Submitted:
22 May 2023
Posted:
23 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Bacterial isolates
2.2. Antimicrobial Susceptibility Testing
2.3. Detection of carbapenemase genes
2.4. Assessment of biofilm formation
2.4.1. Quantitative of biofilm formation assessment
2.4.2. Detection of biofilm-associated genes
2.5. Statistical analysis
3. Results
3.1. Bacterial isolates
3.2. Antimicrobial Susceptibility Testing
3.3. Selected carbapenemases genes
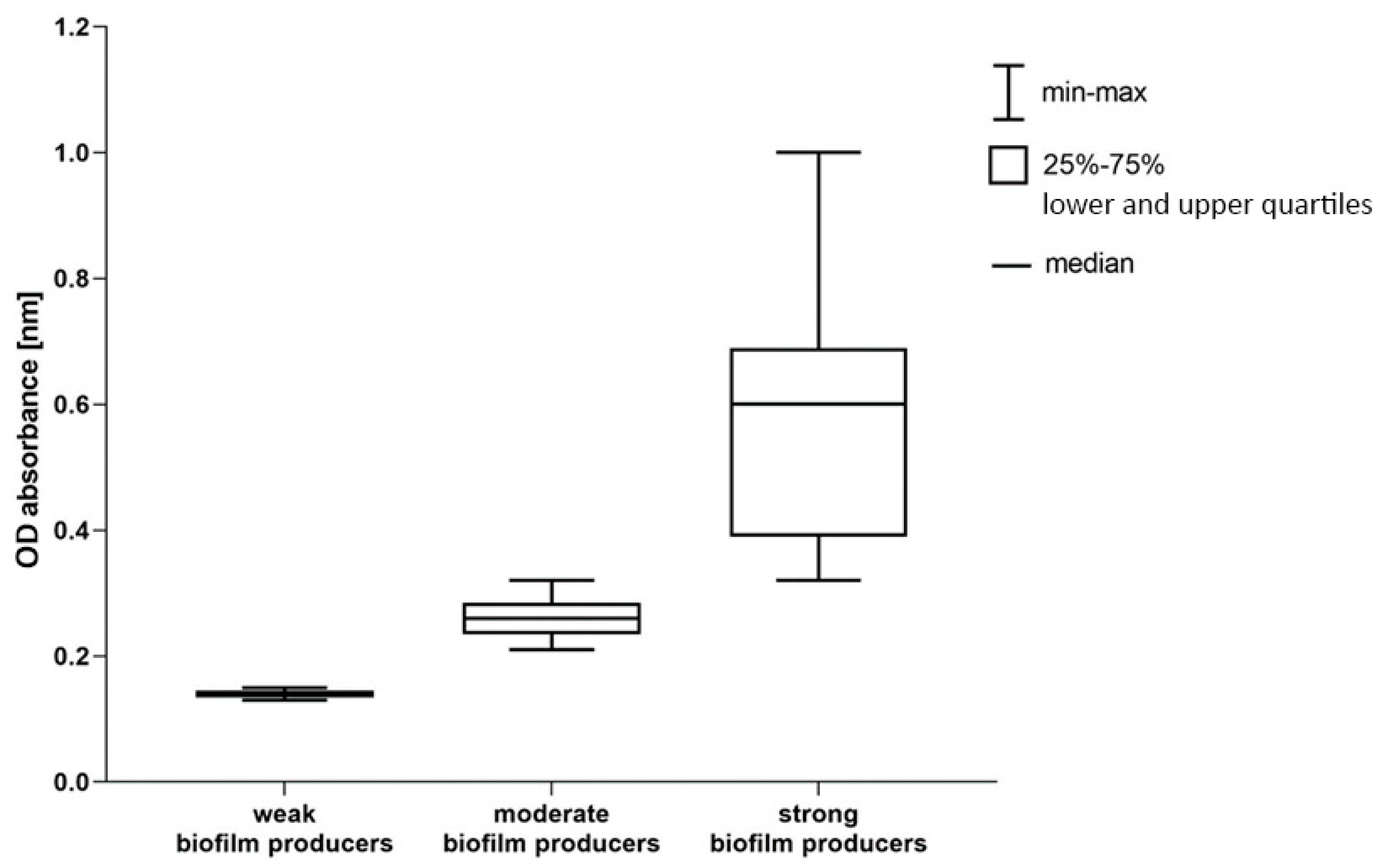
3.4. Quantitative biofilm formation assessment
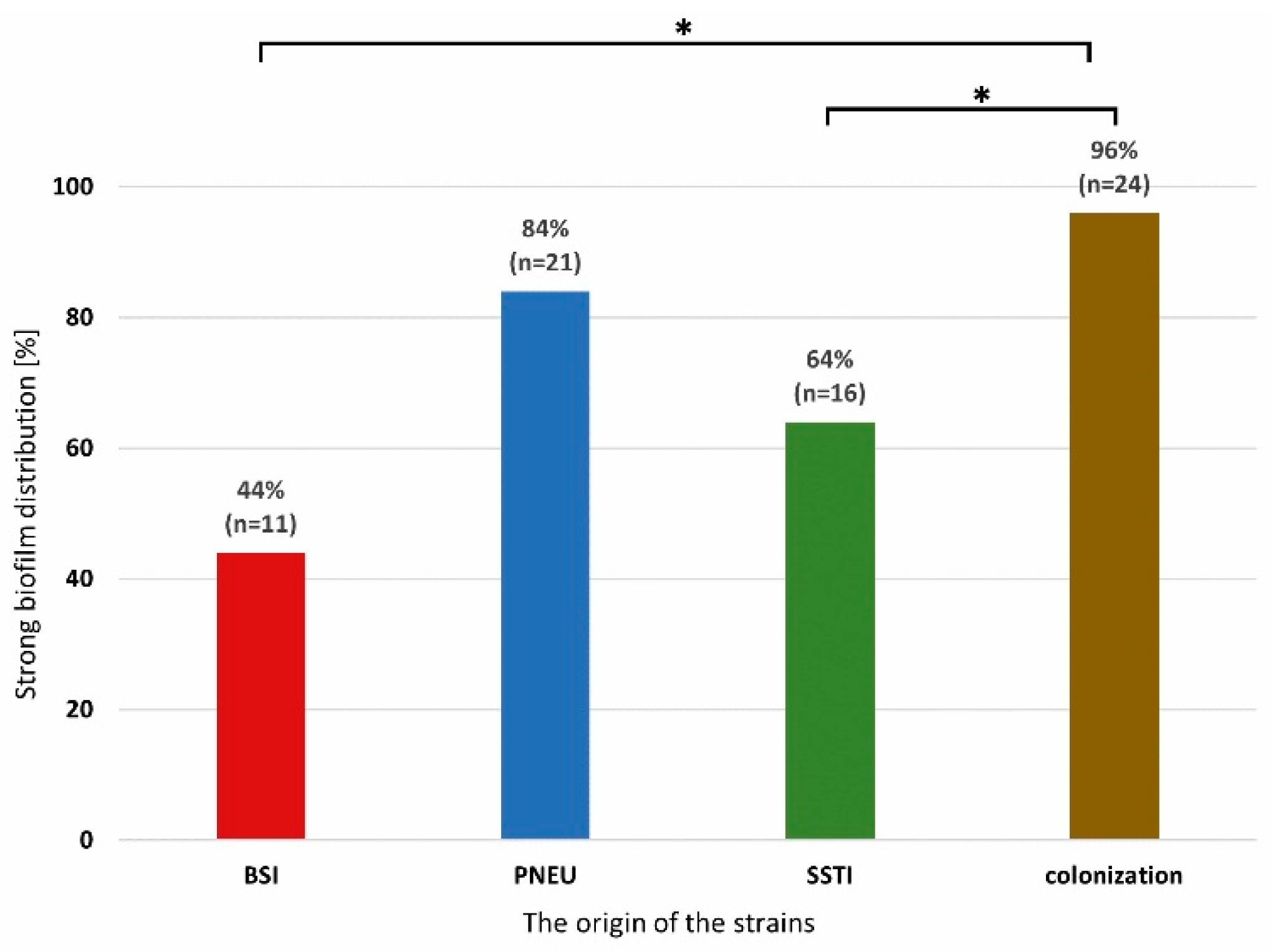
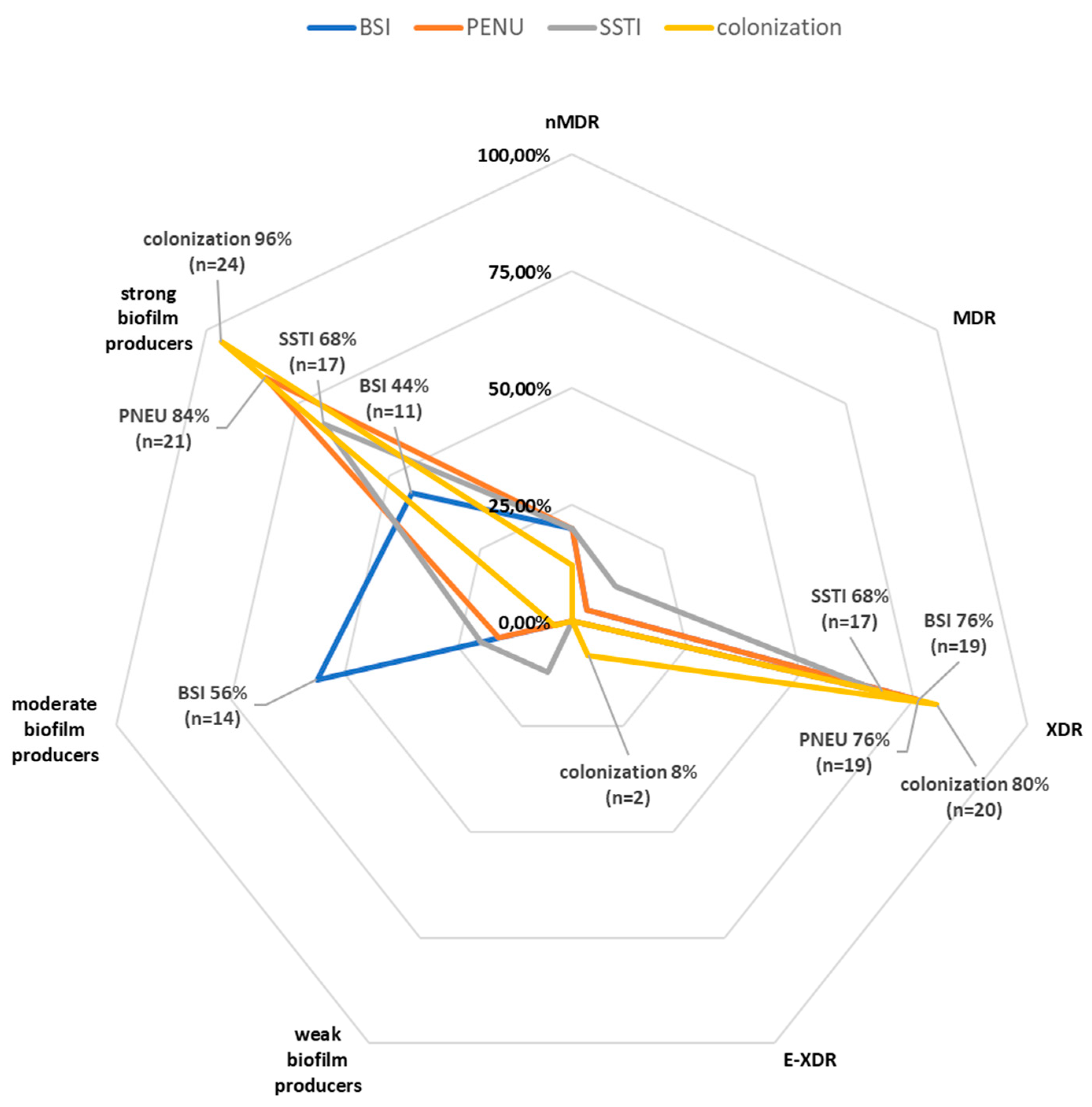
3.4. Correlation between the ability to biofilm formation and resistance among strains with different clinical forms of infection or colonization.
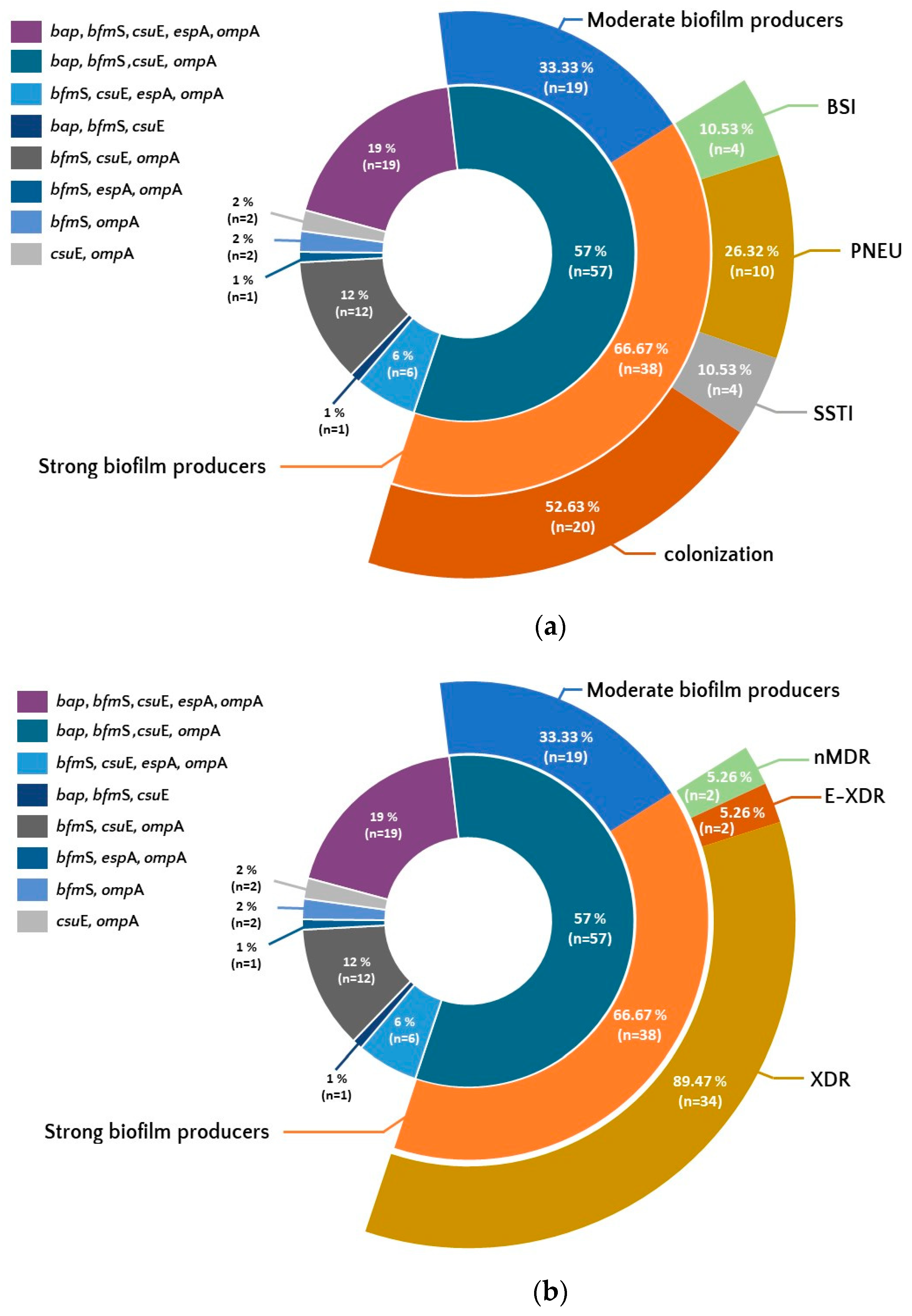
3.5. Selected biofilm associated genes.
4. Discussion
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 7 February 2023).
- Nguyen, M.; Joshi, S.G. Carbapenem Resistance in Acinetobacter Baumannii, and Their Importance in Hospital-Acquired Infections: A Scientific Review. J. Appl. Microbiol. 2021, 131, 2715–2738. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; Lebeaux, D.; Oliver, A.; Ullmann, A.J.; Williams, C. ; ESCMID Study Group for Biofilms (ESGB); Consulting External Expert Werner Zimmerli ESCMID Guideline for the Diagnosis and Treatment of Biofilm Infections 2014. Clin. Microbiol. Infect. 2015, 21 Suppl 1, S1–S25. [Google Scholar] [CrossRef]
- Gedefie, A.; Demsis, W.; Ashagrie, M.; Kassa, Y.; Tesfaye, M.; Tilahun, M.; Bisetegn, H.; Sahle, Z. Acinetobacter Baumannii Biofilm Formation and Its Role in Disease Pathogenesis: A Review. Infect. Drug Resist. 2021, 14, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Surveillance in Europe 2022 - 2020 Data. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2022-2020-data (accessed on 7 February 2023).
- Antimicrobial Resistance in the EU/EEA (EARS-Net) - Annual Epidemiological Report for 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-ears-net-annual-epidemiological-report-2020 (accessed on 7 February 2023).
- Chmielarczyk, A.; Pilarczyk-Zurek, M.; Kamińska, W.; Pobiega, M.; Romaniszyn, D.; Ziółkowski, G.; Wójkowska-Mach, J.; Bulanda, M. Molecular Epidemiology and Drug Resistance of Acinetobacter Baumannii Isolated from Hospitals in Southern Poland: ICU as a Risk Factor for XDR Strains. Microb. Drug Resist. 2016, 22, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Wałaszek, M.; Różańska, A.; Bulanda, M.; Wojkowska-Mach, J. ; Polish Society of Hospital Infections Team Alarming Results of Nosocomial Bloodstream Infections Surveillance in Polish Intensive Care Units. Przegl. Epidemiol. 2018, 72, 33–44. [Google Scholar]
- Azimi, L.; Fallah, F.; Karimi, A.; Shirdoust, M.; Azimi, T.; Sedighi, I.; Rahbar, M.; Armin, S. Survey of Various Carbapenem-Resistant Mechanisms of Acinetobacter Baumannii and Pseudomonas Aeruginosa Isolated from Clinical Samples in Iran. Iran. J. Basic Med. Sci. 2020, 23, 1396–1400. [Google Scholar] [CrossRef]
- Zeighami, H.; Valadkhani, F.; Shapouri, R.; Samadi, E.; Haghi, F. Virulence Characteristics of Multidrug Resistant Biofilm Forming Acinetobacter Baumannii Isolated from Intensive Care Unit Patients. BMC Infect. Dis. 2019, 19. [Google Scholar] [CrossRef]
- Adams-Haduch, J.M.; Paterson, D.L.; Sidjabat, H.E.; Pasculle, A.W.; Potoski, B.A.; Muto, C.A.; Harrison, L.H.; Doi, Y. Genetic Basis of Multidrug Resistance in Acinetobacter Baumannii Clinical Isolates at a Tertiary Medical Center in Pennsylvania. Antimicrob. Agents Chemother. 2008, 52, 3837–3843. [Google Scholar] [CrossRef]
- Falagas, M.E.; Karveli, E.A.; Kelesidis, I.; Kelesidis, T. Community-Acquired Acinetobacter Infections. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 857–868. [Google Scholar] [CrossRef]
- Ababneh, Q.; Al-Rousan, E.; Jaradat, Z. Fresh Produce as a Potential Vehicle for Transmission of Acinetobacter Baumannii. Int. J. Food Contam. 2022, 9, 1–9. [Google Scholar] [CrossRef]
- Carvalheira, A.; Silva, J.; Teixeira, P. Lettuce and Fruits as a Source of Multidrug Resistant Acinetobacter Spp. Food Microbiol. 2017, 64, 119–125. [Google Scholar] [CrossRef]
- Rafei, R.; Hamze, M.; Pailhoriès, H.; Eveillard, M.; Marsollier, L.; Joly-Guillou, M.L.; Dabboussi, F.; Kempf, M. Extrahuman Epidemiology of Acinetobacter Baumannii in Lebanon. Appl. Environ. Microbiol. 2015, 81, 2359–2367. [Google Scholar] [CrossRef]
- Ababneh, Q.; Abu Laila, S.; Jaradat, Z. Prevalence, Genetic Diversity, Antibiotic Resistance and Biofilm Formation of Acinetobacter Baumannii Isolated from Urban Environments. J. Appl. Microbiol. 2022, 133, 3617–3633. [Google Scholar] [CrossRef]
- Thummeepak, R.; Kongthai, P.; Leungtongkam, U.; Sitthisak, S. Distribution of Virulence Genes Involved in Biofilm Formation in Multi-Drug Resistant Acinetobacter Baumannii Clinical Isolates. Int. Microbiol. 2016, 19, 121–129. [Google Scholar] [CrossRef]
- Greene, C.; Vadlamudi, G.; Newton, D.; Foxman, B.; Xi, C. The Influence of Biofilm Formation and Multidrug Resistance on Environmental Survival of Clinical and Environmental Isolates of Acinetobacter Baumannii. Am. J. Infect. Control 2016, 44, e65–e71. [Google Scholar] [CrossRef]
- Lin, M.-F.; Lan, C.-Y. Antimicrobial Resistance in Acinetobacter Baumannii: From Bench to Bedside. World J. Clin. Cases WJCC 2014, 2, 787. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the Mechanisms of Acinetobacter Baumannii Virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Badave, G.K.; Dhananjay, K. Biofilm Producing Multidrug Resistant Acinetobacter Baumannii: An Emerging Challenge. J. Clin. Diagn. Res. 2015, 9, DC08. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.Y.; Baek, W.K.; Kim, H.A. Association of Biofilm Production with Colonization among Clinical Isolates of Acinetobacter Baumannii. Korean J. Intern. Med. 2017, 32, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Rumbo-Feal, S.; Gómez, M.J.; Gayoso, C.; Álvarez-Fraga, L.; Cabral, M.P.; Aransay, A.M.; Rodríguez-Ezpeleta, N.; Fullaondo, A.; Valle, J.; Tomás, M.; Bou, G.; Poza, M. Whole Transcriptome Analysis of Acinetobacter Baumannii Assessed by RNA-Sequencing Reveals Different MRNA Expression Profiles in Biofilm Compared to Planktonic Cells. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Szczypta, A.; Talaga-ćwiertnia, K.; Kielar, M.; Krzyściak, P.; Gajewska, A.; Szura, M.; Bulanda, M.; Chmielarczyk, A. Investigation of Acinetobacter Baumannii Activity in Vascular Surgery Units through Epidemiological Management Based on the Analysis of Antimicrobial Resistance, Biofilm Formation and Genotyping. Int. J. Environ. Res. Public Health 2021, 18, 1–15. [Google Scholar] [CrossRef]
- Eucast: The 13.0 Versions of Breakpoints, Dosing and QC (2023) Published. Available online: https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=518&cHash=2509b0db92646dffba041406dcc9f20c (accessed on 23 April 2023).
- CLSI Publishes M100—Performance Standards for Antimicrobial Susceptibility Testing, 31st Edition. Available online: https://clsi.org/about/press-releases/clsi-publishes-m100-performance-standards-for-antimicrobial-susceptibility-testing-31st-edition/ (accessed on 7 February 2023).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; Paterson, D.L.; Rice, L.B.; Stelling, J.; Struelens, M.J.; Vatopoulos, A.; Weber, J.T.; Monnet, D.L. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Mohamed, H.M.A.; Abd-Elhafeez, H.H.; Al-Jabr, O.A.; El-Zamkan, M.A. Characterization of Acinetobacter Baumannii Isolated from Raw Milk. Biology (Basel). 2022, 11, 1845. [Google Scholar] [CrossRef]
- Cerezales, M.; Biniossek, L.; Gerson, S.; Xanthopoulou, K.; Wille, J.; Wohlfarth, E.; Kaase, M.; Seifert, H.; Higgins, P.G. Novel Multiplex PCRs for Detection of the Most Prevalent Carbapenemase Genes in Gram-Negative Bacteria within Germany. J. Med. Microbiol. 2021, 70. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Sharon Goh, H.M.; Beatson, S.A.; Totsika, M.; Moriel, D.G.; Phan, M.D.; Szubert, J.; Runnegar, N.; Sidjabat, H.E.; Paterson, D.L.; Nimmo, G.R.; Lipman, J.; Schembri, M.A. Molecular Analysis of the Acinetobacter Baumannii Biofilm-Associated Protein. Appl. Environ. Microbiol. 2013, 79, 6535. [Google Scholar] [CrossRef]
- Turton, J.F.; Gabriel, S.N.; Valderrey, C.; Kaufmann, M.E.; Pitt, T.L. Use of Sequence-Based Typing and Multiplex PCR to Identify Clonal Lineages of Outbreak Strains of Acinetobacter Baumannii. Clin. Microbiol. Infect. 2007, 13, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Tayabali, A.F.; Nguyen, K.C.; Shwed, P.S.; Crosthwait, J.; Coleman, G.; Seligy, V.L. Comparison of the Virulence Potential of Acinetobacter Strains from Clinical and Environmental Sources. PLoS One 2012, 7, e37024. [Google Scholar] [CrossRef] [PubMed]
- Liou, M.L.; Soo, P.C.; Ling, S.R.; Kuo, H.Y.; Tang, C.Y.; Chang, K.C. The Sensor Kinase BfmS Mediates Virulence in Acinetobacter Baumannii. J. Microbiol. Immunol. Infect. 2014, 47, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Chmielarczyk, A.; Pobiega, M.; Romaniszyn, D.; Wójkowska-Mach, J. Multi-Locus Sequence Typing (MLST) of Non-Fermentative Gram-Negative Bacilli Isolated from Bloodstream Infections in Southern Poland. Folia Microbiol. (Praha). 2018, 63, 191. [Google Scholar] [CrossRef]
- Ziółkowski, G.; Pawłowska, I.; Krawczyk, L.; Wojkowska-Mach, J. Antibiotic Consumption versus the Prevalence of Multidrug-Resistant Acinetobacter Baumannii and Clostridium Difficile Infections at an ICU from 2014-2015. J. Infect. Public Health 2018, 11, 626–630. [Google Scholar] [CrossRef]
- Inchai, J.; Liwsrisakun, C.; Theerakittikul, T.; Chaiwarith, R.; Khositsakulchai, W.; Pothirat, C. Risk Factors of Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Acinetobacter Baumannii Ventilator-Associated Pneumonia in a Medical Intensive Care Unit of University Hospital in Thailand. J. Infect. Chemother. 2015, 21, 570–574. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoon, J.H.; Kim, S.I.; Hong, K.W.; Kim, J.I.; Choi, J.Y.; Yoon, S.K.; You, Y.K.; Lee, M.D.; Moon, I.S.; Kim, D.G.; Kang, M.W. High Mortality Associated with Acinetobacter Species Infection in Liver Transplant Patients. Transplant. Proc. 2011, 43, 2397–2399. [Google Scholar] [CrossRef]
- Depka, D.; Mikucka, A.; Bogiel, T.; Rzepka, M.; Zawadka, P.; Gospodarek-Komkowska, E. Conventional and Real-Time PCR Targeting BlaOXA Genes as Reliable Methods for a Rapid Detection of Carbapenem-Resistant Acinetobacter Baumannii Clinical Strains. Antibiotics 2022, 11. [Google Scholar] [CrossRef]
- Słoczyńska, A.; Wand, M.E.; Tyski, S.; Laudy, A.E. Analysis of BlaCHDL Genes and Insertion Sequences Related to Carbapenem Resistance in Acinetobacter Baumannii Clinical Strains Isolated in Warsaw, Poland. Int. J. Mol. Sci. 2021, Vol. 22, Page 2486 2021, 22, 2486. [Google Scholar] [CrossRef]
- Yoon, E.J.; Balloy, V.; Fiette, L.; Chignard, M.; Courvalin, P.; Grillot-Courvalin, C. Contribution of the Ade Resistance-Nodulation-Cell Division-Type Efflux Pumps to Fitness and Pathogenesis of Acinetobacter Baumannii. MBio 2016, 7. [Google Scholar] [CrossRef]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of Bacterial Efflux Pumps in Biofilm Formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef]
- Kim, C.M.; Park, G.; Ko, Y.J.; Kang, S.H.; Jang, S.J. Relationships between Relative Expression of RND Efflux Pump Genes, H33342 Efflux Activity, Biofilm-Forming Activity, and Antimicrobial Resistance in Acinetobacter Baumannii Clinical Isolates. Jpn. J. Infect. Dis. 2021, 74, 499–506. [Google Scholar] [CrossRef]
- Yang, C.H.; Su, P.W.; Moi, S.H.; Chuang, L.Y. Biofilm Formation in Acinetobacter Baumannii: Genotype-Phenotype Correlation. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Ranjbar, R.; Farahani, A. Study of Genetic Diversity, Biofilm Formation, and Detection of Carbapenemase, MBL, ESBL, and Tetracycline Resistance Genes in Multidrug-Resistant Acinetobacter Baumannii Isolated from Burn Wound Infections in Iran. Antimicrob. Resist. Infect. Control 2019, 8. [Google Scholar] [CrossRef]
- Celik, B. Evaluation of the Correlation between Biofilm Formation and Drug Resistance in Clinical Isolates of Acinetobacter Baumannii. Int. J. Pathog. Res. 2020, 5, 16–27. [Google Scholar] [CrossRef]
- Asaad, A.M.; Soma, S.A.; Ajlan, E.; Awad, S.M. Epidemiology of Biofilm Producing Acinetobacter Baumannii Nosocomial Isolates from a Tertiary Care Hospital in Egypt: A Cross-Sectional Study. Infect. Drug Resist. 2021, 14, 709–717. [Google Scholar] [CrossRef]
- Krzyściak, P.; Chmielarczyk, A.; Pobiega, M.; Romaniszyn, D.; Wójkowska-Mach, J. Acinetobacter Baumannii Isolated from Hospital-Acquired Infection: Biofilm Production and Drug Susceptibility. APMIS 2017, 125, 1017–1026. [Google Scholar] [CrossRef]
- Qi, L.; Li, H.; Zhang, C.; Liang, B.; Li, J.; Wang, L.; Du, X.; Liu, X.; Qiu, S.; Song, H. Relationship between Antibiotic Resistance, Biofilm Formation, and Biofilm-Specific Resistance in Acinetobacter Baumannii. Front. Microbiol. 2016, 7, 483. [Google Scholar] [CrossRef]
- Ababneh, Q.; Abulaila, S.; Jaradat, Z. Isolation of Extensively Drug Resistant Acinetobacter Baumannii from Environmental Surfaces inside Intensive Care Units. Am. J. Infect. Control 2022, 50, 159–165. [Google Scholar] [CrossRef]
- Ghasemi, E.; Ghalavand, Z.; Goudarzi, H.; Yeganeh, F.; Hashemi, A.; Dabiri, H.; Mirsamadi, E.S.; Foroumand, M. Phenotypic and Genotypic Investigation of Biofilm Formation in Clinical and Environmental Isolates of Acinetobacter Baumannii. Arch. Clin. Infect. Dis. 2018 134 2018, 13, 12914. [Google Scholar] [CrossRef]
- Poulikakos, P.; Tansarli, G.S.; Falagas, M.E. Combination Antibiotic Treatment versus Monotherapy for Multidrug-Resistant, Extensively Drug-Resistant, and Pandrug-Resistant Acinetobacter Infections: A Systematic Review. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1675–1685. [Google Scholar] [CrossRef]
- Papathanakos, G.; Andrianopoulos, I.; Papathanasiou, A.; Koulenti, D.; Gartzonika, K.; Koulouras, V. Pandrug-Resistant Acinetobacter Baumannii Treatment: Still a Debatable Topic with No Definite Solutions. 2020, 75. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Pandrug-Resistant Gram-Negative Bacteria: A Systematic Review of Current Epidemiology, Prognosis and Treatment Options. J. Antimicrob. Chemother. 2020, 75, 271–282. [Google Scholar] [CrossRef]
- Sobouti, B.; Mirshekar, M.; Fallah, S.; Tabaei, A.; Mehrabadi, J.F.; Darbandi, A. Pan Drug-Resistant Acinetobacter Baumannii Causing Nosocomial Infections among Burnt Children. Med. J. Islam. Repub. Iran 2020, 34, 24. [Google Scholar] [CrossRef]
- Wang, Y.C.; Kuo, S.C.; Yang, Y.S.; Lee, Y.T.; Chiu, C.H.; Chuang, M.F.; Lin, J.C.; Chang, F.Y.; Chen, T.L. Individual or Combined Effects of Meropenem, Imipenem, Sulbactam, Colistin, and Tigecycline on Biofilm-Embedded Acinetobacter Baumannii and Biofilm Architecture. Antimicrob. Agents Chemother. 2016, 60, 4670–4676. [Google Scholar] [CrossRef]
- Song, J.Y.; Cheong, H.J.; Noh, J.Y.; Kim, W.J. In Vitro Comparison of Anti-Biofilm Effects against Carbapenem-Resistant Acinetobacter Baumannii: Imipenem, Colistin, Tigecycline, Rifampicin and Combinations. Infect. Chemother. 2015, 47, 27. [Google Scholar] [CrossRef]
- Peng, Q.; Lin, F.; Ling, B. In Vitro Activity of Biofilm Inhibitors in Combination with Antibacterial Drugs against Extensively Drug-Resistant Acinetobacter Baumannii. Sci. Reports 2020 101 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Roberts, K.D.; Zhu, Y.; Azad, M.A.K.; Han, M.L.; Wang, J.; Wang, L.; Yu, H.H.; Horne, A.S.; Pinson, J.A.; Rudd, D.; Voelcker, N.H.; Patil, N.A.; Zhao, J.; Jiang, X.; Lu, J.; Chen, K.; Lomovskaya, O.; Hecker, S.J.; Thompson, P.E.; et al. A Synthetic Lipopeptide Targeting Top-Priority Multidrug-Resistant Gram-Negative Pathogens. Nat. Commun. 2022, 13, 1625. [Google Scholar] [CrossRef]
- Selvaraj, A.; Valliammai, A.; Sivasankar, C.; Suba, M.; Sakthivel, G.; Pandian, S.K. Antibiofilm and Antivirulence Efficacy of Myrtenol Enhances the Antibiotic Susceptibility of Acinetobacter Baumannii. Sci. Reports 2020 101 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Vukotic, G.; Obradovic, M.; Novovic, K.; Di Luca, M.; Jovcic, B.; Fira, D.; Neve, H.; Kojic, M.; McAuliffe, O. Characterization, Antibiofilm, and Depolymerizing Activity of Two Phages Active on Carbapenem-Resistant Acinetobacter Baumannii. Front. Med. 2020, 7, 426. [Google Scholar] [CrossRef]
- Grygorcewicz, B.; Wojciuk, B.; Roszak, M.; Łubowska, N.; Blstrokaejczak, P.; Jursa-Kulesza, J.; Rakoczy, R.; Masiuk, H.; Dogowska, B. Environmental Phage-Based Cocktail and Antibiotic Combination Effects on Acinetobacter Baumannii Biofilm in a Human Urine Model. Microb. Drug Resist. 2021, 27, 25–35. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; Tsuji, M.; Wise, M.G.; Hackel, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, against a Recent Collection of Clinically Relevant Carbapenem-Non-Susceptible Gram-Negative Bacilli, Including Serine Carbapenemase- and Metallo-β-Lactamase-Producing Isolates (SIDERO-WT-2014 Study). Int. J. Antimicrob. Agents 2019, 53, 177–184. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Castaldo, N.; Giacobbe, D.R.; Peghin, M.; Grossi, P.A. Clinical Evidence Supporting Cefiderol for Serious Acinetobacter Baumannii Infections. Curr. Opin. Infect. Dis. 2022, 35, 545–551. [Google Scholar] [CrossRef]
- European Medicines Agency Fetcroja | European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/fetcroja (accessed on 16 March 2023).
- RPL. Available online: https://rejestrymedyczne.ezdrowie.gov.pl/rpl/search/public (accessed on 16 March 2023).
- Kinross, P.; Gagliotti, C.; Merk, H.; Plachouras, D.; Monnet, D.L.; Högberg, L.D. ; EARS-Net Study Group; EARS-Net Study Group participants Large Increase in Bloodstream Infections with Carbapenem-Resistant Acinetobacter Species during the First 2 Years of the COVID-19 Pandemic, EU/EEA, 2020 and 2021. Euro Surveill. 2022, 27, 2200845. [Google Scholar] [CrossRef]
| Detected genes | Primer sequences (5’-3’)1 | Product size (bp) | Annealing temperature | Reference |
|---|---|---|---|---|
| blaOXA-23 | F: TCTGGTTGTACGGTTCAGCA | 718 | 58 °C | [29] |
| R: GCATTTCTGACCGCATTTCC | ||||
| blaOXA-40 | F: GCATTGTCAGCAGTTCCAGT | 402 | 58 °C | [29] |
| R: AGAACCAGACATTCCTTCTTTCA | ||||
| blaNDM | F: GTTTGATCGTCAGGGATGGC | 517 | 58 °C | [29] |
| R: CTCATCACGATCATGCTGGC | ||||
| blaOXA-58 | F: ATCAAGAATTGGCACGTCGT | 303 | 58 °C | [29] |
| R: CCACATACCAACCCACTTGC |
| Detected genes | Primer sequences (5’-3’)1 | Product size (bp) | Annealing temperature | Reference |
|---|---|---|---|---|
| bap | F: TACTTCCAATCCAATGCTAGGGAGGGTACCAATGCAG | 1225 | 56.5 °C | [31] |
| R: TTATCCACTTCCAATGATCAGCAACCAAACCGCTAC | ||||
| csuE | F: ATGCATGTTCTCTGGACTGATGTTGAC | 976 | 57 °C | [32] |
| R: CGACTTGTACCGTGACCGTATCTTGATAAG | ||||
| ompA | F: CGCTTCTGCTGGTGCTGAAT | 531 | 55 °C | [33] |
| R: CGTGCAGTAGCGTTAGGGTA | ||||
| bfmS | F: TTGCTCGAACTTCCAATTTATTATAC | 1368 | 55 °C | [34] |
| R: TTATGCAGGTGCTTTTTTATTGGTC | ||||
| espA | F: AGCAAGTGGTTATCCAATCG | 451 | 55 °C | [33] |
| R: ACCAGACTCACCCATTACAT |
| The origin of the strains |
ICU |
non-ICU |
||
|---|---|---|---|---|
| n (%) | % of total N=100 |
n (%) | % of total N=100 |
|
| BSI | 11 (20.7) | 44% | 14 (29.8) | 56% |
| PNEU | 14 (26.4) | 56% | 11 (23.4) | 44% |
| SSTI | 3 (5.7) | 12% | 22 (46.8) | 88% |
| colonization | 25 (47.2) | 100% | 0 (0) | 0% |
| Total | 53 (100) | 53% | 47 (100) | 47% |
| Antibiotic Classes | Antimicrobial | Resistant Isolate; number (%) | ||||
|---|---|---|---|---|---|---|
| Total | The origin of the strains | |||||
| BSI | PNEU | SSTI | colonization | |||
| Penicillin | ampicillin/sulbactam piperacillin/tazobactam |
65 (65%) 86 (86%) |
16 (64%) 21 (84%) |
17 (68%) 21 (84%) |
12 (48%) 22 (88%) |
20 (80%) 22 (88%) |
| Cephalosporins | cefoperazone/sulbactam | 95 (95%) | 23 (92%) | 24 (96%) | 23 (92%) | 25 (100%) |
| Carbapenems | Imipenem* meropenem* |
69 (69%) 69 (69%) |
18 (72%) 18 (72%) |
17 (68%) 17 (68%) |
12 (48%) 12 (48%) |
22 (88%) 22 (88%) |
| Fluoroquinolones | ciprofloxacin levofloxacin |
87 (87%) 80 (80%) |
23 (92%) 19 (76%) |
23 (92%) 21 (82%) |
18 (68%) 18 (72%) |
22 (88%) 22 (88%) |
| Aminoglycosides | amikacin gentamycin** tobramycin |
69 (69%) 55 (55%) 70 (70%) |
19 (76%) 19 (76%) 17 (68%) |
18 (72%) 14 (56%) 19 (76%) |
14 (56%) 15 (60%) 16 (64%) |
18 (72%) 7 (28%) 18 (72%) |
| Tetracyclines | tigecycline | 71 (71%) | 18 (72%) | 19 (76%) | 15 (60%) | 19 (76%) |
| Miscellaneous agents | colistin trimethoprim/sulfamethoxazole |
8 (8%) 77 (77%) |
0 (0%) 20 (80%) |
0 (0%) 19 (76%) |
0 (0%) 16 (64%) |
8 (32%) 22 (88%) |
| Antibiotic Patterns* | No. of Isolates | MAR Index |
|---|---|---|
| SAM, TZP, SCF, IMP, MEM, CIP, LEV, AMI, GEN, TN, TIG, SXT, | 37 | 0,92 |
| SAM, TZP, SCF, IMP, MEM, CIP, LEV, AMI, TN, TIG, SXT, | 10 | 0,85 |
| TZP, SCF, IMP, MEM, CIP, LEV, AMI, GEN, TN, TIG, SXT, | 5 | 0,85 |
| SCF, | 4 | 0,08 |
| SCF, CIP, | 4 | 0,15 |
| SAM, TZP, SCF, IMP, MEM, CIP, LEV, AMI, TN, TIG, SXT, CL | 3 | 0,92 |
| CIP, | 2 | 0,08 |
| SAM, TZP, SCF, CIP, LEV, AMI, TN, TIG, SXT, | 2 | 0,69 |
| SAM, TZP, SCF, CIP, LEV, GEN, TIG, SXT, | 2 | 0,62 |
| SAM, TZP, SCF, IMP, MEM, CIP, LEV, AMI, GEN, TN, TIG, SXT, CL | 2 | 1,00 |
| SAM, TZP, SCF, IMP, MEM, CIP, LEV, AMI, TN, SXT, | 2 | 0,77 |
| SAM, TZP, SCF, IMP, MEM, CIP, LEV, TIG, SXT, CL | 2 | 0,77 |
| TZP, | 2 | 0,08 |
| TZP, SCF, | 2 | 0,15 |
| Group of resistance | ICU | non-ICU | ||
|---|---|---|---|---|
| n (%) | % of total N=100 |
n (%) | % of total N=100 |
|
| nMDR | 5 (9.4) | 27.8% | 13 (27.6) | 72.2% |
| MDR | 2 (3.8) | 40% | 3 (6.4) | 60% |
| XDR | 44 (83) | 58.7% | 31 (66) | 42.3% |
| E-XDR | 2 (3.8) | 100% | 0 (0) | 0% |
| Total | 53 (100) | 53% | 47 (100) | 47% |
|
blaOXA-23 N (%) |
blaOXA-40 N (%) |
blaNDM N (%) |
None of the tested genes N (%) |
|
|---|---|---|---|---|
| Type of unit | ||||
| Non-ICU | 13 (27.6) | 23 (48,9) | 0 | 13 (27.6) |
| ICU | 13 (24.5) | 19 (35.8) | 3 (5.7) | 19 (35.8) |
| The origin of the strains | ||||
| BSI | 11 (44) | 5 (20) | 0 | 9 (36) |
| PNEU | 7 (28) | 5 (20) | 0 | 13 (52) |
| SSTI | 3 (12) | 18 (72) | 0 | 4 (16) |
| colonization | 5 (20) | 14 (56) | 3 (12) | 6 (24) |
| Group of resistance | ||||
| nMDR | 2 (11.1) | 7 (38.9) | 0 | 9 (50) |
| MDR | 0 | 2 (40) | 0 | 3 (60) |
| XDR | 24 (32) | 31 (41.3) | 3 (4) | 20 (26.7) |
| E-XDR | 0 | 2 (100) | 0 | 0 |
| Both carbanenems resistant strains | 21 (30.4) | 31 (44.9) | 3 (4.3) | 7 (10.1) |
| Total | 26 (26) | 42 (42) | 3 (3) | 32 (32) |
| Group of biofilm producers | ICU | non-ICU | ||
|---|---|---|---|---|
| n(%) | % of total N=100 |
n (%) | % of total N=100 |
|
| Weak+moderate biofilm producers | 12 (22.6) | 42.8% | 16 (34) | 57.2% |
| Strong biofilm producers | 41 (77) | 57% | 31 (66) | 43% |
| Total | 53 (100) | 53% | 47 (100) | 47% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





