Submitted:
23 May 2023
Posted:
23 May 2023
You are already at the latest version
Abstract
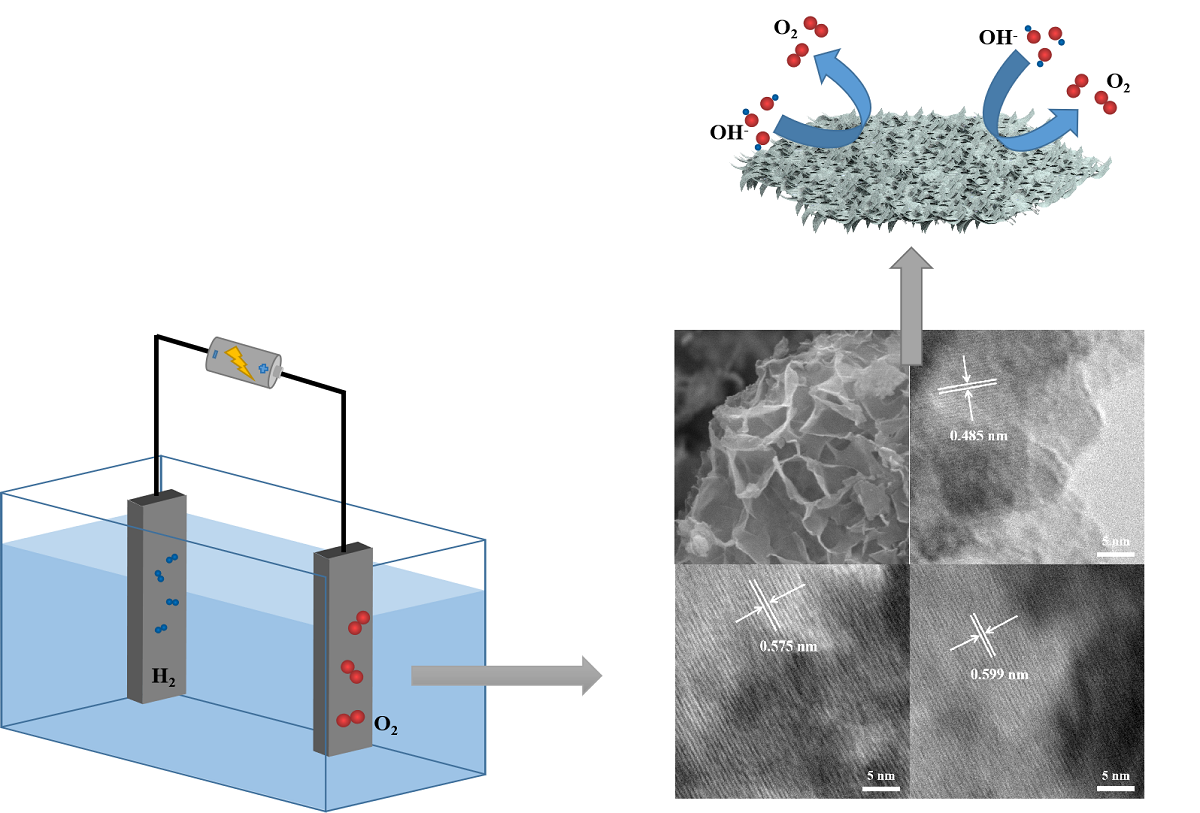
Keywords:
1. Introduction
2. Experimental
2.1. Preparation of N-doped carbon quantum dots solution
2.2. Preparation of Co9Se8/Ni3Se4/Fe3O4@C
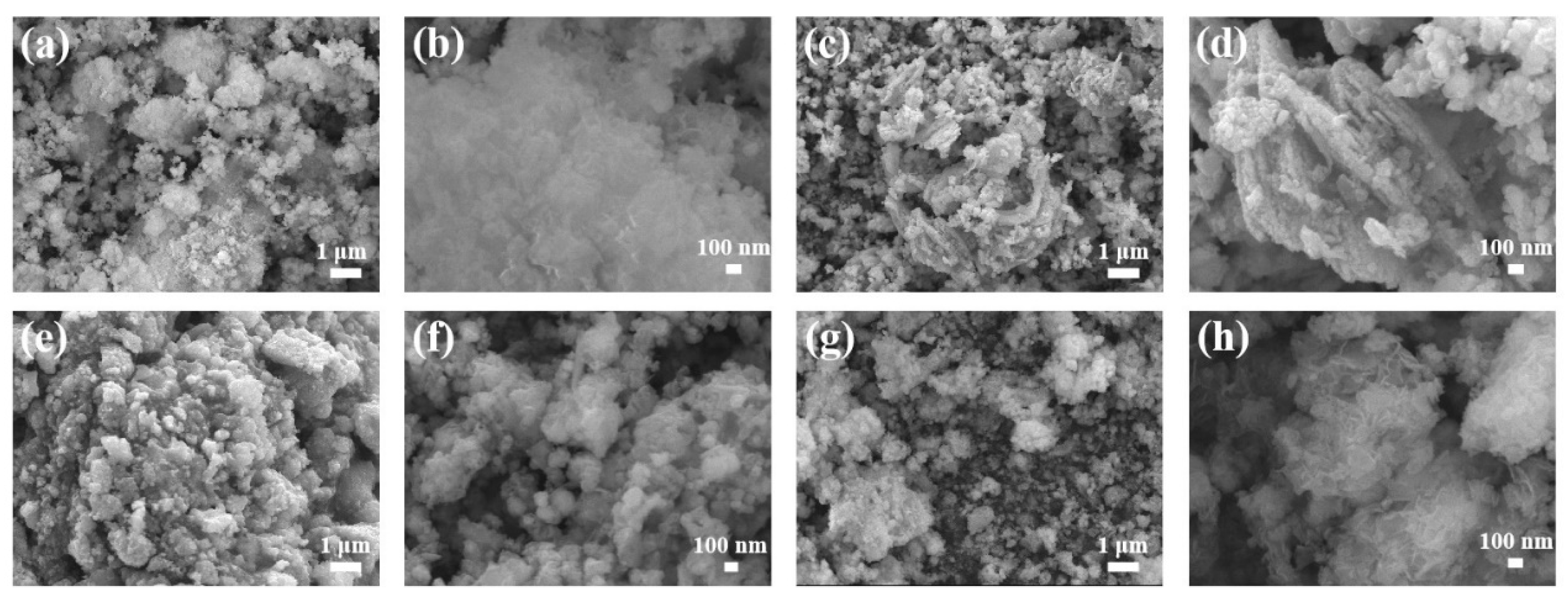
2.3. Characterization
2.4. Electrochemical measurement
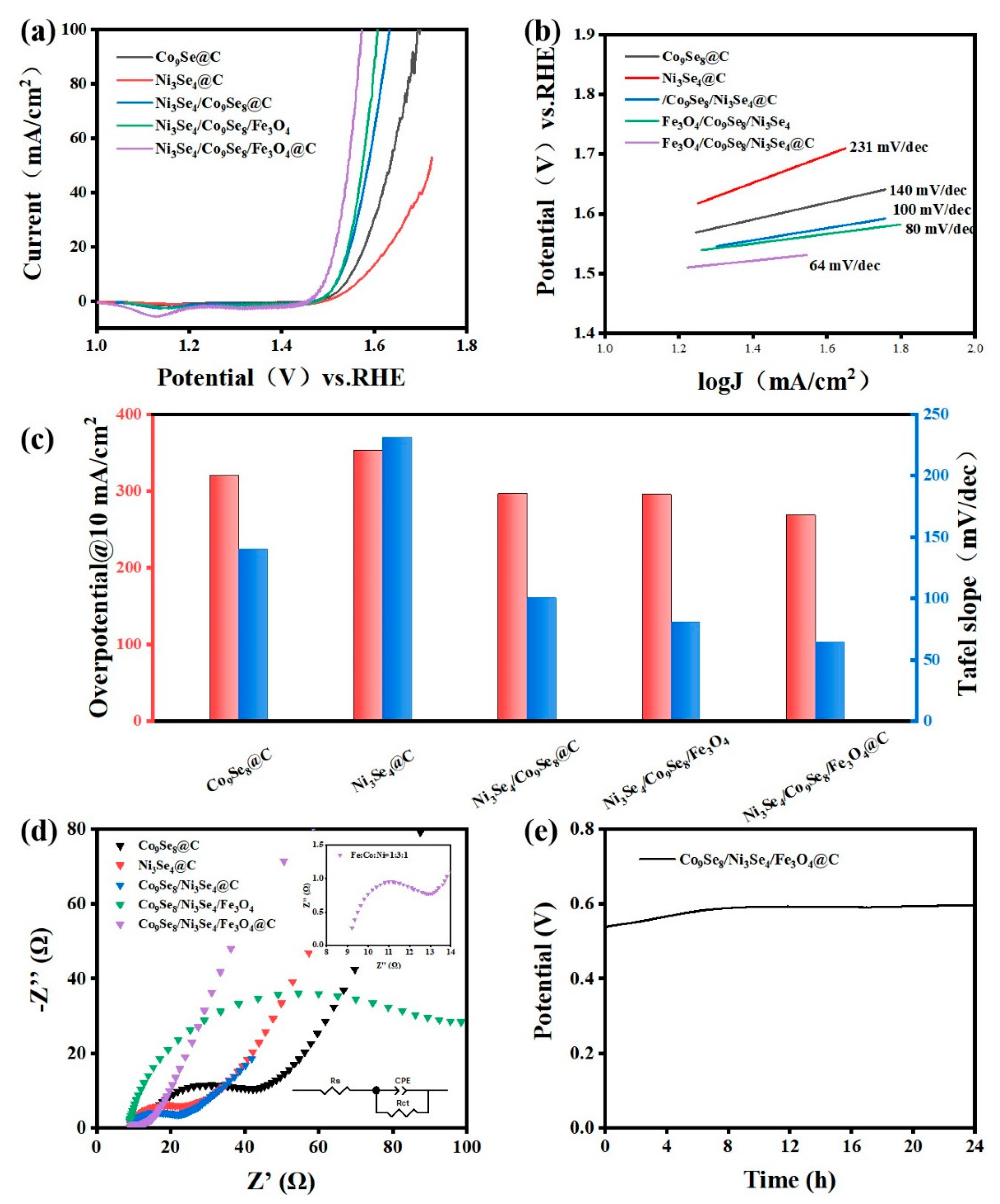
3. Results and Discussion
4. Conclusion
Acknowledgments
References
- Tian, X.; Lu, X.F.; Xia, B.Y.; Lou, X.W. Advanced Electrocatalysts for the Oxygen Reduction Reaction in Energy Conversion Technologies. Joule 2020, 4, 45–68. [Google Scholar] [CrossRef]
- Chang, H.; Shi, L.-N.; Chen, Y.-H.; Wang, P.-F.; Yi, T.-F. Advanced MOF-derived carbon-based non-noble metal oxygen electrocatalyst for next-generation rechargeable Zn-air batteries. Co-ord. Chem. Rev. 2022, 473, 214839. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, S.; Deng, J.; Zhang, W.; Feng, Y.; Ma, J. Transition metal carbides in electrocatalytic oxygen evolution reaction. Chin. Chem. Lett. 2021, 32, 291–298. [Google Scholar] [CrossRef]
- Lu, X.F.; Fang, Y.; Luan, D.; Lou, X.W.D. Metal–Organic Frameworks Derived Functional Materials for Electrochemical Energy Storage and Conversion: A Mini Review. Nano Lett. 2021, 21, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Cao, K.W.; He, J.W.; Li, F.M.; Huang, H.; Chen, P.; Chen, Y. Nitrogen-doped graphene aerogel-supported ruthenium nanocrystals for pH-universal hydrogen evolution reaction. Chin. J. Catal. 2022, 43, 1535–1543. [Google Scholar] [CrossRef]
- Xue, Q.; Bai, X.-Y.; Zhao, Y.; Li, Y.-N.; Wang, T.-J.; Sun, H.-Y.; Li, F.-M.; Chen, P.; Jin, P.; Yin, S.-B.; et al. Au core-PtAu alloy shell nanowires for formic acid electrolysis. J. Energy Chem. 2022, 65, 94–102. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, Y.; He, J.; Li, F.; Ding, Y.; Chen, P.; Chen, Y. Porous palladium phosphide nanotubes for formic acid electrooxidation. Carbon Energy 2022, 4, 283–293. [Google Scholar] [CrossRef]
- Li, L.; Cao, X.; Huo, J.; Qu, J.; Chen, W.; Liu, C.; Zhao, Y.; Liu, H.; Wang, G. High valence metals engineering strategies of Fe/Co/Ni-based catalysts for boosted OER electrocatalysis. J. Energy Chem. 2023, 76, 195–213. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J. A review on NiFe-based electrocatalysts for efficient alkaline oxygen evolution reaction. J. Power Sources 2020, 448, 227375. [Google Scholar] [CrossRef]
- Hoang, V.C.; Dave, K.; Gomes, V.G. Carbon quantum dot-based composites for energy storage and electrocatalysis: Mechanism, applications and future prospects. Nano Energy 2019, 66, 104093. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Xie, T.; Chen, Q.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Heterointerface enhanced NiFe LDH/V–Co4N electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A 2022, 10, 21523–21530. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Huang, Y.; Li, Z.; Liang, Z.; Cao, G. Nickel induced electronic structural regulation of cobalt hydroxide for enhanced water oxidation. J. Mater. Chem. A 2020, 8, 6699–6708. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, S.; Zhu, C.; Shi, J.; Su, C.; Yang, J.; Wang, M.; Li, J.; Li, J.; Liu, P.; et al. Rational construction of high-active Co3O4 electrocatalysts for oxygen evolution reaction. Nano Res. 2022, 16, 624–633. [Google Scholar] [CrossRef]
- Alegre, C.; Busacca, C.; Di Blasi, A.; Di Blasi, O.; Aricò, A.S.; Antonucci, V.; Baglio, V. Electrocatalysis of Oxygen on Bifunctional Nickel-Cobaltite Spinel. ChemElectroChem 2020, 7, 124–130. [Google Scholar] [CrossRef]
- Yang, J.; Xuan, H.; Yang, J.; Meng, L.; Wang, J.; Liang, X.; Li, Y.; Han, P. Metal-organic framework-derived FeS2/CoNiSe2 heterostructure nanosheets for highly-efficient oxygen evolution reaction. Appl. Surf. Sci. 2021, 578, 152016. [Google Scholar] [CrossRef]
- Li, W.; Wu, L.; Wu, X.; Shi, C.; Li, Y.; Zhang, L.; Mi, H.; Zhang, Q.; He, C.; Ren, X. Regulation and mechanism study of the CoS2/Cu2S-NF heterojunction as highly-efficient bifunctional electrocatalyst for oxygen reactions. Appl. Catal. B Environ. 2022, 303, 120849. [Google Scholar] [CrossRef]
- Wang, R.; Liu, B.; You, S.; Li, Y.; Zhang, Y.; Wang, D.; Tang, B.; Sun, Y.; Zou, J. Three-dimensional Ni3Se4 flowers integrated with ultrathin carbon layer with strong electronic interactions for boosting oxygen reduction/evolution reactions. Chem. Eng. J. 2021, 430, 132720. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, H.W.; Jia, Y.B.; Zhang, Y.; Jiang, Z.G.; Li, C.J.; Li, X.; Bao, L.H.; Ma, R.G.; Wang, K.K. Flower-like MOF-74 nanocomposites directed by selenylation towards high-efficient oxygen evolution. J. Colloid Interface Sci. 2022, 623, 552–560. [Google Scholar] [CrossRef]
- Fan, X.M.; Ma, Y.X.; Sun, A.K.; Zhang, X.; Tang, L.; Guo, J.X. Engineering heterogeneous nickel-iron oxide/iron phosphate on P, N co-doped carbon fibers for efficient oxygen evolution reaction in neutral and alkaline solutions. Surf Interfaces 2021, 25, 101193. [Google Scholar] [CrossRef]
- Jia, Y.; Zhu, L.; Pan, H.; Liao, Y.; Zhang, Y.; Zhang, X.; Jiang, Z.; Chen, M.; Wang, K. Excellent electrocatalytic hydrogen evolution performance of hexagonal NiCoP porous nanosheets in alkaline solution. Appl. Surf. Sci. 2022, 580, 152314. [Google Scholar] [CrossRef]
- Lu, Z.; Cao, Y.; Xie, J.; Hu, J.; Wang, K.; Jia, D. Construction of Co2P/CoP@Co@NCNT rich-interface to synergistically promote overall water splitting. Chem. Eng. J. 2022, 430, 132877. [Google Scholar] [CrossRef]
- Zhang, G.G.; Wang, X.C. Oxysulfide semiconductors for photocatalytic overall water splitting with visible light. Angew. Chem. Int. Ed. Engl. 2019, 58, 15580–15582. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Takata, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K. Oxysulfide Sm2Ti2S2O5 as a Stable Photocatalyst for Water Oxidation and Reduction under Visible Light Irradiation (λ ≤ 650 nm). J. Am. Chem. Soc. 2002, 124, 13547–13553. [Google Scholar] [CrossRef]
- Mukherjee, P.; Sathiyan, K.; Vijay, A.K.; Bar-Ziv, R.; Zidki, T. Hybrid Nanostructure of Mixed Transition Metal Oxysulfides Supported by Porous PBA as Efficient Electrocatalysts for the Oxygen Evolution Reaction. Isr. J. Chem. 2021, 62, e202100110. [Google Scholar] [CrossRef]
- Wang, B.; Hu, Y.; Yu, B.; Zhang, X.; Yang, D.; Chen, Y. Heterogeneous CoFe–Co8FeS8 nanoparticles embedded in CNT networks as highly efficient and stable electrocatalysts for oxygen evolution reaction. J. Power Sources 2019, 433, 126688. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, Y.; Zheng, X.; Cai, M.; Zhang, J.; Wang, J.; Hu, W. Engineering Interface and Oxygen Vacancies of NixCo1–xSe2 to Boost Oxygen Catalysis for Flexible Zn–Air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 27964–27972. [Google Scholar] [CrossRef] [PubMed]
- Moysiadou, A.; Lee, S.; Hsu, C.-S.; Chen, H.M.; Hu, X. Mechanism of Oxygen Evolution Catalyzed by Cobalt Oxyhydroxide: Cobalt Superoxide Species as a Key Intermediate and Dioxygen Release as a Rate-Determining Step. J. Am. Chem. Soc. 2020, 142, 11901–11914. [Google Scholar] [CrossRef]
- Zhuang, L.; Jia, Y.; Liu, H.; Wang, X.; Hocking, R.K.; Liu, H.; Chen, J.; Ge, L.; Zhang, L.; Li, M.; et al. Defect-Induced Pt–Co–Se Coordinated Sites with Highly Asymmetrical Electronic Distribution for Boosting Oxygen-Involving Electrocatalysis. Adv. Mater. 2019, 31, e1805581. [Google Scholar] [CrossRef]
- Qin, J.-F.; Yang, M.; Chen, T.-S.; Dong, B.; Hou, S.; Ma, X.; Zhou, Y.-N.; Yang, X.-L.; Nan, J.; Chai, Y.-M. Ternary metal sulfides MoCoNiS derived from metal organic frameworks for efficient oxygen evolution. Int. J. Hydrogen Energy 2020, 45, 2745–2753. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.; Qiao, Z.; Yang, Y.; Deng, L.; Li, C.; Chen, X.; Wang, S.; Huang, Y.; Zhang, X.; et al. Facile synthesis of Fe2P/Co embedded trifunctional electrocatalyst for high-performance anion exchange membrane fuel cells, rechargeable Zn–air batteries, and overall water splitting. J. Mater. Chem. A 2022, 10, 16037–16045. [Google Scholar] [CrossRef]
- Su, H.; Song, S.; Gao, Y.; Li, N.; Fu, Y.; Ge, L.; Song, W.; Liu, J.; Ma, T. In Situ Electronic Redistribution Tuning of NiCo2S4 Nanosheets for Enhanced Electrocatalysis. Adv. Funct. Mater. 2022, 32, 2109731. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. “The Fe Effect”: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, H.; Li, A.; Li, Z.; Liu, S.; Zhang, Q.; Gong, Y.; Zheng, L.; Zhu, Y.; Chen, C.; et al. Constructing NiCo/Fe3O4 Heteroparticles within MOF-74 for Efficient Oxygen Evolution Reactions. J. Am. Chem. Soc. 2018, 140, 15336–15341. [Google Scholar] [CrossRef]
- Gao, W.-K.; Yang, M.; Chi, J.-Q.; Zhang, X.-Y.; Xie, J.-Y.; Guo, B.-Y.; Wang, L.; Chai, Y.-M.; Dong, B. In situ construction of surface defects of carbon-doped ternary cobalt-nickel-iron phosphide nanocubes for efficient overall water splitting. Sci. China Mater. 2019, 62, 1285–1296. [Google Scholar] [CrossRef]
- Du, J.; You, S.; Li, X.; Tang, B.; Jiang, B.; Yu, Y.; Cai, Z.; Ren, N.; Zou, J. In Situ Crystallization of Active NiOOH/CoOOH Heterostructures with Hydroxide Ion Adsorption Sites on Velutipes-like CoSe/NiSe Nanorods as Catalysts for Oxygen Evolution and Cocatalysts for Methanol Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 686–697. [Google Scholar] [CrossRef]
- Liu, T.; Asiri, A.M.; Sun, X. Electrodeposited Co-doped NiSe2nanoparticles film: A good electrocatalyst for efficient water splitting. Nanoscale 2016, 8, 3911–3915. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zou, Z.; Liu, C.; Xu, C. Hierarchical Fe-doped Ni3Se4 ultrathin nanosheets as an efficient electrocatalyst for oxygen evolution reaction. Nanoscale 2018, 10, 5163–5170. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, G.; Liu, Y.; Xia, J.; Ji, Z.; Shen, X.; Wu, S. Fe3O4-Decorated Co9S8 Nanoparticles In Situ Grown on Reduced Graphene Oxide: A New and Efficient Electrocatalyst for Oxygen Evolution Reaction. Adv. Funct. Mater. 2016, 26, 4712–4721. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, Y.; Sun, Y.; Zhu, C.; Hu, L.; Gao, J.; Huang, H.; Liu, Y.; Kang, Z. Cobalt phosphide/carbon dots composite as an efficient electrocatalyst for oxygen evolution reaction. Dalton Trans. 2018, 47, 5459–5464. [Google Scholar] [CrossRef]
- Yuan, M.; Dipazir, S.; Wang, M.; Sun, Y.; Gao, D.; Bai, Y.; Zhang, M.; Lu, P.; He, H.; Zhu, X.; et al. Polyoxometalate-assisted formation of CoSe/MoSe2 heterostructures with enhanced oxygen evolution activity. J. Mater. Chem. A 2019, 7, 3317–3326. [Google Scholar] [CrossRef]
- Thambidurai, M.; Kim, J.Y.; Song, J.; Ko, Y.; Muthukumarasamy, N.; Velauthapillai, D.; Lee, C. Nanocrystalline Ga-doped ZnO thin films for inverted polymer solar cells. Sol. Energy 2014, 106, 95–101. [Google Scholar] [CrossRef]
- Xu, X.J.; Li, J.Y.; Zhang, C.H.; Zhang, S.C.; Su, G.; Shi, Z.C.; Wang, H.L.; Huang, M.H. Controllable transition engineering from homogeneous NiSe2 nanowrinkles to heterogeneous Ni3Se4/NiSe2 rod-like nanoarrays for promoted urea-rich water oxidation at large current densities. Appl. Catal. B. 2022, 319, 121949. [Google Scholar] [CrossRef]
- Chen, H.; Fan, M.; Li, C.; Tian, G.; Lv, C.; Chen, D.; Shu, K.; Jiang, J. One-pot synthesis of hollow NiSe–CoSe nanoparticles with improved performance for hybrid supercapacitors. J. Power Sources 2016, 329, 314–322. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, R.; Chen, F.; Yan, G.; Tian, W.; Zhang, J.; Zhang, J. Electrochemical process of early-stage corrosion detection based on N-doped carbon dots with superior Fe3+ responsiveness. J. Colloid Interface Sci. 2021, 606, 567–576. [Google Scholar] [CrossRef]
- Li, N.; Bediako, D.K.; Hadt, R.G.; Hayes, D.; Kempa, T.J.; von Cube, F.; Bell, D.C.; Chen, L.X.; Nocera, D.G. Influence of iron doping on tetravalent nickel content in catalytic oxygen evolving films. Proc. Natl. Acad. Sci. USA 2017, 114, 1486–1491. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Y.; Zhang, G.; Wu, M.; Chen, Z.; Sun, S.; Shi, Z. MnOx-Decorated Nickel-Iron Phosphides Nanosheets: Interface Modifications for Robust Overall Water Splitting at Ultra-High Current Densities. Small 2022, 18, 2105803. [Google Scholar] [CrossRef]
- Wang, P.; Qi, J.; Chen, X.; Li, C.; Li, W.; Wang, T.; Liang, C. Three-Dimensional Heterostructured NiCoP@NiMn-Layered Double Hydroxide Arrays Supported on Ni Foam as a Bifunctional Electrocatalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 4385–4395. [Google Scholar] [CrossRef]
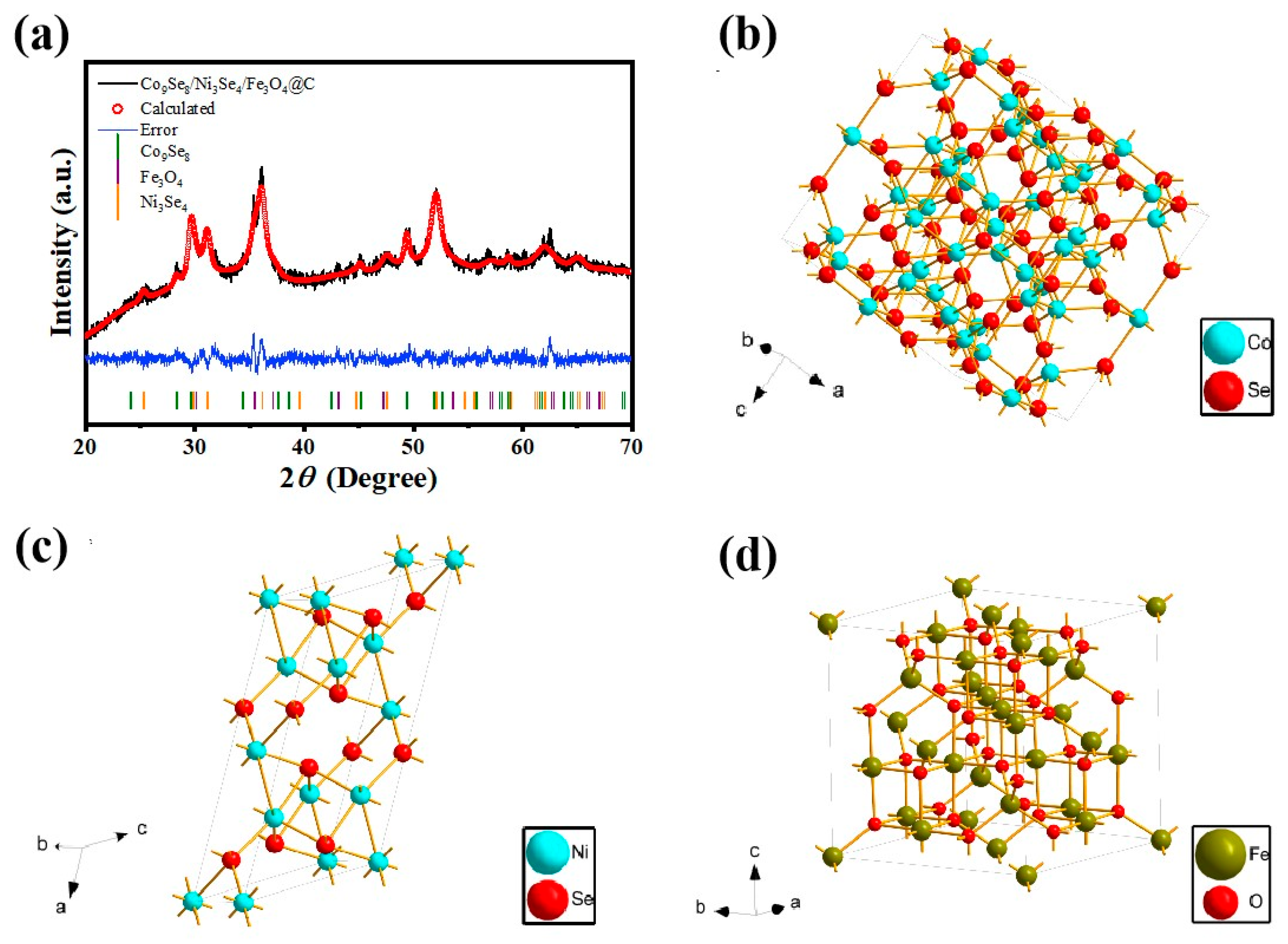
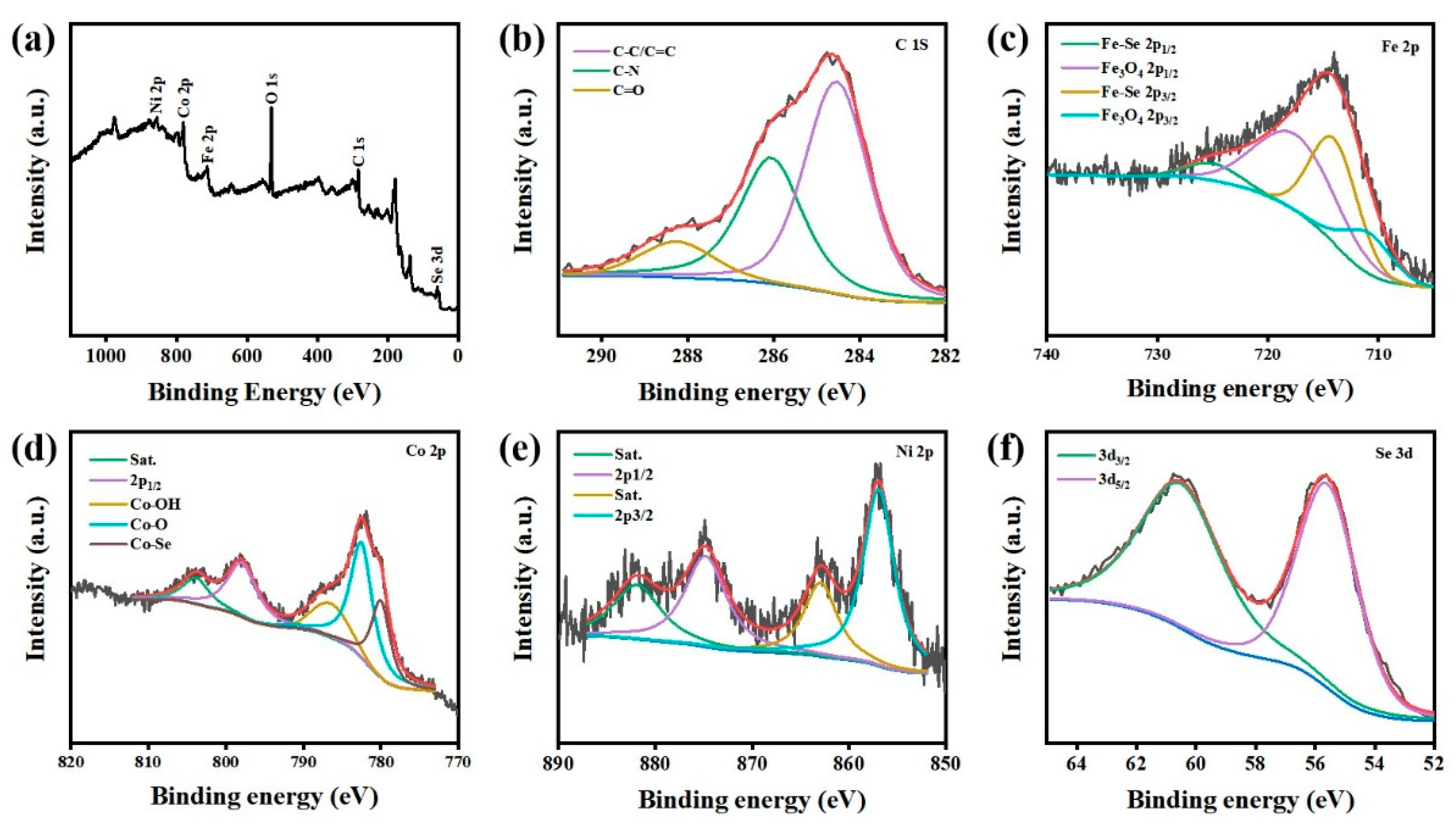

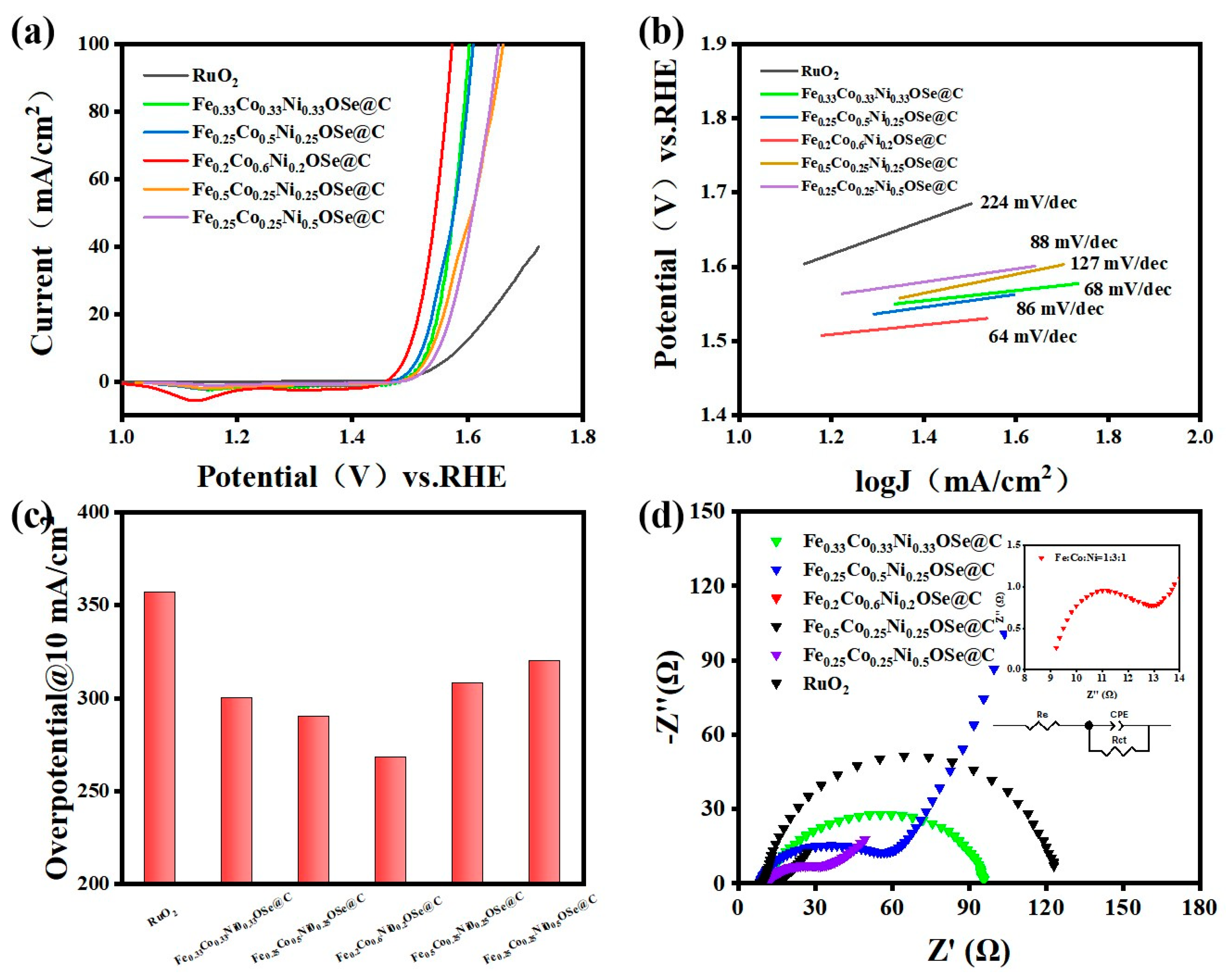
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




