. Fate of Mycobacterium tuberculosis in peroxidase-loaded resting murine macrophages. Int. J. mycobacteriology 2013, 2, 3–13. [Google Scholar] [CrossRef]
- Stephan, A.; Batinica, M.; Steiger, J.; Hartmann, P.; Zaucke, F.; Bloch, W.; Fabri, M. LL37:DNA complexes provide antimicrobial activity against intracellular bacteria in human macrophages. Immunology 2016, 148, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Braian, C.; Hogea, V.; Stendahl, O. Mycobacterium tuberculosis- induced neutrophil extracellular traps activate human macrophages. J. Innate Immun. 2013, 5, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Schechter, M.C.; Buac, K.; Adekambi, T.; Cagle, S.; Celli, J.; Ray, S.M.; Mehta, C.C.; Rada, B.; Rengarajan, J.; Delgado-Rizo, V.; et al. Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis. Front. Immunol. 2013, 7, 1–13. [Google Scholar] [CrossRef]
- Moreira-Teixeira, L.; Stimpson, P.J.; Stavropoulos, E.; Hadebe, S.; Chakravarty, P.; Ioannou, M.; Aramburu, I.V.; Herbert, E.; Priestnall, S.L.; Suarez-Bonnet, A.; et al. Type I IFN exacerbates disease in tuberculosis-susceptible mice by inducing neutrophil-mediated lung inflammation and NETosis. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Su, R.; Peng, Y.P.; Deng, Z.; Deng, Y.T.; Ye, J.Q.; Guo, Y.; Huang, Z.K.; Luo, Q.; Jiang, H.; Li, J.M. Mycobacterium tuberculosis Infection Induces Low-Density Granulocyte Generation by Promoting Neutrophil Extracellular Trap Formation via ROS Pathway. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Francis, R.J.; Butler, R.E.; Stewart, G.R. Mycobacterium tuberculosis ESAT-6 is a leukocidin causing Ca2+ influx, necrosis and neutrophil extracellular trap formation. Cell Death Dis. 2014, 5. [Google Scholar] [CrossRef]
- Hunter, R.L. The Pathogenesis of Tuberculosis-The Koch Phenomenon Reinstated. Pathog. (Basel, Switzerland) 2020, 9, 1–25. [Google Scholar] [CrossRef]
- Ong, C.W.M.; Fox, K.; Ettorre, A.; Elkington, P.T.; Friedland, J.S. Hypoxia increases neutrophil-driven matrix destruction after exposure to Mycobacterium tuberculosis. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Schechter, M.C.; Buac, K.; Adekambi, T.; Cagle, S.; Celli, J.; Ray, S.M.; Mehta, C.C.; Rada, B.; Rengarajan, J. Neutrophil extracellular trap (NET) levels in human plasma are associated with active TB. PLoS One 2017, 12. [Google Scholar] [CrossRef]
- De Melo, M.G.M.; Mesquita, E.D.D.; Oliveira, M.M.; Da Silva-Monteiro, C.; Silveira, A.K.A.; Malaquias, T.S.; Dutra, T.C.P.; Galliez, R.M.; Kritski, A.L.; Silva, E.C. Imbalance of NET and Alpha-1-Antitrypsin in Tuberculosis Patients Is Related With Hyper Inflammation and Severe Lung Tissue Damage. Front. Immunol. 2019, 9. [Google Scholar] [CrossRef]
- Meier, S.; Seddon, J.A.; Maasdorp, E.; Kleynhans, L.; du Plessis, N.; Loxton, A.G.; Malherbe, S.T.; Zak, D.E.; Thompson, E.; Duffy, F.J.; et al. Neutrophil degranulation, NETosis and platelet degranulation pathway genes are co-induced in whole blood up to six months before tuberculosis diagnosis. PLoS One 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Ríos-López, A.L.; González, G.M.; Hernández-Bello, R.; Sánchez-González, A. Avoiding the trap: Mechanisms developed by pathogens to escape neutrophil extracellular traps. Microbiol. Res. 2021, 243, 126644. [Google Scholar] [CrossRef] [PubMed]
- Arazna, M.; Pruchniak, M.P.; Demkow, U. Neutrophil extracellular traps in bacterial infections: strategies for escaping from killing. Respir. Physiol. Neurobiol. 2013, 187, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Manda, A.; Pruchniak, M.P.; Araźna, M.; Demkow, U.A. Neutrophil extracellular traps in physiology and pathology. Cent. J. Immunol. 2014, 39, 116–121. [Google Scholar] [CrossRef]
- Lauth, X.; Von Köckritz-Blickwede, M.; McNamara, C.W.; Myskowski, S.; Zinkernagel, A.S.; Beall, B.; Ghosh, P.; Gallo, R.L.; Nizet, V. M1 Protein Allows Group A Streptococcal Survival in Phagocyte Extracellular Traps through Cathelicidin Inhibition. J. Innate Immun. 2009, 1, 202. [Google Scholar] [CrossRef]
- Brennan, P.J. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 2003, 83, 91–97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




