1. Introduction
An ecological disaster is characterized by the World Health Organization as an unprecedented catastrophic event exacerbated by human activity. The recent floods which devastated KwaZulu-Natal in South Africa during 2022 are an example of such an event [
1]. The consequences are compounded in lower- and middle-income countries where an existing burden of disease is coupled with a poorly resourced and fragile health care system. The interaction between environment, pathogen and host aptly describes the epidemiology and inter-relationship of disease and its consequence. Of particular focus, skin and soft tissue infection (SSTI) following environmental water exposure presents a challenge in terms of surgical management and antimicrobial usage. Accordingly, a guideline-based diagnostic approach, which identifies and treats common causative organisms in addition to a multi-disciplinary team approach will aid the treating surgeon in providing optimal care.
2. Principles of Infectious Disease after Hydrological Disaster
SSTI following flooding occurs commonly and is associated with major morbidity. The “One Health” concept which includes the triad of pathogen, environment and host, can be used to aptly describe the dynamics involved in infection after hydrological disaster [
2]. Typically, inoculation occurs following direct contact with a break in the skin integrity and environmental exposure to water either fresh, salt or brackish. A chain of events ensues which causes disease. Infective agents each with their individual virulence features, characterize the typical invading pathogens. They include viruses, bacteria, fungi and parasites. This article will selectively focus on fungal and bacterial agents, highlighting the diagnostic approach and treatment options [
3].
The susceptible host is typically of poor socio-economic standing, at extremes of age, who had limited access to healthcare and suffered physical trauma following the ecological disaster. Open wounds in communication with effluent provide a common mechanism for disease transmission [
3]. SSTI can affect any part of the body although the abdominal wall, perineum and the lower limb are more commonly affected. Successful management hinges on prompt recognition and source control aided by the use of appropriate antimicrobial therapy.
A chief consequence of ecological disasters is damage to infrastructure. Access to life-saving healthcare intervention is limited and underpins the challenges faced by a strained health care system. Major infrastructural installments like dams and roads are destroyed. Impaired access to critically ill victims, due to entrapment, physical infrastructure and health service collapse, poses a challenge to healthcare providers in terms of transport and logistics. Additionally, proper management of wastewater in treatment facilities is pivotal in protecting public health [
4]. Unfortunately, an ecological disaster and in particular flooding, destroys infrastructure and consequently untreated effluent adds to the virulence of multi-drug resistant organisms.
SSTI following an ecological disaster typically results in a polymicrobial systemic infection. Appropriate use of antibiotics is crucial with misuse having the potential to result in antimicrobial resistance [
2,
5].
For this summative review the literature was reviewed via a Pubmed and Google scholar search with access to the EBSCO, Elsevier, Springer and Proquest databases. The keywords and MeSH terms used included “soft tissue” and “sepsis” and / or “necrotizing infection” and or “water related skin sepsis”. The current literature of NSTI and in particular the diagnosis and management of water-related NSTI was reviewed and summarized. This is an underreported aspect of NSTI and makes this review timely in the light of the increased incidence of floods and climatic emergencies across the world.
3. Pathophysiology of Infection
There is a chain of events that leads to infections, involving several steps including the infectious agent (pathogens), a reservoir, the entering of a susceptible host, subsequent exit and transmission to a new host, with entry of the new host. The known agents include bacteria, viruses, fungi, parasites, arthropods and prions [
6].
Traditionally, necrotizing soft tissue infections have been classified into four groups, namely: type 1 being the classic polymicrobial type with facultative aerobes and anaerobes; type 2 being the monomicrobial beta-hemolytic streptococcus type (occasionally with
Staph aureus); type 3 water related bacterial organisms are involved and with type 4 fungal infection is the cause of the NSTI. [
7] This discussion will be restricted to bacteria and fungi focusing on water-related sepsis, thus focusing on types 3 and 4 NSTI.
The infective process begins when one of these agents successfully enters the body of the host and multiplies. The entrance to the host and the host pathogen interface usually occurs through mucosa or compromised skin such as in the case of open wounds. Entry doesn’t always result in infection but it may just lead to colonization. The difference between infection and colonization is often a matter of circumstance governed by host factors (age, diabetes mellitus, immunodeficiency), agent factors (virulence), and the external environment.
Injuries associated with water exposure include: Lacerations following cuts by objects that can be found in the water, puncture wounds secondary to items such as fish hooks, bites from aquatic animals and in patients with pre-existing wounds being exposed to the water with contaminants.
Individuals that are at risk include people who spend a lot of time in these waters including swimmers, fishermen, boaters, flood victims (as evident with the two presented cases), rescue workers following floods, people undergoing leech therapy and patients who are immunocompromised i.e. HIV, Diabetes, Chronic liver disease.
When considering skin and soft tissue sepsis it is pivotal to understand that human skin serves as the first line of defense against pathogens and when it is compromised, infecting pathogens may cause tissue necrosis/damage and incite an inflammatory response [
5,
8]. Some of these pathogens are capable of producing virulence proteins and toxins that are more potent and responsible for the process or sequela. There are two main classes of toxins, namely endotoxins and exotoxins.
Endotoxins are lipopolysaccharide chains mainly in gram negative bacterial cell walls, which may be beneficial by activating the immune system and enhancing T lymphocyte activities [
9]. Exotoxins are actively secreted proteins that cause tissue damage or dysfunction [
5,
9]. These bind conserved portions of the T cell receptors and are able to achieve a large number of T lymphocytes. A massive release of cytokines results, leading to an exaggerated overstimulation of the host’s inflammatory response.
Part of the inflammatory process involves the host response to tissue invasion and damage, the aim of which is to remove the offending pathogen and begin tissue repair. In some circumstances this response continues unrestrained and may be a cause of ongoing tissue damage. Tissue becomes devitalized and hypoxia ensues which predisposes to anaerobic infections.
4. Site and Classification of SSTIs
Skin and soft tissue infections (SSTIs) result from compromise in the skin that leads to pathogen invasion of skin, subcutaneous tissue, fascia and muscles. The World Society of Emergency Surgery classifies SSTIs into three main groups: [
10]
Surgical site infections (SSIs), which are further classified into incisional and organ/organ space infections (not truly SSTIs)
Non-necrotizing SSTIs
Necrotizing SSTIs (NSSTIs)
Invasion is further categorized into superficial (skin and subcutaneous tissue) and Deep (Fascia and muscle). Non-necrotizing SSTI’S include erysipelas impetigo, folliculitis, simple abscesses and complex abscesses. Necrotizing SSTI’S include necrotizing cellulitis, necrotizing fasciitis, Fournier’s gangrene and necrotizing myositis. (All describing different depths and sites of necrosis) [
10,
11].
Soft tissue infections following water exposure are relatively uncommon and are associated with high infection rates and morbidity or mortality compared to non-water exposed infections [
5,
12,
13]. The broad and infrequent array of microorganisms following water exposure are said to be the drive behind the complexity of these infections.
The first case reports (Individual cases and small case series) on trauma associated water exposed infections were published around the 1980’s and 1990’s [
13]. The 2004 Indian ocean Tsunami and the 2005 Hurricane Katrina disasters demonstrated the potential impact of water exposed infections on a much larger scale. The floods in the Sub Saharan Africa have also proven to have similar impacts [
14].
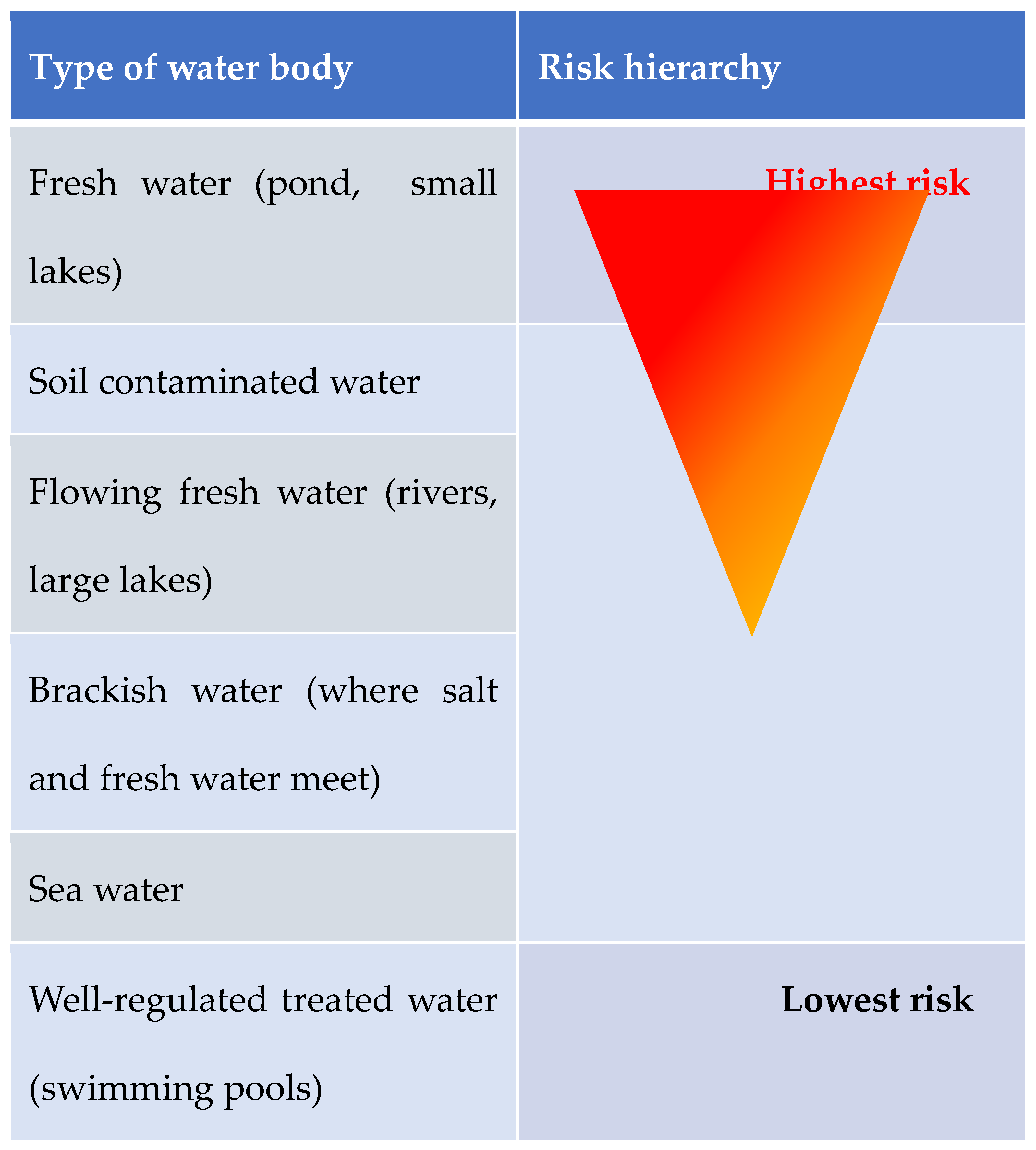
Different water exposure types hold different infection risk simply because of the different microorganism found in different bodies of water [
12,
13,
15]. Water bodies can be classified into 6 main groups: 1- fresh water (ponds, small lakes), 2- flowing fresh water (rivers large lakes), 3- brackish water (where salt and fresh water meet), 4- soil contaminated water, 5- sea water, and 6- well-regulated treated water (swimming pools). One can appreciate the higher infection risk in fresh waters.
Figure 1 illustrates the increasing infection risk depending on water-type.
5. Common Microorganisms Linked to Water Exposed Infections Found in Different Water Bodies
The focus in this discussion is SSTIs in water exposed wounds. However, it is important to bear in mind the role of colonization and infection by gram positive cocci (Staph Aureus and Group B Streptococcus) and Pseudomonas apart from the organisms that will be discussed below. This also helps to add valuable insight into the management of these patients.
Aeromonas species (Fresh and Brackish Water)
These are gram negative, facultatively anaerobic fermentative rods that morphologically resemble the
Enterobacteriaciae family [
9].
Aeromonas hydrophilia is the most common species that causes water-exposure associated SSTIs [
13,
15]. They usually grow at temperatures ranging from 0 to 42 degrees Celsius [
9,
13], These are highly virulent pathogens which cause wound infection to develop within 8 – 24 hours of exposure. The infection progresses rapidly to involve fascia, bone, muscle and joints.
Aeromonas is inherently resistant to early generation penicillin and cephalosporins, and preferentially susceptible to ciprofloxacin.
Edwardsiella tarda (Fresh Water)
These are part of the
Enterobacteriaciae family, and are anaerobic gram-negative rods that usually occur in patients with Liver disease or iron overload conditions [
15]. They are usually sensitive to most antimicrobials that act against gram negative bacteria.
Vibrio vulnificus (Salt Water, Brackish Water)
These are gram negative, motile and curved bacteria that are usually associated with consumption of raw oysters [
12]. Infection usually presents with hemorrhagic bullous skin lesions with underlying erythema that can rapidly progress to necrotic ulcers along with severe septic presentation [
12,
13,
16]. Signs usually develop 12 hours after wound exposure, and 25% of these tend to develop into necrotizing infections, osteomyelitis and or bacteraemia. More than 50% mortality is observed in this scenario [
13,
16].
Mycobacterium marinum (Salt and Fresh Water)
An atypical mycobacterium that commonly affects fish and rarely humans [
12]. This usually presents weeks after wound exposure and presents as granulomatous ulcerated papules. No systemic disease is usually associated with these bacteria. Surgical intervention is not often required, and these infections are commonly managed with long term antibiotics.
Eryspelothrix rhusiopathiae (Salt Water)
These are gram positive bacilli that cause infections that are usually self-limited in their course [
13]. It commonly affects fishermen or seafood handlers and usually resolves with antibiotic use. Usually no surgical intervention is needed.
Clostridium tetani
This is a large motile spore forming bacterial rod. The primary virulence factor is tetanospasmin which is a heat labile neurotoxin that blocks neurotransmitter release for inhibitory synapses. The spores are found mostly in soil and the tetanus disease is more common in places where access to vaccination or anti-tetanus toxoid (ATT) is poor [
9].
Prevention is essential and involves the use of ATT as prophylaxis in wound management specifically that of trauma-associated wounds. It is always important to review the immunization status of all patients with trauma-related wounds [
17].
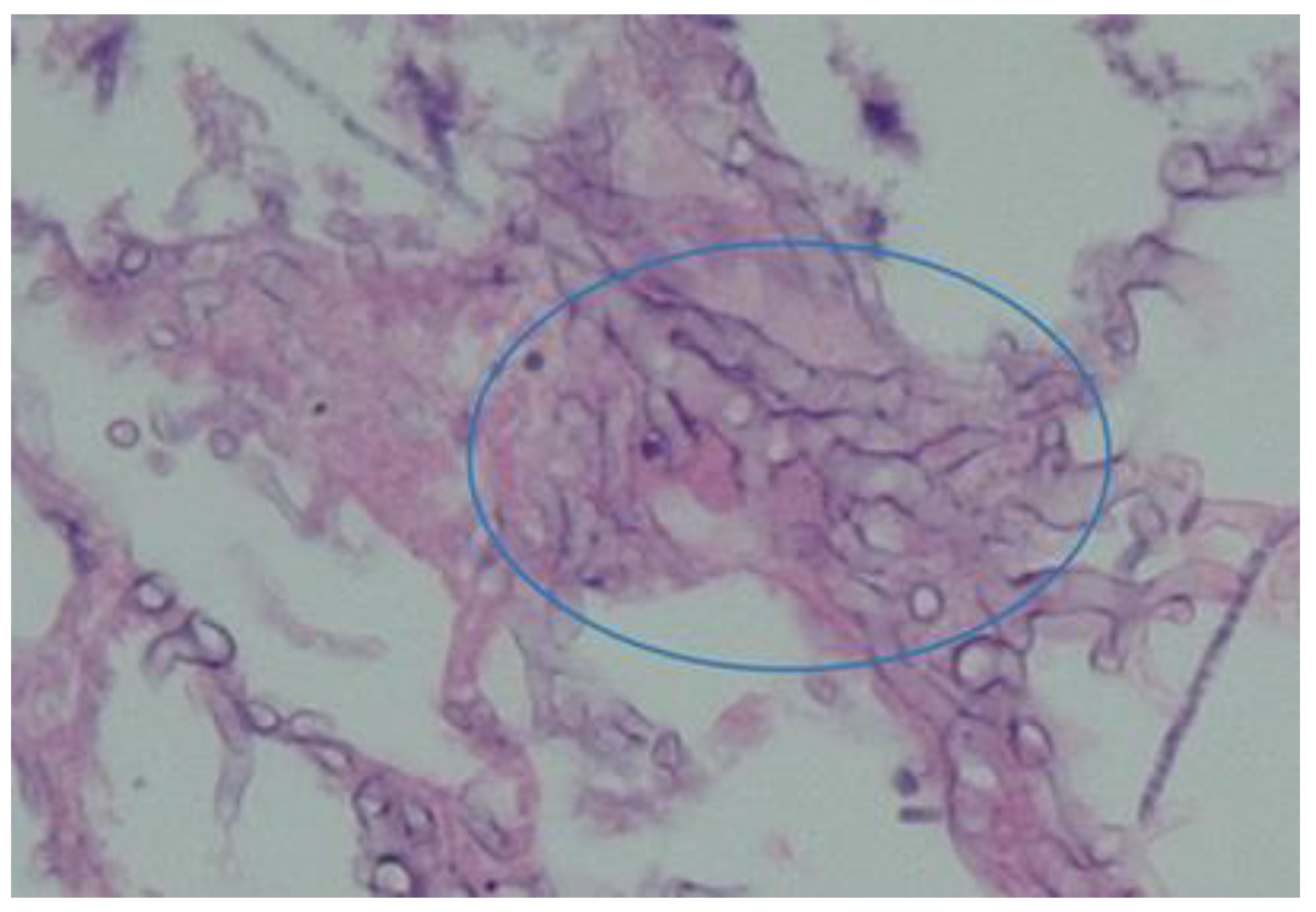
Aspergillus Species
These are opportunistic mycoses (fungal pathogens). They include multiple species, approximately nineteen in number. They grow as branched septate hyphae producing conidial heads which differ in shape according to the species [
9]. This is illustrated in
Figure 2.
These pathogens mostly affect patients with immune deficiency and cause a wide spectrum of disease including pulmonary infection, abdominal conditions (after ingestion), and superficial cutaneous infections.
Aspergillus water colonization may involve air conditioning systems, potentially causing nosocomial infections. They can also grow in water distribution systems such as water pipes [
18].
Prevention consists of preventing exposure in high risk patients. Decreasing immunosuppression and reconstitution of host immune defenses is also important [
9]. An example of a recent patient treated by the authors is illustrated in
Figure 3.
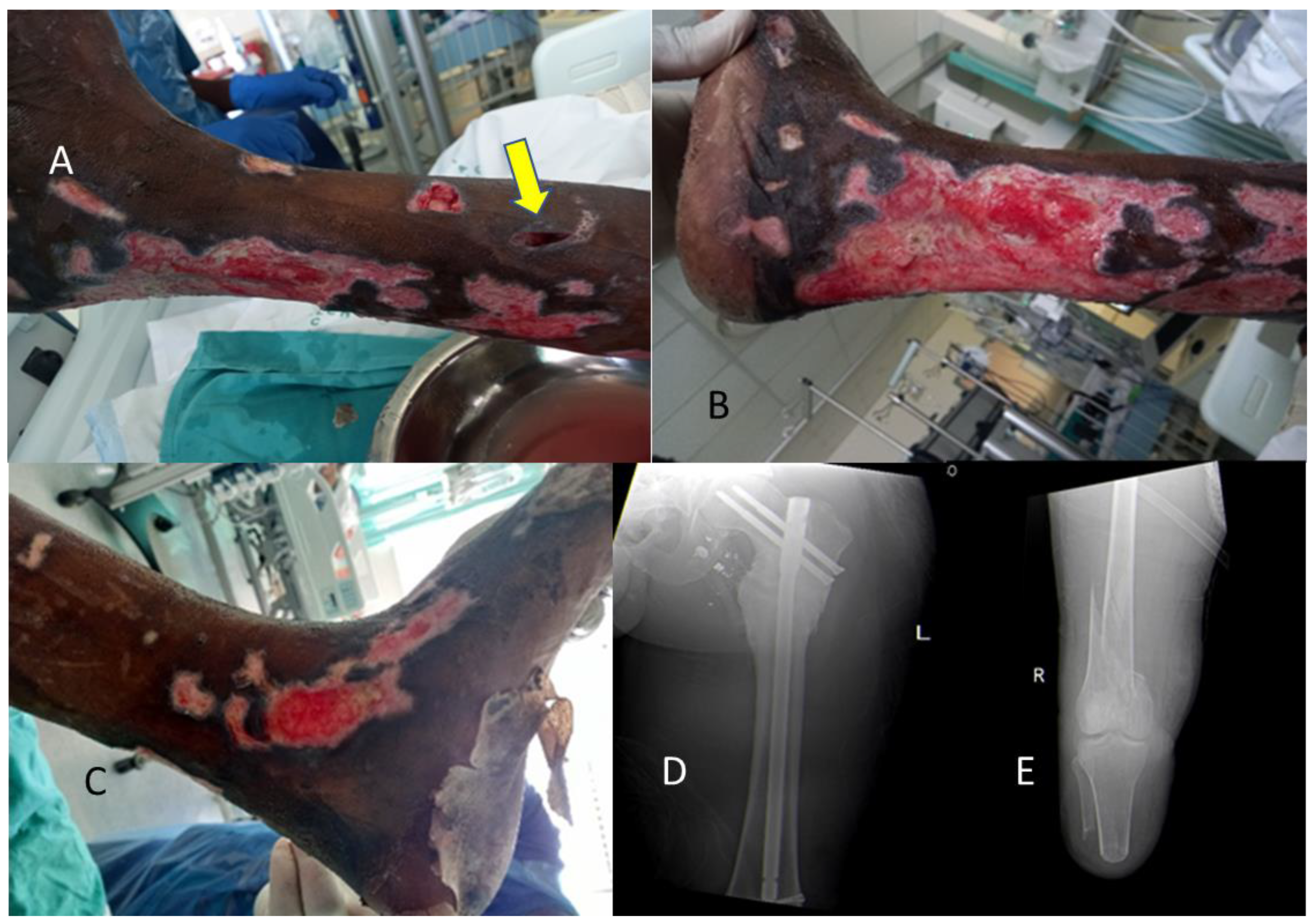
Rhizopus Species
These fungi originate from the class of zygomycetes that causes zygomycosis. These appear as ribbon-like, aseptate, nonpigmented hyphae under the microscope (see
Figure 4). Infection with these pathogens is rare however where it occurs, it results in very high mortality rate ranging from 70-100% [
9].
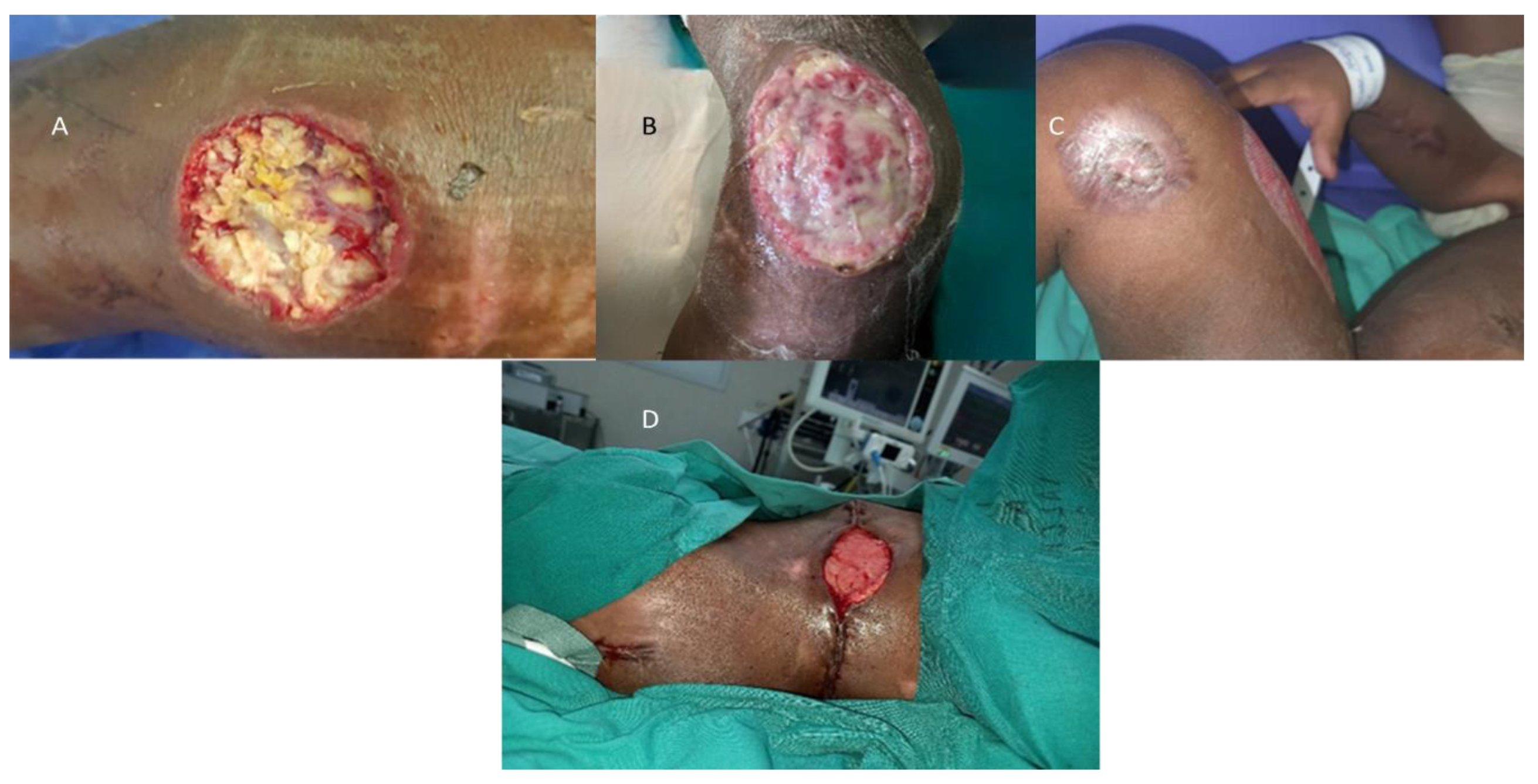
Infection results in multiple clinical syndromes which can be pulmonary, angioinvasive and cutaneous zygomycosis. The source of wound infection is from the contamination of wounds with sporangiospores from the environment, including from fresh water. Cutaneous disease causes nodular lesions with an ecchymotic center. Primary cutaneous zygomycoses occur following traumatic injury, surgical dressings, and burn wound colonization. Early diagnosis is important involving tissue biopsy for microscopy, histology and culture (Low sensitivity, and false negative up to 50% of mucormycosis cases) [
17,
19,
20,
21].
An example of such wounds from a recent flood-related admission are demonstrated in
Figure 5A–D.
Zoonoses and Vector Borne Diseases
For completion it is worth mentioning the effect of flood associated water exposure that leads to zoonosis and vector borne diseases. Zoonotic disease taeniasis has been highly associated with flooding. The floodwaters spread disease by transporting teaniasis species eggs to areas not previously affected. Flooding may also create breeding sites for disease vectors where stagnant water pools result [
14].
There are various other negative health consequences that result from fresh water exposure following flooding, but the focus in this article is SSTIs.
6. Diagnosis
Diagnosis is based on three elements, namely: clinical examination, imaging and confirmation of causative agent (MCS); however, the diagnosis of NSTIs remains primarily clinical while upholding a high index of suspicion. For clinically uninfected wounds, routine laboratory studies and cultures are not indicated. In clinically
infected wounds, laboratory studies (full blood count, erythrocyte sedimentation rate, C- reactive protein and procalcitonin) are reasonable as part of the diagnostic process [
17].
Some of these tests also play a significant role in the monitoring of the disease process i.e.: procalcitonin. Blood cultures should be obtained in the setting of fever or haemodynamic instability, as well as in patients with increased risk of systemic infection. Beta-D glucan indicates an invasive fungal infection; however, sensitivity and specificity of this marker vary substantially between different patient populations and the test is usually unable to detect zygomycetes such as
Mucor and
Rhizopus [
19,
20].
Galactomannan immunoassay was designed specifically for the easy diagnosis of invasive aspergillosis (IA). The assay is the oldest major fungal biomarker test; however, there is relatively little data on its use in children [
22,
23,
24].
Multiple publications exist reporting the utility of nucleic acid-based detection of fungal DNA through PCR, focusing most commonly on Aspergillus spp or Candida spp as pathogens of interest. Advancement in PCR technology for the diagnosis of candidiasis is the T2 Candida assay. After the DNA amplification step, T2 weighted magnetic resonance is used to detect the amplified products within 3-5 hours [
22,
23,
24]. Wound cultures remain the gold standard to establish the microbiology of the infection and to guide antimicrobial therapy (pus and tissue specimens) [
10,
11,
12,
13,
15,
17].
Different radiological imaging modalities may assist in providing useful information when the diagnosis is uncertain, however these must not delay definitive surgical management [
10]. On x-ray gas can be seen tracking along fascia and subcutaneous planes, however, the absence thereof cannot exclude SSTIs [
6,
10]. Ultrasound has the advantage of being rapidly performed on the bedside. Point of care ultrasound (POCUS) can improve the diagnosis accuracy for necrotizing soft tissue infections in combination with clinical evaluation [
6,
10,
11]. CT has higher sensitivity than plain x-rays in identifying necrotizing soft tissue infection and MRI is the imaging modality of choice, however limited by emergency availability [
10]. The LRINEC score was proposed in predicting the presence of necrotizing infection, with a score of 8 and higher having a 75% risk of Necrotizing Soft Tissue Infection. However, recent evidence demonstrates that it lacks sensitivity to be a useful adjunct for the diagnosis of necrotizing infection (Sensitivity of 40.8%) [
10].
7. Principles of Treatment of Severe SSTI’s (NSTI’s)
For the management of water exposed infection the same basic management principles of skin and soft tissue apply: timely diagnosis identifying patients at risk, appropriate antimicrobials and timely and appropriate surgical interventions where applicable.
Upon presentation it must be borne in mind that the majority of these patients with water related SSTIs are trauma patients that have experienced an injury. The approach would then be initial assessment according to the Advance Trauma Life Support principles and the secondary survey guiding the focused assessment of these wounds/ SSTIs [
25]. A high index of suspicion is of paramount importance as all water exposed wounds are at higher risk for severe SSTIs [
10,
12,
15].
Early detection of shock and prompt aggressive treatment of the underlying organ dysfunction as per the current Surviving Sepsis guidelines remains an essential component of improving the outcome of critically ill patients [
10,
26]
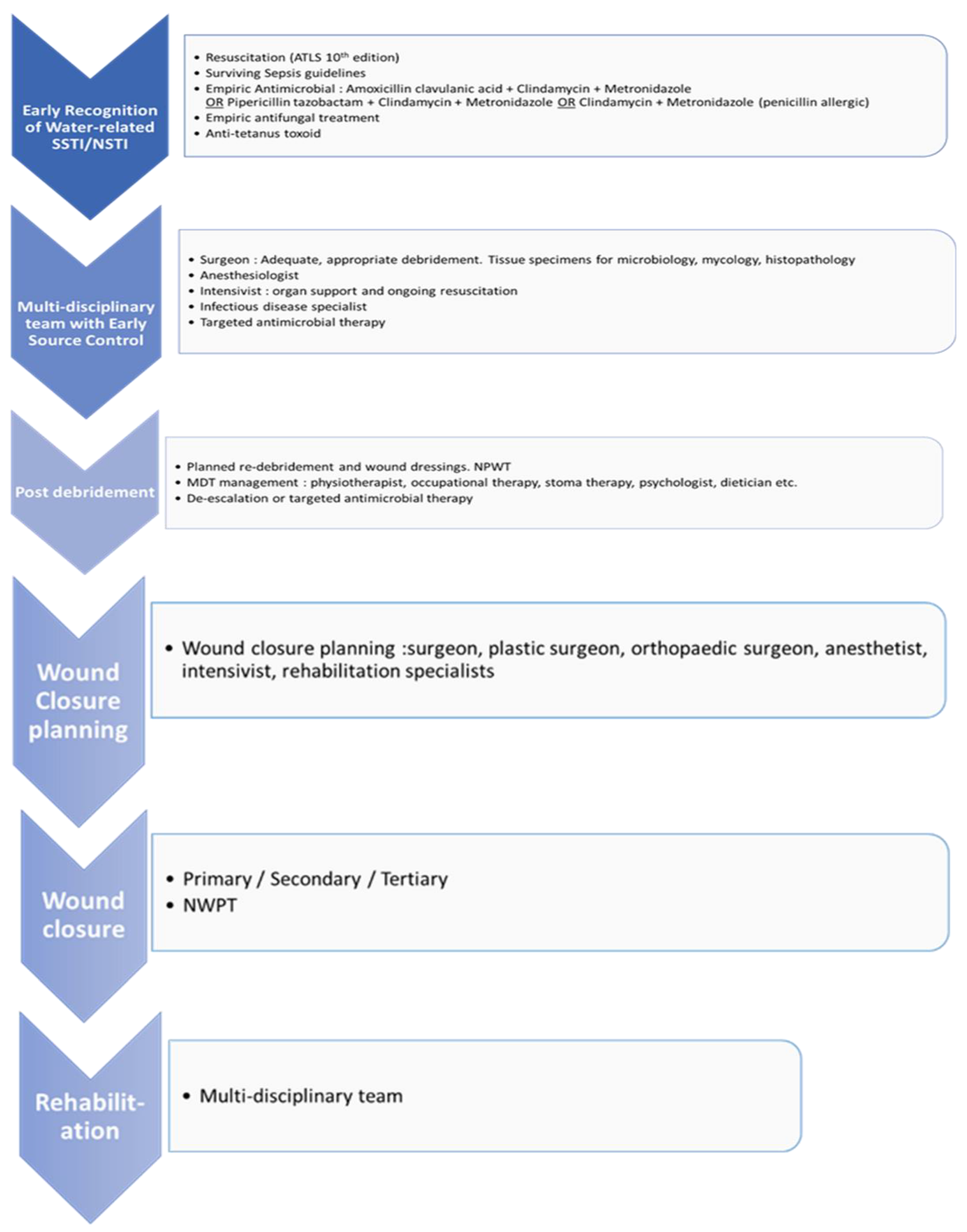
Different management strategies have been implemented at different phases. One of the many approaches is provided as flowchart 1 that may guide the diagnosis and treatment of such patients.
Flowchart 1.
A clinical pathway for the management of necrotizing soft tissue injury.
Flowchart 1.
A clinical pathway for the management of necrotizing soft tissue injury.
Acute phase management
Resuscitative measures
Resuscitation should follow the currently accepted “Surviving Sepsis” guidelines and standard trauma or surgical wound management principles. [
25,
26] Early multi-disciplinary care and intensive care admission are recommended.
Prophylactic and empiric antimicrobial therapy
The National Antimicrobial Resistance Strategy Framework is a comprehensive approach which encompasses surveillance, infection prevention and control, and antimicrobial stewardship to combat resistance [
27,
28]. This is a multidisciplinary team approach and is paramount in the management of any infectious condition, including the topic at hand [
27,
28].
There is currently no clinical trial data evaluating the benefit of antimicrobial prophylaxis (single dose administration) for patients with open wounds associated with water exposure. Therefore, the use of prophylaxis remains uncertain [
17]. Prophylaxis is reasonable in deep wounds beyond the dermis, wounds requiring surgical repair or primary closure and those associated with crush injury. Prophylaxis is also advised in wounds with associated vascular or lymphatic compromise, wounds in close proximity to bones or joints, those in special areas (genital or face) and those in immunocompromised patients.
Empiric therapy should be based on the epidemiology of the exposure and patient factors and usually is given for duration of 3 to 5 days [
17]. Regimens are based on the most likely organisms to cause infection [
12,
13,
21]. Initiation is recommended within 1 hour as delay prolongs hospital stay. The lack of active therapy within 48 hours of admission is associated with treatment failure [
13,
29]. Most empiric therapy guidelines are tailored for antibacterial cover utilizing appropriate broad-spectrum antimicrobial choice which covers Gram positive, Gram negative and anaerobic organisms, as follows:
Amoxicillin clavulanate / Piperacillin tazobactam with Metronidazole
or Ceftriaxone + Metronidazole
and consider adding (NB. if Strep pyogenes causation has been excluded) Clindamycin (anti-toxin activity in the event the NSSTI is a monomicrobial Group A Streptococcus or Clostridium species).
If penicillin allergy is present, then use Clindamycin and Metronidazole. No guidelines currently include anti-fungal cover, despite the evidence that wounds exposed to water are colonized with fungi (with or without soil contamination) [
12,
13,
14,
15,
30,
31], which is a short-coming of the current guidelines. The knowledge of antibiotic pharmacokinetic and pharmacodynamic principles may allow more rational determination of optimal dosing regimens in terms of the dose and dosing interval [
32,
33].
When considering recent experience with flood victims, it is suggested to include an antifungal agent in the empirical management of these severe (polymicrobial) SSTIs. Further research into this aspect of SSTIs is required.
The achievement of appropriate concentration of the antibiotic at the target site is essential to eradicate the pathogens and this may be achieved by administration of the first dose via a loading dose. This is essential in the presence of sepsis as the volume distribution of hydrophilic agents (e.g. Beta-lactams, aminoglycosides) may be altered by change in the permeability of the microvascular endothelium and alterations in extracellular body water. Loading doses should be appropriate for age and weight e.g.: 2.4g IVI Amoxicillin Clavulanate in adults. Dose frequency is also related to the concept of tissue dependent versus concentration dependent killing. Antimicrobial exhibiting time dependent activity, it’s important that the serum concentration exceed the MIC for appropriate duration of the dosing interval. Higher frequency dosing, prolonged infusion & continuous infusions can be utilized to achieve this effect.
Early surgical source control
Source control includes drainage of infected fluid collections, debridement of necrotic, non-viable, septic tissue, removal of infected foreign bodies, amputations and even correction of any anatomical derangement resulting in ongoing contamination [
10,
11,
13]. Source control is the most important determinant of outcomes in necrotizing soft tissue infections. A systematic review and meta-analysis demonstrated that mortality was significantly lower in patients that had surgical intervention within 6 hours of presentation compared to patients delayed more than 6 hours. Mandatory re-exploration and repeat debridement should be performed every 12 -24 hours until the wounds demonstrate little necrosis or no debridement is required [
10,
13].
Appropriate, adequate and efficient debridement saves lives. All devitalized or infarcted skin is removed and healthy, well-perfused skin spared. In areas of dubious skin viability, skin preservation and reassessment at the second-look operation is indicated [
7,
10,
11]. All pockets of murky “dishwater” fluid or pus must be explored, de-loculated and irrigated with copious warm saline. The ‘finger test’ may assist intra-operatively to provide tactile evidence of dubiously appearing tissue [
9,
10]. For perineal wounds fecal diversion is a useful option. One can make use of closed suction or corrugated drains to allow for draining, irrigation and easier access to multiple wound tract and/or cavities. Drains should be anchored on healing skin using simple non-absorbable sutures. Tissue specimens should always be sent for microbiological culture in normal saline, histopathological investigation in formalin, and for mycology culture in normal saline.
Early involvement of critical care team
Part of the surviving sepsis campaign includes early admission of patients who are septic or in septic shock is recommended within 6 hours [
26]. Selective patients may benefit from pre-operative resuscitation in a critical care environment prior to source control. This is not always be possible, particularly in LMIC’s. In this latter scenario, regular assessment, evaluation and appropriate treatment should not be delayed, and should occur independent of patient location. Using dynamic measures to guide resuscitation, over physical examination, or static perimeters alone, and also using capillary time as an adjunct is recommended [
26]. Critical care aims to optimize organ support by means of mechanical ventilation if required, cardiac support and renal replacement therapy if needed.
Post Debridement
During this phase the wound may need frequent re-exposure and further debridement, thus more short-term dressings may be used. A multi-disciplinary approach is of paramount importance in the management of these patients at all stages of wound care [
10]. Depending on the timeline, various specialties are involved. Initial treatment always requires coordination between the surgeons, intensivists, and infectious disease specialists. Early rehabilitation is an essential and integral component of recovery and it would be impossible without the combined efforts by physiotherapy, occupational therapy, dietetics, social worker and psychological support.
Microbiology results should be available and directed antimicrobial therapy can be initiated and tailored to microbiology data when available. Severe SSTIs warrant in hospital management with IV antimicrobials. The switch from IV to oral depends on the clinical response to therapy, their ability to tolerate the switch and the microbiological etiology. Advantages of the switch are early hospital discharge, reduced isolation of the patient and improved quality of life. Patients who are afebrile for over 24 hours, who have received IV antibiotics for over 24 hours, who are clinically stable, with no cardiovascular abnormalities and a normal white cell count can be changed to oral therapy [
28]. Importantly, ongoing organ support may be required from all disciplines concerned.
Below is a table indicating organisms associated with water exposed SSTI’s and the options for antimicrobials likely to cover the organisms [
8,
12,
13,
17,
18,
21].
Table 1.
Common waterborne organisms and likely antimicrobial options.
Table 1.
Common waterborne organisms and likely antimicrobial options.
| Pathogen |
Antimicrobial |
| Aeromonas Species |
Fluoroquinolones: Ciprofloxacin, Levofloxacin
3rd or 4th generation cephalosporins: Ceftazidime/Cefepime |
| Edwardsiella Tarda |
Ampicillin
Trimethoprim sulfamethoxazole
Chloramphenicol |
| Vibrio vulnificus |
Doxycycline
3rd or 4th generation cephalosporins
Fluoroquinolones |
| Mycobactyerium marinum |
Clarithromycin or Trimethoprim Sulfamethoxazole
Rifampicin
Ethambutol |
| Erysipelothrix rhusiopathiae |
Usually self-limiting
If required: Penicillin, Cephalexin or ciprofloxacin |
| Clostridium tetani |
Prevention with ATT- anti-tetanus toxoid
Metronidazole
Penicillin
Botulinum toxin for tetanus spasms. |
| Aspergillus Species |
Amphotericin B
Voriconazole for resistant cases |
| Rhizopus Species |
Amphotericin B.
Posaconazole |
Wound Closure Post-Surgical Debridement
Open wounds are managed by making use of 3 principles: healing by Primary, Secondary or Tertiary intention [
34,
36]. Primary intention wound closure is usually advocated in clean wounds presenting less than 8 hours, however this is not the case in wounds that are water exposed due to the high risk of infection. Secondary intention encompasses chemical debridement by means of short- and long-term dressings. NPWT has proven to be of great benefit in large, open and/or exudative wounds. Tertiary intention or delayed closure can be by means of approximating sutures, split-skin grafts, full thickness grafts, flaps and skin substitutes.
8. Conclusions
SSTI following ecological disaster presents a major challenge to healthcare providers and epidemiologists. Medical treatment involves recognition, host optimization and surgical management. Administration of appropriate antibiotics and strict adherence to antibiotic stewardship principles is crucial. The suggested algorithmic approach may assist in implementation of these pivotal and inter-related aspects in management.
Understanding how components of the epidemiological triad influence each other can aid role-players in developing and implementing interventions to lessen disease burden. The effects of an ecological disaster are devastating; further research with emphasis on a multidisciplinary team approach, appropriate medical management and infrastructural development can reduce morbidity and mortality. Following a diagnostic pathway and knowledge of the likely organisms will result in treatment success.
Author Contributions
All authors contributed equally to this work. (I) Conception and design: S.N. and A.M.Z (II) administrative support: none; (III) provision of study materials or patients: S.N., A.M.Z, A.P. and TCH; (IV) collection and assembly of data: S.N. and TCH; (V) data analysis and interpretation: S.N.; (VI) manuscript writing: S.N., A.M.Z, A.P. and TCH; (VII) final approval of manuscript: all authors approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All clinical data, laboratory and clinical images are covered by the Trauma Unit University of KwaZulu-Natal BREC Class Approval, BCA207/09. Patient consent for images was obtained under that class approval.
Informed Consent Statement
See Ethics approval above. Waiver of approval for retrospective clinical data.
Data Availability Statement
The study only includes new data as images to illustrate the review.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wikipedia. 2022 KwaZulu-Natal floods. available from https://en.wikipedia.org/wiki/2022_KwaZulu-Natal_floods accessed April 2023.
- Iskander K, Molinier L, Hallit S, Sartelli M, Catena F, Coccolini F, Hardcastle TC, Roques C, Salameh P. Drivers of Antibiotic Resistance Transmission in Low- and Middle-Income Countries from a “One Health” Perspective—A Review. Antibiotics 2020, 9(7), 372. https://www.mdpi.com/2079-6382/9/7/372/pdf. [CrossRef]
- Rose N, Matthäus-Krämer C, Schwarzkopf D, Scherag A, Born S, Reinhart K, Fleischmann-Struzek C. Association between sepsis incidence and regional socioeconomic deprivation and health care capacity in Germany - an ecological study. BMC Pub Health. 2021;21(1): 1636. [CrossRef] [PubMed]
- Grab SW, Nash DJ. A new flood chronology for KwaZulu-Natal (1836–2022): the April 2022 Durban floods in historical context. S Afr Geogr J, 2023; 1. [CrossRef]
- Liang SY, Messenger N. Infectious Diseases After Hydrologic Disasters. Emerg Med Clin North Am. 2018; (4): 835-851. [CrossRef] [PubMed]
- Kotra LP. Infectious Diseases. xPharm: The Comprehensive Pharmacology Reference. 2007: 1–2. [CrossRef]
- Bonne SL, Kadri SS. Evaluation and Management of Necrotizing Soft Tissue Infections. Infect Dis Clin North Am. 2017; 31(3): 497-511. [CrossRef] [PubMed]
- Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can. J. Infect. Dis. Med. Microbiol. 2008; 19(2): 173–184. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2605859/.
- Murray PR, Rosenthal KS, Pfaller MA. Medical microbiology. 6th ed. Philadelphia, Pa: Elsevier; 2008.
- Sartelli M, Guirao X, Hardcastle TC, Kluger Y, Boermeester MarjaA, Raşa K, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018; 13:58. [CrossRef]
- Sartelli M Coccolini F, Kluger Y, Egastra E, Abu-Zidan FM, et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J Emerg Surg, 2022; 17:3. [CrossRef]
- Lewis S, Collins D, Bressler A. Bacterial Soft Tissue Infections Following Water Exposure. 2017. Available from: http://www.podiatryinstitute.com/pdfs/Update_2017/Chapter23_final.pdf accessed Apr 17 2023.
- Emigh B, Trust MD. Contaminated Wounds: Fresh Water, Salt Water, and Agricultural Contamination. Curr Trauma Rep. 2018; 4(4): 309–315.
- Suhr F, Steinert JI. Epidemiology of floods in sub-Saharan Africa: a systematic review of health outcomes. BMC PublicHealth. 2022; 22(1):268. [CrossRef] [PubMed]
- Ribeiro NFF, Heath CH, Kierath J, Rea S, Duncan-Smith M, Wood FM. Burn wounds infected by contaminated water: Case reports, review of the literature and recommendations for treatment. Burns. 2010; 36(1): 9–22.
- Ruppert J, Panzig B, Guertler L, Hinz P, Schwesinger G, Felix SB, et al. Two cases of severe sepsis due to Vibrio vulnificus wound infection acquired in the Baltic Sea. Eur J Clin Microbiol Infect Dis. 2004; 23: 912–915.
- Baddour L, Sexton D, Hall K. UpToDate. www.uptodate.com. 2021. Available from: https://www.uptodate.com/contents/soft-tissue-infections-following-water-exposure accessed April 2023.
- Richardson M, Rautemaa-Richardson R. Exposure to Aspergillus in Home and Healthcare Facilities’ Water Environments: Focus on Biofilms. Microorganisms. 2019; 7(1): 7.
- Von Lilienfeld-Toal M, Wagener J, Einsele H, Cornely OA, Kurzai O. Invasive Fungal Infection. Deutsches Arzteblatt International. 2019; 116(16): 271–278. Available from: https://pubmed.ncbi.nlm.nih.gov/31159914/.
- Haydour Q, Hage CA, Carmona EM, Epelbaum O, Evans SE, Gabe LM, et al. Diagnosis of Fungal Infections. A Systematic Review and Meta-Analysis Supporting American Thoracic Society Practice Guideline. Ann Am Thor Soc. 2019; 16(9): 1179–1188.
- Diaz JH, Lopez FA. Skin Soft Tissue and Systemic Bacterial Infections Following Aquatic Injuries and Exposures. Am J Med Sci. 2015; 349(3): 269–275.
- Thompson GR, Boulware DR, Bahr NC, Clancy CJ, Harrison TS, Kauffman CA, Le T, Miceli MH, Mylonakis E, Nguyen MH, Ostrosky-Zeichner L, Patterson TF, Perfect JR, Spec A, Kontoyiannis DP, Pappas PG. Noninvasive Testing and Surrogate Markers in Invasive Fungal Diseases. Open Forum Infect Dis. 2022; 9(6): ofac112. [CrossRef]
- Peetermans M, de Prost N, Eckmann C, Norrby-Teglund A, Skrede S, De Waele JJ. Necrotizing skin and soft-tissue infections in the intensive care unit. Clin Microbiol Infec. 2020; 26(1): 8–17.
- Huppler AR, Fisher BT, Lehrnbecher T, Walsh TJ, Steinbach WJ. Role of Molecular Biomarkers in the Diagnosis of Invasive Fungal Diseases in Children. J Pediatr Infect Dis Soc. 2017; 6 (suppl1): S32–44.
- Committee on Trauma. ATLS Student manual, 10th Ed, 2019. American College of Surgeons, Chicago Il.
- Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021; 49(11): e1063-e1143. [CrossRef]
- South Africa: South African antimicrobial resistance national strategy framework [Internet]. www.who.int. 2018 [cited 2023 Apr 17]. Available from: https://www.who.int/publications/m/item/south-africa-south-african-antimicrobial-resistance-national-strategy-framework-a-one-health-approach accessed April 2023.
- Sartelli M, Kluger Y, Ansaloni L, Carlet J, Brink A, Hardcastle TC, et al. A global declaration on the appropriate use of antimicrobials across the surgical pathway. Surg Infect, 2017, 18(8): 846-853 http://online.liebertpub.com/doi/abs/10.1089/sur.2017.219.
- Leong HN, Kurup A, Tan MY, Kwa ALH, Liau KH, Wilcox M. Management of complicated skin and soft tissue infections with a special focus on the role of newer antibiotics. Infect Drug Resist. 2018; 11: 1959–1974.
- Jenkin A, Mantha P, Palamuthusingam P. Do we need to change empiric antibiotic use following natural disasters? A reflection on the Townsville flood. ANZ J Surg. 2021; 92(1-2): 195–199.
- Baumgardner DJ. Freshwater Fungal Infections. J. Patient Cent. Res. Rev. 2017;4(1): 32–38.
- Sartelli M. Antibiotics dosing in critically ill patients with sepsis and septic shock [Internet]. Global Alliance for Infections in Surgery. 2017. Available from: https://infectionsinsurgery.org/antibiotics-dosing-in-critically-ill-patients/ accessed April 2023.
- Urbina T, Razazi K, Ourghanlian C, Woerther PL, Chosidow O, Lepeule R, et al. Antibiotics in Necrotizing Soft Tissue Infections. Antibiotics. 2021; 10(9): 1104. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8466904/ accessed April 2023.
- Diehm YF, Fischer S, Wirth GA, Haug V, Orgill DP, Momeni A, et al. Management of Acute and Traumatic Wounds With Negative-Pressure Wound Therapy With Instillation and Dwell Time. Plast Recon Surg. 2021; 147(1S-1):43S. Available from: https://journals.lww.com/plasreconsurg/Abstract/2021/01001/Management_of_Acute_and_Traumatic_Wounds_With.8.aspx access April 2023.
- Pelletier J, Gottlieb M, Long B, Perkins JC. Necrotizing Soft Tissue Infections (NSTI): Pearls and Pitfalls for the Emergency Clinician. J Emerg Med. 2022;62(4): 480–491. Available from: https://pubmed.ncbi.nlm.nih.gov/35115188/ accessed April 2023.
- Chhabra S, Chhabra N, Kaur A, Gupta N. Wound Healing Concepts in Clinical Practice of OMFS. J J. Maxillofac. Surg. 2016; 16(4): 403–423.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).