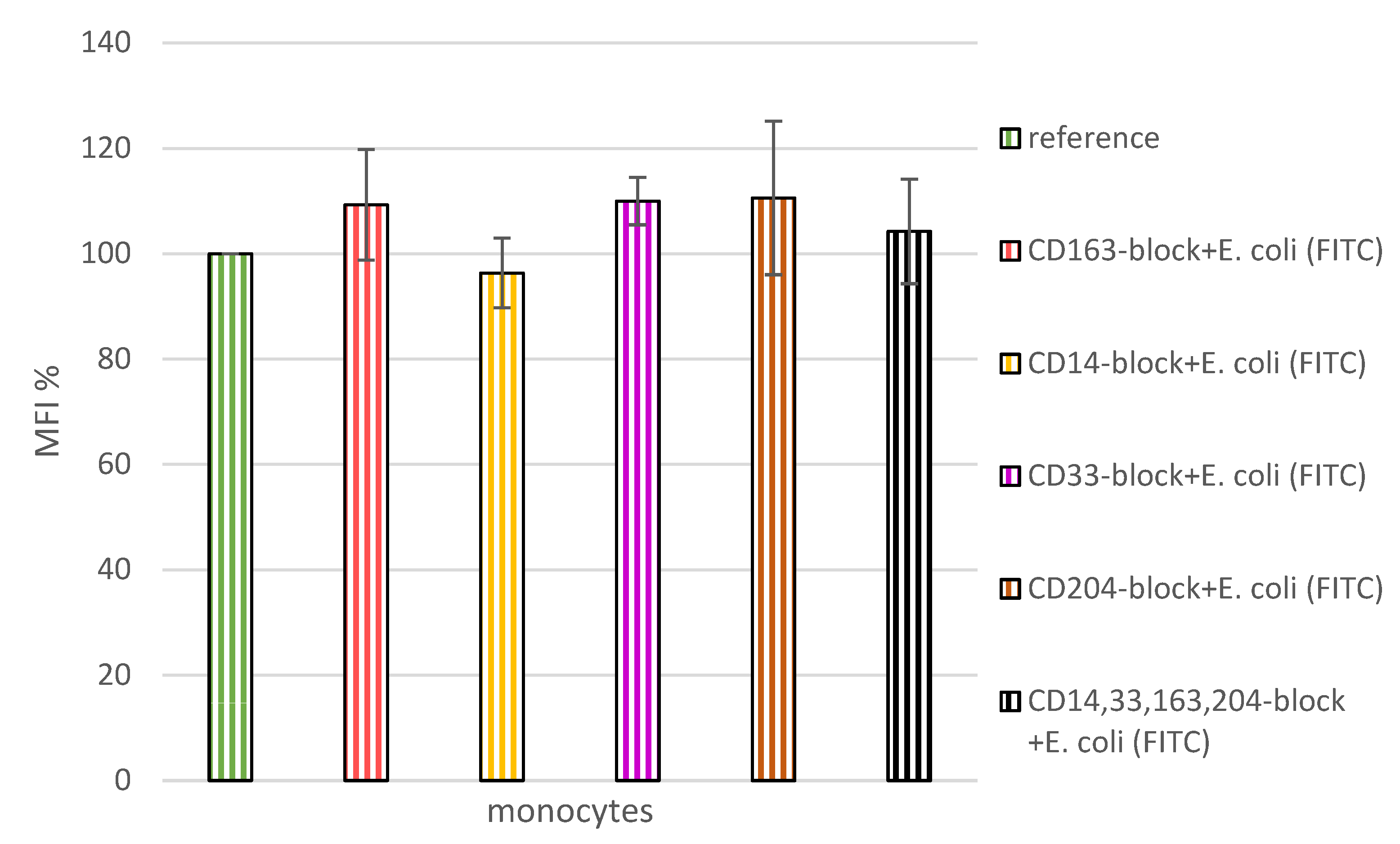1. Introduction
Blood transfusions save lives every day and have become an indispensable part of clinical practice in modern medicine. However, there have always been numerous limitations and problems with their use: dependence on donor readiness, short shelf life [
1]risk of infection [
2]transfusion reaction in the case of non-blood group-specific transfusion [
3], immunization with the formation of antibodies [
4]and an overall high personnel and financial expense [
5].
For decades, science has been trying to overcome these problems [
6]. Although erythrocytes also pose as a CO
2 transporter and native hemoglobin as a buffer-system [
7], research is being focused on their function as an oxygen-transporter. The search is on for a laboratory-produced oxygen carrier that is relatively simple and inexpensive to produce, can be stored for a long time, administered universally, is safe to use and has few to none adverse drug effects.
One approach associated with great hopes is such an oxygen carrier based on hemoglobin (HBOC, hemoglobin-based oxygen carrier). Initially, however, serious side effects were encountered during development. Nitrogen-monoxide (NO) scavenging and associated hypertensive crises, massive renal damage due to tubular reabsorption of Hemoglobin (Hb), decayed into dimers [8-13], and oxidative stress [
14,
15]. Various approaches of intra- as well as inter-molecular modifications of HBOC have been devised. Crosslinking of different Hb chains, polymerization of Hb molecules [16-18], surface-modification [
19] and techniques for encapsulation [
20,
21] already brought us somewhat closer towards a safe to use HBOC [
22]. However, a persistent problem is the severely limited retention time of oxygen carriers in the circulatory system. Erythrocytes and therefore the Hb they contain have a mean survival time of about 120 days within the human body [
7]. HBOCs, on the other hand, have been eliminated within minutes to a few hours in previous experiments [
5,
23]. Much of this process appears to occur in the liver [24-26]. The question of the mechanisms by which HBOCs are sequestered remains partly unclear though.
Possible degradation pathways include haptoglobin (Hp), which, depending on the size and surface properties of HBOCs, could bind its physiological target protein hemoglobin [
16,
19]. CD163, which as the receptor for Hp-Hb complexes also shows some affinity for Hb [
16,
27,
28]. The binding site at the receptor for this appears to be the same as for the binding of Hp-Hb complexes, according to Schaer et al. [
27]; whereas within Hb, the binding site for direct interaction with CD163 is probably within the β-chain of Hb (binding of Hb to Hp via a binding site within the Hb-α-chain) [
27]. Furthermore, not only cells of the monocyte/macrophage lineage appear to be involved in sequestering Hb, but also hepatocytes [
25,
26]. It can be speculated that the same could be true for HBOC. This fits with a case report by Drieghe et al. which suggests that hemopexin (Hpx) may also play a role in the elimination of HBOC [
25,
29]. In the case described, Hpx was depleted before a change in Hp levels could be observed, when catecholamine-requiring patients were treated with an HBOC for intentional NO scavenging and consecutive increase in peripheral vascular resistance. Whether binding between cell-surface proteins and Hb/HBOC can occur and with what affinity, probably depends on modifications, made to the Hb. Intramolecular crosslinking has an impact, depending on whether the binding site within the Hb-α chain is freely accessible (exclusively β-crosslinked-Hb vs. α-crosslinked-Hb) [
27]. Intermolecular modifications, changing the molecular or polymer size of the HBOC [
16,
19,
30] are also relevant for protein-binding.
Hemoglobin sub-micron particles (HbMP) using the CCD technique (Coprecipitation-Crosslinking-Dissolution) represent a promising strategy as HBOC ([19,31-33]). It could be shown that neither NO-scavenging nor vasoconstriction can be detected when using such HbMP as an oxygen carrier [
34]. In addition to transporting oxygen, HbMP can also be used as a drug carrier. However, in a pharmacokinetic study with the HbMP, accumulation of the particles in the sinusoids of the liver, where the Kupffer cells are located, was observed [
35]. The mechanisms of the interaction of liver macrophages with HbMP have also not yet been systematically investigated.
In this study, we screened several monocytic surface receptors for a possible influence on the uptake of HbMP by monocytes, precursor cells of macrophages. We chose to screen for a CD14- as well as CD33-dependant HbMP-uptake by Monocytes, since both proteins are specific for this cell-line. CD163, the Hp-Hb – complex receptor, was tested due to its direct affinity to Hb and therefore other HBOC, that has been observed in various other studies. Also, we tested the effect of blocking CD204 (scavenger receptor A / SR-A). SR-A is a membrane-protein, occurring in the monocyte/macrophage lineage. Playing an important role in host-defense it presents a long list of possible ligands, amongst them chemically modified serum Albumin, which might also occur within our HBMP [
36]. The aim of this work is to contribute to a further step towards the development of HbMP as a complete, safe blood substitute for volume expansion and oxygen distribution. The interaction of HbMP (with different surface modifications) with Hp, as well as with anti-Hb antibodies has already been studied by Prapan et al [
19]. With this work, the investigation of the role of CD14, CD33, CD163 and CD204 in the uptake of HbMP by monocytes now followed. We hypothesize that CD163 plays an important role in the uptake of our HbMP into monocytes, while there might be additional other mechanisms at play. Therefore, we compared the effects of CD163 with those of the other (scavenger-) receptors.
2. Materials and Methods
2.1. chemicals
0.9% Sodium-chloride was purchased from B. Braun SE, Melsungen, Germany. Phosphate buffered saline (PBS), 10-fold solution was purchased from Fisher scientific, Fair Lawn, New Jersey, USA. Human Serum Albumin (Plasbumin®20) was purchased from Grifols Deutschland GmbH, Frankfurt am Main, Germany. Trypan-blue, 0,4% solution was purchased from PAN-Biotech GmbH, Aidenbach, Germany. Manganese chloride (MnCl2), sodium-hydroxide (NaOH), sodium-borohydride, sodium carbonate (Na2CO3), Ethylenediaminetetraacetic acid (EDTA), Glutaraldehyde solution, grade II, 25% in H2O as well as Triton™ X-100 were purchased from Sigma-Aldrich, Steinheim, Germany. Pronase was purchased from Roche diagnostics GmbH, Mannheim, Germany. Bovine hemoglobin (Acto2Hem®) was provided by Biophyll GmbH, Dietersburg, Germany.
PHAGOTEST ™ and PHAGOBURST ™ test-kits were purchased from Glycotope Biotechnology GmbH, Berlin, Germany Mouse derived, monoclonal anti-human CD14 antibody (IgG2a, Κ), conjugated with Fluorescein isothiocyanate (FITC) was purchased at BD Biosciences, Heidelberg, Germany. Mouse derived, monoclonal anti-human CD14 antibody (IgG2a, Κ) was purchased at BioLegend, San Diego, USA. Mouse derived, monoclonal anti-human CD33 antibody (IgG1, K) was purchased at BioLegend, San Diego, USA. Mouse derived, monoclonal anti-human CD163-antibody (IgG1, clone: GHI61), conjugated with Allophycocyanin (APC) was purchased at antibodies-online GmbH, Aachen, Germany. Mouse derives anti-human CD204-antibody (IgG2a, K) was purchased at BioLegend, San Diego, USA.
2.2. Donated Blood
Peripheral venous blood was collected from healthy donors and anticoagulated with lithium heparin according to the requirements of the German law regulating transfusion. Written informed consent was obtained from all donors, as well as a positive vote of the Ethics Committee of the Charité - Universitätsmedizin Berlin (EA4/023/22). Blood samples were processed immediately after blood collection and cell metabolism was maximally slowed down by storage at 0°C.
2.3. Particle Preparation
For the preparation of hemoglobin-based sub-micron-particles (HbMP), the protocol of particle preparation using the 'CCD-technique' as previously described ([
24]) has been followed: 0.5% bovine hemoglobin-solution was mixed with 0.125 M manganese-chloride solution (MnCl
2). While stirring rapidly, 0.125 M Na
2CO
3 was added and stirred further for 30 seconds to let Hb-MnCO
3-particles form. 20% HSA solution was added and allowed to incubate with the particles for 5 minutes. After washing in 0.9% NaCl-solution, particles were centrifuged (3000g, 3 min.) and the supernatant decanted. Particles were then resuspended in 0.9% NaCl. For crosslinking, 0.02% glutaraldehyde (GA) was added to the particle-suspension, which was then incubated for 1 hour at room temperature, while stirring. After another washing in aqua dest. the MnCO
3-matrix was dissolved, using 0.25 M EDTA. After a 30-minute incubation time, 0.2 mg/mL NaBH
4 in 0.1 M NaOH was added to prevent Hb-oxidation to met-Hb. Incubation for another 30 minutes followed. After triplicate washing in a washing-solution of 0.9% NaCl, containing 0.2% HSA, particles were resuspended in 0.9% NaCl, checked via light-microscopy for aggregation and stored at 4°C.
2.4. Particle Characterization
To ensure that the subsequent experiments would be carried out on particles that also met the quality requirements for potential use as oxygen transporters in vivo, the particles were subjected to various measurements for their characterization. In this way, comparability was achieved between different particle batches, in different experiments. All experiments within this work have been performed with HbMP from the same batch.
2.4.1. Concentration Determination
HbMP suspension was placed in glass capillaries for hematocrit determination and then centrifuged at 15000g for 10 minutes. The amount of particle sediment was determined manually and the remaining suspension was diluted with 0.9% NaCl to a concentration of 2% HbMP.
2.4.2. Size, Zeta Potential and Conductivity
After dilution of the particle suspension to 0.13% (V/V) with NaCl, the average size and conductivity as well as the zeta potential of the particles, were determined via zetasizer (zetasizer Nano ZS, Malvern Instruments, Malvern, United Kingdom), each measured in triplicate.
2.4.3. Hemoglobin Content
To determine the HbMP’s hemoglobin content, the modified alkaline hematin-D method (AHD method) was used, as described in detail elsewhere ([
33]). Pronase solution (0.5mg/mL) was added to the particle suspension and incubated at 45°C for 30 minutes. The AHD reagent (25mg/mL Triton™ X-100 in 0.1M NaOH) was added to the particle sample (final concentration: 12.5 mg/mL), mixed and incubated at room temperature for 15 minutes while stirring. After centrifugation (20,000g; 10min.) the supernatant was taken off and immediately the absorbance at a wavelength of 574nm was determined photometrically in it, in three single measurements (cytation 3 imaging reader, BioTek Instruments GmbH, Bad Friedrichshall, Germany). The hemoglobin concentration of the particles was then calculated from the measured values according to the following formula, as published by Smuda et al. [
37]:
2.4.4. Percentage of Functional Hemoglobin
Hemoglobin contains an Fe
2+ ion, on which the ability of Hb to bind oxygen is based. Methemoglobin has undergone an oxidation process. The central iron ion now presents itself as trivalent and the Hb derivative has lost its ability to bind oxygen. Therefore, only bivalent Hb is functional. The 'oxygen-release method' was used to determine the percentage of functional hemoglobin, as previously described in detail by Kloypan et al. [
33]. In brief: The oxygen content of the suspension was measured in three individual measurements in 500µL each of the particle suspension diluted to a total Hb of 0.5mg/mL, under steady, gentle stirring (Microx 4, PreSens, Regensburg, Germany). After reaching a stable initial value, 50µL of 10% potassium ferricyanide (K
3[Fe(CN)
6]) was added and the stirring speed was increased. The oxygen bound to Hb was thus released and the peak value of O
2 within the suspension was determined. From the difference between the initial and peak values, the proportion of functional Hb was calculated.
2.5. Indirect Phagocytosis Test
In the development of a suitable experimental set-up, a strong influence of direct fluorescent labeling of HbMP, on the phagocytosis activity of monocytes was found. Consequently, we chose an indirect phagocytosis assay for our experiments, which did not require any labeling of the HbMP potentially to be phagocytosed. The method of indirect phagocytosis assay is described in detail elsewhere [
22].
In brief: (indirect phagocytosis-test No 1) Samples were prepared each with and without an insert of 5µL each of anti-CD163-AB (APC conjugated), to block the monocytic membrane protein, as formerly described by Schaer et al. [
27]. For a reference-sample, the phagocytic capacity of monocytes was fully utilized for the uptake of FITC-labeled E. coli lysate, over a period of 10 minutes at 37°C. For comparison, samples were prepared in which the cells were able to phagocytose either unlabeled E. coli lysate, or HbMP in a pre-feeding step (approximately 100 HbMP per leukocyte). The incubation period here was 120 minutes. In addition, samples with HbMP pre-feeding were also prepared at a shortened incubation time of only 30 minutes to obtain information of the course of phagocytic activity over time. Identical negative controls were carried along, which remained at 0°C at all times (supplementary data:
Figure S1).
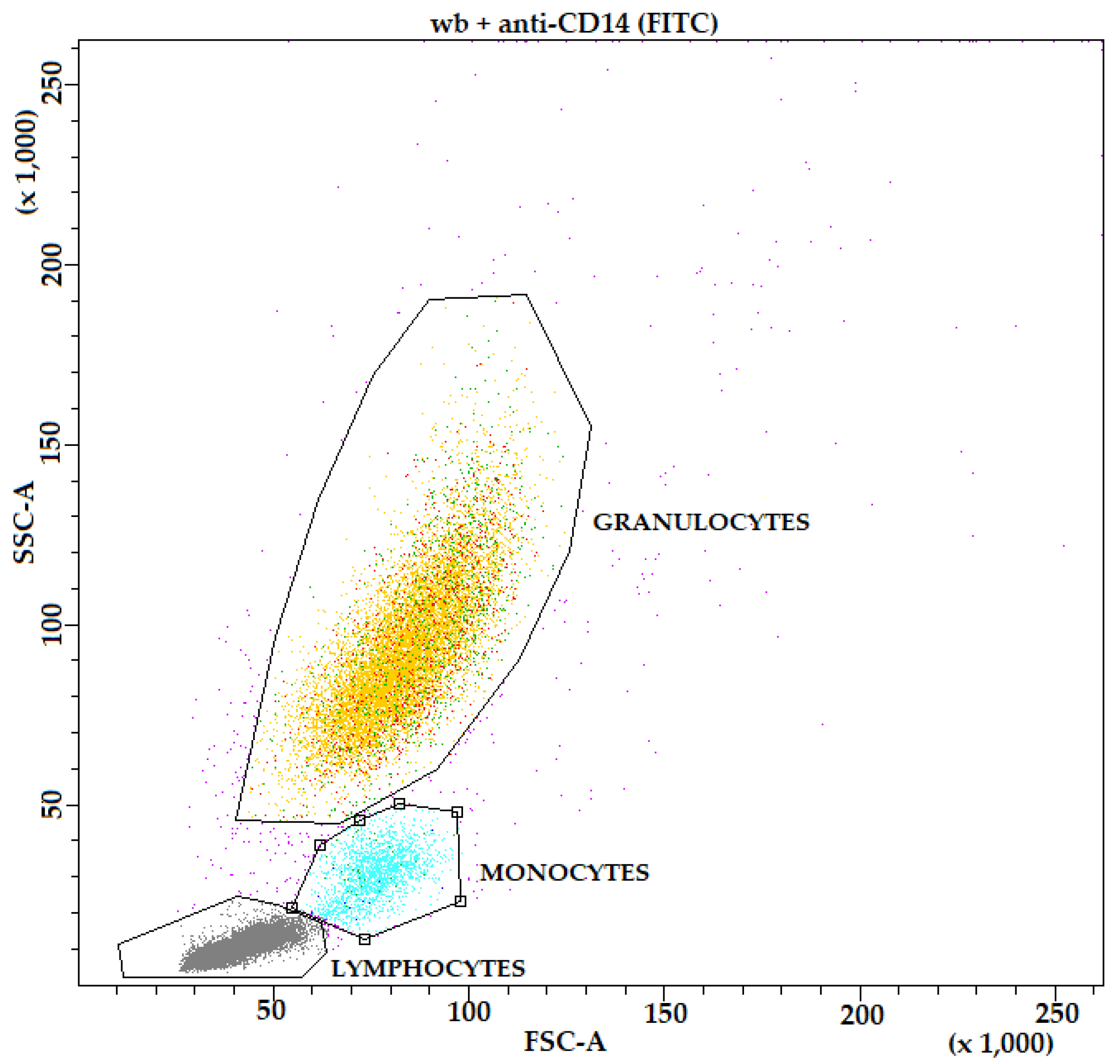
Analogous to CD163, samples were prepared in which CD14, CD33, CD204 or all of the monocytic surface proteins mentioned were blocked using specific antibodies (indirect phagocytosis-test No 2). These samples allowed an assessment of the dependence of HbMP-uptake on these receptors. Incubation-time with HbMP was 30 minutes. All samples were analyzed by flowcytometry: Leucocytes were identified by DNA-staining with propidium iodide (PI) or diamidino-phenylindole (DAPI). The closely spaced emission maxima of DAPI and FITC, or PI and APC, necessitated the use of different dyes for DNA staining, depending on which signal, FITC or APC was of interest in each sample. In each run, 2000 monocytes were analyzed. The mean fluorescence intensity (MFI) of the reference-sample, as well as its reduction in the remaining samples, provided information about the extent of phagocytosis in the pre-feeding step. For each experiment-run, blood from a different donor was used. Due to the donor’s variability (different cell-counts, immune-cell-activity, surface-receptor-density etc.), a relatively wide spread of MFI values between the single runs was observed, while relations between the different samples and their respective reference sample within one run were rather stable. Data were therefore normalized by calculating each sample’s MFI, relative to its reference within one run (expressed as percentages). Then the arithmetic-means over three experiment-runs were calculated from these normalized MFI-values.
Furthermore, to identify the monocyte population in FACS analysis, whole blood samples were each incubated with FITC-labeled mouse anti-human CD14 antibody (anti-CD14-wb) and APC-labeled mouse anti-human CD163 antibody (blocking antibody) (anti-CD163-wb), and a whole blood sample lysed and DNA-stained only was prepared for comparison (wb). In addition, a whole blood sample, which has been kept at 37°C for 120 min. (as the pre-fed samples were) was prepared to check for an influence of continued active cell metabolism on the MFI.
Table 1.
Samples for exclusion of confounders and identification of leucocyte-sub-populations.
Table 1.
Samples for exclusion of confounders and identification of leucocyte-sub-populations.
| Sample *
|
Incubation with |
Purpose |
| HbMP |
- |
HbMP autofluorescence |
| DAPI-HbMP |
DAPI |
DAPI-impact |
| PI-HbMP |
PI |
PI-impact |
| wb |
- |
wb/background-noise |
| incub-wb |
- |
Impact of incubation-time |
| DAPI-wb |
DAPI |
DAPI-impact |
| PI-wb |
PI |
PI-impact |
| anti-CD14-wb |
Anti-CD14, FITC |
Identify monocyte population |
| anti-CD163-wb |
Anti-CD163, APC |
Identify monocyte population |
Table 2.
Samples for indirect phagocytosis-test No 1, role of CD163.
Table 2.
Samples for indirect phagocytosis-test No 1, role of CD163.
| Sample 1
|
AB-use 2
|
pre-fed with |
|
Purpose |
| reference |
no |
no |
|
Max. MFI |
| EC-prefed-120’ 3
|
no |
E. coli, untagged |
|
Extent of E. coli-phagocytosis |
| CD163-block |
yes |
no |
|
AB-impact on FITC-E. coli phagocytosis |
| CD163-block+EC-prefed-120’ |
yes |
E. coli, untagged |
|
AB-impact on untagged E. coli phagocytosis |
| HbMP-prefed-30’ |
no |
HbMP |
|
Unhindered uptake of HbMP, 30 min. |
| HbMP-prefed-120’ |
no |
HbMP |
|
Unhindered uptake of HbMP, 120 min. |
| CD163-block+HbMP-prefed-30’ |
yes |
HbMP |
|
uptake of HbMP with blocked CD163, 30 min. |
| CD163-block+HbMP-prefed-120’ |
yes |
HbMP |
|
uptake of HbMP with blocked CD163, 120 min. |
Table 3.
Samples for indirect phagocytosis-test No 2, role of CD14, CD33, CD204.
Table 3.
Samples for indirect phagocytosis-test No 2, role of CD14, CD33, CD204.
| Sample 1
|
AB-use 2
|
pre-fed with |
|
Purpose |
| reference |
no |
no |
|
Max. MFI |
| HbMP-prefed-30’ |
no |
HbMP |
|
Unhindered uptake of HbMP, 30 min. |
| CD14-block |
yes |
no |
|
AB-impact on FITC-E. coli phagocytosis |
| CD33-block |
yes |
no |
|
AB-impact on FITC-E. coli phagocytosis |
| CD204-block |
yes |
no |
|
AB-impact on FITC-E. coli phagocytosis |
| CD14,33,163,204-block |
yes |
no |
|
AB-impact on FITC-E. coli phagocytosis |
| CD14-block+HbMP-prefed-30’ |
yes |
HbMP |
|
uptake of HbMP with blocked CD14, 30 min. |
| CD33-block+HbMP-prefed-30’ |
yes |
HbMP |
|
uptake of HbMP with blocked CD33, 30 min. |
| CD204-block+HbMP-prefed-30’ |
yes |
HbMP |
|
uptake of HbMP with blocked CD204, 30 min. |
| CD14, 33, 163, 204-block+HbMP-prefed-30’ |
yes |
HbMP |
|
uptake of HbMP with blockedCD14, 33, 163, 204, 30 min. |
4. Discussion
The indirect phagocytosis assays were carried out with whole blood in current studies. Monocytes are therefore less stressed and the entire test system can better simulate the in vivo situation. For the negative control samples, it can be assumed that keeping the samples on ice, effectively reduced the cell metabolism and no phagocytosis occurred in these samples. As expected, the reference-sample (
reference and
CD163-block, respectively) showed by far the highest MFI reading. This allowed the remaining values to be considered in their relation to this positive control. Blockade of any of the tested monocytic surface-antigens by antibody-binding (
CD14-block; CD33-block; CD163-block; CD204-block; all antibodies simultaneously CD14,33,163,204-block), had no inhibitory effect on the ability of monocytes to phagocytose FITC-labelled E. coli (
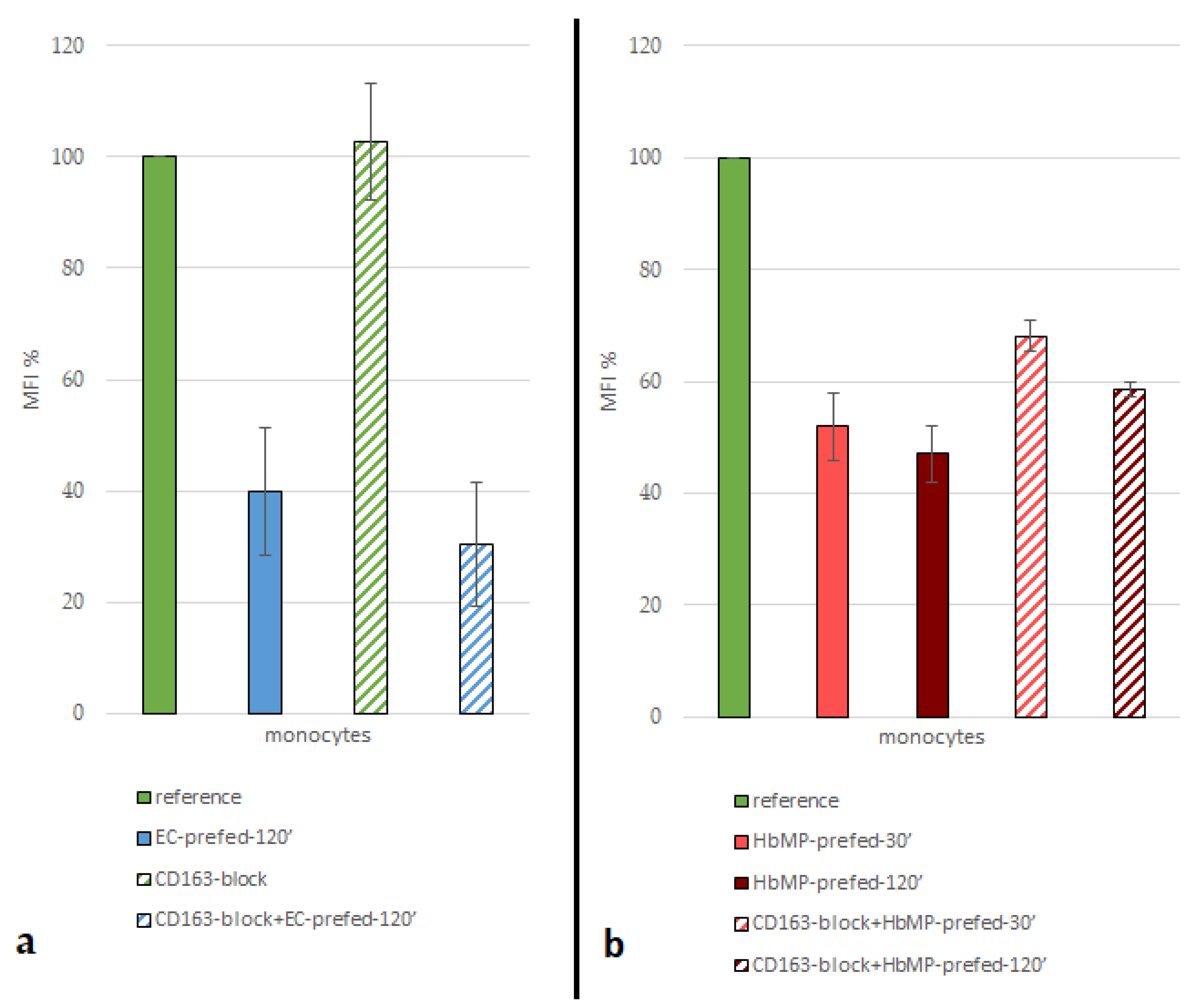
Figure 4). This observation confirmed the soundness of the indirect measurement method chosen to study the phagocytosis of HbMP and the role of CD163 in comparison with the other tested receptors in it. When monocytes with blocked CD163 were pre-fed with untagged E. coli lysate (
CD163-block+EC-prefed-120’), they took up non-significantly less FITC-labelled E. coli lysate compared with non CD163 blocked monocytes. It can be concluded from the data that antibody blockade of CD163 was in any case not an obstacle to phagocytosis of untagged E. coli in the incubation step in question. Pre-feeding of monocytes with HbMP, at an incubation time of only 30 minutes (
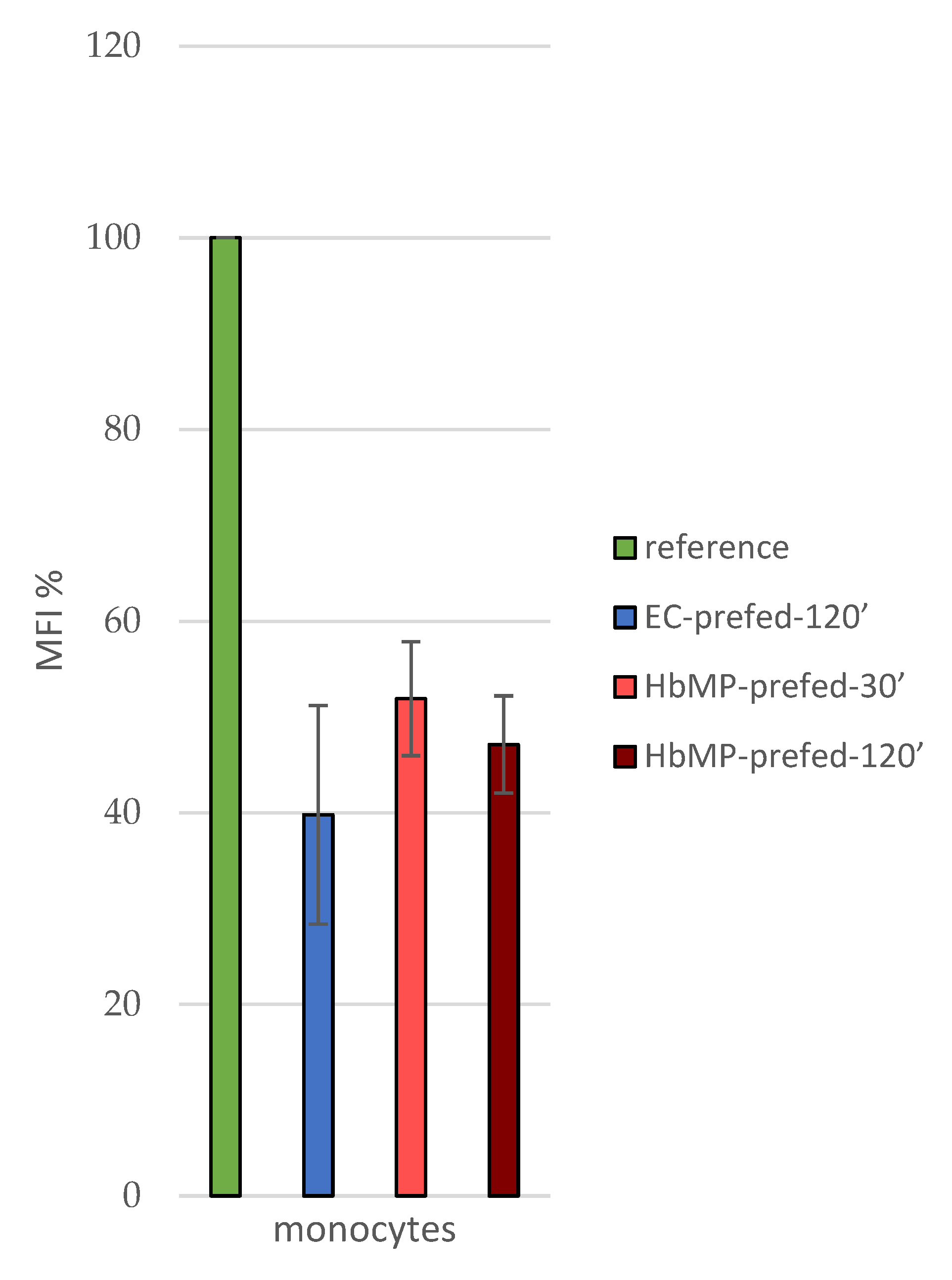
HbMP-prefed-30’), already caused a reduction of MFI to 50.7 ± 5.1% (mean over indirect phagocytosis-test No1 and 2) compared to the
reference sample. A longer incubation period of 120 minutes (
HbMP-prefed-120’) resulted in an MFI of 47.1 ± 5.1% (indirect phagocytosis-test No 1) of the reference-value (
Figure 5). This suggests that the Hb particles were taken up with great affinity by the monocytes; a large proportion already within the first 30 minutes of incubation of cells with HbMP.
Blockade of CD163 prior to incubation of the cells with HbMP, resulted in an increased MFI after feeding with FITC-labeled E. coli (
CD163-block+HbMP-prefed-120’ vs.
HbMP-prefed-120’) (
Figure 2; Table 4). It can be concluded, that less HbMP have been taken up by monocytes in pre-feeding due to the Blockade of CD163, with a maximum reduction of uptake of 31.3 ± 10.4%. Thus, CD163 does appear to play an important role in the phagocytosis of our HbMP by monocytes. These results are also in line with previous research. In several other studies, using various other HBOCs, a direct Hb-CD163 interaction was observed [
5,
27,
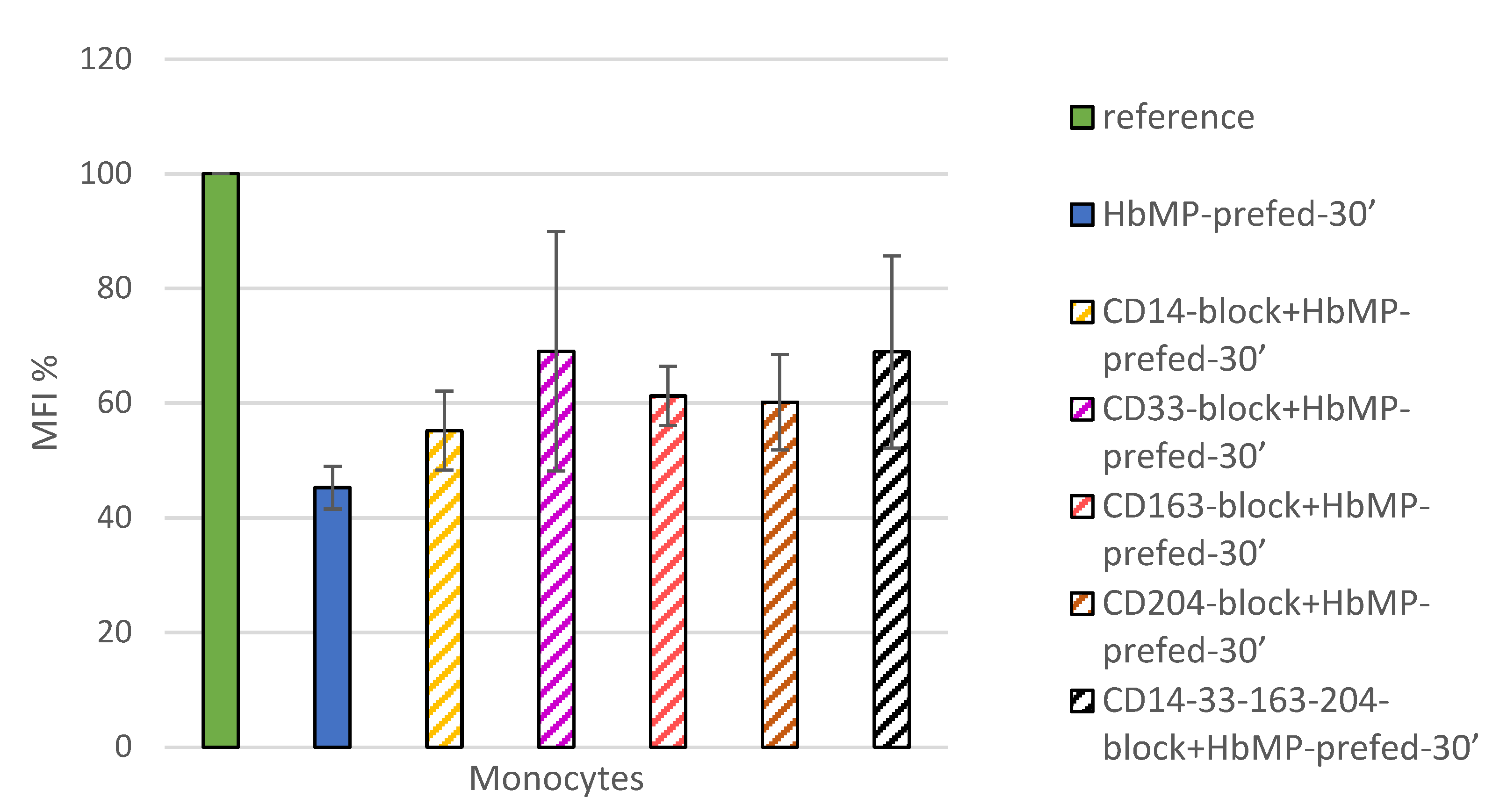
38]. However, the blockade of CD163 could not completely inhibit the uptake of HbMP, which suggests the existence of at least one further mechanism for HbMP-phagocytosis. When screening for possible other players in the monocytic uptake of HbMP, we observed, that CD14, CD33 and CD204 all showed an effect on phagocytic activity, when blocked by antibodies, that ranged from 10% (
CD14-block+HbMP-prfed-30’) to 24% (
CD33-block+HbMP-prefed-30’) higher HbMP uptake, compared to unhindered HbMP uptake (
HbMP-prefed-30’) (
Figure 3). However, the observed effects seem to not cumulate when all tested Antigens were blocked all at once (CD14,33,163,204-block+HbMP-prefed-30’) . Overall, while each one of the receptors we tested thus far seem to contribute in some way to the phagocytosis of HbMP, neither one of them alone provides a satisfactory explanation for the full extent of this process. The overall extent of phagocytosis of HbMP appears to be slightly lower to that of untagged E. coli lysate, as
Figure 5 shows. Since whole blood samples were used for the phagocytosis test, all physiologically available plasma proteins were always present. It could be hypothesized that one of these might show an affinity for Hb and triggers uptake by monocytes. One possibility here would be hemopexin (Hpx), which physiologically binds free heme [
39] but showed Hb affinity in a case-study described by Drieghe et al [
29]. The receptor of the Hpx-heme complex’, CD91 is expressed by numerous cell types including hepatocytes as well as monocytes. It is also conceivable that as CD163, as a receptor of the Hp-Hb complex’ shows affinity for Hb, analogously CD91 could also show similar behavior, with a possible binding site for the receptor within Hb being unknown. In their work from 2013, Yan et al. [
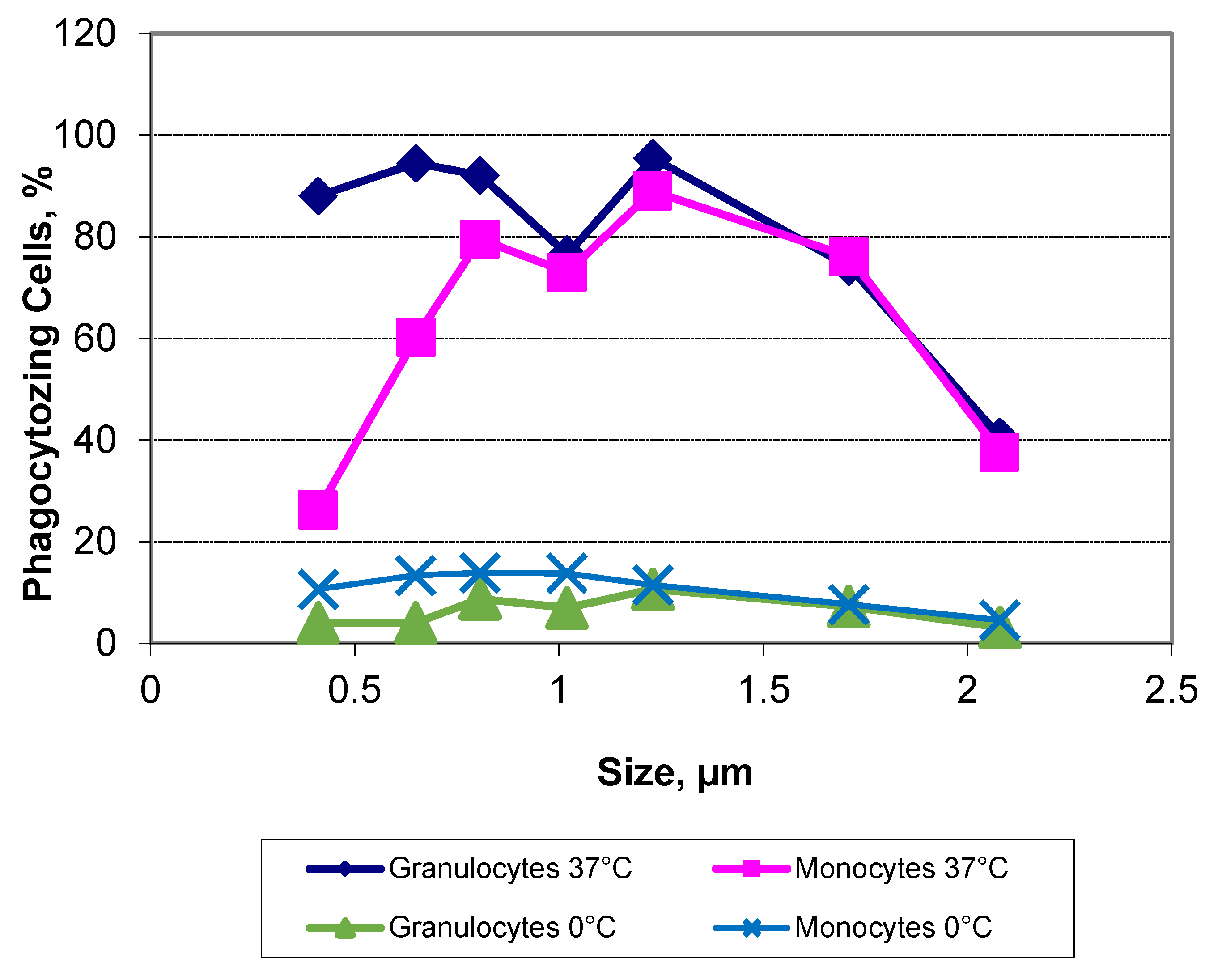
40] showed how the formation of a protein corona influences particle-cell interaction. Especially bovine serum albumin (BSA) showed an ambivalent effect: On the one hand, the corona, which consisted mainly of BSA, reduced the direct cell surface adhesion of the test particles, on the other hand, binding to the particles caused a conformational change within the structure of the BSA. This denatured BSA promoted binding by scavenger receptor A (SR-A/CD204) and thus induced internalization of the protein-particle-receptor complex. In the case of our HbMP containing human serum albumin, we assume that a conformational change within the HSA might occur when the molecules are cross-linked by glutaraldehyde. Detection of this denatured protein by receptors of phagocytic cells would also be conceivable in this case to explain CD204’s part in the uptake of HbMP. Further research on this topic will be conducted in the future. To be considered as well is a possible mechanism leading to particle uptake by phagocytes, based less on specific recognition of Hb, rather than on other recognition properties. There exists a fundamental relationship between particle size and their phagocytosis. Our own results with PMMA-FluoroGreen-COOH particles (microparticles GmbH, Berlin, Germany), diameter between 0.4 and 2.1 µm demonstrate this relationship (Incubation of heparin blood with 2 x 10
8 MP/mL) (
Figure 6,
Figure 7) [
41].
Champion et al. researched this in a model as well [
42]. Test particles (polystyrene microspheres) in the range of 3µm of diameter were phagocytosed significantly more than both smaller and larger particles in this experiment. According to the authors, this is based on the number of possible contact points with the respective cell, depending on the morphology of the cell surface. In addition, the particle charge, i.e., the hydrophilicity/-phobia, also played a role. This together with the particle composition might be a possible explanation for the difference in the results of Champion et al. and our own. Furthermore size dependence also probably exists in antibody-induced phagocytosis, as Montel et al. were able to show [
43]. Firstly, however, its remains unclear whether or not the various mechanisms that may be at play follow a hierarchy. Second, what processes might be responsible for the overall extent of phagocytosis and in what period of time they take place, and third, whether the processes take place in parallel. Other receptors and ways of particle-recognition might affect the affinity with which monocytes take up HbMP. Another mechanism that has received little attention in research to date could be the opsonization of particles by complement factors. Especially factor C3b, which can arise spontaneously from C3 by hydrolysis, could be considered here. Moghimi et al. report numerous possible interactions of the complement system with, among others, nanoparticles as a 'drug delivery system' [
44]. The authors also discussed the possible effects of forming water shells and emerging hydrogen bonds, altered surface structures and possible interactions between proteins and particles and in particular also polymers, such as HbMP are [
45,
46]. This adsorption of various Plasma-Proteins seems to not only enhance the chance of complement-activation but also to promote phagocytosis directly, as Zhang describes [
47]. Lück et al. showed by two-dimensional electrophoresis that upon serum incubation, protein accumulation occurred on latex particles with an average size of 660nm that led to complement activation [
48]. At this time, it is unclear, whether that mechanism could also be playing a role for HbMP-sequestration, as the HSA on our particle’s surface has shown to attenuate the adsorption of plasma-proteins [
19].
Kloypan et al. however, observed granulocytic uptake of albumin particles in an indirect phagocytosis assay [
22]. An observation, that was repeatedly made again in the present study. Thus, it appears that Hb is not necessarily the key factor that triggers uptake into cells and, in any case, CD163 does not appear to be the single key protein for this either. Xiong et al. observed in a rat-model, after intravenous injection of a HBOC solution, agglomeration of HBOC in the liver of the test-animal became visible after a short while in the MRI-scan. While the authors hypothesized that the HBOC was taken up by CD163-expressing Kupffer-cells / Macrophages, Chow et al. reported that when isolated rat livers were perfused with a HBOC solution, hepatocytes also took up abundant hemin, as determined by heme oxygenase-1 expression [
25]. Goldfischer et al. observed immunohistochemically the presence of Hb in phagocytic but also hepatocytic lysosomes [
49]. Hepatocytes, however, do not express CD163 and therefore must have a currently still unknown mechanism, potentially for Hb recognition, but surely for HBOC-uptake. Future research will have to show whether this mechanism might also be responsible for a part of the uptake of HBOC in monocytes and macrophages. Anyhow, with this study, the role of CD163 in the phagocytosis of hemins by monocytes, could be confirmed also for our HbMP, a GA-polymerized bovine Hb-sub-micron-particle, coated in HSA. Moreover, our data suggest the involvement of other monocytic surface proteins in this process as well.
Author Contributions
Conceptualization, Hans Bäumler and Jonathan Nimz; methodology, Jonathan Nimz, Yu Xiong, Pichayut Rerkshanandana, Chiraphat Kloypan, Saranya Chaiwaree and Radostina Georgieva; validation, Hans Bäumler, Radostina Georgieva and Yu Xiong; formal analysis, Jonathan Nimz, Ullrich Kalus, Saranya Chaiwaree, Pichayut Rerkshanandana; investigation Jonathan Nimz, Pichayut Rerkshanandana and Chiraphat Kloypan; writing—original draft preparation, Jonathan Nimz; writing—review and editing, Hans Bäumler, Radostina Georgieva, Yu Xiong and Jonathan Nimz; visualization, Jonathan Nimz, Radostina Georgieva and Ulrich Kalus; supervision, Hans Bäumler, Radostina Georgieva and Axel Pruß; project administration, Hans Bäumler; funding acquisition, Hans Bäumler. All authors have read and agreed to the published version of the manuscript.